Lipocalin-2, Matrix Metalloproteinase-9, and MMP-9/NGAL Complex in Upper Aerodigestive Tract Carcinomas: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants’ Selection and Sampling
2.2. Hematologic Parameters
2.3. RNA Extraction
2.4. Reverse Transcription
2.5. Digital Droplet PCR
2.6. Serum LCN-2, MMP-9, and MMP-9/NGAL Complex Analysis
2.7. Statistical Analysis
3. Results
3.1. General Description of the Enrolled Individuals
3.2. Hematologic Parameters
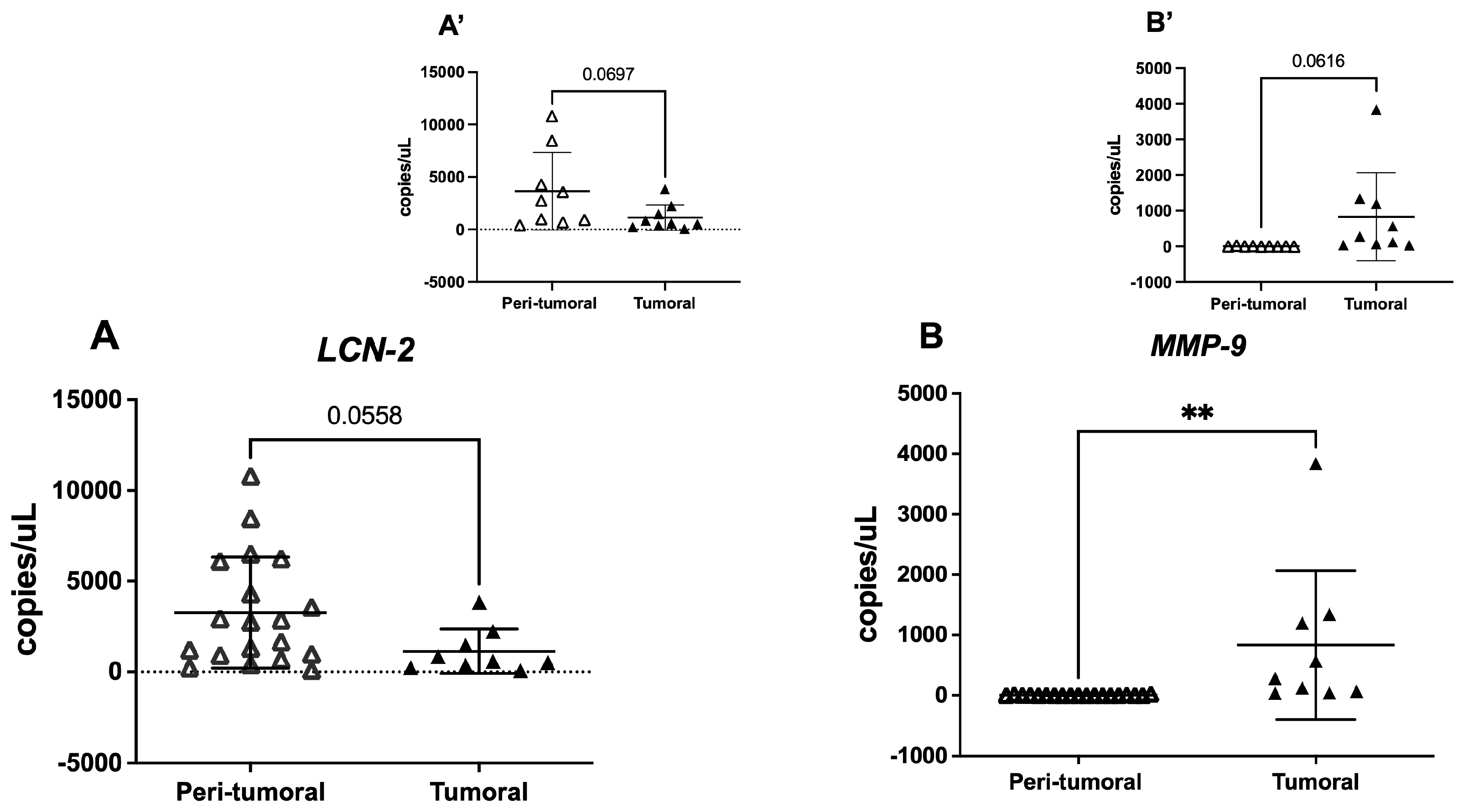
3.3. Tissue LCN-2 and MMP-9 Gene Expression
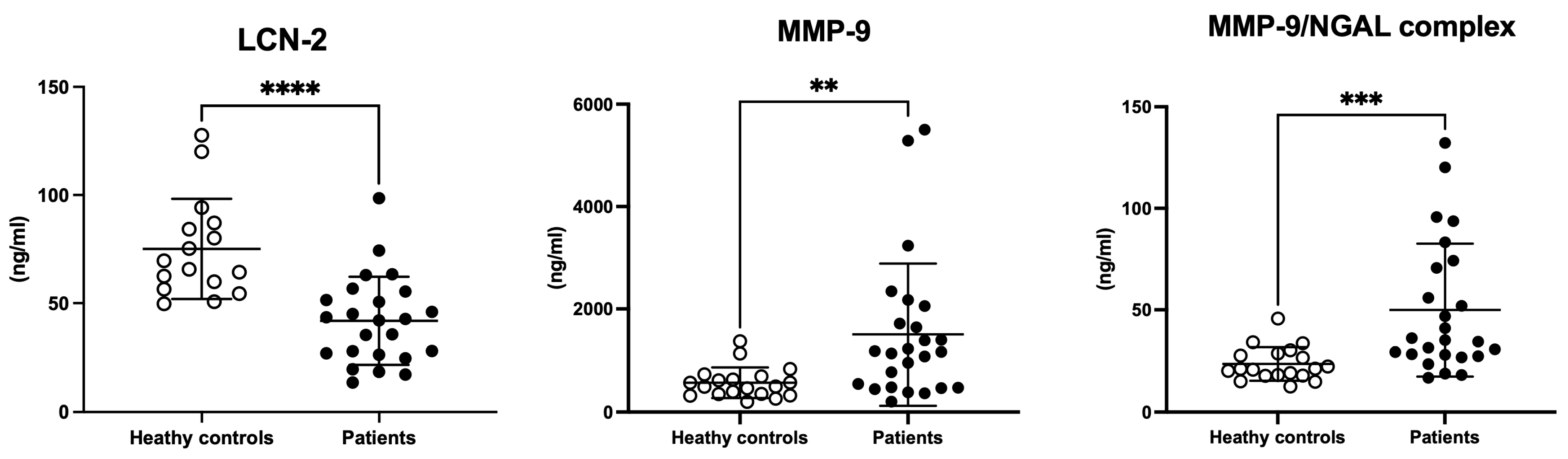
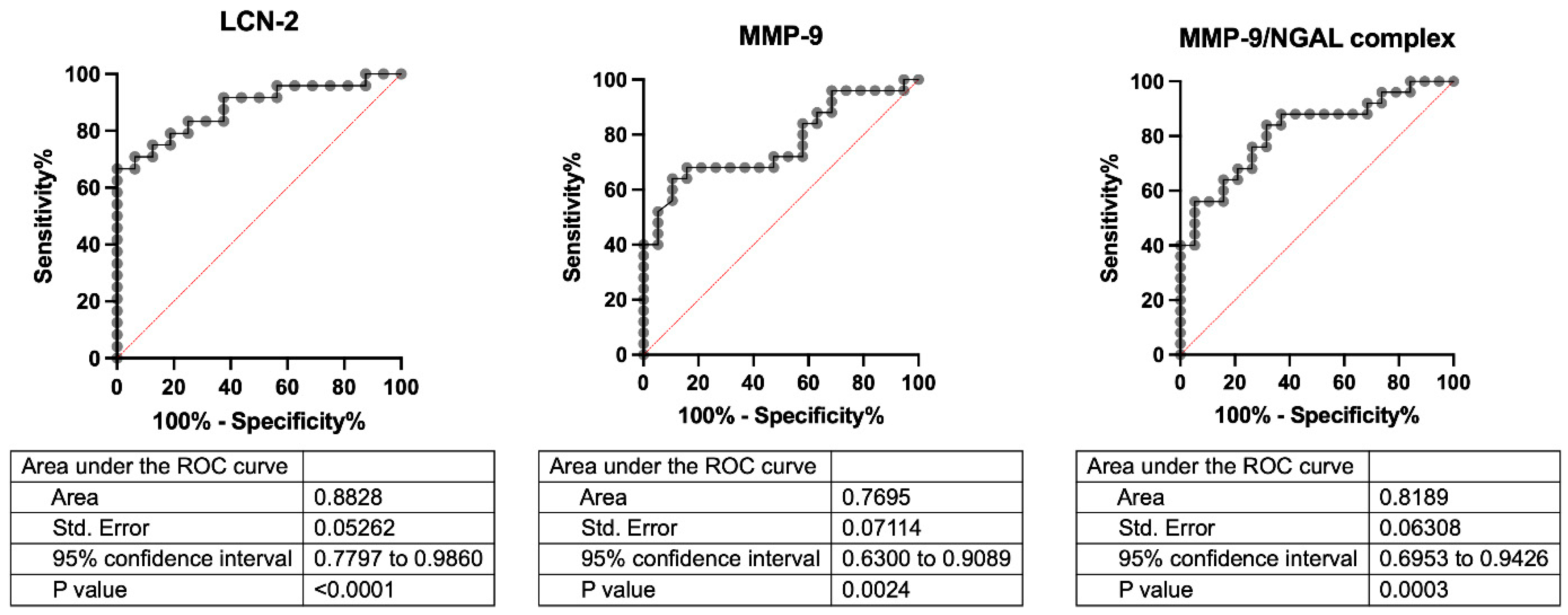
3.4. Serum LCN-2, MMP-9, and MMP-9/NGAL Complex
3.5. Spearman’s Correlation
4. Discussion
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, B.; Lalloo, R.; Johnson, N.W. Life course models for upper aero-digestive tract cancer. Int. Dent. J. 2015, 65, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.; Eveson, J.W.; Reichart, P.; Sidransky, D. Pathology and Genetics of Head and Neck Tumours; IARC: Lyon, France, 2005; Volume 9. [Google Scholar]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Sawabe, M.; Ito, H.; Oze, I.; Hosono, S.; Kawakita, D.; Tanaka, H.; Hasegawa, Y.; Murakami, S.; Matsuo, K. Heterogeneous impact of alcohol consumption according to treatment method on survival in head and neck cancer: A prospective study. Cancer Sci. 2017, 108, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Fedewa, S.; Chen, A.Y. Epidemiology and Demographics of the Head and Neck Cancer Population. Oral Maxillofac. Surg. Clin. N. Am. 2018, 30, 381–395. [Google Scholar] [CrossRef]
- Warren, G.W.; Arnold, S.M.; Valentino, J.P.; Gal, T.J.; Hyland, A.J.; Singh, A.K.; Rangnekar, V.M.; Cummings, K.M.; Marshall, J.R.; Kudrimoti, M.R. Accuracy of self-reported tobacco assessments in a head and neck cancer treatment population. Radiother. Oncol. 2012, 103, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Pandruvada, S.; Kessler, R.; Thai, A. Head and neck cancer treatment in the era of molecular medicine. Adv. Cancer Res. 2023, 160, 205–252. [Google Scholar] [CrossRef]
- Mesia, R.; Iglesias, L.; Lambea, J.; Martínez-Trufero, J.; Soria, A.; Taberna, M.; Trigo, J.; Chaves, M.; García-Castaño, A.; Cruz, J. SEOM clinical guidelines for the treatment of head and neck cancer (2020). Clin. Transl. Oncol. 2021, 23, 913–921. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2016; Volume 91, pp. 386–396. [Google Scholar] [CrossRef]
- Meliante, P.G.; Petrella, C.; Fiore, M.; Minni, A.; Barbato, C. Antioxidant Use after Diagnosis of Head and Neck Squamous Cell Carcinoma (HNSCC): A Systematic Review of Application during Radiotherapy and in Second Primary Cancer Prevention. Antioxidants 2023, 12, 1753. [Google Scholar] [CrossRef]
