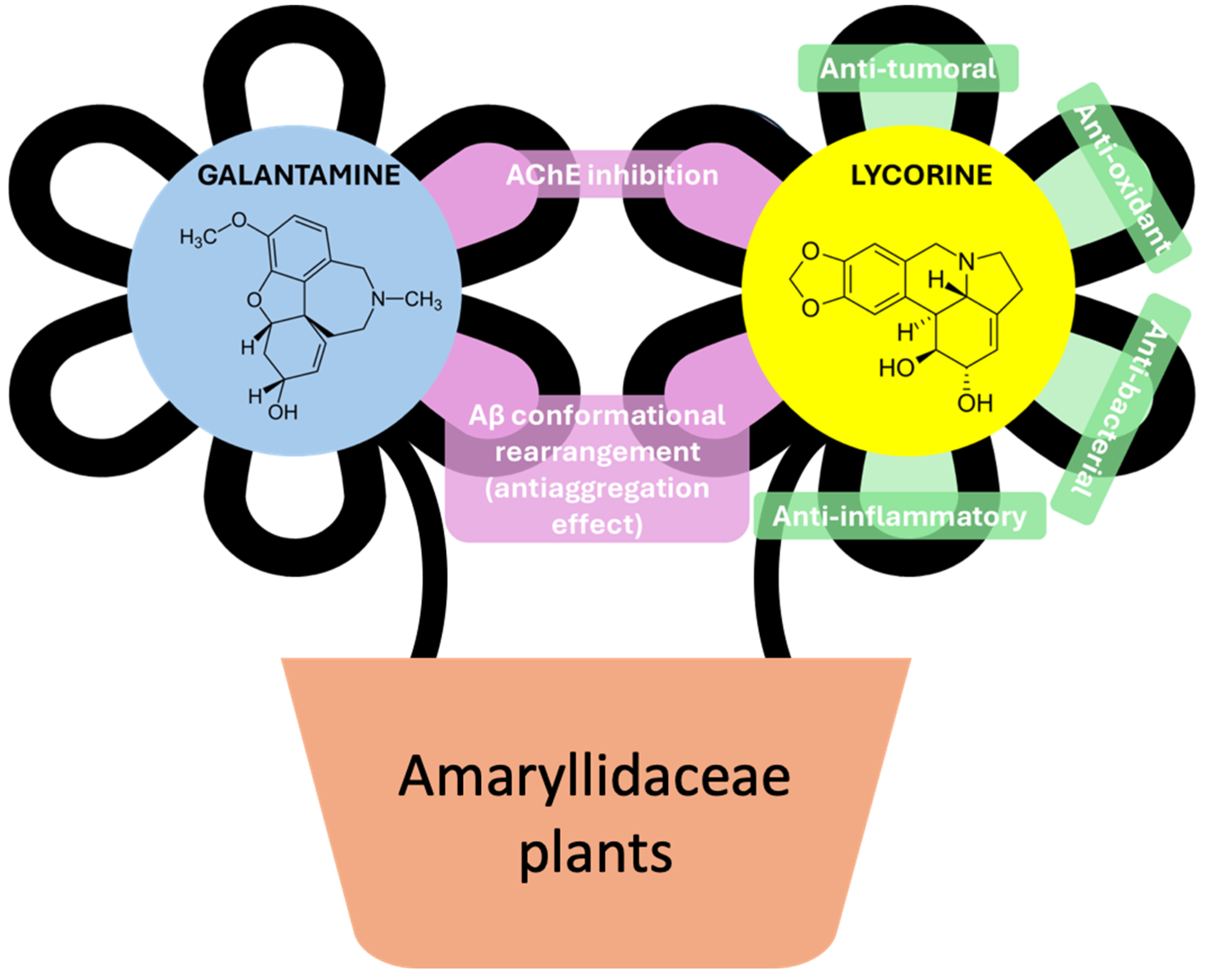
NMR Metabolomic Profiling of Differentiated SH-SY5Y Neuronal Cells: Amyloid-β Toxicity and Protective Effects of Galantamine and Lycorine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. NMR Metabolomics
2.3.1. Aβ, Lycorine, and Galantamine Exposure of SH-SY5Y
2.3.2. NMR Data Acquisition and Processing
2.3.3. Multivariate Analysis and Spectral Integration
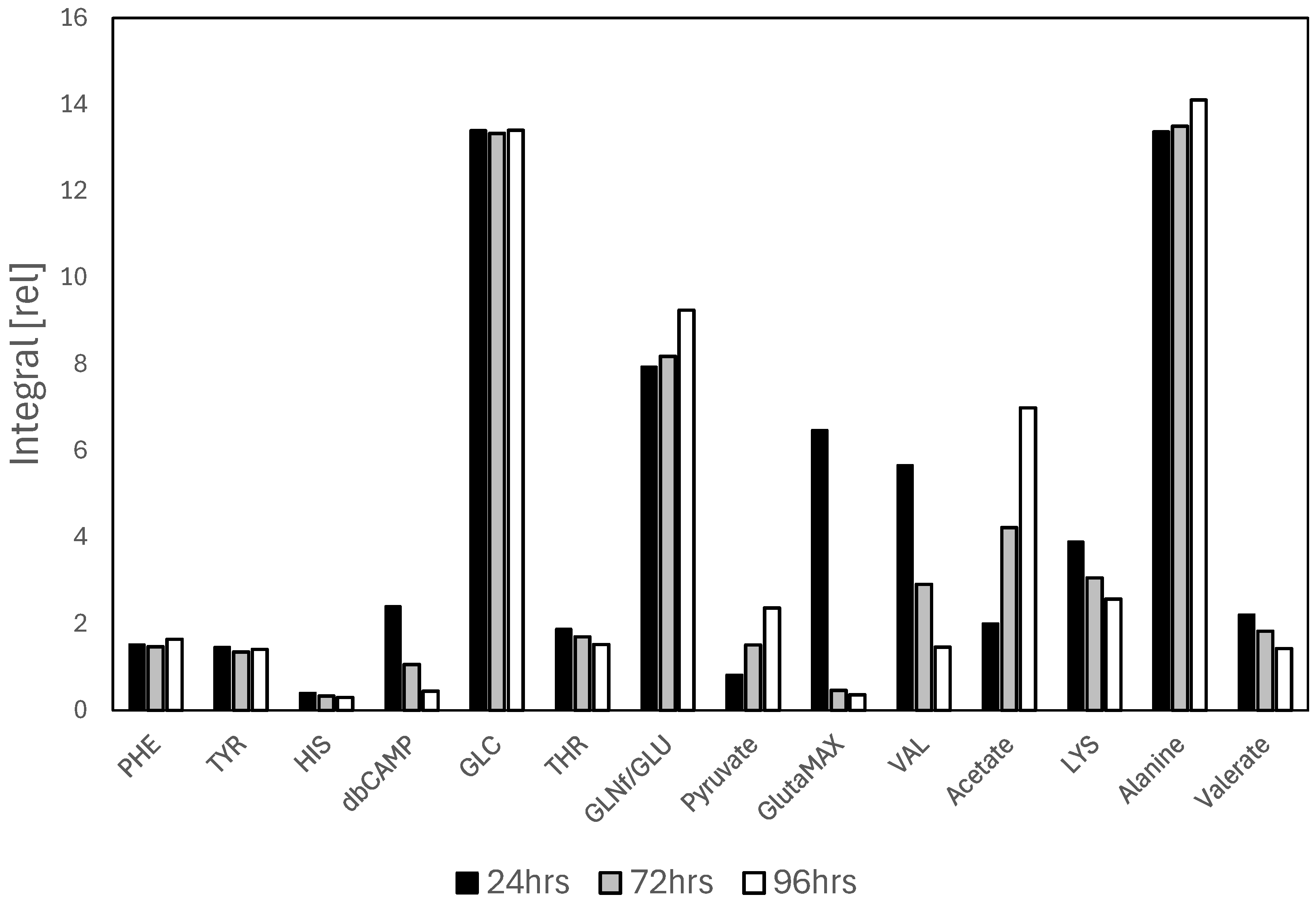
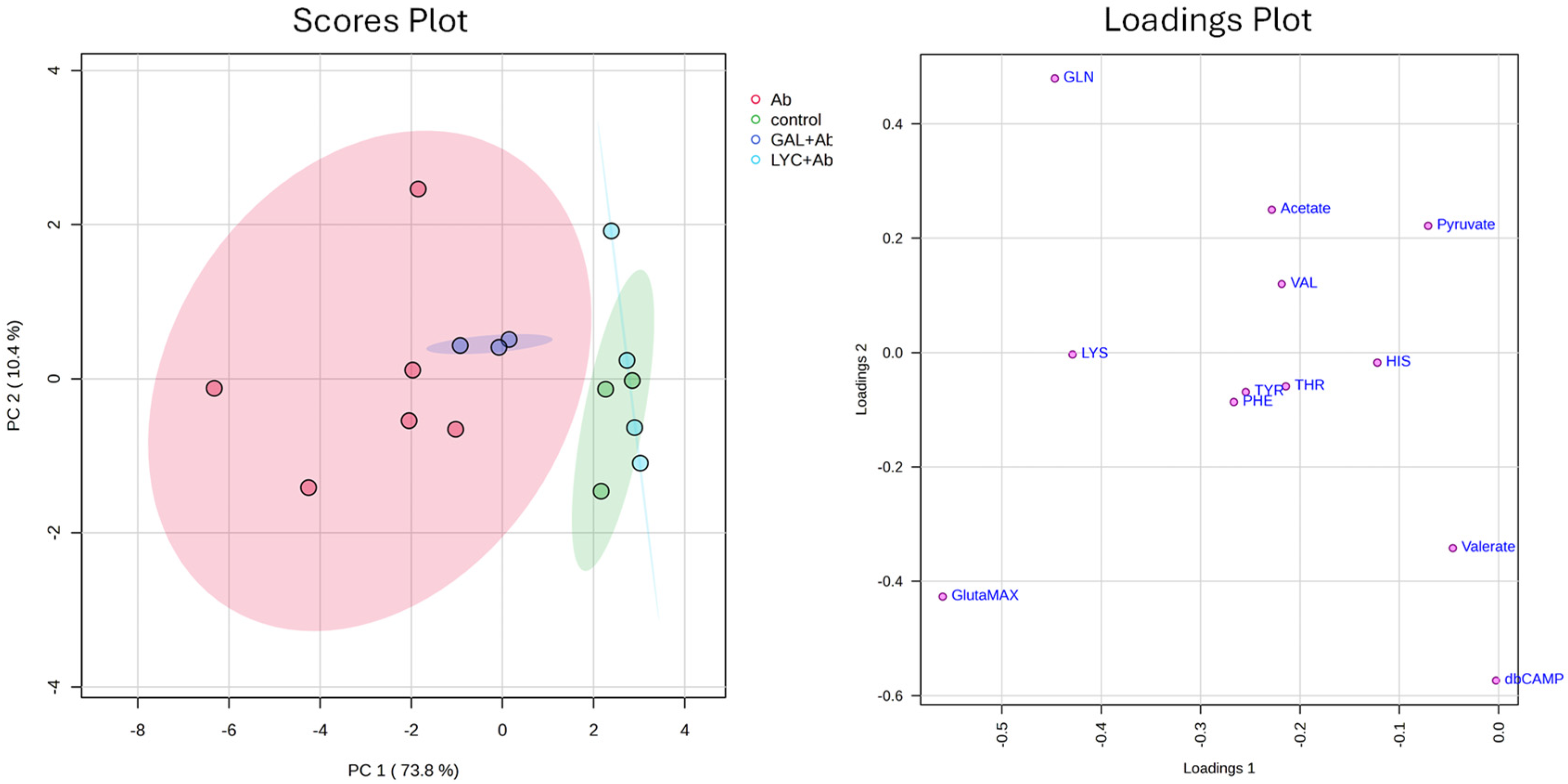
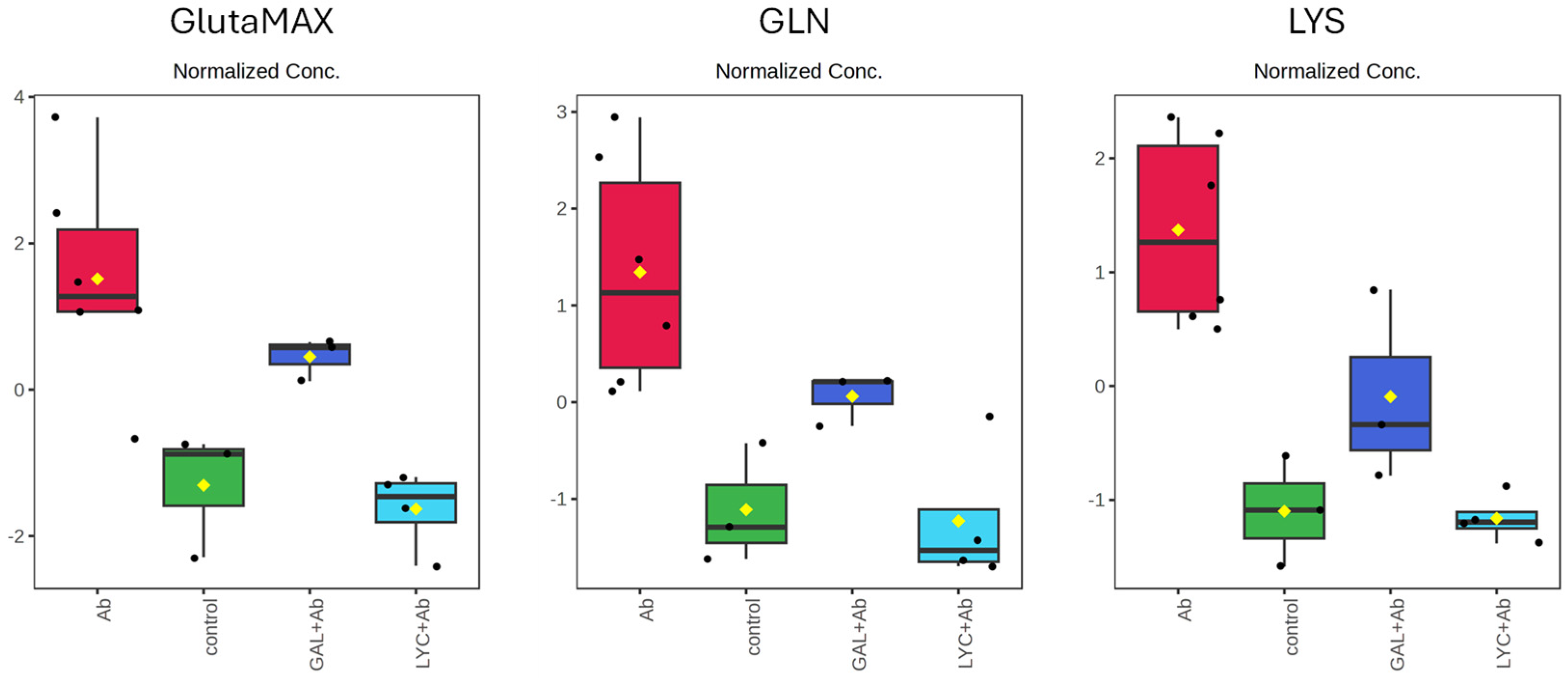
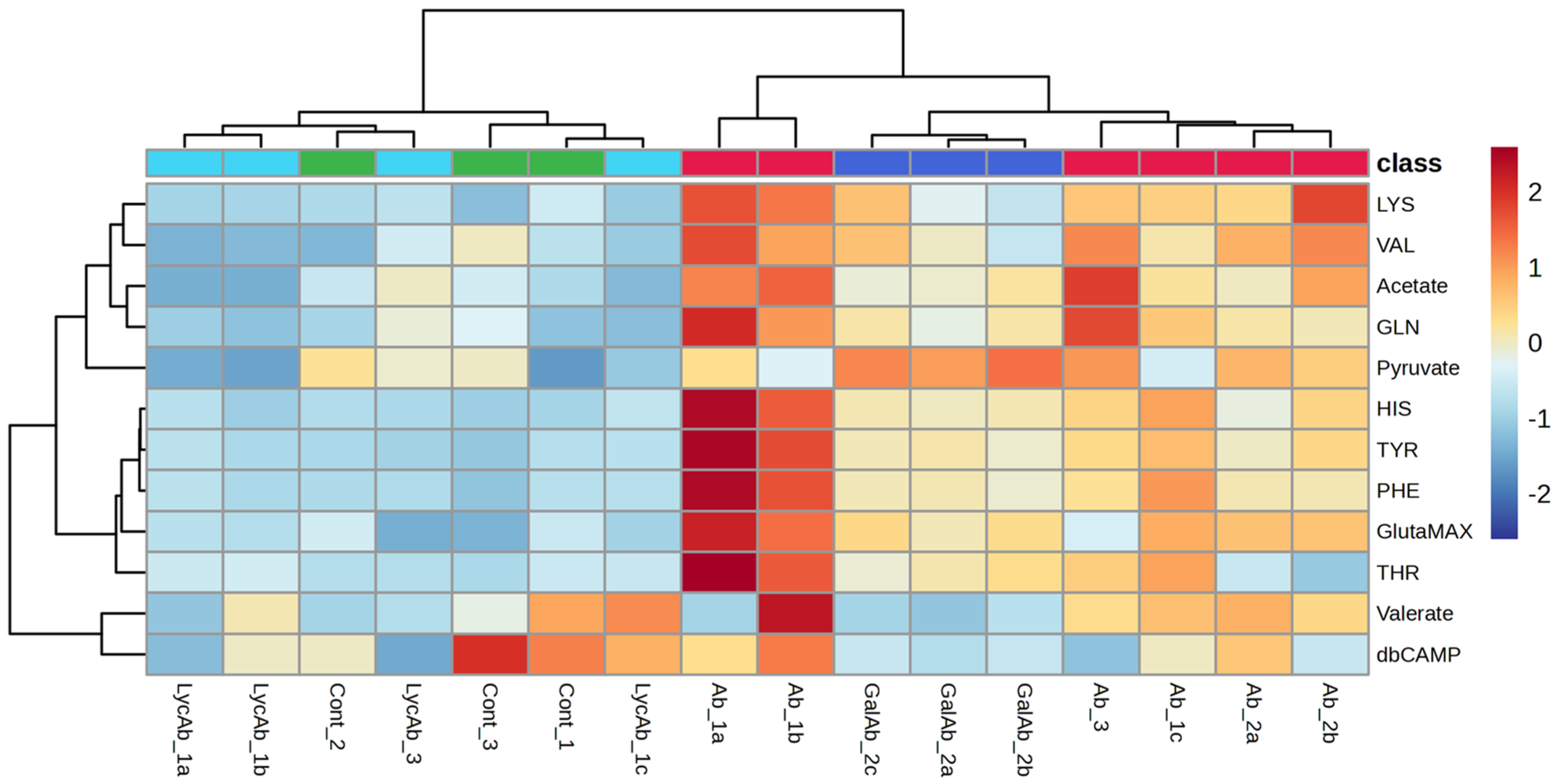
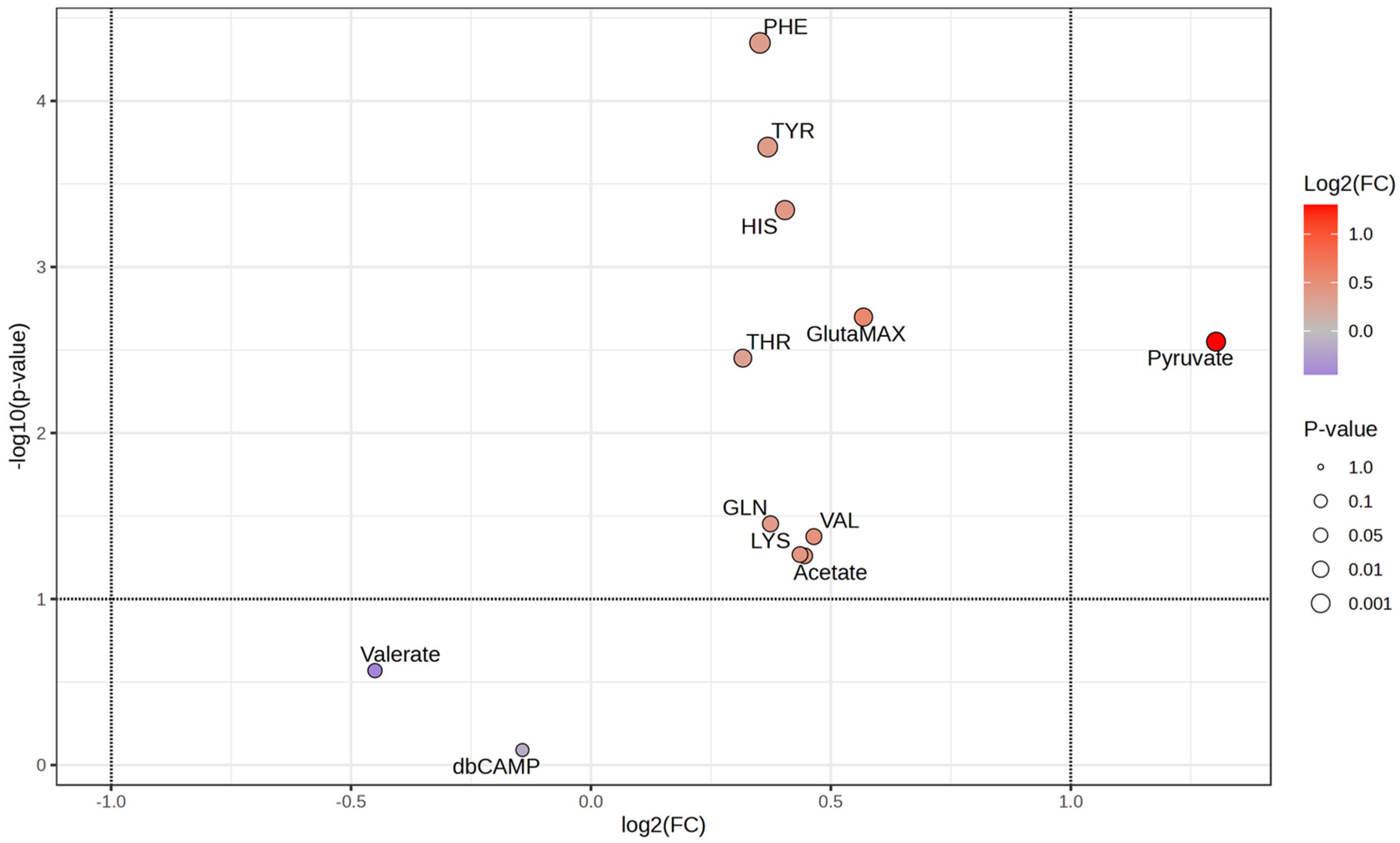
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| GAL | Galantamine |
| LYC | Lycorine |
| Aβ | Amyloid-β |
| AChE | Acetylcholinesterase |
| ROS | Reactive Oxygen Species |
| FDA | Food and Drug Administration |
| EMA | European Medicines Agency |
| BGM | Basic Growth Medium |
| DM | Differentiation Medium |
| ECM | Extracellular Matrix |
| EMEM | Eagle’s Minimum Essential Medium |
| BDNF | Brain-Derived Neurotrophic Factor |
| db-cAMP | Dibutyryl cyclic AMP |
| RA | Retinoic Acid |
| TMSP-d4 | 3-(Trimethylsilyl)-2,2,3,3-tetradeuteropropionic Acid Sodium Salt |
| NOESY | Nuclear Overhauser Enhancement Spectroscopy |
| PCA | Principal Component Analysis |
| PLS-DA | Partial Least Squares Discriminant Analysis |
| Ortho-PLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| ANOVA | Analysis of Variance |
| PC | Principal Component |
| TCA | Tricarboxylic Acid |
| CSF | Cerebrospinal Fluid |
| MIC | Mild Cognitive Impairment |
| PDHC | Pyruvate-Dehydrogenase Complex |
| α-KGDHC | α-ketoglutarate Dehydrogenase |
| CNS | Central Nervous System |
| EPO | Erythropoietin |
References
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Poulson, B.G.; Szczepski, K.; Lachowicz, J.I.; Jaremko, L.; Emwas, A.-H.; Jaremko, M. Aggregation of Biologically Important Peptides and Proteins: Inhibition or Acceleration Depending on Protein and Metal Ion Concentrations. RSC Adv. 2019, 10, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Polo, A.D.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving Neurodegeneration: Common Mechanisms and Strategies for New Treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef]
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L.; Perry, G.; Avila, J.; Moreira, P.I.; Sorensen, A.A.; Tabaton, M. The Amyloid-β Oligomer Hypothesis: Beginning of the Third Decade. J. Alzheimer’s Dis. 2018, 64, S567–S610. [Google Scholar] [CrossRef]
