The Impact of TRPM8 on Prostate Cancer Transcriptomic Dynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Housing Conditions
2.3. RNA Sequencing
2.4. Data Analysis
2.5. Pathway Enrichment Analysis
2.6. Data Visualization Statistical Analysis
3. Results
3.1. TRPM8 and AR mRNA Expression in Prostate Cancer and Implications for Androgen-Deprivation Therapies
3.2. Role of TRPM8 in Prostate Cancer via RNA Sequencing of TRPM8 Knockout and Wild-Type Prostate Tissues
3.3. Differential Gene Expression and Pathway Alterations in TRPM8 Knockout Prostates
3.4. Pathway Enrichment Analysis of Upregulated Genes in TRPM8 Knockout Prostates
3.5. Pathway Enrichment of Downregulated Genes in TRPM8 Knockout Prostates
3.6. Functional Enrichment Analysis of Differentially Expressed Genes in TRPM8 Knockout Prostates Using Gene Ontology
3.7. Gene Set Enrichment Analysis (GSEA) of TRPM8 Knockout Prostates
3.8. Differential Expression Profile and Correlation Analysis of TRPM8 Knockout Prostates
3.9. Coding Potential Analysis in TRPM8 Knockout Prostates
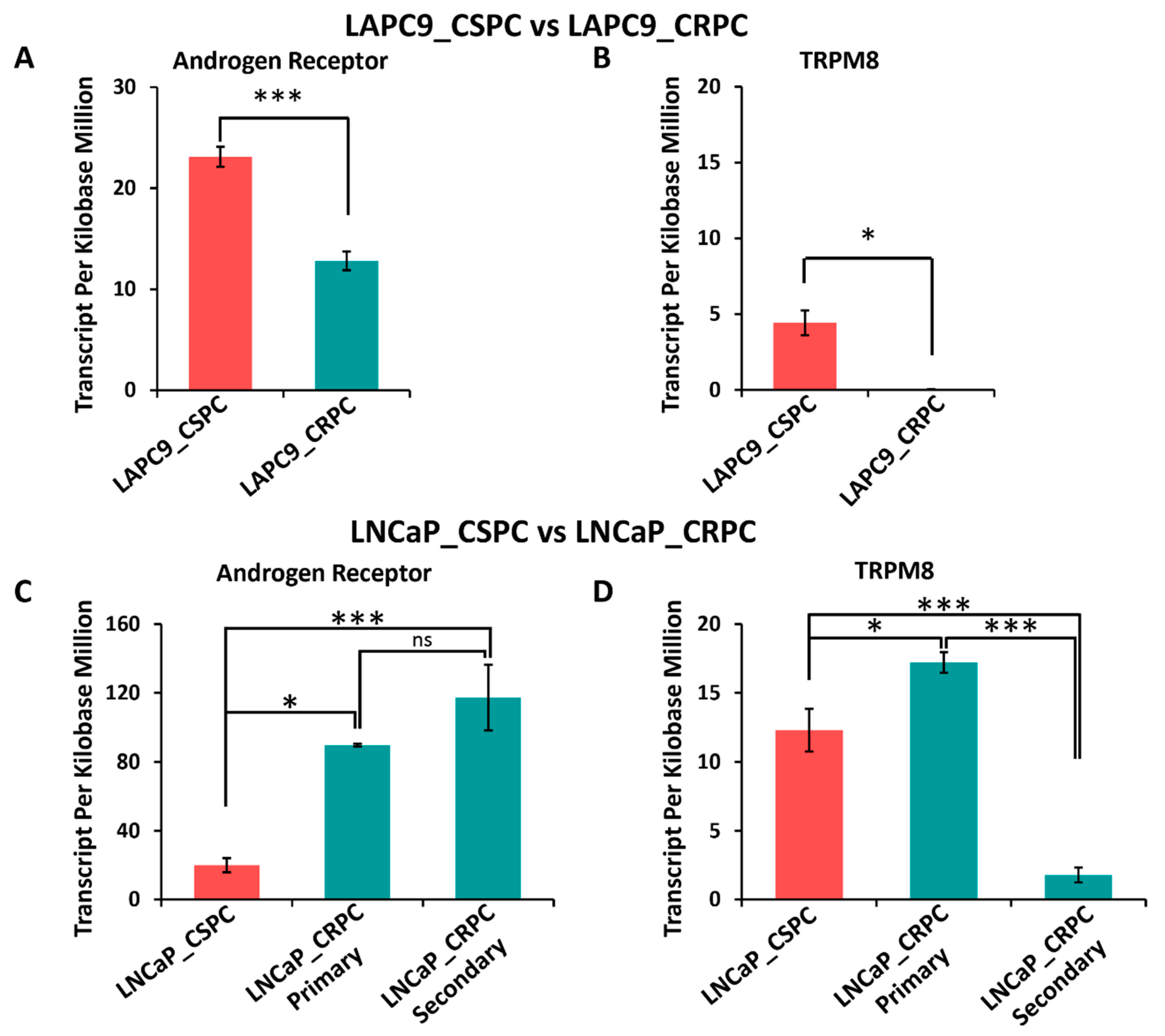
3.10. Differential Expression of AR and TRPM8 in Castration-Sensitive and Castration-Resistant Prostate Cancer Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, M.H.E.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [PubMed] [PubMed Central]
- Jacob, A.; Raj, R.; Allison, D.B.; Myint, Z.W. Androgen receptor signaling in prostate cancer and therapeutic strategies. Cancers 2021, 13, 5417. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Rajwa, P.; Quhal, F.; Kawada, T.; Bekku, K.; Laukhtina, E.; von Deimling, M.; Chlosta, M.; Karakiewicz, P.I.; Kimura, T.; et al. Neoadjuvant androgen receptor signaling inhibitors before radical prostatectomy for non-metastatic advanced prostate cancer: A systematic review. J. Pers. Med. 2023, 13, 641. [Google Scholar] [CrossRef] [PubMed]
- Pagliarulo, V.; Bracarda, S.; Eisenberger, M.A.; Mottet, N.; Schroder, F.H.; Sternberg, C.N.; Studer, U.E. Contemporary role of androgen deprivation therapy for prostate cancer. Eur. Urol. 2012, 61, 11–25. [Google Scholar] [PubMed Central]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85. [Google Scholar] [PubMed Central]
- Asuthkar, S.; Velpula, K.K.; Elustondo, P.A.; Demirkhanyan, L.; Zakharian, E. TRPM8 channel as a novel molecular target in androgen-regulated prostate cancer cells. Oncotarget 2015, 6, 17221–17236. [Google Scholar] [PubMed Central]