- Riccardi, G.; Bellizzi, M.G.; Fatuzzo, I.; Zoccali, F.; Cavalcanti, L.; Greco, A.; de Vincentiis, M.; Ralli, M.; Fiore, M.; Petrella, C.; et al. Salivary Biomarkers in Oral Squamous Cell Carcinoma: A Proteomic Overview. Proteomes 2022, 10, 37. [Google Scholar] [CrossRef]
- Meliante, P.G.; Zoccali, F.; de Vincentiis, M.; Ralli, M.; Petrella, C.; Fiore, M.; Minni, A.; Barbato, C. Diagnostic Predictors of Immunotherapy Response in Head and Neck Squamous Cell Carcinoma. Diagnostics 2023, 13, 862. [Google Scholar] [CrossRef]
- Al Jaberi, S.; Cohen, A.; D’souza, C.; Abdulrazzaq, Y.M.; Ojha, S.; Bastaki, S.; Adeghate, E.A. Lipocalin-2: Structure, function, distribution and role in metabolic disorders. Biomed. Pharmacother. 2021, 142, 112002. [Google Scholar] [CrossRef]
- Romejko, K.; Markowska, M.; Niemczyk, S. The Review of Current Knowledge on Neutrophil Gelatinase-Associated Lipocalin (NGAL). Int. J. Mol. Sci. 2023, 24, 10470. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, L.; Johnsen, A.; Sengeløv, H.; Borregaard, N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, L.; Bainton, D.F.; Sengeløv, H.; Borregaard, N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 1994, 83, 799–807. [Google Scholar]
- Wang, Y.; Lam, K.S.L.; Kraegen, E.W.; Sweeney, G.; Zhang, J.; Tso, A.W.; Chow, W.-S.; Wat, N.M.; Xu, J.Y.; Hoo, R.L.; et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 2007, 53, 34–41. [Google Scholar] [CrossRef]
- Santiago-Sánchez, G.S.; Pita-Grisanti, V.; Quiñones-Díaz, B.; Gumpper, K.; Cruz-Monserrate, Z.; Vivas-Mejía, P.E. Biological Functions and Therapeutic Potential of Lipocalin 2 in Cancer. Int. J. Mol. Sci. 2020, 21, 4365. [Google Scholar] [CrossRef] [PubMed]
- Meyers, K.; López, M.; Ho, J.; Wills, S.; Rayalam, S.; Taval, S. Lipocalin-2 deficiency may predispose to the progression of spontaneous age-related adiposity in mice. Sci. Rep. 2020, 10, 14589. [Google Scholar] [CrossRef]
- Monisha, J.; Roy, N.K.; Padmavathi, G.; Banik, K.; Bordoloi, D.; Khwairakpam, A.D.; Arfuso, F.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; et al. NGAL is Downregulated in Oral Squamous Cell Carcinoma and Leads to Increased Survival, Proliferation, Migration and Chemoresistance. Cancers 2018, 10, 228. [Google Scholar] [CrossRef]
- Rashid, Z.A.; Bardaweel, S.K. Novel Matrix Metalloproteinase-9 (MMP-9) Inhibitors in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 12133. [Google Scholar] [CrossRef]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260. [Google Scholar] [CrossRef]
- Eilenberg, W.; Stojkovic, S.; Kaider, A.; Kozakowski, N.; Domenig, C.M.; Burghuber, C.; Nanobachvili, J.; Huber, K.; Klinger, M.; Neumayer, C.; et al. NGAL and MMP-9/NGAL as biomarkers of plaque vulnerability and targets of statins in patients with carotid atherosclerosis. Clin. Chem. Lab. Med. (CCLM) 2017, 56, 147–156. [Google Scholar] [CrossRef]