- Cacabelos, R.; Martínez-Iglesias, O.; Cacabelos, N.; Carrera, I.; Corzo, L.; Naidoo, V. Therapeutic Options in Alzheimer’s Disease: From Classic Acetylcholinesterase Inhibitors to Multi-Target Drugs with Pleiotropic Activity. Life 2024, 14, 1555. [Google Scholar] [CrossRef]
- da Rosa, M.M.; de Amorim, L.C.; de Oliveira Alves, J.V.; da Silva Aguiar, I.F.; da Silva Oliveira, F.G.; da Silva, M.V.; dos Santos, M.T.C. The Promising Role of Natural Products in Alzheimer’s Disease. Brain Disord. 2022, 7, 100049. [Google Scholar] [CrossRef]
- Qin, W.; Pang, Y.; Nie, S.; Quan, M.; Jia, J. Alzheimer’s Disease and Immunotherapy. Curr. Med. 2024, 3, 8. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Y.; Ruan, S.; Hu, Y. Current Anti-Amyloid-β Therapy for Alzheimer’s Disease Treatment: From Clinical Research to Nanomedicine. Int. J. Nanomed. 2023, 18, 7825–7845. [Google Scholar] [CrossRef]
- Rai, S.N.; Singh, C.; Singh, A.; Singh, M.P.; Singh, B.K. Mitochondrial Dysfunction: A Potential Therapeutic Target to Treat Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 3075–3088. [Google Scholar] [CrossRef]
- Self, W.K.; Holtzman, D.M. Emerging Diagnostics and Therapeutics for Alzheimer Disease. Nat. Med. 2023, 29, 2187–2199. [Google Scholar] [CrossRef]
- Bouyahya, A.; Bakrim, S.; Aboulaghras, S.; El Kadri, K.; Aanniz, T.; Khalid, A.; Abdalla, A.N.; Abdallah, A.A.; Ardianto, C.; Ming, L.C.; et al. Bioactive Compounds from Nature: Antioxidants Targeting Cellular Transformation in Response to Epigenetic Perturbations Induced by Oxidative Stress. Biomed. Pharmacother. 2024, 174, 116432. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rapposelli, S.; Sestito, S.; Herrera-Bravo, J.; Arancibia-Diaz, A.; Salazar, L.A.; Yeskaliyeva, B.; Beyatli, A.; Leyva-Gómez, G.; González-Contreras, C.; et al. Multi-Target Mechanisms of Phytochemicals in Alzheimer’s Disease: Effects on Oxidative Stress, Neuroinflammation and Protein Aggregation. J. Pers. Med. 2022, 12, 1515. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Khadija, J.F.; Harun-Or-Rashid, M.; Rahaman, M.S.; Nafady, M.H.; Islam, M.R.; Akter, A.; Emran, T.B.; Wilairatana, P.; Mubarak, M.S. Bioactive Compounds and Their Derivatives: An Insight into Prospective Phytotherapeutic Approach against Alzheimer’s Disease. Oxid. Med. Cell Longev. 2022, 2022, 5100904. [Google Scholar] [CrossRef]
- Kola, A.; Vigni, G.; Lamponi, S.; Valensin, D. Protective Contribution of Rosmarinic Acid in Rosemary Extract Against Copper-Induced Oxidative Stress. Antioxidants 2024, 13, 1419. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Nafady, M.H.; Islam, M.R.; Saha, S.; Rashid, S.; Akter, A.; Or-Rashid, M.H.; Akhtar, M.F.; Perveen, A.; Ashraf, G.M.; et al. Resveratrol and Neuroprotection: An Insight into Prospective Therapeutic Approaches against Alzheimer’s Disease from Bench to Bedside. Mol. Neurobiol. 2022, 59, 4384–4404. [Google Scholar] [CrossRef]