- Asuthkar, S.; Demirkhanyan, L.; Mueting, S.R.; Cohen, A.; Zakharian, E. High-throughput proteome analysis reveals targeted TRPM8 degradation in prostate cancer. Oncotarget 2017, 8, 12877–12890. [Google Scholar] [PubMed Central]
- Atala, A. Re: Ion channel TRPM8 promotes hypoxic growth of prostate cancer cells via an O2-independent and RACK1-mediated mechanism of HIF-1α stabilization. J. Urol. 2015, 194, 260. [Google Scholar]
- Bidaux, G.; Roudbaraki, M.; Merle, C.; Crepin, A.; Delcourt, P.; Slomianny, C.; Thebault, S.; Bonnal, J.L.; Benahmed, M.; Cabon, F.; et al. Evidence for specific TRPM8 expression in human prostate secretory epithelial cells: Functional androgen receptor requirement. Endocr.-Relat. Cancer 2005, 12, 367–382. [Google Scholar]
- Asuthkar, S.; Demirkhanyan, L.; Sun, X.; Elustondo, P.A.; Krishnan, V.; Baskaran, P.; Velpula, K.K.; Thyagarajan, B.; Pavlov, E.V.; Zakharian, E. The TRPM8 protein is a testosterone receptor: II. functional evidence for an ionotropic effect of testosterone on TRPM8. J. Biol. Chem. 2015, 290, 2670–2688. [Google Scholar] [PubMed Central]
- Munkley, J.; Vodak, D.; Livermore, K.E.; James, K.; Wilson, B.T.; Knight, B.; Mccullagh, P.; Mcgrath, J.; Crundwell, M.; Harries, L.W.; et al. Glycosylation is an androgen-regulated process essential for prostate cancer cell viability. eBioMedicine 2016, 8, 103–116. [Google Scholar] [PubMed] [PubMed Central]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar]
- Grolez, G.P.; Chinigo, G.; Barras, A.; Hammadi, M.; Noyer, L.; Kondratska, K.; Bulk, E.; Oullier, T.; Marionneau-Lambot, S.; Le Mee, M.; et al. TRPM8 as an anti-tumoral target in prostate cancer growth and metastasis dissemination. Int. J. Mol. Sci. 2022, 23, 6672. [Google Scholar] [CrossRef] [PubMed]
- Grolez, G.P.; Gordiendko, D.V.; Clarisse, M.; Hammadi, M.; Desruelles, E.; Fromont, G.; Prevarskaya, N.; Slomianny, C.; Gkika, D. TRPM8-androgen receptor association within lipid rafts promotes prostate cancer cell migration. Cell Death Dis. 2019, 10, 652–658. [Google Scholar] [PubMed Central]
- Mohandass, A.; Krishnan, V.; Gribkova, E.D.; Asuthkar, S.; Baskaran, P.; Nersesyan, Y.; Hussain, Z.; Wise, L.M.; George, R.E.; Stokes, N.; et al. TRPM8 as the rapid testosterone signaling receptor: Implications in the regulation of dimorphic sexual and social behaviors. FASEB J. 2020, 34, 10887–10906. [Google Scholar] [PubMed Central]
- Faraldi, M.; Mangiavini, L.; Conte, C.; Banfi, G.; Napoli, N.; Lombardi, G. A novel methodological approach to simultaneously extract high-quality total RNA and proteins from cortical and trabecular bone. Open Biol. 2022, 12, 210387. [Google Scholar] [PubMed Central]
