B7H3 in Gastrointestinal Tumors: Role in Immune Modulation and Cancer Progression: A Review of the Literature
Abstract
1. Introduction
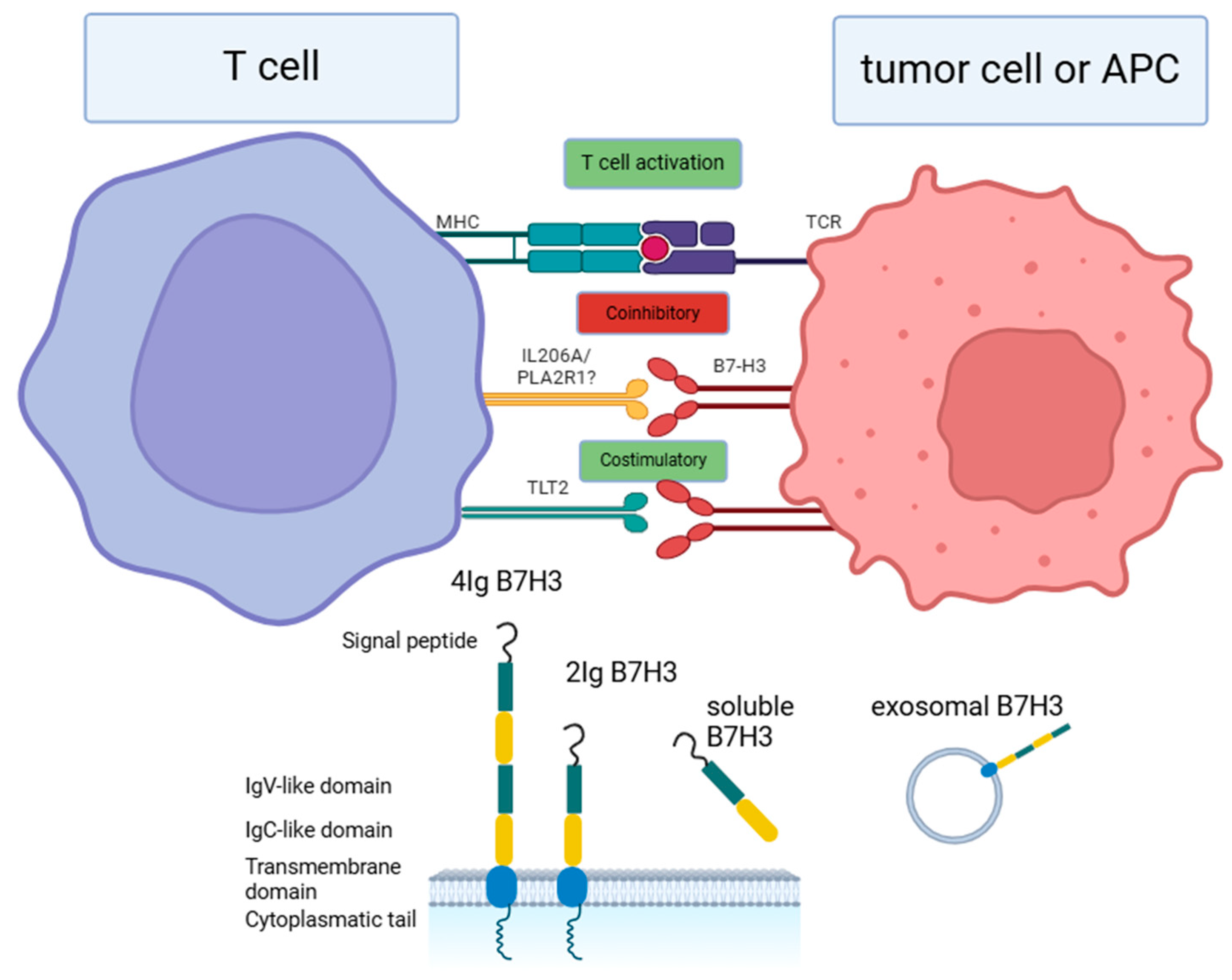
2. Receptors for B7-H3
3. Signaling Pathways
3.1. JAK2/STAT3 Pathway
3.2. TLR4/NF-κB Pathway
3.3. PI3K/AKT Pathway
3.4. Ras/Raf/MEK/MAPK
3.5. NRF2/ROS
4. Immune Functions of B7-H3 in Gastrointestinal Tumors
4.1. B7-H3 in the Regulation of TILs and Cytokine Secretion
4.2. B7-H3 in the Regulation of Other Immune Cells
4.3. Other Immune Processes Regulated by B7-H3
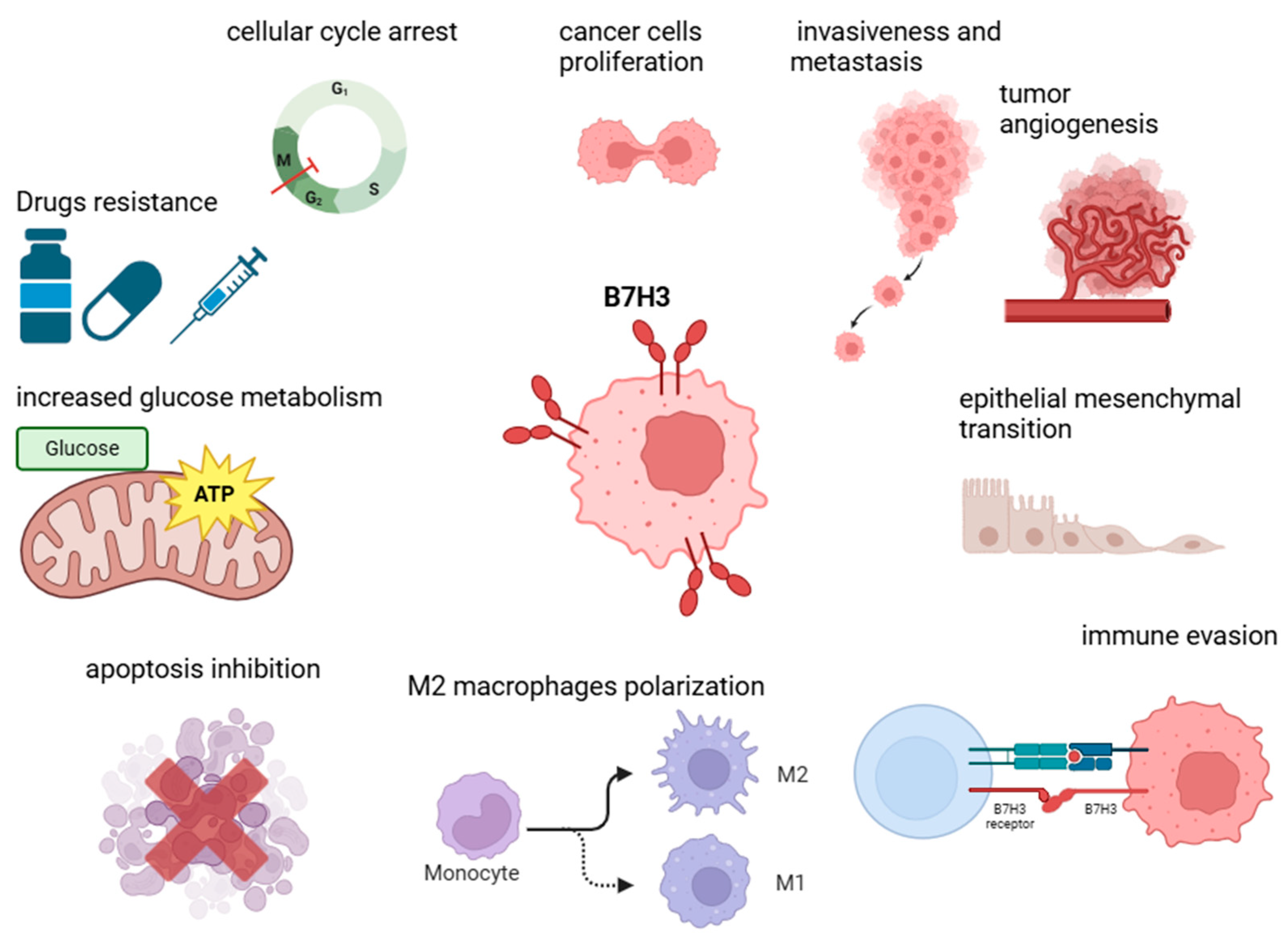
5. Non-Immune Functions of B7-H3 in Tumorigenesis
5.1. Proliferation, Invasiveness, Migration, and Epithelial–Mesenchymal Transition (EMT)
5.2. Metabolism and Angiogenesis Regulation
5.3. Apoptosis Inhibition
6. B7-H3 in Gastrointestinal Tumors
6.1. Colorectal Cancer (CRC)
6.1.1. B7-H3 Expression in CRC
6.1.2. B7-H3 Influence on Clinicopathological Characteristics, Immune Responses, and Tumorigenesis in CRC
6.1.3. Possible Therapeutic Approaches Involving B7-H3
6.1.4. The Clinical Significance of ICIs for CRC Treatment
6.2. Esophageal Cancer
6.2.1. B7-H3 Expression in EC
6.2.2. Influence on Immunity and Therapeutic Options
6.3. Gastric Cancer
6.3.1. B7-H3 Expression in GC
6.3.2. B7-H3 Influence on Immune Responses in GC
6.3.3. Potential Therapeutic Options
6.4. Hepatocellular Carcinoma and Cholangiocarcinoma
6.4.1. B7-H3 Expression in Hepatocellular Carcinoma and Cholangiocarcinoma
6.4.2. Effect on Immunity and TME
6.5. Pancreatic Cancer
6.5.1. B7-H3 Expression in Pancreatic Cancer
6.5.2. B7-H3 Influence on Prognosis and Immune Responses in Pancreatic Cancer
6.5.3. Regulation of B7-H3 Expression in Pancreatic Tumors and Potential Therapeutic Strategies
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Getu, A.A.; Tigabu, A.; Zhou, M.; Lu, J.; Fodstad, Ø.; Tan, M. New Frontiers in Immune Checkpoint B7-H3 (CD276) Research and Drug Development. Mol. Cancer 2023, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.M. The Immune Checkpoint Inhibitors: Where Are We Now? Nat. Rev. Drug Discov. 2014, 13, 883–884. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune Checkpoint Signaling and Cancer Immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tian, S.; Lin, Z.; Fu, Z.; Li, C. Costimulatory and Coinhibitory Molecules of B7-CD28 Family in Cardiovascular Atherosclerosis: A Review. Medicine 2022, 101, e31667. [Google Scholar] [CrossRef]
- Bolandi, N.; Derakhshani, A.; Hemmat, N.; Baghbanzadeh, A.; Asadzadeh, Z.; Afrashteh Nour, M.; Brunetti, O.; Bernardini, R.; Silvestris, N.; Baradaran, B. The Positive and Negative Immunoregulatory Role of B7 Family: Promising Novel Targets in Gastric Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 10719. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and Hot Tumors: From Molecular Mechanisms to Targeted Therapy. Sig Transduct. Target. Ther. 2024, 9, 1–65. [Google Scholar] [CrossRef]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A Costimulatory Molecule for T Cell Activation and IFN-γ Production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef]
- Sun, M.; Richards, S.; Prasad, D.V.R.; Mai, X.M.; Rudensky, A.; Dong, C. Characterization of Mouse and Human B7-H3 Genes1. J. Immunol. 2002, 168, 6294–6297. [Google Scholar] [CrossRef]
- Zhou, Y.-H.; Chen, Y.-J.; Ma, Z.-Y.; Xu, L.; Wang, Q.; Zhang, G.-B.; Xie, F.; Ge, Y.; Wang, X.-F.; Zhang, X.-G. 4IgB7-H3 Is the Major Isoform Expressed on Immunocytes as Well as Malignant Cells. Tissue Antigens 2007, 70, 96–104. [Google Scholar] [CrossRef]
- Vigdorovich, V.; Ramagopal, U.A.; Lázár-Molnár, E.; Sylvestre, E.; Lee, J.S.; Hofmeyer, K.A.; Zang, X.; Nathenson, S.G.; Almo, S.C. Structure and T Cell Inhibition Properties of B7 Family Member, B7-H3. Structure 2013, 21, 707–717. [Google Scholar] [CrossRef]
- Kovaleva, O.V.; Gratchev, A.N.; Sokolov, N.Y.; Maslennikov, V.V.; Kuzmin, Y.B.; Gershtein, E.S.; Alferov, A.A.; Mamedli, Z.Z.; Stilidi, I.S.; Kushlinskii, N.E. Soluble B7-H3 in Colorectal Cancer. Bull. Exp. Biol. Med. 2023, 176, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, M.; Kobori, H.; Ritprajak, P.; Kamimura, Y.; Kozono, H.; Azuma, M. Triggering Receptor Expressed on Myeloid Cell-like Transcript 2 (TLT-2) Is a Counter-Receptor for B7-H3 and Enhances T Cell Responses. Proc. Natl. Acad. Sci. USA 2008, 105, 10495–10500. [Google Scholar] [CrossRef] [PubMed]
- Koumprentziotis, I.-A.; Theocharopoulos, C.; Foteinou, D.; Angeli, E.; Anastasopoulou, A.; Gogas, H.; Ziogas, D.C. New Emerging Targets in Cancer Immunotherapy: The Role of B7-H3. Vaccines 2024, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Husain, B.; Ramani, S.R.; Chiang, E.; Lehoux, I.; Paduchuri, S.; Arena, T.A.; Patel, A.; Wilson, B.; Chan, P.; Franke, Y.; et al. A Platform for Extracellular Interactome Discovery Identifies Novel Functional Binding Partners for the Immune Receptors B7-H3/CD276 and PVR/CD155. Mol. Cell Proteom. 2019, 18, 2310–2323. [Google Scholar] [CrossRef]
- Flem-Karlsen, K.; Fodstad, Ø.; Tan, M.; Nunes-Xavier, C.E. B7-H3 in Cancer—Beyond Immune Regulation. Trends Cancer 2018, 4, 401–404. [Google Scholar] [CrossRef]
- Ingebrigtsen, V.A.; Boye, K.; Tekle, C.; Nesland, J.M.; Flatmark, K.; Fodstad, Ø. B7-H3 Expression in Colorectal Cancer: Nuclear Localization Strongly Predicts Poor Outcome in Colon Cancer. Int. J. Cancer 2012, 131, 2528–2536. [Google Scholar] [CrossRef]