- D’Amico, F.; Candido, S.; Libra, M. Interaction between matrix metalloproteinase-9 (MMP-9) and neutrophil gelatinase-associated lipocalin (NGAL): A recent evolutionary event in primates. Dev. Comp. Immunol. 2021, 116, 103933. [Google Scholar] [CrossRef] [PubMed]
- Candido, S.; Abrams, S.L.; Steelman, L.S.; Lertpiriyapong, K.; Fitzgerald, T.L.; Martelli, A.M.; Cocco, L.; Montalto, G.; Cervello, M.; Polesel, J.; et al. Roles of NGAL and MMP-9 in the tumor microenvironment and sensitivity to targeted therapy. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 438–448. [Google Scholar] [CrossRef]
- Tsakogiannis, D.; Kalogera, E.; Zagouri, F.; Zografos, E.; Balalis, D.; Bletsa, G. Determination of FABP4, RBP4 and the MMP-9/NGAL complex in the serum of women with breast cancer. Oncol. Lett. 2021, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.M.; Blaconà, G.; Cicero, S.L.; Castelli, G.; Virgulti, M.; Testino, G.; Pierandrei, S.; Fuso, A.; Cimino, G.; Ferraguti, G.; et al. Quantitative Evaluation of CFTR Gene Expression: A Comparison between Relative Quantification by Real-Time PCR and Absolute Quantification by Droplet Digital PCR. Genes 2023, 14, 1781. [Google Scholar] [CrossRef] [PubMed]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Glastonbury, C.M. Head and Neck Squamous Cell Cancer: Approach to Staging and Surveillance. In Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging; Springer: Berlin/Heidelberg, Germany, 2020; pp. 215–222. [Google Scholar] [CrossRef]
- Sano, D.; Yabuki, K.; Arai, Y.; Tanabe, T.; Chiba, Y.; Nishimura, G.; Takahashi, H.; Yamanaka, S.; Oridate, N. The applicability of new TNM classification for humanpapilloma virus-related oropharyngeal cancer in the 8th edition of the AJCC/UICC TNM staging system in Japan: A single-centre study. Auris Nasus Larynx 2018, 45, 558–565. [Google Scholar] [CrossRef]
- Asaf, S.; Maqsood, F.; Jalil, J.; Sarfraz, Z.; Sarfraz, A.; Mustafa, S.; Ojeda, I.C. Lipocalin 2—not only a biomarker: A study of current literature and systematic findings of ongoing clinical trials. Immunol. Res. 2023, 71, 287–313. [Google Scholar] [CrossRef]
- Živalj, M.; Van Ginderachter, J.A.; Stijlemans, B. Lipocalin-2: A Nurturer of Tumor Progression and a Novel Candidate for Targeted Cancer Therapy. Cancers 2023, 15, 5159. [Google Scholar] [CrossRef]
- Bao, Y.; Yan, Z.; Shi, N.; Tian, X.; Li, J.; Li, T.; Cheng, X.; Lv, J. LCN2: Versatile players in breast cancer. Biomed. Pharmacother. 2024, 171, 116091. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.T.; Min, I.S.; Park, Y.R.; Lee, J.H.; Kim, D.; Kim, S. Lipocalin 2 negatively regulates cell proliferation and epithelial to mesenchymal transition through changing metabolic gene expression in colorectal cancer. Cancer Sci. 2017, 108, 2176–2186. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, K.; Zhou, J.; Li, M.; Fan, M.; Gao, H.; Ma, R.; Gao, L.; Chen, M. Bioinformatics and experimental approach identify lipocalin 2 as a diagnostic and prognostic indicator for lung adenocarcinoma. Int. J. Biol. Macromol. 2024, 272, 132797. [Google Scholar] [CrossRef]