- Cui, X.; Lin, Q.; Liang, Y. Plant-Derived Antioxidants Protect the Nervous System from Aging by Inhibiting Oxidative Stress. Front. Aging Neurosci. 2020, 12, 209. [Google Scholar] [CrossRef]
- Sridhar, G.R. Acetylcholinesterase Inhibitors (Galantamine, Rivastigmine, and Donepezil). In NeuroPsychopharmacotherapy; Riederer, P., Laux, G., Nagatsu, T., Le, W., Riederer, C., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 2709–2721. ISBN 978-3-030-62059-2. [Google Scholar]
- Dhouafli, Z.; Cuanalo-Contreras, K.; Hayouni, E.A.; Mays, C.E.; Soto, C.; Moreno-Gonzalez, I. Inhibition of Protein Misfolding and Aggregation by Natural Phenolic Compounds. Cell. Mol. Life Sci. 2018, 75, 3521. [Google Scholar] [CrossRef]
- Lin, M.-W.; Chen, Y.-H.; Yang, H.-B.; Lin, C.C.; Hung, S.-Y. Galantamine Inhibits Aβ1–42-Induced Neurotoxicity by Enhancing α7nAChR Expression as a Cargo Carrier for LC3 Binding and Aβ1–42 Engulfment During Autophagic Degradation. Neurotherapeutics 2019, 17, 676. [Google Scholar] [CrossRef]
- Kola, A.; Lamponi, S.; Currò, F.; Valensin, D. A Comparative Study between Lycorine and Galantamine Abilities to Interact with AMYLOID β and Reduce In Vitro Neurotoxicity. Int. J. Mol. Sci. 2023, 24, 2500. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Li, T.; Xu, C.-C.; Qian, J.-Y.; Guo, H.; Zhang, X.; Zhan, Z.-J.; Lu, J.-J. Uncover the Anticancer Potential of Lycorine. Chin. Med. 2024, 19, 121. [Google Scholar] [CrossRef]
- Phelan, M.M.; Caamaño-Gutiérrez, E.; Gant, M.S.; Grosman, R.X.; Madine, J. Using an NMR Metabolomics Approach to Investigate the Pathogenicity of Amyloid-Beta and Alpha-Synuclein. Metabolomics 2017, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Gao, R.; Chen, Z.; Lin, D.; Liu, Z.; Wang, L.; Lin, L.; Liu, X.; Liu, X.; Liu, L. Liraglutide Reduces Oxidative Stress and Improves Energy Metabolism in Methylglyoxal-Induced SH-SY5Y Cells. NeuroToxicology 2022, 92, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Gallego, R.; Valdés, A.; Sánchez-Martínez, J.D.; Suárez-Montenegro, Z.J.; Ibáñez, E.; Cifuentes, A.; Herrero, M. Study of the Potential Neuroprotective Effect of Dunaliella Salina Extract in SH-SY5Y Cell Model. Anal. Bioanal. Chem. 2022, 414, 5357–5371. [Google Scholar] [CrossRef] [PubMed]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. J. Vis. Exp. 2016, 108, 53193. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Valeria, R.; Luisa, S.; Adele, M.; Stefania, B.; Fabio, T.; Nicoletta, B.; Carmine, P.M.; Silvia, A. Changes in the NMR Metabolic Profile of Live Human Neuron-Like SH-SY5Y Cells Exposed to Interferon-A2. J. Neuroimmune Pharmacol. 2016, 11, 142–152. [Google Scholar] [CrossRef]