- Perez-Portela, R.; Riesgo, A. Optimizing preservation protocols to extract high-quality RNA from different tissues of echinoderms for next-generation sequencing. Mol. Ecol. Resour. 2013, 13, 884–889. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [PubMed Central]
- Yang, Z.H.; Wang, X.H.; Wang, H.P.; Hu, L.Q. Effects of TRPM8 on the proliferation and motility of prostate cancer PC-3 cells. Asian J. Androl. 2009, 11, 157–165. [Google Scholar] [PubMed Central]
- Fallerini, C.; Baldassarri, M.; Trevisson, E.; Morbidoni, V.; La Manna, A.; Lazzarin, R.; Pasini, A.; Barbano, G.; Pinciaroli, A.R.; Garosi, G.; et al. Alport syndrome: Impact of digenic inheritance in patients management. Clin. Genet. 2017, 92, 34–44. [Google Scholar]
- Bao, B.; Zheng, C.; Yang, B.; Jin, Y.; Hou, K.; Li, Z.; Zheng, X.; Yu, S.; Zhang, X.; Fan, Y.; et al. Identification of subtype-specific three-gene signature for prognostic prediction in diffuse type gastric cancer. Front. Oncol. 2019, 9, 1243. [Google Scholar] [CrossRef] [PubMed Central]
- Cheng, Y.; Wang, G.; Zhao, L.; Dai, S.; Han, J.; Hu, X.; Zhou, C.; Wang, F.; Ma, H.; Li, B.; et al. Periplocymarin induced colorectal cancer cells apoptosis via impairing PI3K/AKT pathway. Front. Oncol. 2021, 11, 753598. [Google Scholar] [CrossRef] [PubMed Central]
- Lu, X.; Song, X.; Hao, X.; Liu, X.; Zhang, X.; Yuan, N.; Ma, H.; Zhang, Z. miR-186-3p attenuates the tumorigenesis of cervical cancer via targeting insulin-like growth factor 1 to suppress PI3K-akt signaling pathway. Bioengineered 2021, 12, 7079–7092. [Google Scholar] [CrossRef] [PubMed Central]
- Koti, M.; Gooding, R.J.; Nuin, P.; Haslehurst, A.; Crane, C.; Weberpals, J.; Childs, T.; Bryson, P.; Dharsee, M.; Evans, K.; et al. Identification of the IGF1/PI3K/NF κB/ERK gene signalling networks associated with chemotherapy resistance and treatment response in high-grade serous epithelial ovarian cancer. BMC Cancer 2013, 13, 549. [Google Scholar] [CrossRef] [PubMed Central]
- Goc, A.; Al-Husein, B.; Kochuparambil, S.T.; Liu, J.; Heston, W.W.D.; Somanath, P.R. PI3 kinase integrates akt and MAP kinase signaling pathways in the regulation of prostate cancer. Int. J. Oncol. 2011, 38, 267–277. [Google Scholar]
- Mulholland, D.J.; Kobayashi, N.; Ruscetti, M.; Zhi, A.; Tran, L.M.; Huang, J.; Gleave, M.; Wu, H. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012, 72, 1878–1889. [Google Scholar] [PubMed Central]