- Provatopoulou, X.; Gounaris, A.; Kalogera, E.; Zagouri, F.; Flessas, I.; Goussetis, E.; Nonni, A.; Papassotiriou, I.; Zografos, G. Circulating levels of matrix metalloproteinase-9 (MMP-9), neutrophil gelatinase-associated lipocalin (NGAL) and their complex MMP-9/NGAL in breast cancer disease. BMC Cancer 2009, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Yeh, T.S.; Wu, R.C.; Pang, J.H.S.; Cheng, C.T.; Wang, S.Y.; Yeh, C.N. Lipocalin 2 (LCN2) is a promising target for cholangiocarcinoma treatment and bile LCN2 level is a potential cholangiocarcinoma diagnostic marker. Sci. Rep. 2016, 6, 36138. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Eickhoff, J.C.; Gould, M.N.; Mundhenke, C.; Maass, N.; Friedl, A. Neutrophil gelatinase-associated lipocalin (NGAL) is a predictor of poor prognosis in human primary breast cancer. Breast Cancer Res. Treat. 2008, 108, 389–397. [Google Scholar] [CrossRef]
- Iannetti, A.; Pacifico, F.; Acquaviva, R.; Lavorgna, A.; Crescenzi, E.; Vascotto, C.; Tell, G.; Salzano, A.M.; Scaloni, A.; Vuttariello, E.; et al. The neutrophil gelatinase-associated lipocalin (NGAL), a NF-κB-regulated gene, is a survival factor for thyroid neoplastic cells. Proc. Natl. Acad. Sci. USA 2008, 105, 14058–14063. [Google Scholar] [CrossRef]
- Gomez-Chou, S.B.; Swidnicka-Siergiejko, A.K.; Badi, N.; Chavez-Tomar, M.; Lesinski, G.B.; Bekaii-Saab, T.; Farren, M.R.; Mace, T.A.; Schmidt, C.; Liu, Y.; et al. Lipocalin-2 Promotes Pancreatic Ductal Adenocarcinoma by Regulating Inflammation in the Tumor Microenvironment. Cancer Res. 2017, 77, 2647–2660. [Google Scholar] [CrossRef]
- Noguchi, K.; Hiromoto, T.; Yamamura, M.; Zushi, Y.; Segawa, E.; Takaoka, K.; Moridera, K.; Kishimoto, H.; Urade, M. Up-regulation of neutrophil gelatinase-associated lipocalin in oral squamous cell carcinoma: Relation to cell differentiation. Oncol. Rep. 2011, 26, 1415–1421. [Google Scholar] [CrossRef]
- Lin, C.W.; Tseng, S.W.; Yang, S.F.; Ko, C.P.; Lin, C.H.; Wei, L.H.; Hsieh, Y.S. Role of lipocalin 2 and its complex with matrix metalloproteinase-9 in oral cancer. Oral Dis. 2012, 18, 734–740. [Google Scholar] [CrossRef]
- Huang, Z.; Rui, X.; Yi, C.; Chen, Y.; Chen, R.; Liang, Y.; Wang, Y.; Yao, W.; Xu, X.; Huang, Z. Silencing LCN2 suppresses oral squamous cell carcinoma progression by reducing EGFR signal activation and recycling. J. Exp. Clin. Cancer Res. 2023, 42, 60, Erratum in J. Exp. Clin. Cancer Res. 2023, 42, 104. https://doi.org/10.1186/s13046-023-02679-0. [Google Scholar] [CrossRef]