- da Silva, G.H.R.; Mendes, L.F.; de Carvalho, F.V.; de Paula, E.; Duarte, I.F. Comparative Metabolomics Study of the Impact of Articaine and Lidocaine on the Metabolism of SH-SY5Y Neuronal Cells. Metabolites 2022, 12, 581. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Sig. Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Varma, V.R.; Oommen, A.M.; Varma, S.; Casanova, R.; An, Y.; Andrews, R.M.; O’Brien, R.; Pletnikova, O.; Troncoso, J.C.; Toledo, J.; et al. Brain and Blood Metabolite Signatures of Pathology and Progression in Alzheimer Disease: A Targeted Metabolomics Study. PLOS Med. 2018, 15, e1002482. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.V.; Liu, M.; Beric, A.; Johnson, M.; Cetin, A.; Patel, M.; Budde, J.; Kohlfeld, P.; Bergmann, K.; Lowery, J.; et al. Genetic and Multi-Omic Resources for Alzheimer Disease and Related Dementia from the Knight Alzheimer Disease Research Center. Sci. Data 2024, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J. Treating Neurodegenerative Diseases with Multitarget Drugs: An Interview with Maria João Matos. Future Med. Chem. 2022, 14, 847–850. [Google Scholar] [CrossRef]
- Kumar, D.; Ashraf, G.M.; Bilgrami, A.L.; Imtaiyaz Hassan, M. Emerging Therapeutic Developments in Neurodegenerative Diseases: A Clinical Investigation. Drug Discov. Today 2022, 27, 103305. [Google Scholar] [CrossRef]
- Bell, M.; Zempel, H. SH-SY5Y-Derived Neurons: A Human Neuronal Model System for Investigating TAU Sorting and Neuronal Subtype-Specific TAU Vulnerability. Rev. Neurosci. 2022, 33, 1–15. [Google Scholar] [CrossRef]
- Langerscheidt, F.; Bell-Simons, M.; Zempel, H. Differentiating SH-SY5Y Cells into Polarized Human Neurons for Studying Endogenous and Exogenous Tau Trafficking: Four Protocols to Obtain Neurons with Noradrenergic, Dopaminergic, and Cholinergic Properties. In Tau Protein: Methods and Protocols; Smet-Nocca, C., Ed.; Springer: New York, NY, USA, 2024; pp. 521–532. ISBN 978-1-07-163629-9. [Google Scholar]
- Kovalevich, J.; Santerre, M.; Langford, D. Considerations for the Use of SH-SY5YSH-SY5Y NeuroblastomaNeuroblastoma Cells in Neurobiology. In Neuronal Cell Culture: Methods and Protocols; Amini, S., White, M.K., Eds.; Springer: New York, NY, USA, 2021; pp. 9–23. ISBN 978-1-07-161437-2. [Google Scholar]