- Zhao, B.; Li, H.; Xia, Y.; Wang, Y.; Wang, Y.; Shi, Y.; Xing, H.; Qu, T.; Wang, Y.; Ma, W. Immune Checkpoint of B7-H3 in Cancer: From Immunology to Clinical Immunotherapy. J. Hematol. Oncol. 2022, 15, 153. [Google Scholar] [CrossRef]
- Park, R.; Yu, J.; Shahzad, M.; Lee, S.; Ji, J.D. The Immune Regulatory Function of B7-H3 in Malignancy: Spotlight on the IFN-STAT1 Axis and Regulation of Tumor-Associated Macrophages. Immunol. Res. 2024, 72, 526–537. [Google Scholar] [CrossRef]
- Sukocheva, O.; Menschikowski, M.; Hagelgans, A.; Yarla, N.S.; Siegert, G.; Reddanna, P.; Bishayee, A. Current Insights into Functions of Phospholipase A2 Receptor in Normal and Cancer Cells: More Questions than Answers. Semin. Cancer Biol. 2019, 56, 116–127. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, M.; Zhao, A.; Shi, T.; Xi, Q. B7-H3 Regulates Anti-Tumor Immunity and Promotes Tumor Development in Colorectal Cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2024, 1879, 189031. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, G.; Li, H.; Yang, S.; Xu, Y.; Pan, B.; Lai, W.; Chen, G.; Liao, W.; Zhang, X. B7-H3 Promotes Nasopharyngeal Carcinoma Progression by Regulating CD8+ T Cell Exhaustion. Immun. Inflamm. Dis. 2024, 12, e70005. [Google Scholar] [CrossRef] [PubMed]
- Kobori, H.; Hashiguchi, M.; Piao, J.; Kato, M.; Ritprajak, P.; Azuma, M. Enhancement of Effector CD8+ T-Cell Function by Tumour-Associated B7-H3 and Modulation of Its Counter-Receptor Triggering Receptor Expressed on Myeloid Cell-like Transcript 2 at Tumour Sites. Immunology 2010, 130, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, C.; Xiang, Y.; Hong, Z.; He, D.; Zhong, H.; Liu, Y.; Wu, Y.; Zheng, X.; Yin, H.; et al. TLT2 Suppresses Th1 Response by Promoting IL-6 Production in Monocyte Through JAK/STAT3 Signal Pathway in Tuberculosis. Front. Immunol. 2020, 11, 2031. [Google Scholar] [CrossRef]
- Yan, R.; Yang, S.; Gu, A.; Zhan, F.; He, C.; Qin, C.; Zhang, X.; Feng, P. Murine B7-H3 Is a Co-Stimulatory Molecule for T Cell Activation. Monoclon. Antib. Immunodiagn. Immunother. 2013, 32, 395–398. [Google Scholar] [CrossRef]
- Leitner, J.; Klauser, C.; Pickl, W.F.; Stöckl, J.; Majdic, O.; Bardet, A.F.; Kreil, D.P.; Dong, C.; Yamazaki, T.; Zlabinger, G.; et al. B7-H3 Is a Potent Inhibitor of Human T-Cell Activation: No Evidence for B7-H3 and TREML2 Interaction. Eur. J. Immunol. 2009, 39, 1754–1764. [Google Scholar] [CrossRef]
- Augert, A.; Payré, C.; de Launoit, Y.; Gil, J.; Lambeau, G.; Bernard, D. The M-Type Receptor PLA2R Regulates Senescence through the P53 Pathway. EMBO Rep. 2009, 10, 271–277. [Google Scholar] [CrossRef]
- Griveau, A.; Devailly, G.; Eberst, L.; Navaratnam, N.; Le Calvé, B.; Ferrand, M.; Faull, P.; Augert, A.; Dante, R.; Vanacker, J.M.; et al. The PLA2R1-JAK2 Pathway Upregulates ERRα and Its Mitochondrial Program to Exert Tumor-Suppressive Action. Oncogene 2016, 35, 5033–5042. [Google Scholar] [CrossRef]
- Cao, S.; Peterson, S.M.; Müller, S.; Reichelt, M.; McRoberts Amador, C.; Martinez-Martin, N. A Membrane Protein Display Platform for Receptor Interactome Discovery. Proc. Natl. Acad. Sci. USA 2021, 118, e2025451118. [Google Scholar] [CrossRef]
- Rutz, S.; Wang, X.; Ouyang, W. The IL-20 Subfamily of Cytokines—From Host Defence to Tissue Homeostasis. Nat. Rev. Immunol. 2014, 14, 783–795. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Ji, W.-D.; Jiao, H.-M.; Lu, A.; Chen, K.-F.; Liu, Q.-B. Targeting 4-1BB for Tumor Immunotherapy from Bench to Bedside. Front. Immunol. 2022, 13, 975926. [Google Scholar] [CrossRef]
- Goodwin, R.G.; Din, W.S.; Davis-Smith, T.; Anderson, D.M.; Gimpel, S.D.; Sato, T.A.; Maliszewski, C.R.; Brannan, C.I.; Copeland, N.G.; Jenkins, N.A.; et al. Molecular Cloning of a Ligand for the Inducible T Cell Gene 4-1BB: A Member of an Emerging Family of Cytokines with Homology to Tumor Necrosis Factor. Eur. J. Immunol. 1993, 23, 2631–2641. [Google Scholar] [CrossRef]
- Hu, J.; Qiu, J.; Zheng, Y.; Zhang, T.; Yin, T.; Xie, X.; Wang, G. AAMP Regulates Endothelial Cell Migration and Angiogenesis Through RhoA/Rho Kinase Signaling. Ann. Biomed. Eng. 2016, 44, 1462–1474. [Google Scholar] [CrossRef]
- Yao, S.; Shi, F.; Mu, N.; Li, X.; Ma, G.; Wang, Y.; Sun, X.; Liu, X.; Su, L. Angio-Associated Migratory Cell Protein (AAMP) Interacts with Cell Division Cycle 42 (CDC42) and Enhances Migration and Invasion in Human Non-Small Cell Lung Cancer Cells. Cancer Lett. 2021, 502, 1–8. [Google Scholar] [CrossRef]
- Ciprut, S.; Berberich, A.; Knoll, M.; Pusch, S.; Hoffmann, D.; Furkel, J.; Ward Gahlawat, A.; Kahlert-Konzelamnn, L.; Sahm, F.; Warnken, U.; et al. AAMP Is a Binding Partner of Costimulatory Human B7-H3. Neurooncol. Adv. 2022, 4, vdac098. [Google Scholar] [CrossRef]
- Wang, R.; Ma, Y.; Zhan, S.; Zhang, G.; Cao, L.; Zhang, X.; Shi, T.; Chen, W. B7-H3 Promotes Colorectal Cancer Angiogenesis through Activating the NF-κB Pathway to Induce VEGFA Expression. Cell Death Dis. 2020, 11, 55. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, B.; Zou, S.-T.; Liu, F.; Hua, D. Overexpression of B7-H3 Augments Anti-Apoptosis of Colorectal Cancer Cells by Jak2-STAT3. World J. Gastroenterol. 2015, 21, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ouyang, S.; Li, J.; Huang, X.; Ai, X.; Zeng, Y.; Lv, Y.; Cai, M. The Novel Non-Immunological Role and Underlying Mechanisms of B7-H3 in Tumorigenesis. J. Cell. Physiol. 2019, 234, 21785–21795. [Google Scholar] [CrossRef]