- Moghaddam, S.J.K.; Roushandeh, A.M.; Hamidi, M.; Nemati, S.; Jahanian-Najafabadi, A.; Roudkenar, M.H. Lipocalin-2 Upregulation in Nasopharyngeal Carcinoma: A Novel Potential Diagnostic Biomarker. Iran. J. Med. Sci. 2023, 48, 268–276. [Google Scholar] [CrossRef]
- Lin, C.-W.; Yang, W.-E.; Lee, W.-J.; Hua, K.-T.; Hsieh, F.-K.; Hsiao, M.; Chen, C.-C.; Chow, J.-M.; Chen, M.-K.; Yang, S.-F.; et al. Lipocalin 2 prevents oral cancer metastasis through carbonic anhydrase IX inhibition and is associated with favourable prognosis. Carcinog. 2016, 37, 712–722. [Google Scholar] [CrossRef]
- Li, J.-P.; Lin, C.-W.; Huang, C.-C.; Lu, Y.-T.; Ho, Y.-T.; Yang, S.-F.; Hsin, C.-H. Lipocalin 2 Reduces MET Levels by Inhibiting MEK/ERK Signaling to Inhibit Nasopharyngeal Carcinoma Cell Migration. Cancers 2022, 14, 5707. [Google Scholar] [CrossRef]
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in translation: From molecule to brain physiology, pathology, and therapy. J. Neurochem. 2016, 139, 91–114. [Google Scholar] [CrossRef]
- Mittal, R.; Patel, A.P.; Debs, L.H.; Nguyen, D.; Patel, K.; Grati, M.; Mittal, J.; Yan, D.; Chapagain, P.; Liu, X.Z. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. J. Cell. Physiol. 2016, 231, 2599–2621. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.B.; Ashraf, N.S.; Mahjabeen, I. Deregulation of MMP-2 and MMP-9 in laryngeal cancer: A retrospective observational study. Medicine 2024, 103, e38362. [Google Scholar] [CrossRef]
- Song, Z.; Wang, J.; Su, Q.; Luan, M.; Chen, X.; Xu, X. The role of MMP-2 and MMP-9 in the metastasis and development of hypopharyngeal carcinoma. Braz. J. Otorhinolaryngol. 2021, 87, 521–528. [Google Scholar] [CrossRef]
- Xia, Q.; Du, Z.; Chen, M.; Zhou, X.; Bai, W.; Zheng, X.; Lin, L.; Zhao, Y.; Ding, J.; Wu, Z.; et al. A protein complex of LCN2, LOXL2 and MMP9 facilitates tumour metastasis in oesophageal cancer. Mol. Oncol. 2023, 17, 2451–2471. [Google Scholar] [CrossRef]
- Liu, M.-F.; Hu, Y.-Y.; Jin, T.; Xu, K.; Wang, S.-H.; Du, G.-Z.; Wu, B.-L.; Li, L.-Y.; Xu, L.-Y.; Li, E.-M.; et al. Matrix Metalloproteinase-9/Neutrophil Gelatinase-Associated Lipocalin Complex Activity in Human Glioma Samples Predicts Tumor Presence and Clinical Prognosis. Dis. Markers 2015, 2015, 138974. [Google Scholar] [CrossRef]
- Gao, G.; Li, J.; Zhang, Y.; Chang, Y.-Z. Cellular Iron Metabolism and Regulation. In Brain Iron Metabolism and CNS Diseases; Springer: Berlin/Heidelberg, Germany, 2019; pp. 21–32. [Google Scholar] [CrossRef]
- Ni, S.; Yuan, Y.; Kuang, Y.; Li, X. Iron Metabolism and Immune Regulation. Front. Immunol. 2022, 13, 816282. [Google Scholar] [CrossRef]
- Gamage, S.M.; Lee, K.T.; Dissabandara, D.L.O.; Lam, A.K.-Y.; Gopalan, V. Dual role of heme iron in cancer; promotor of carcinogenesis and an inducer of tumour suppression. Exp. Mol. Pathol. 2021, 120, 104642. [Google Scholar] [CrossRef]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. 2017, 21 (Suppl. S1), S6–S20. [Google Scholar] [CrossRef] [PubMed]