- Agholme, L.; Lindström, T.; Kågedal, K.; Marcusson, J.; Hallbeck, M. An In Vitro Model for Neuroscience: Differentiation of SH-SY5Y Cells into Cells with Morphological and Biochemical Characteristics of Mature Neurons. J. Alzheimer’s Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y Cell Line in Parkinson’s Disease Research: A Systematic Review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Dwane, S.; Durack, E.; Kiely, P.A. Optimising Parameters for the Differentiation of SH-SY5Y Cells to Study Cell Adhesion and Cell Migration. BMC Res. Notes 2013, 6, 366. [Google Scholar] [CrossRef]
- Krishna, A.; Biryukov, M.; Trefois, C.; Antony, P.M.; Hussong, R.; Lin, J.; Heinäniemi, M.; Glusman, G.; Köglsberger, S.; Boyd, O.; et al. Systems Genomics Evaluation of the SH-SY5Y Neuroblastoma Cell Line as a Model for Parkinson’s Disease. BMC Genom. 2014, 15, 1154. [Google Scholar] [CrossRef]
- Encinas, M.; Iglesias, M.; Liu, Y.; Wang, H.; Muhaisen, A.; Ceña, V.; Gallego, C.; Comella, J.X. Sequential Treatment of SH-SY5Y Cells with Retinoic Acid and Brain-Derived Neurotrophic Factor Gives Rise to Fully Differentiated, Neurotrophic Factor-Dependent, Human Neuron-Like Cells. J. Neurochem. 2000, 75, 991–1003. [Google Scholar] [CrossRef]
- Kume, T.; Kawato, Y.; Osakada, F.; Izumi, Y.; Katsuki, H.; Nakagawa, T.; Kaneko, S.; Niidome, T.; Takada-Takatori, Y.; Akaike, A. Dibutyryl Cyclic AMP Induces Differentiation of Human Neuroblastoma SH-SY5Y Cells into a Noradrenergic Phenotype. Neurosci. Lett. 2008, 443, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.-R.; Gandawijaya, J.; Oguro-Ando, A. A Novel Method for Generating Glutamatergic SH-SY5Y Neuron-like Cells Utilizing B-27 Supplement. Front. Pharmacol. 2022, 13, 943627. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, R.; Mulholland, M.T.; Sedensky, M.; Morgan, P.; Johnson, S.C. Glutamine Metabolism in Diseases Associated with Mitochondrial Dysfunction. Mol. Cell. Neurosci. 2023, 126, 103887. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Markussen, K.H.; Jakobsen, E.; Schousboe, A.; Waagepetersen, H.S.; Rosenberg, P.A.; Aldana, B.I. Glutamate Metabolism and Recycling at the Excitatory Synapse in Health and Neurodegeneration. Neuropharmacology 2021, 196, 108719. [Google Scholar] [CrossRef]
- Zhang, D.; Hua, Z.; Li, Z. The Role of Glutamate and Glutamine Metabolism and Related Transporters in Nerve Cells. CNS Neurosci. Ther. 2024, 30, e14617. [Google Scholar] [CrossRef]