- Ye, Z.; Zheng, M.; Zeng, Y.; Wei, S.; Huang, H.; Wang, Y.; Liu, Q.; Lin, Z.; Chen, S.; Zheng, Q.; et al. A 13-gene metabolic prognostic signature is associated with clinical and immune features in stomach adenocarcinoma. Front. Oncol. 2021, 11, 612952. [Google Scholar] [CrossRef] [PubMed Central]
- Dhuli, K.; Medori, M.C.; Donato, K.; Maltese, P.E.; Tanzi, B.; Tezzele, S.; Mareso, C.; Miertus, J.; Generali, D.; Donofrio, C.A.; et al. Omics sciences and precision medicine in thyroid cancer. Clin. Ter. 2023, 174 (Suppl. S2), 11–20. [Google Scholar]
- Wang, T.K.; Xu, S.; Fan, Y.; Wu, J.; Wang, Z.; Chen, Y.; Zhang, Y. The synergistic effect of proanthocyanidin and HDAC inhibitor inhibit breast cancer cell growth and promote apoptosis. Int. J. Mol. Sci. 2023, 24, 10476. [Google Scholar] [CrossRef] [PubMed Central]
- Huang, P.; Yang, Y.; Wang, C.; Hsiao, P.; Chiang, H.; Chen, T. Increase in akkermansiaceae in gut microbiota of prostate cancer-bearing mice. Int. J. Mol. Sci. 2021, 22, 9626. [Google Scholar] [CrossRef] [PubMed Central]
- Knuuttila, M.; Yatkin, E.; Kallio, J.; Savolainen, S.; Laajala, T.D.; Aittokallio, T.; Oksala, R.; Hakkinen, M.; Keski-Rahkonen, P.; Auriola, S.; et al. Castration induces up-regulation of intratumoral androgen biosynthesis and androgen receptor expression in an orthotopic VCaP human prostate cancer xenograft model. Am. J. Pathol. 2014, 184, 2163–2173. [Google Scholar]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [PubMed Central]
- El-Kenawi, A.; Dominguez-Viqueira, W.; Liu, M.; Awasthi, S.; Abraham-Miranda, J.; Keske, A.; Steiner, K.K.; Noel, L.; Serna, A.N.; Dhillon, J.; et al. Macrophage-derived cholesterol contributes to therapeutic resistance in prostate cancer. Cancer Res. 2021, 81, 5477–5490. [Google Scholar] [PubMed Central]
- Martinez-Breijo, S.; Chantada-Abal, V.; Aller-Rodriguez, M.; Bohorquez-Cruz, M.; Sacristan-Lista, F.; Ponce-Diaz, J.; Vazquez-Martul, D.; Lamas-Diaz, L. Castration resistance mechanisms in prostate cancer. Arch. Esp. Urol. 2018, 71, 628–638. [Google Scholar]
- Yang, J.C.; Xu, P.; Ning, S.; Wasielewski, L.J.; Adomat, H.; Hwang, S.H.; Morisseau, C.; Gleave, M.; Corey, E.; Gao, A.C.; et al. Novel inhibition of AKR1C3 and androgen receptor axis by PTUPB synergizes enzalutamide treatment in advanced prostate cancer. Oncogene 2023, 42, 693–707. [Google Scholar] [PubMed Central]
- Helenius, A.; Aebi, M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004, 73, 1019–1049. [Google Scholar]
- Vajaria, B.N.; Patel, P.S. Glycosylation: A hallmark of cancer? Glycoconj. J. 2017, 34, 147–156. [Google Scholar]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar]