- Salnikow, K. Role of iron in cancer. Semin. Cancer Biol. 2021, 76, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Mertens, C.; Bauer, R.; Rehwald, C.; Brüne, B. Lipocalin-2 and iron trafficking in the tumor microenvironment. Pharmacol. Res. 2017, 120, 146–156. [Google Scholar] [CrossRef]
- Xiao, X.; Yeoh, B.S.; Vijay-Kumar, M. Lipocalin 2: An Emerging Player in Iron Homeostasis and Inflammation. Annu. Rev. Nutr. 2017, 37, 103–130. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.D. Iron metabolism at the host pathogen interface: Lipocalin 2 and the pathogen-associated iroA gene cluster. Int. J. Biochem. Cell Biol. 2007, 39, 1776–1780. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef]
- Deng, L.; He, S.; Guo, N.; Tian, W.; Zhang, W.; Luo, L. Molecular mechanisms of ferroptosis and relevance to inflammation. Inflamm. Res. 2023, 72, 281–299. [Google Scholar] [CrossRef]
| Patient ID# | Sex | Age (Years) | Cancer District | p/cTNM | Serum Sampling (Yes/No) | Tissue Sampling (Yes/No) | |
|---|---|---|---|---|---|---|---|
| Peri- Tumoral | Tumoral | ||||||
| 1 | M | 60 | Hypopharynx | cT4bN3bM0 | Yes | No | No |
| 2 | F | 57 | Hypopharynx | pT4aN0M0 | Yes | No | No |
| 3 | M | 72 | Larynx | pT4aN0M0 | Yes | No | No |
| 4 | M | 58 | Hypopharynx | pT3N3bM0 | Yes | No | No |
| 5 | M | 54 | Larynx | pT3N3bM0 | Yes | No | No |
| 6 | M | 63 | Larynx | pT4aN0M0 | Yes | Yes | No |
| 7 | M | 57 | Tongue | pT3N1M0 | Yes | Yes | No |
| 8 | M | 49 | Larynx | pT3N0M0 | Yes | Yes | No |
| 9 | M | 66 | Oropharynx | cT4N2bM0 | Yes | No | No |
| 10 | F | 58 | Tongue | pT3N3bM0 | Yes | Yes | No |
| 11 | M | 59 | Upper Gingiva | cT4bN2cM1 | Yes | No | No |
| 12 | M | 60 | Tongue | cT4aN2bM0 | Yes | No | No |
| 13 | M | 53 | Oropharynx | cT4aN3bM0 | Yes | No | No |
| 14 | M | 46 | Tongue | pT1N0M0 | Yes | No | No |
| 15 | M | 65 | Larynx | pT3N0M0 | Yes | No | No |
| 16 | M | 70 | Larynx | pT4aN3bM0 | Yes | Yes | No |
| 17 | F | 75 | Larynx | pT3N0M0 | Yes | Yes | No |
| 18 | M | 56 | Larynx | pT3N0M0 | Yes | Yes | No |
| 19 | M | 71 | Larynx | pT3N3bM0 | Yes | Yes | No |
| 20 | F | 60 | Larynx | pT2N0M0 | Yes | Yes | No |
| 21 | M | 67 | Larynx | pT4aN0M0 | Yes | Yes | No |
| 22 | M | 78 | Larynx | pT3N0M0 | Yes | Yes | Yes |
| 23 | M | 56 | Larynx | pT4aN1M0 | Yes | Yes | Yes |
| 24 | F | 73 | Larynx | pT3N3bM0 | Yes | Yes | Yes |
| 25 | M | 77 | Larynx | pT3N0M0 | Yes | Yes | Yes |
| 26 | F | 62 | Larynx | pT2N0M0 | No | Yes | Yes |
| 27 | M | 69 | Larynx | pT4aN0M0 | No | Yes | Yes |
| 28 | M | 76 | Larynx | pT2N0M0 | No | Yes | Yes |
| 29 | M | 65 | Larynx | pT4aN2bM0 | No | Yes | Yes |
| 30 | M | 60 | Larynx | pT4aN3bM0 | No | Yes | Yes |