- Baek, J.H.; Park, H.; Kang, H.; Kim, R.; Kang, J.S.; Kim, H.J. The Role of Glutamine Homeostasis in Emotional and Cognitive Functions. Int. J. Mol. Sci. 2024, 25, 1302. [Google Scholar] [CrossRef]
- Schneider, L.; Giordano, S.; Zelickson, B.R.; Johnson, M.S.; Benavides, G.A.; Ouyang, X.; Fineberg, N.; Darley-Usmar, V.M.; Zhang, J. Differentiation of SH-SY5Y Cells to a Neuronal Phenotype Changes Cellular Bioenergetics and the Response to Oxidative Stress. Free Radic. Biol. Med. 2011, 51, 2007–2017. [Google Scholar] [CrossRef]
- Sakagami, H.; Suzuki, R.; Shirataki, Y.; Iwama, S.; Nakagawa, M.; Suzuki, H.; Tanaka, K.; Tamura, N.; Takeshima, H. Re-Evaluation of Culture Condition of PC12 and SH-SY5Y Cells Based on Growth Rate and Amino Acid Consumption. In Vivo 2017, 31, 1089–1095. [Google Scholar]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef]
- Griffin, J.W.D.; Bradshaw, P.C. Amino Acid Catabolism in Alzheimer’s Disease Brain: Friend or Foe? Oxidative Med. Cell. Longev. 2017, 2017, 5472792. [Google Scholar] [CrossRef]
- Rupsingh, R.; Borrie, M.; Smith, M.; Wells, J.L.; Bartha, R. Reduced Hippocampal Glutamate in Alzheimer Disease. Neurobiol. Aging 2011, 32, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Batra, R.; Krumsiek, J.; Wang, X.; Allen, M.; Blach, C.; Kastenmüller, G.; Arnold, M.; Ertekin-Taner, N.; Kaddurah-Daouk, R.; Alzheimer’s Disease Metabolomics Consortium (ADMC). Comparative Brain Metabolomics Reveals Shared and Distinct Metabolic Alterations in Alzheimer’s Disease and Progressive Supranuclear Palsy. Alzheimer’s Dement. 2024, 20, 8294–8307. [Google Scholar] [CrossRef]
- Jasbi, P.; Shi, X.; Chu, P.; Elliott, N.; Hudson, H.; Jones, D.; Serrano, G.; Chow, B.; Beach, T.G.; Liu, L.; et al. Metabolic Profiling of Neocortical Tissue Discriminates Alzheimer’s Disease from Mild Cognitive Impairment, High Pathology Controls, and Normal Controls. J. Proteome Res. 2021, 20, 4303–4317. [Google Scholar] [CrossRef] [PubMed]
- Trushina, E.; Dutta, T.; Persson, X.-M.T.; Mielke, M.M.; Petersen, R.C. Identification of Altered Metabolic Pathways in Plasma and CSF in Mild Cognitive Impairment and Alzheimer’s Disease Using Metabolomics. PLoS ONE 2013, 8, e63644. [Google Scholar] [CrossRef]
- Huang, Z.; Yan, Q.; Wang, Y.; Zou, Q.; Li, J.; Liu, Z.; Cai, Z. Role of Mitochondrial Dysfunction in the Pathology of Amyloid-β. J. Alzheimer’s Dis. 2020, 78, 505–514. [Google Scholar] [CrossRef]
- Gibson, G.E.; Xu, H.; Chen, H.-L.; Chen, W.; Denton, T.; Zhang, S. Alpha-Ketoglutarate Dehydrogenase Complex-Dependent Succinylation of Proteins in Neurons and Neuronal Cell Lines. J. Neurochem. 2015, 134, 86–96. [Google Scholar] [CrossRef]