- Mengie Ayele, T.; Tilahun Muche, Z.; Behaile Teklemariam, A.; Bogale Kassie, A.; Chekol Abebe, E. Role of JAK2/STAT3 Signaling Pathway in the Tumorigenesis, Chemotherapy Resistance, and Treatment of Solid Tumors: A Systemic Review. J. Inflamm. Res. 2022, 15, 1349–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, L.; Han, S.; Li, Y.; Qian, Q.; Zhang, Q.; Zhang, H.; Yang, Z.; Zhang, Y. B7-H3 Is Related to Tumor Progression in Ovarian Cancer. Oncol. Rep. 2017, 38, 2426–2434. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, T.; Zou, S.; Jiang, B.; Hua, D. B7-H3 Promotes Cell Migration and Invasion through the Jak2/Stat3/MMP9 Signaling Pathway in Colorectal Cancer. Mol. Med. Rep. 2015, 12, 5455–5460. [Google Scholar] [CrossRef]
- Liu, H.; Tekle, C.; Chen, Y.-W.; Kristian, A.; Zhao, Y.; Zhou, M.; Liu, Z.; Ding, Y.; Wang, B.; Mælandsmo, G.M.; et al. B7-H3 Silencing Increases Paclitaxel Sensitivity by Abrogating Jak2/Stat3 Phosphorylation. Mol. Cancer Ther. 2011, 10, 960–971. [Google Scholar] [CrossRef] [PubMed]
- ZHAO, X.; ZHANG, G.-B.; GAN, W.-J.; XIONG, F.; LI, Z.; ZHAO, H.; ZHU, D.-M.; ZHANG, B.; ZHANG, X.-G.; LI, D.-C. Silencing of B7-H3 Increases Gemcitabine Sensitivity by Promoting Apoptosis in Pancreatic Carcinoma. Oncol. Lett. 2013, 5, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Tao, B.; Chen, Y.; Guo, Z.; Yang, X.; Peng, L.; Xia, X.; Chen, L. B7-H3 Regulates Glioma Growth and Cell Invasion Through a JAK2/STAT3/Slug-Dependent Signaling Pathway. Oncol. Targets Ther. 2020, 13, 2215–2224. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Wu, Y.; Zhao, K.; Ye, Z.; Zhu, J.; Xu, X.; Zhao, X.; Xing, C. B7-H3 Promotes Gastric Cancer Cell Migration and Invasion. Oncotarget 2017, 8, 71725–71735. [Google Scholar] [CrossRef]
- Fan, T.-F.; Deng, W.-W.; Bu, L.-L.; Wu, T.-F.; Zhang, W.-F.; Sun, Z.-J. B7-H3 Regulates Migration and Invasion in Salivary Gland Adenoid Cystic Carcinoma via the JAK2/STAT3 Signaling Pathway. Am. J. Transl. Res. 2017, 9, 1369–1380. [Google Scholar]
- Zhao, J.; Meng, Z.; Xie, C.; Yang, C.; Liu, Z.; Wu, S.; Wang, B.; Fan, P.; Jin, X.; Wu, H. B7-H3 Is Regulated by BRD4 and Promotes TLR4 Expression in Pancreatic Ductal Adenocarcinoma. Int. J. Biochem. Cell Biol. 2019, 108, 84–91. [Google Scholar] [CrossRef]
- Napetschnig, J.; Wu, H. Molecular Basis of NF-κB Signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, H.; Gao, X.; Yu, G.; Zhang, X.; Jin, H.; Xu, R.; Wang, Z.; Zhang, G. High Expression of B7-H3 on Monocyte/Macrophages in Tumor Microenvironment Promotes Lung Cancer Progression by Inhibiting Apoptosis. Transl. Oncol. 2024, 41, 101874. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, D.; Chen, Q.; Yang, C.; Wang, B.; Wu, H. Soluble B7-H3 Promotes the Invasion and Metastasis of Pancreatic Carcinoma Cells through the TLR4/NF-κB Pathway. Sci. Rep. 2016, 6, 27528. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, T.; Liu, F.; Sun, Z.; Shi, H.; Hua, D.; Yang, C. The Co-Stimulatory Molecule B7-H3 Promotes the Epithelial-Mesenchymal Transition in Colorectal Cancer. Oncotarget 2016, 7, 31755–31771. [Google Scholar] [CrossRef]
- Li, Y.; Guo, G.; Song, J.; Cai, Z.; Yang, J.; Chen, Z.; Wang, Y.; Huang, Y.; Gao, Q. B7-H3 Promotes the Migration and Invasion of Human Bladder Cancer Cells via the PI3K/Akt/STAT3 Signaling Pathway. J. Cancer 2017, 8, 816–824. [Google Scholar] [CrossRef]
- Qi, M.; Elion, E.A. MAP Kinase Pathways. J. Cell Sci. 2005, 118, 3569–3572. [Google Scholar] [CrossRef] [PubMed]
- Flem-Karlsen, K.; Tekle, C.; Øyjord, T.; Flørenes, V.A.; Mælandsmo, G.M.; Fodstad, Ø.; Nunes-Xavier, C.E. P38 MAPK Activation through B7-H3-Mediated DUSP10 Repression Promotes Chemoresistance. Sci. Rep. 2019, 9, 5839. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Phillips, J.B.; Arora, R.; McClellan, S.; Li, J.; Kim, J.-H.; Sobol, R.W.; Tan, M. Immunoregulatory Protein B7-H3 Regulates Cancer Stem Cell Enrichment and Drug Resistance through MVP-Mediated MEK Activation. Oncogene 2019, 38, 88–102. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Chen, J.; Liu, X.-M.; Zhao, R.; Zhe, H. Nrf2-Mediated Metabolic Reprogramming in Cancer. Oxidative Med. Cell. Longev. 2018, 2018, 9304091. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.; Chen, L.; Qian, Z.; Zhang, Y. A Promising Target for Breast Cancer: B7-H3. BMC Cancer 2024, 24, 182. [Google Scholar] [CrossRef]
- Inamura, K.; Amori, G.; Yuasa, T.; Yamamoto, S.; Yonese, J.; Ishikawa, Y. Relationship of B7-H3 Expression in Tumor Cells and Tumor Vasculature with FOXP3+ Regulatory T Cells in Renal Cell Carcinoma. CMAR 2019, 11, 7021–7030. [Google Scholar] [CrossRef]
- Yim, J.; Koh, J.; Kim, S.; Song, S.G.; Ahn, H.K.; Kim, Y.A.; Jeon, Y.K.; Chung, D.H. Effects of B7-H3 Expression on Tumour-Infiltrating Immune Cells and Clinicopathological Characteristics in Non–Small-Cell Lung Cancer. Eur. J. Cancer 2020, 133, 74–85. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Jia, L.; Kim, J.k.; Li, J.; Deng, P.; Zhang, W.; Krebsbach, P.H.; Wang, C.-Y. CD276 Expression Enables Squamous Cell Carcinoma Stem Cells to Evade Immune Surveillance. Cell Stem Cell 2021, 28, 1597–1613.e7. [Google Scholar] [CrossRef]
- Miyamoto, T.; Murakami, R.; Hamanishi, J.; Tanigaki, K.; Hosoe, Y.; Mise, N.; Takamatsu, S.; Mise, Y.; Ukita, M.; Taki, M.; et al. B7-H3 Suppresses Antitumor Immunity via the CCL2–CCR2–M2 Macrophage Axis and Contributes to Ovarian Cancer Progression. Cancer Immunol. Res. 2022, 10, 56–69. [Google Scholar] [CrossRef]