| Parameter (Range) | Sex | Healthy Controls (n. 20; M16/F4) | Cancer Patients (n. 25; M20/F5) | p-Value | (df), F |
|---|---|---|---|---|---|
| Glucose (70.3–100.9 mg/dL) | tot | 88.31 ± 2.23 | 113.32 ± 6.46 | 0.002 | (1,42), 10.591 |
| M | 87.6 ± 2.51 | 111 ± 19.4 | p(disease) 0.007 p(gender) 0.464 | (1,40) F(disease) 7.99 F(gender) 0.646 | |
| F | 91 ± 5.35 | 122 ± 6.71 | |||
| UREA (10.20–49.80 mg/dL) | tot | 16.94 ± 1.15 | 31.8 ± 3.51 | <0.001 | (1,42), 12.749 |
| M | 17.45 ± 1.29 | 30.47 ± 3.85 | p(disease) 0.002 p(gender) 0.686 | (1,40) F(disease) 11.41 F(gender) 0.16 | |
| F | 15 ± 2.64 | 37.18 ± 8.92 | |||
| Phosphate (2.80–4.60 mg/dL) | tot | 3.12 ± 0.10 | 3.52 ± 0.10 | 0.01 | (1,42), 7.364 |
| M | 2.98 ± 0.09 | 3.46 ± 0.103 | p(disease) 0.081 p(gender) 0.006 | (1,40) F(disease) 3.215 F(gender) 8.468 | |
| F | 3.65 ± 0.17 | 3.78 ± 0.32 | |||
| Protein (60–82 g/L) | tot | 72.05 ± 0.83 | 66.6 ± 1.58 | 0.007 | (1,42), 7.911 |
| M | 71.2 ± 0.87 | 66.35 ± 1.86 | p(disease) 0.013 p(gender) 0.301 | (1,40) F(disease) 6.806 F(gender) 1.097 | |
| F | 72.25 ± 1.55 | 67.4 ± 2.89 | |||
| Albumin (35–55 g/L) | tot | 44.16 ± 0.57 | 40.64 ± 1.19 | 0.03 | (1,42), 5.072 |
| M | 43.9 ± 0.71 | 40.1 ± 1.44 | p(disease) 0.130 p(gender) 0.340 | (1,40) F(disease) 2.393 F(gender) 0.933 | |
| F | 45 ± 0.41 | 42.8 ± 3.0 | |||
| Bilirubin–Direct (<0.20 mg/dL) | tot | 0.15 ± 0.02 | 0.31 ± 0.04 | 0.002 | (1,42), 10.481 |
| M | 0.15 ± 0.03 | 0.32 ± 0.05 | p(disease) 0.013 p(gender) 0.832 | (1,40) F(disease) 6.729 F(gender) 0.05 | |
| F | 0.13 ± 0.02 | 0.31 ± 0.05 | |||
| Iron (64.8–174.90 ug/dL) | tot | 97.89 ± 5.84 | 75.14 ± 9.17 | 0.059 | (1,42), 3.775 |
| M | 99.73 ± 7.24 | 78.28 ± 10.70 | p(disease) 0.09 p(gender) 0.410 | (1,40) F(disease) 2.883 F(gender) 0.694 | |
| F | 91 ± 6.10 | 78.28 ± 10.70 | |||
| PCR (100–6000 ug/L) | tot | 1392 ± 319 | 29212± 11387 | 0.04 | (1,42), 4.372 |
| M | 1673 ± 274 | 32955 ± 13990 | p(disease) 0.197 p(gender) 0.587 | (1,40) F(disease) 1.721 F(gender) 0.300 | |
| F | 2350 ± 1211 | 14240 ± 10081 | |||
| Transferrin (2.15–3.65 g/L) | tot | 2.76 ± 0.10 | 2.23 ± 0.11 | 0.001 | (1,42), 2.999 |
| M | 2.70 ± 0.11 | 2.24 ± 0.13 | p(disease) 0.002 p(gender) 0.617 | (1,40) F(disease) 10.808 F(gender) 0.254 | |
| F | 2.97 ± 0.19 | 2.17 ± 0.21 | |||
| Creatinine (M: 0.70–1.20 mg/Dl F: 0.50–0.9 mg/dL) | M | 1.04 ± 0.02 | 0.85 ± 0.06 | 0.012 | (1,33), 7.112 |
| F | 0.78 ± 0.05 | 0.64 ± 0.08 | 0.189 | (1,7), 2.117 | |
| gGT (M: 10.00–40.00 U/L F: 7.00–35.00 U/L) | M | 17.88 ± 1.54 | 55 ± 18.24 | 0.01 | (1,33), 3.073 |
| F | 16.25 ± 4.25 | 26.6 ± 3.72 | 0.109 | (1,7), 3.374 | |
| LDH (M: 135.00–225.00 U/L F: 135.00–214.00 U/L) | M | 161.26 ± 8.14 | 129.95 ± 9.28 | 0.02 | (1,33), 5.950 |
| F | 173.5 ± 12.63 | 138 ± 28.53 | 0.334 | (1,7), 1.077 | |