- Newington, J.T.; Rappon, T.; Albers, S.; Wong, D.Y.; Rylett, R.J.; Cumming, R.C. Overexpression of Pyruvate Dehydrogenase Kinase 1 and Lactate Dehydrogenase A in Nerve Cells Confers Resistance to Amyloid β and Other Toxins by Decreasing Mitochondrial Respiration and Reactive Oxygen Species Production. J. Biol. Chem. 2012, 287, 37245–37258. [Google Scholar] [CrossRef]
- Desagher, S.; Glowinski, J.; Prémont, J. Pyruvate Protects Neurons against Hydrogen Peroxide-Induced Toxicity. J. Neurosci. 1997, 17, 9060–9067. [Google Scholar] [CrossRef]
- Wang, X.; Perez, E.; Liu, R.; Yan, L.-J.; Mallet, R.T.; Yang, S.-H. Pyruvate Protects Mitochondria from Oxidative Stress in Human Neuroblastoma SK-N-SH Cells. Brain Res. 2007, 1132, 1–9. [Google Scholar] [CrossRef]
- Wang, Q.; van Hoecke, M.; Tang, X.N.; Lee, H.; Zheng, Z.; Swanson, R.A.; Yenari, M.A. Pyruvate Protects against Experimental Stroke via an Anti-Inflammatory Mechanism. Neurobiol. Dis. 2009, 36, 223–231. [Google Scholar] [CrossRef]
- Ryou, M.-G.; Liu, R.; Ren, M.; Sun, J.; Mallet, R.T.; Yang, S.-H. Pyruvate Protects the Brain Against Ischemia–Reperfusion Injury by Activating the Erythropoietin Signaling Pathway. Stroke 2012, 43, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Cheng, J.; Zhang, J.; Ye, L.; Zhang, F.; Dong, Q.; Wang, H.; Fu, F. Protection of Pyruvate against Glutamate Excitotoxicity Is Mediated by Regulating DAPK1 Protein Complex. PLoS ONE 2014, 9, e95777. [Google Scholar] [CrossRef] [PubMed]
- Fedotova, E.I.; Dolgacheva, L.P.; Abramov, A.Y.; Berezhnov, A.V. Lactate and Pyruvate Activate Autophagy and Mitophagy That Protect Cells in Toxic Model of Parkinson’s Disease. Mol. Neurobiol. 2022, 59, 177–190. [Google Scholar] [CrossRef]
- Maffioli, E.; Murtas, G.; Rabattoni, V.; Badone, B.; Tripodi, F.; Iannuzzi, F.; Licastro, D.; Nonnis, S.; Rinaldi, A.M.; Motta, Z.; et al. Insulin and Serine Metabolism as Sex-Specific Hallmarks of Alzheimer’s Disease in the Human Hippocampus. Cell Rep. 2022, 40, 111271. [Google Scholar] [CrossRef]
- Ambeskovic, M.; Hopkins, G.; Hoover, T.; Joseph, J.T.; Montina, T.; Metz, G.A.S. Metabolomic Signatures of Alzheimer’s Disease Indicate Brain Region-Specific Neurodegenerative Progression. Int. J. Mol. Sci. 2023, 24, 14769. [Google Scholar] [CrossRef]
- Parnetti, L.; Gaiti, A.; Polidori, M.C.; Brunetti, M.; Palumbo, B.; Chionne, F.; Cadini, D.; Cecchetti, R.; Senin, U. Increased Cerebrospinal Fluid Pyruvate Levels in Alzheimer’s Disease. Neurosci. Lett. 1995, 199, 231–233. [Google Scholar] [CrossRef]