- Ouyang, P.; Wang, L.; Wu, J.; Tian, Y.; Chen, C.; Li, D.; Yao, Z.; Chen, R.; Xiang, G.; Gong, J.; et al. Overcoming Cold Tumors: A Combination Strategy of Immune Checkpoint Inhibitors. Front. Immunol. 2024, 15, 1344272. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, D.; Saw, P.E.; Song, E. Turning Cold Tumors Hot: From Molecular Mechanisms to Clinical Applications. Trends Immunol. 2022, 43, 523–545. [Google Scholar] [CrossRef]
- Miller, C.D.; Lozada, J.R.; Zorko, N.A.; Elliott, A.; Makovec, A.; Radovich, M.; Heath, E.I.; Agarwal, N.; Mckay, R.R.; Garje, R.; et al. Pan-Cancer Interrogation of B7-H3 (CD276) as an Actionable Therapeutic Target Across Human Malignancies. Cancer Res. Commun. 2024, 4, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-J.; Du, H.; Khabibullin, D.; Zarei, M.; Wei, K.; Freeman, G.J.; Kwiatkowski, D.J.; Henske, E.P. mTORC1 Upregulates B7-H3/CD276 to Inhibit Antitumor T Cells and Drive Tumor Immune Evasion. Nat. Commun. 2023, 14, 1214. [Google Scholar] [CrossRef]
- Shen, B.; Mei, J.; Xu, R.; Cai, Y.; Wan, M.; Zhou, J.; Ding, J.; Zhu, Y. B7-H3 Is Associated with the Armored-Cold Phenotype and Predicts Poor Immune Checkpoint Blockade Response in Melanoma. Pathol. Res. Pract. 2024, 256, 155267. [Google Scholar] [CrossRef]
- Cattaneo, G.; Ventin, M.; Arya, S.; Kontos, F.; Michelakos, T.; Sekigami, Y.; Cai, L.; Villani, V.; Sabbatino, F.; Chen, F.; et al. Interplay between B7-H3 and HLA Class I in the Clinical Course of Pancreatic Ductal Adenocarcinoma. Cancer Lett. 2024, 587, 216713. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhao, Z.-X.; Cheng, P.; Huang, F.; Guan, X.; Zhang, M.-G.; Chen, H.-P.; Liu, Z.; Jiang, Z.; Zheng, Z.-X.; et al. B7-H3 Immune Checkpoint Expression Is a Poor Prognostic Factor in Colorectal Carcinoma. Mod. Pathol. 2020, 33, 2330–2340. [Google Scholar] [CrossRef]
- Ahrends, T.; Borst, J. The Opposing Roles of CD4+ T Cells in Anti-tumour Immunity. Immunology 2018, 154, 582–592. [Google Scholar] [CrossRef]
- Cai, D.; Li, J.; Liu, D.; Hong, S.; Qiao, Q.; Sun, Q.; Li, P.; Lyu, N.; Sun, T.; Xie, S.; et al. Tumor-Expressed B7-H3 Mediates the Inhibition of Antitumor T-Cell Functions in Ovarian Cancer Insensitive to PD-1 Blockade Therapy. Cell Mol. Immunol. 2020, 17, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Fan, T.-F.; Wu, L.; Yu, G.-T.; Deng, W.-W.; Chen, L.; Bu, L.-L.; Ma, S.-R.; Liu, B.; Bian, Y.; et al. Selective Blockade of B7-H3 Enhances Antitumour Immune Activity by Reducing Immature Myeloid Cells in Head and Neck Squamous Cell Carcinoma. J. Cell. Mol. Med. 2017, 21, 2199–2210. [Google Scholar] [CrossRef]
- Meng, F.; Yang, M.; Chen, Y.; Chen, W.; Wang, W. miR-34a Induces Immunosuppression in Colorectal Carcinoma through Modulating a SIRT1/NF-κB/B7-H3/TNF-α Axis. Cancer Immunol. Immunother. 2021, 70, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Peuker, K.; Strigli, A.; Tauriello, D.V.F.; Hendricks, A.; von Schönfels, W.; Burmeister, G.; Brosch, M.; Herrmann, A.; Krüger, S.; Nitsche, J.; et al. Microbiota-Dependent Activation of the Myeloid Calcineurin-NFAT Pathway Inhibits B7H3- and B7H4-Dependent Anti-Tumor Immunity in Colorectal Cancer. Immunity 2022, 55, 701–717.e7. [Google Scholar] [CrossRef]
- Zhou, X.; Mao, Y.; Zhu, J.; Meng, F.; Chen, Q.; Tao, L.; Li, R.; Fu, F.; Liu, C.; Hu, Y.; et al. TGF-Β1 Promotes Colorectal Cancer Immune Escape by Elevating B7-H3 and B7-H4 via the miR-155/miR-143 Axis. Oncotarget 2016, 7, 67196–67211. [Google Scholar] [CrossRef]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef] [PubMed]
- Pathania, A.S.; Chava, H.; Chaturvedi, N.K.; Chava, S.; Byrareddy, S.N.; Coulter, D.W.; Challagundla, K.B. The miR-29 Family Facilitates the Activation of NK-Cell Immune Responses by Targeting the B7-H3 Immune Checkpoint in Neuroblastoma. Cell Death Dis. 2024, 15, 428. [Google Scholar] [CrossRef]
- Xiong, G.; Chen, Z.; Liu, Q.; Peng, F.; Zhang, C.; Cheng, M.; Ling, R.; Chen, S.; Liang, Y.; Chen, D.; et al. CD276 Regulates the Immune Escape of Esophageal Squamous Cell Carcinoma through CXCL1–CXCR2 Induced NETs. J. Immunother. Cancer 2024, 12, e008662. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Leader, A.M.; Merad, M. MDSC: Markers, Development, States, and Unaddressed Complexity. Immunity 2021, 54, 875–884. [Google Scholar] [CrossRef]
- Maggs, L.; Sadagopan, A.; Moghaddam, A.S.; Ferrone, S. HLA Class I Antigen Processing Machinery Defects in Antitumor Immunity and Immunotherapy. Trends Cancer 2021, 7, 1089–1101. [Google Scholar] [CrossRef]
- Mielcarska, S.; Dawidowicz, M.; Kula, A.; Kiczmer, P.; Skiba, H.; Krygier, M.; Chrabańska, M.; Piecuch, J.; Szrot, M.; Ochman, B.; et al. B7H3 Role in Reshaping Immunosuppressive Landscape in MSI and MSS Colorectal Cancer Tumours. Cancers 2023, 15, 3136. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, L.; Wang, F.; Zhu, D.; Ge, X.; Hua, D.; Sun, J. Cancer Cell-Expressed B7-H3 Regulates the Differentiation of Tumor-Associated Macrophages in Human Colorectal Carcinoma. Oncol. Lett. 2017, 14, 6177–6183. [Google Scholar] [CrossRef]
- Wang, L.; Cao, N.; Wang, S.; Man, H.; Li, P.; Shan, B. Roles of Coinhibitory Molecules B7-H3 and B7-H4 in Esophageal Squamous Cell Carcinoma. Tumor. Biol. 2016, 37, 2961–2971. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Xu, B.; Wang, Q.; Zhou, W.; Zhang, G.; Sun, J.; Shi, L.; Pei, H.; Wu, C.; et al. B7-H3 Expression Associates with Tumor Invasion and Patient’s Poor Survival in Human Esophageal Cancer. Am. J. Transl. Res. 2015, 7, 2646–2660. [Google Scholar] [PubMed]