| CPK (M: 20–200 U/L F:20–180 U/L) | M | 139.27 ± 27.68 | 51.90 ± 12.91 | 0.004 | (1,33), 9.627 |
| F | 89.75 ± 13.70 | 30.20 ± 5.40 | 0.003 | (1,7), 19.454 | |
| Ferritin (M: 30–400 ug/L F: 15–150 ug/L) | M | 67 ± 10.89 | 402.95 ± 87.45 | 0.002 | (1,33), 10.891 |
| F | 61.50 ± 18.40 | 313 ± 76.35 | 0.02 | (1,7), 8.154 |
| Spearman’s Correlations in Healthy Controls | ||||||
|---|---|---|---|---|---|---|
| Variable | LCN-2 | MMP-9/NGAL | MMP-9 | Transferrin | Ferritin | |
| LCN-2 | Spearman’s rho | — | ||||
| p-value | — | |||||
| MMP-9/NGAL | Spearman’s rho | 0.236 | — | |||
| p-value | 0.397 | — | ||||
| MMP-9 | Spearman’s rho | −0.443 | 0.751 *** | — | ||
| p-value | 0.100 | <0.001 | — | |||
| Transferrin | Spearman’s rho | 0.524 * | 0.203 | −0.106 | — | |
| p-value | 0.040 | 0.418 | 0.674 | — | ||
| Ferritin | Spearman’s rho | −0.680 ** | −0.338 | −0.137 | −0.688 ** | — |
| p-value | 0.004 | 0.171 | 0.587 | 0.001 | — | |
| Spearman’s Correlations in UADT Cancer Patients | ||||||
| Variable | LCN-2 | MMP-9/NGAL | MMP-9 | Transferrin | Ferritin | |
| LCN-2 | Spearman’s rho | — | ||||
| p-value | — | |||||
| MMP-9/NGAL | Spearman’s rho | 0.598 ** | — | |||
| p-value | 0.002 | — | ||||
| MMP-9 | Spearman’s rho | 0.242 | 0.674 *** | — | ||
| p-value | 0.242 | <0.001 | — | |||
| Transferrin | Spearman’s rho | 0.155 | 0.130 | 0.053 | — | |
| p-value | 0.459 | 0.534 | 0.800 | — | ||
| Ferritin | Spearman’s rho | −0.400 * | −0.479 * | −0.254 | −0.425 * | — |
| p-value | 0.049 | 0.016 | 0.220 | 0.034 | — | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalcanti, L.; Francati, S.; Ferraguti, G.; Fanfarillo, F.; Peluso, D.; Barbato, C.; Greco, A.; Minni, A.; Petrella, C. Lipocalin-2, Matrix Metalloproteinase-9, and MMP-9/NGAL Complex in Upper Aerodigestive Tract Carcinomas: A Pilot Study. Cells 2025, 14, 506. https://doi.org/10.3390/cells14070506
Cavalcanti L, Francati S, Ferraguti G, Fanfarillo F, Peluso D, Barbato C, Greco A, Minni A, Petrella C. Lipocalin-2, Matrix Metalloproteinase-9, and MMP-9/NGAL Complex in Upper Aerodigestive Tract Carcinomas: A Pilot Study. Cells. 2025; 14(7):506. https://doi.org/10.3390/cells14070506
Chicago/Turabian StyleCavalcanti, Luca, Silvia Francati, Giampiero Ferraguti, Francesca Fanfarillo, Daniele Peluso, Christian Barbato, Antonio Greco, Antonio Minni, and Carla Petrella. 2025. "Lipocalin-2, Matrix Metalloproteinase-9, and MMP-9/NGAL Complex in Upper Aerodigestive Tract Carcinomas: A Pilot Study" Cells 14, no. 7: 506. https://doi.org/10.3390/cells14070506
APA StyleCavalcanti, L., Francati, S., Ferraguti, G., Fanfarillo, F., Peluso, D., Barbato, C., Greco, A., Minni, A., & Petrella, C. (2025). Lipocalin-2, Matrix Metalloproteinase-9, and MMP-9/NGAL Complex in Upper Aerodigestive Tract Carcinomas: A Pilot Study. Cells, 14(7), 506. https://doi.org/10.3390/cells14070506