- Lee, M.W.; Kim, D.S.; Lee, J.H.; Lee, B.S.; Lee, S.H.; Jung, H.L.; Sung, K.W.; Kim, H.T.; Yoo, K.H.; Koo, H.H. Roles of AKT1 and AKT2 in non-small cell lung cancer cell survival, growth, and migration. Cancer Sci. 2011, 102, 1822–1828. [Google Scholar] [PubMed Central]
- Li, P.; Zhou, L.; Zhao, T.; Liu, X.; Zhang, P.; Liu, Y.; Zheng, X.; Li, Q. Caspase-9: Structure, mechanisms and clinical application. Oncotarget 2017, 8, 23996–24008. [Google Scholar] [PubMed Central]
- Engelmann, D.; Putzer, B.M. The dark side of E2F1: In transit beyond apoptosis. Cancer Res. 2012, 72, 571–575. [Google Scholar]
- Chodon, D.; Guilbert, A.; Dhennin-Duthille, I.; Gautier, M.; Telliez, M.S.; Sevestre, H.; Ouadid-Ahidouch, H. Estrogen regulation of TRPM8 expression in breast cancer cells. BMC Cancer 2010, 10, 212. [Google Scholar] [PubMed Central]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [PubMed Central]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [PubMed Central]
- Luo, Z.; Tian, M.; Yang, G.; Tan, Q.; Chen, Y.; Li, G.; Zhang, Q.; Li, Y.; Wan, P.; Wu, J. Hypoxia signaling in human health and diseases: Implications and prospects for therapeutics. Signal Transduct. Target. Ther. 2022, 7, 218. [Google Scholar] [CrossRef] [PubMed Central]
- Luongo, F.; Colonna, F.; Calapa, F.; Vitale, S.; Fiori, M.E.; De Maria, R. PTEN tumor-suppressor: The dam of stemness in cancer. Cancers 2019, 11, 1076. [Google Scholar] [CrossRef] [PubMed Central]
- Gonzalez-Billalabeitia, E.; Seitzer, N.; Song, S.J.; Song, M.S.; Patnaik, A.; Liu, X.; Epping, M.T.; Papa, A.; Hobbs, R.M.; Chen, M.; et al. Vulnerabilities of PTEN-TP53-deficient prostate cancers to compound PARP-PI3K inhibition. Cancer Discov. 2014, 4, 896–904. [Google Scholar] [PubMed Central]
- Chen, Z.; Trotman, L.C.; Shaffer, D.; Lin, H.K.; Dotan, Z.A.; Niki, M.; Koutcher, J.A.; Scher, H.I.; Ludwig, T.; Gerald, W.; et al. Crucial role of p53-dependent cellular senescence in suppression of pten-deficient tumorigenesis. Nature 2005, 436, 725–730. [Google Scholar] [PubMed Central]
- Chang, C.; Sue, Y.; Yang, N.; Lee, Y.; Juan, S. 3-methylcholanthrene, an AhR agonist, caused cell-cycle arrest by histone deacetylation through a RhoA-dependent recruitment of HDAC1 and pRb2 to E2F1 complex. PLoS ONE 2014, 9, e92793. [Google Scholar] [CrossRef] [PubMed Central]
- Mori, K.; Uchida, T.; Fukumura, M.; Tamiya, S.; Higurashi, M.; Sakai, H.; Ishikawa, F.; Shibanuma, M. Linkage of E2F1 transcriptional network and cell proliferation with respiratory chain activity in breast cancer cells. Cancer Sci. 2016, 107, 963–971. [Google Scholar] [CrossRef] [PubMed Central]