- Guo, L.; Liu, Z.; Zhang, Y.; Quan, Q.; Huang, L.; Xu, Y.; Cao, L.; Zhang, X. Association of Increased B7 Protein Expression by Infiltrating Immune Cells with Progression of Gastric Carcinogenesis. Medicine 2019, 98, e14663. [Google Scholar] [CrossRef]
- Chen, S.; Zhan, S.; Ding, S.; Zhang, Q.; Xuan, H.; Zhang, X.; Cao, L. B7-H3 and CD47 Co-Expression in Gastric Cancer Is a Predictor of Poor Prognosis and Potential Targets for Future Dual-Targeting Immunotherapy. J. Cancer Res. Clin. Oncol. 2023, 149, 16609–16621. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Wang, J.-T.; Chen, G.; Shan, Z.-G.; Wang, T.-T.; Shen, Y.; Chen, J.; Yan, Z.-B.; Peng, L.-S.; Mao, F.-Y.; et al. Expression, Regulation and Clinical Significance of B7-H3 on Neutrophils in Human Gastric Cancer. Clin. Immunol. 2021, 227, 108753. [Google Scholar] [CrossRef] [PubMed]
- Kang, F.-B.; Wang, L.; Li, D.; Zhang, Y.-G.; Sun, D.-X. Hepatocellular Carcinomas Promote Tumor-Associated Macrophage M2-Polarization via Increased B7-H3 Expression. Oncol. Rep. 2015, 33, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, G.; Liu, T.; Yu, G.; Zhang, G.; Luan, X. B7-H3 Was Highly Expressed in Human Primary Hepatocellular Carcinoma and Promoted Tumor Progression. Cancer Investig. 2014, 32, 262–271. [Google Scholar] [CrossRef]
- Loos, M.; Hedderich, D.M.; Ottenhausen, M.; Giese, N.A.; Laschinger, M.; Esposito, I.; Kleeff, J.; Friess, H. Expression of the Costimulatory Molecule B7-H3 Is Associated with Prolonged Survival in Human Pancreatic Cancer. BMC Cancer 2009, 9, 463. [Google Scholar] [CrossRef]
- Si, S.; Wang, L.; Cao, H.; Xu, Y.; Zhan, Q. Co-Deficiency of B7-H3 and B7-H4 Identifies High CD8 + T Cell Infiltration and Better Prognosis in Pancreatic Cancer. BMC Cancer 2022, 22, 211. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Hui, K.; Jiang, X. Non-Immune Functions of B7-H3: Bridging Tumor Cells and the Tumor Vasculature. Front. Oncol. 2024, 14, 1408051. [Google Scholar] [CrossRef]
- Kang, F.; Wang, L.; Jia, H.; Li, D.; Li, H.; Zhang, Y.; Sun, D. B7-H3 Promotes Aggression and Invasion of Hepatocellular Carcinoma by Targeting Epithelial-to-Mesenchymal Transition via JAK2/STAT3/Slug Signaling Pathway. Cancer Cell Int. 2015, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Xie, J.; Zhang, D.; Chen, C.; Lin, S.; Chen, Y.; Zhang, G. B7-H3 Inhibits Apoptosis of Gastric Cancer Cell by Interacting with Fibronectin. J. Cancer 2021, 12, 7518–7526. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Zhu, X.; Wu, H.; Wang, J.; Zhang, M.; Xiang, J.; Xia, S.; Shi, T.; Xi, Q. B7-H3 Promotes the Migration and Invasion of Colorectal Cancer Cells via Regulating the Actin Cytoskeleton and RhoA/ROCK1/LIMK1 Signaling Pathway. Tissue Cell. 2024, 90, 102518. [Google Scholar] [CrossRef]
- Chen, L.-C.; Yang, P.-C.; Chen, C.-Y.; Chiang, S.-F.; Chen, T.-W.; Chen, W.T.-L.; Ke, T.-W.; Liang, J.-A.; Shiau, A.; Chao, K.S.C.; et al. Dual Inhibition of B7-H3 and EGFR Overcomes Acquired Chemoresistance in Colon Adenocarcinoma. J. Cancer 2024, 15, 1750–1761. [Google Scholar] [CrossRef]
- Yue, G.; Tang, J.; Zhang, L.; Niu, H.; Li, H.; Luo, S. CD276 Suppresses CAR-T Cell Function by Promoting Tumor Cell Glycolysis in Esophageal Squamous Cell Carcinoma. J. Gastrointest. Oncol. 2021, 12, 38–51. [Google Scholar] [CrossRef]
- Lim, S.; Liu, H.; Madeira da Silva, L.; Arora, R.; Liu, Z.; Phillips, J.B.; Schmitt, D.C.; Vu, T.; McClellan, S.; Lin, Y.; et al. Immunoregulatory Protein B7-H3 Reprograms Glucose Metabolism in Cancer Cells by ROS-Mediated Stabilization of HIF1α. Cancer Res. 2016, 76, 2231–2242. [Google Scholar] [CrossRef]
- Gao, Q.; Huang, C.; Liu, T.; Yang, F.; Chen, Z.; Sun, L.; Zhao, Y.; Wang, M.; Luo, L.; Zhou, C.; et al. Gastric Cancer Mesenchymal Stem Cells Promote Tumor Glycolysis and Chemoresistance by Regulating B7H3 in Gastric Cancer Cells. J. Cell Biochem. 2024, 125, e30521. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhang, Y.; Xu, X.; You, Q.; Yu, C.; Wang, W.; Mao, Y. Exosomal B7-H3 Facilitates Colorectal Cancer Angiogenesis and Metastasis through AKT1/mTOR/VEGFA Pathway. Cell. Signal. 2023, 109, 110737. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, S.; Wang, J.; Ruan, X.; Wu, L.; Zhang, Z.; Wang, X.; Zhang, J.; Liu, Y.; Li, Y.; et al. B7-H3 Enhances Colorectal Cancer Progression by Regulating HB-EGF via HIF-1α. J. Gastrointest. Oncol. 2024, 15, 1035–1049. [Google Scholar] [CrossRef]
- Wang, R.; Sun, L.; Xia, S.; Wu, H.; Ma, Y.; Zhan, S.; Zhang, G.; Zhang, X.; Shi, T.; Chen, W. B7-H3 Suppresses Doxorubicin-Induced Senescence-like Growth Arrest in Colorectal Cancer through the AKT/TM4SF1/SIRT1 Pathway. Cell Death Dis. 2021, 12, 453. [Google Scholar] [CrossRef]
- Yamato, M.; Hasegawa, J.; Maejima, T.; Hattori, C.; Kumagai, K.; Watanabe, A.; Nishiya, Y.; Shibutani, T.; Aida, T.; Hayakawa, I.; et al. DS-7300a, a DNA Topoisomerase I Inhibitor, DXd-Based Antibody-Drug Conjugate Targeting B7-H3, Exerts Potent Antitumor Activities in Preclinical Models. Mol. Cancer Ther. 2022, 21, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Ju, E.J.; Park, J.; Ko, E.J.; Kwon, M.R.; Lee, H.W.; Son, G.W.; Park, Y.-Y.; Kim, Y.J.; Song, S.Y.; et al. ITC-6102RO, a Novel B7-H3 Antibody-Drug Conjugate, Exhibits Potent Therapeutic Effects against B7-H3 Expressing Solid Tumors. Cancer Cell Int. 2023, 23, 172. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Y.; Xu, M.; Zhou, F.; Yan, J. Serum miR-1301-3p, miR-335-5p, miR-28-5p, and Their Target B7-H3 May Serve as Novel Biomarkers for Colorectal Cancer. J. BUON 2019, 24, 1120–1127. [Google Scholar]