- Nielsen, J.E.; Andreassen, T.; Gotfredsen, C.H.; Olsen, D.A.; Vestergaard, K.; Madsen, J.S.; Kristensen, S.R.; Pedersen, S. Serum Metabolic Signatures for Alzheimer’s Disease Reveal Alterations in Amino Acid Composition: A Validation Study. Metabolomics 2024, 20, 12. [Google Scholar] [CrossRef]

| Day | Procedure |
|---|---|
| Day 1 | Replace BGM with Differentiation Medium 1 (DM1) *. |
| Day 3 | Change DM1 with new medium. |
| Day 6 | Change DM1 with new medium. |
| Day 7 | Split cells, suspend in DM1, and replate into new 35 mm2 Petri dishes. |
| Day 8 | Replace DM1 with Differentiation Medium 2 (DM2) **. |
| Day 10 | Split cells, suspend in DM2, and seed into extracellular matrix (ECM)-coated 35 mm2 plates. |
| Day 13 | Replace DM2 with Differentiation Medium 3 (DM3) ***. |
| Day 14 | Change DM3 with new medium. |
| Day 17 | Change DM3 with new medium. |
| Day 18 | Neuronal cultures are ready for experiments. |
| Compound | Concentrations |
|---|---|
| Aβ | 2 µM |
| LYC | 8 µM |
| 1.6 µM | |
| GAL | 500 µM |
| 250 µM |
| Sample | % of Viable Differentiated SH-SY5Y Cells |
|---|---|
| LYCORINE | |
| Aβ42 2 µM | 54 ± 2 |
| Aβ42 2 µM + LYC 8 µM | 94 ± 2 |
| Aβ42 2 µM + LYC 1.6 µM | 98 ± 3 |
| GALANTAMINE | |
| Aβ42 2 µM | 56 ± 2 |
| Aβ42 2 µM + GAL 500 µM | 77 ± 2 |
| Aβ42 2 µM + GAL 250 µM | 51 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kola, A.; Costanti, F.; Kahfi, J.; Emwas, A.-H.; Jaremko, M.; Valensin, D. NMR Metabolomic Profiling of Differentiated SH-SY5Y Neuronal Cells: Amyloid-β Toxicity and Protective Effects of Galantamine and Lycorine. Cells 2025, 14, 525. https://doi.org/10.3390/cells14070525
Kola A, Costanti F, Kahfi J, Emwas A-H, Jaremko M, Valensin D. NMR Metabolomic Profiling of Differentiated SH-SY5Y Neuronal Cells: Amyloid-β Toxicity and Protective Effects of Galantamine and Lycorine. Cells. 2025; 14(7):525. https://doi.org/10.3390/cells14070525
Chicago/Turabian StyleKola, Arian, Filippo Costanti, Jordan Kahfi, Abdul-Hamid Emwas, Mariusz Jaremko, and Daniela Valensin. 2025. "NMR Metabolomic Profiling of Differentiated SH-SY5Y Neuronal Cells: Amyloid-β Toxicity and Protective Effects of Galantamine and Lycorine" Cells 14, no. 7: 525. https://doi.org/10.3390/cells14070525
APA StyleKola, A., Costanti, F., Kahfi, J., Emwas, A.-H., Jaremko, M., & Valensin, D. (2025). NMR Metabolomic Profiling of Differentiated SH-SY5Y Neuronal Cells: Amyloid-β Toxicity and Protective Effects of Galantamine and Lycorine. Cells, 14(7), 525. https://doi.org/10.3390/cells14070525