- Wang, S.; Pang, L.; Liu, Z.; Meng, X. SERPINE1 associated with remodeling of the tumor microenvironment in colon cancer progression: A novel therapeutic target. BMC Cancer 2021, 21, 1–13. [Google Scholar] [PubMed Central]
- Pavón, M.A.; Arroyo-Solera, I.; Téllez-Gabriel, M.; León, X.; Virós, D.; López, M.; Gallardo, A.; Céspedes, M.V.; Casanova, I.; López-Pousa, A.; et al. Enhanced cell migration and apoptosis resistance may underlie the association between high SERPINE1 expression and poor outcome in head and neck carcinoma patients. Oncotarget 2015, 6, 29016–29033. [Google Scholar] [PubMed Central]
- Arroyo-Solera, I.; Pavon, M.A.; Leon, X.; Lopez, M.; Gallardo, A.; Cespedes, M.V.; Casanova, I.; Pallares, V.; Lopez-Pousa, A.; Mangues, M.A.; et al. Effect of serpinE1 overexpression on the primary tumor and lymph node, and lung metastases in head and neck squamous cell carcinoma. Head Neck 2019, 41, 429–439. [Google Scholar]
- Lee, H.; Mullins, S.R.; Franco-Barraza, J.; Valianou, M.; Cukierman, E.; Cheng, J.D. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer 2011, 11, 245. [Google Scholar] [PubMed Central]
- Jezierska, A.; Motyl, T. Matrix metalloproteinase-2 involvement in breast cancer progression: A mini-review. Med. Sci. Monit. 2009, 15, RA32–RA40. [Google Scholar]
- Almutairi, S.; Kalloush, H.M.; Manoon, N.A.; Bardaweel, S.K. Matrix metalloproteinases inhibitors in cancer treatment: An updated review (2013–2023). Molecules 2023, 28, 5567. [Google Scholar] [CrossRef]
- Carew, N.T.; Schmidt, H.M.; Yuan, S.; Galley, J.C.; Hall, R.; Altmann, H.M.; Hahn, S.A.; Miller, M.P.; Wood, K.C.; Gabris, B.; et al. Loss of cardiomyocyte CYB5R3 impairs redox equilibrium and causes sudden cardiac death. J. Clin. Investig. 2022, 132, e147120. [Google Scholar] [CrossRef] [PubMed Central]
- Moreb, J.S.; Baker, H.V.; Chang, L.; Amaya, M.; Lopez, M.C.; Ostmark, B.; Chou, W. ALDH isozymes downregulation affects cell growth, cell motility and gene expression in lung cancer cells. Mol. Cancer 2008, 7, 87. [Google Scholar] [PubMed Central]
- Kim, S.S.; Cho, H.J.; Kang, J.Y.; Kang, H.K.; Yoo, T.K. Inhibition of androgen receptor expression with small interfering RNA enhances cancer cell apoptosis by suppressing survival factors in androgen insensitive, late stage LNCaP cells. Sci. World J. 2013, 2013, 519397. [Google Scholar] [PubMed Central]
- Sun, Y.; Wang, B.; Leong, K.G.; Yue, P.; Li, L.; Jhunjhunwala, S.; Chen, D.; Seo, K.; Modrusan, Z.; Gao, W.; et al. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: Implications for androgen-deprivation therapy. Cancer Res. 2012, 72, 527–536. [Google Scholar]