- Wu, J.; Wang, F.; Liu, X.; Zhang, T.; Liu, F.; Ge, X.; Mao, Y.; Hua, D. Correlation of IDH1 and B7H3 Expression with Prognosis of CRC Patients. Eur. J. Surg. Oncol. 2018, 44, 1254–1260. [Google Scholar] [CrossRef]
- Zekri, L.; Lutz, M.; Prakash, N.; Manz, T.; Klimovich, B.; Mueller, S.; Hoerner, S.; Hagelstein, I.; Engel, M.; Chashchina, A.; et al. An Optimized IgG-Based B7-H3xCD3 Bispecific Antibody for Treatment of Gastrointestinal Cancers. Mol. Ther. 2023, 31, 1033–1045. [Google Scholar] [CrossRef]
- Sun, J.; Chen, L.; Zhang, G.; Jiang, J.; Zhu, M.; Tan, Y.; Wang, H.; Lu, B.; Zhang, X. Clinical Significance and Regulation of the Costimulatory Molecule B7-H3 in Human Colorectal Carcinoma. Cancer Immunol. Immunother. 2010, 59, 1163–1171. [Google Scholar] [CrossRef]
- Bin, Z.; Guangbo, Z.; Yan, G.; Huan, Z.; Desheng, L.; Xueguang, Z. Overexpression of B7-H3 in CD133+ Colorectal Cancer Cells Is Associated with Cancer Progression and Survival in Human Patients. J. Surg. Res. 2014, 188, 396–403. [Google Scholar] [CrossRef]
- Lupu, C.; Eisenbach, C.; Lupu, A.; Kuefner, M.; Hoyler, B.; Stremmel, W.; Encke, J. Adenoviral B7-H3 Therapy Induces Tumor Specific Immune Responses and Reduces Secondary Metastasis in a Murine Model of Colon Cancer. Oncol. Rep. 2007, 18, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhan, S.; Lu, H.; Wang, R.; Xu, Y.; Zhang, G.; Cao, L.; Shi, T.; Zhang, X.; Chen, W. B7-H3 Regulates KIF15-Activated ERK1/2 Pathway and Contributes to Radioresistance in Colorectal Cancer. Cell Death Dis. 2020, 11, 824. [Google Scholar] [CrossRef]
- Sahin, I.H.; Akce, M.; Alese, O.; Shaib, W.; Lesinski, G.B.; El-Rayes, B.; Wu, C. Immune Checkpoint Inhibitors for the Treatment of MSI-H/MMR-D Colorectal Cancer and a Perspective on Resistance Mechanisms. Br. J. Cancer 2019, 121, 809–818. [Google Scholar] [CrossRef]
- Edin, S.; Gylling, B.; Li, X.; Stenberg, Å.; Löfgren-Burström, A.; Zingmark, C.; Van Guelpen, B.; Ljuslinder, I.; Ling, A.; Palmqvist, R. Opposing Roles by KRAS and BRAF Mutation on Immune Cell Infiltration in Colorectal Cancer—Possible Implications for Immunotherapy. Br. J. Cancer 2024, 130, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Pei, L.; Xia, H.; Tang, Q.; Bi, F. Role of Oncogenic KRAS in the Prognosis, Diagnosis and Treatment of Colorectal Cancer. Mol. Cancer 2021, 20, 143. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Liu, H.; Wei, C.; Ru, H.; Qin, H.; Lai, H.; Meng, Y.; Wu, G.; Xie, W.; et al. Immune Landscape and Prognostic Immune-Related Genes in KRAS-Mutant Colorectal Cancer Patients. J. Transl. Med. 2021, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Cathomas, G. PIK3CA in Colorectal Cancer. Front. Oncol. 2014, 4, 35. [Google Scholar] [CrossRef]
- Ahn, A.R.; Kim, K.M.; Jang, K.Y.; Moon, W.S.; Ha, G.W.; Lee, M.R.; Chung, M.J. Correlation of PIK3CA Mutation with Programmed Death Ligand-1 (PD-L1) Expression and Their Clinicopathological Significance in Colorectal Cancer. Ann. Transl. Med. 2021, 9, 1406. [Google Scholar] [CrossRef]
- Chowdhury, S.; Ferdous Ara, S.J.; Mili, S.M.; Momotaz, T.; Ahmed Molla, M.M.; Anwar, S.; Saleh, A.A. Mutational Profile of KRAS, NRAS, BRAF, PIK3CA, and AKT1 Genes in Colorectal Cancer Patients in a Tertiary Care Hospital, Dhaka. Adv. Cancer Biol. Metastasis 2022, 5, 100054. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Q.; Wang, Z.; Shi, L.; Wu, C.; Jiang, J. Assessment of Combined Expression of B7-H3 and B7-H4 as Prognostic Marker in Esophageal Cancer Patients. Oncotarget 2016, 7, 77237–77243. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Lu, P.; Gao, M.-C.; Wang, R.; Li, Y.-Y.; Guo, R.-Q.; Zhang, W.-S.; Song, J.-X. Nomogram Based on Multimodal Magnetic Resonance Combined with B7-H3mRNA for Preoperative Lymph Node Prediction in Esophagus Cancer. World J. Clin. Oncol. 2024, 15, 419–433. [Google Scholar] [CrossRef]
- Song, J.; Shi, W.; Zhang, Y.; Sun, M.; Liang, X.; Zheng, S. Epidermal Growth Factor Receptor and B7-H3 Expression in Esophageal Squamous Tissues Correlate to Patient Prognosis. Onco Targets Ther. 2016, 9, 6257–6263. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wu, Y.; Wang, J.; Zhou, W.; Wang, J.; Guo, H.; Zhang, N.; Zhang, L.; Hu, X.; et al. A Novel Microenvironment Regulated System CAR-T (MRS.CAR-T) for Immunotherapeutic Treatment of Esophageal Squamous Carcinoma. Cancer Lett. 2023, 568, 216303. [Google Scholar] [CrossRef]
- Wu, H.; Liu, C.; Yuan, Q.; Qiao, Y.; Ding, Y.; Duan, L.; Li, W.; Zhang, M.; Zhang, X.; Jiang, Y.; et al. A Novel Fc-Enhanced Humanized Monoclonal Antibody Targeting B7-H3 Suppresses the Growth of ESCC. Oncoimmunology 2023, 12, 2282250. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Yu, X.; Ju, R.; Wang, Z.; Wang, Y. Antitumor Responses in Gastric Cancer by Targeting B7H3 via Chimeric Antigen Receptor T Cells. Cancer Cell Int. 2022, 22, 50. [Google Scholar] [CrossRef]
- Ulase, D.; Behrens, H.-M.; Krüger, S.; Zeissig, S.; Röcken, C. Gastric Carcinomas with Stromal B7-H3 Expression Have Lower Intratumoural CD8+ T Cell Density. Int. J. Mol. Sci. 2021, 22, 2129. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Liu, Z.; Zhang, M.; Guo, T.; Quan, Q.; Huang, L.; Guo, L.; Cao, L.; Zhang, X. Overexpression of B7-H3 in α-SMA-Positive Fibroblasts Is Associated With Cancer Progression and Survival in Gastric Adenocarcinomas. Front. Oncol. 2020, 9, 1466. [Google Scholar] [CrossRef]
- Arigami, T.; Uenosono, Y.; Hirata, M.; Yanagita, S.; Ishigami, S.; Natsugoe, S. B7-H3 Expression in Gastric Cancer: A Novel Molecular Blood Marker for Detecting Circulating Tumor Cells. Cancer Sci. 2011, 102, 1019–1024. [Google Scholar] [CrossRef]
- Xia, L.; Chen, Y.; Li, J.; Wang, J.; Shen, K.; Zhao, A.; Jin, H.; Zhang, G.; Xi, Q.; Xia, S.; et al. B7-H3 Confers Stemness Characteristics to Gastric Cancer Cells by Promoting Glutathione Metabolism through AKT/pAKT/Nrf2 Pathway. Chin. Med. J. 2023, 136, 1977–1989. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.; Yao, P.; Shen, W.; Wu, Y.; Ye, Z.; Zhao, K.; Chen, H.; Cao, J.; Xing, C. B7-H3 Increases the Radioresistance of Gastric Cancer Cells through Regulating Baseline Levels of Cell Autophagy. Am. J. Transl. Res. 2019, 11, 4438–4449. [Google Scholar]
- Lutz, M.S.; Zekri, L.; Weßling, L.; Berchtold, S.; Heitmann, J.S.; Lauer, U.M.; Jung, G.; Salih, H.R. IgG-Based B7-H3xCD3 Bispecific Antibody for Treatment of Pancreatic, Hepatic and Gastric Cancer. Front. Immunol. 2023, 14, 1163136. [Google Scholar] [CrossRef]
- Zhao, L.; Xie, C.; Liu, D.; Li, T.; Zhang, Y.; Wan, C. Early Detection of Hepatocellular Carcinoma in Patients with Hepatocirrhosis by Soluble B7-H3. J. Gastrointest. Surg. 2017, 21, 807–812. [Google Scholar] [CrossRef]
- Xu, F.; Yi, J.; Wang, F.; Wang, W.; Wang, Z.; Xue, J.; Luan, X. Involvement of Soluble B7-H3 in Combination with the Serum Inflammatory Cytokines Interleukin-17, −8 and −6 in the Diagnosis of Hepatocellular Carcinoma. Oncol. Lett. 2017, 14, 8138. [Google Scholar] [CrossRef]
- Zheng, Y.; Liao, N.; Wu, Y.; Gao, J.; Li, Z.; Liu, W.; Wang, Y.; Li, M.; Li, X.; Chen, L.; et al. High Expression of B7-H2 or B7-H3 Is Associated with Poor Prognosis in Hepatocellular Carcinoma. Mol. Med. Rep. 2019, 19, 4315–4325. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yu, X.; Chen, Y.; Tan, X.; Liu, W.; Hua, W.; Chen, L.; Zhang, W. Inhibition of the B7-H3 Immune Checkpoint Limits Hepatocellular Carcinoma Progression by Enhancing T Lymphocyte-Mediated Immune Cytotoxicity In Vitro and In Vivo. Clin. Transl. Oncol. 2023, 25, 1067–1079. [Google Scholar] [CrossRef]
- Shrestha, R.; Bridle, K.R.; Crawford, D.H.G.; Jayachandran, A. TNF-α-Mediated Epithelial-to-Mesenchymal Transition Regulates Expression of Immune Checkpoint Molecules in Hepatocellular Carcinoma. Mol. Med. Rep. 2020, 21, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Bridle, K.R.; Crawford, D.H.G.; Jayachandran, A. Immune Checkpoint Molecules Are Regulated by Transforming Growth Factor (TGF)-Β1-Induced Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma. Int. J. Med. Sci. 2021, 18, 2466–2479. [Google Scholar] [CrossRef]
- Cao, G.; Zhang, G.; Liu, M.; Liu, J.; Wang, Q.; Zhu, L.; Wan, X. GPC3-Targeted CAR-T Cells Secreting B7H3-Targeted BiTE Exhibit Potent Cytotoxicity Activity against Hepatocellular Carcinoma Cell in the in Vitro Assay. Biochem. Biophys. Rep. 2022, 31, 101324. [Google Scholar] [CrossRef]
- Inamura, K.; Takazawa, Y.; Inoue, Y.; Yokouchi, Y.; Kobayashi, M.; Saiura, A.; Shibutani, T.; Ishikawa, Y. Tumor B7-H3 (CD276) Expression and Survival in Pancreatic Cancer. J. Clin. Med. 2018, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Geerdes, E.E.; Sideras, K.; Aziz, M.H.; van Eijck, C.H.; Bruno, M.J.; Sprengers, D.; Boor, P.P.C.; Kwekkeboom, J. Cancer Cell B7-H3 Expression Is More Prevalent in the Pancreato-Biliary Subtype of Ampullary Cancer Than in Pancreatic Cancer. Front. Oncol. 2021, 11, 615691. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, J.; Liu, Y.; Zheng, X.; Feng, J.; Chen, X.; Jiang, T.; Li, Y.; Chen, L. Prognostic Values of B7-H3, B7-H4, and HHLA2 Expression in Human Pancreatic Cancer Tissues Based on mIHC and Spatial Distribution Analysis. Pathol. Res. Pract. 2022, 234, 153911. [Google Scholar] [CrossRef]
- Mo, S.; Zong, L.; Chen, X.; Ban, X.; Li, M.; Lu, Z.; Yu, S.; Chen, J. Expression and Prognostic Value of B7 Family Immune Checkpoints in Pancreatic Neuroendocrine Tumors. Arch. Pathol. Lab. Med. 2023, 147, 193–201. [Google Scholar] [CrossRef]
- Zhong, Y.; Tian, Y.; Wang, Y.; Bai, J.; Long, Q.; Yan, L.; Gong, Z.; Gao, W.; Tang, Q. Small Extracellular Vesicle piR-Hsa-30937 Derived from Pancreatic Neuroendocrine Neoplasms Upregulates CD276 in Macrophages to Promote Immune Evasion. Cancer Immunol. Res. 2024, 12, 840–853. [Google Scholar] [CrossRef]
- Lutz, M.S.; Wang, K.; Jung, G.; Salih, H.R.; Hagelstein, I. An Fc-Modified Monoclonal Antibody as Novel Treatment Option for Pancreatic Cancer. Front. Immunol. 2024, 15, 1343929. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, K.; You, F.; Ma, R.; Yang, N.; Tian, S.; An, G.; Yang, L. Preconditioning of Radiotherapy Enhances Efficacy of B7-H3-CAR-T in Treating Solid Tumor Models. Life Sci. 2023, 331, 122024. [Google Scholar] [CrossRef]
- Kula, A.; Koszewska, D.; Kot, A.; Dawidowicz, M.; Mielcarska, S.; Waniczek, D.; Świętochowska, E. The Importance of HHLA2 in Solid Tumors—A Review of the Literature. Cells 2024, 13, 794. [Google Scholar] [CrossRef]
- Dawidowicz, M.; Kot, A.; Mielcarska, S.; Psykała, K.; Kula, A.; Waniczek, D.; Świętochowska, E. B7H4 Role in Solid Cancers: A Review of the Literature. Cancers 2024, 16, 2519. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.W.; Bledsoe, J.R.; Morales-Oyarvide, V.; Huynh, T.G.; Mino-Kenudson, M. PD-L1 Expression in Colorectal Cancer Is Associated with Microsatellite Instability, BRAF Mutation, Medullary Morphology and Cytotoxic Tumor-Infiltrating Lymphocytes. Mod. Pathol. 2016, 29, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-T.; Jin, W.-L. B7-H3/CD276: An Emerging Cancer Immunotherapy. Front. Immunol. 2021, 12, 701006. [Google Scholar] [CrossRef]
- Ye, Z.; Zheng, Z.; Li, X.; Zhu, Y.; Zhong, Z.; Peng, L.; Wu, Y. B7-H3 Overexpression Predicts Poor Survival of Cancer Patients: A Meta-Analysis. Cell Physiol. Biochem. 2016, 39, 1568–1580. [Google Scholar] [CrossRef]
- Kula, A.; Dawidowicz, M.; Mielcarska, S.; Świętochowska, E.; Waniczek, D. Prognostic Value of HHLA2 in Patients with Solid Tumors: A Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 4760. [Google Scholar] [CrossRef]
- Dawidowicz, M.; Kula, A.; Mielcarska, S.; Świętochowska, E.; Waniczek, D. Prognostic Value of B7H4 Expression in Patients with Solid Cancers: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 5045. [Google Scholar] [CrossRef]
- Gu, L.; Chen, M.; Guo, D.; Zhu, H.; Zhang, W.; Pan, J.; Zhong, X.; Li, X.; Qian, H.; Wang, X. PD-L1 and Gastric Cancer Prognosis: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0182692. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, D.; Zhao, B.; Ren, L.; Huang, R.; Feng, B.; Chen, H. The Predictive Value of PD-L1 Expression in Patients with Advanced Hepatocellular Carcinoma Treated with PD -1/ PD-L1 Inhibitors: A Systematic Review and Meta-analysis. Cancer Medicine 2023, 12, 9282–9292. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, F.; Liu, L. Prognostic Significance of PD-L1 in Solid Tumor: An Updated Meta-Analysis. Medicine 2017, 96, e6369. [Google Scholar] [CrossRef]
- Cai, L.; Chen, A.; Tang, D. A New Strategy for Immunotherapy of Microsatellite-Stable (MSS)-Type Advanced Colorectal Cancer: Multi-Pathway Combination Therapy with PD-1/PD-L1 Inhibitors. Immunology 2024, 173, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Guo, S.; Guan, X.; Kang, Y.; Liu, J.; Yang, X. Immunological Classification of Tumor Types and Advances in Precision Combination Immunotherapy. Front. Immunol. 2022, 13, 790113. [Google Scholar] [CrossRef] [PubMed]
| Influence of B7H3 Upregulation on the Immune Landscape | References | ||
|---|---|---|---|
| Increase in the Following: | Decrease in the Following: | ||
| Colorectal cancer | TAMs (M2 macrophages), Tregs, eosinophils, and neutrophils, Th1 scores | TILs: CD8 T-cells and CD4 memory T-cells, Th2 scores | [72,79,80] |
| Esophageal cancer | Tregs, TAMs, neutrophils | TILs/CD8+ T-cells, NK cells | [76,81,82] |
| Gastric cancer | TAMs (B7H3 in immune cells)/M2 macrophages, neutrophils | CD8 cells (B7H3 in cancer cells) | [83,84,85] |
| Hepatocellular carcinoma | TAMs, Tregs | CD8 T-cells | [86,87] |
| Pancreatic cancer | CD8 and CD4 T-cells, neutrophils, macrophages, DCs | - | [88,89] |
| B7H3 | HHLA2 | B7H4 | PD-L1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor Type | Positive Rate | Cutoff for Positive Expression | Source | Expression Rate | Cutoff for Positive Expression | Source | Expression Rate | Cutoff for Positive Expression | Source | Expression Rate | Cutoff for Positive Expression | Source |
| CRC | 32–87% | >1%, >10% | [79,80] | 83.7% | >1%, >H score median | [143] | 29.1–80% | >1%, H score > 85, final score > 3 | [144] | 9% * | >5% | [145] |
| GC | 39.47–69.2% | median | [146,147] | 53.2% (high expression) | final score ≥ 8 | [143,148] | 44.9–80% | staining 0, +/++, +++, final score > 2 | [144,149] | 11–69.4% | ≥5% | [150] |
| HCC | 70–93.75% | H-score ≥ 2 | [17,147] | 49.0–67.7% | IRS > 3, H-score ≥ 5 | [143,148] | 1–73% | - | [144,149] | 24.06–34.5% | >1%, ≥20%, ≥75% | [151] |
| PC | 41.18–77.78% | final score > 3 | [147] | 77% | H score > 80 | [143,148] | 22.1–76% | >0%, >1%, >10% | [144,149] | 45% | ≥10% | [152] |
| EC | 55.75–69.7% | H-score > 185 | [146,147] | - | - | - | 53.8–95.5% | IHC score > 1, H score > 160 | [144,149] | 82.17% | score >0 | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielcarska, S.; Kot, A.; Kula, A.; Dawidowicz, M.; Sobków, P.; Kłaczka, D.; Waniczek, D.; Świętochowska, E. B7H3 in Gastrointestinal Tumors: Role in Immune Modulation and Cancer Progression: A Review of the Literature. Cells 2025, 14, 530. https://doi.org/10.3390/cells14070530
Mielcarska S, Kot A, Kula A, Dawidowicz M, Sobków P, Kłaczka D, Waniczek D, Świętochowska E. B7H3 in Gastrointestinal Tumors: Role in Immune Modulation and Cancer Progression: A Review of the Literature. Cells. 2025; 14(7):530. https://doi.org/10.3390/cells14070530
Chicago/Turabian StyleMielcarska, Sylwia, Anna Kot, Agnieszka Kula, Miriam Dawidowicz, Piotr Sobków, Daria Kłaczka, Dariusz Waniczek, and Elżbieta Świętochowska. 2025. "B7H3 in Gastrointestinal Tumors: Role in Immune Modulation and Cancer Progression: A Review of the Literature" Cells 14, no. 7: 530. https://doi.org/10.3390/cells14070530
APA StyleMielcarska, S., Kot, A., Kula, A., Dawidowicz, M., Sobków, P., Kłaczka, D., Waniczek, D., & Świętochowska, E. (2025). B7H3 in Gastrointestinal Tumors: Role in Immune Modulation and Cancer Progression: A Review of the Literature. Cells, 14(7), 530. https://doi.org/10.3390/cells14070530