- Gkika, D.; Flourakis, M.; Lemonnier, L.; Prevarskaya, N. PSA reduces prostate cancer cell motility by stimulating TRPM8 activity and plasma membrane expression. Oncogene 2010, 29, 4611–4616. [Google Scholar]
- Chelaru, N.; Chiosa, A.; Sorop, A.; Spiridon, A.; Cojocaru, F.; Domocos, D.; Cucu, D.; Popescu, I.; Dima, S. The association between TRP channels expression and clinicopathological characteristics of patients with pancreatic adenocarcinoma. Int. J. Mol. Sci. 2022, 23, 9045. [Google Scholar] [CrossRef]
- Huang, Y.; Li, S.; Jia, Z.; Zhao, W.; Zhou, C.; Zhang, R.; Ali, D.W.; Michalak, M.; Chen, X.; Tang, J. Transient receptor potential melastatin 8 (TRPM8) channel regulates proliferation and migration of breast cancer cells by activating the AMPK-ULK1 pathway to enhance basal autophagy. Front. Oncol. 2020, 10, 573127. [Google Scholar] [PubMed Central]
- Alaimo, A.; Lorenzoni, M.; Ambrosino, P.; Bertossi, A.; Bisio, A.; Macchia, A.; Zoni, E.; Genovesi, S.; Cambuli, F.; Foletto, V.; et al. Calcium cytotoxicity sensitizes prostate cancer cells to standard-of-care treatments for locally advanced tumors. Cell Death Dis. 2020, 11, 1039. [Google Scholar] [PubMed Central]
- Gkika, D.; Lolignier, S.; Grolez, G.P.; Bavencoffe, A.; Shapovalov, G.; Gordienko, D.; Kondratskyi, A.; Meleine, M.; Prival, L.; Chapuy, E.; et al. Testosterone-androgen receptor: The steroid link inhibiting TRPM8-mediated cold sensitivity. FASEB J. 2020, 34, 7483–7499. [Google Scholar]
- Grolez, G.P.; Hammadi, M.; Barras, A.; Gordienko, D.; Slomianny, C.; Volkel, P.; Angrand, P.O.; Pinault, M.; Guimaraes, C.; Potier-Cartereau, M.; et al. Encapsulation of a TRPM8 agonist, WS12, in lipid nanocapsules potentiates PC3 prostate cancer cell migration inhibition through channel activation. Sci. Rep. 2019, 9, 7926. [Google Scholar] [PubMed Central]
- Zhu, G.; Wang, X.; Yang, Z.; Cao, H.; Meng, Z.; Wang, Y.; Chen, D. Effects of TRPM8 on the proliferation and angiogenesis of prostate cancer PC-3 cells in vivo. Oncol. Lett. 2011, 2, 1213–1217. [Google Scholar] [PubMed Central]
- Bidaux, G.; Borowiec, A.-S.; Dubois, C.; Delcourt, P.; Schulz, C.; Abeele, F.V.; Lepage, G.; Desruelles, E.; Bokhobza, A.; Dewailly, E.; et al. Targeting of short TRPM8 isoforms induces 4TM-TRPM8-dependent apoptosis in prostate cancer cells. Oncotarget 2016, 7, 29063–29080. [Google Scholar] [PubMed Central]
- Peng, M.; Wang, Z.; Yang, Z.; Tao, L.; Liu, Q.; Yi, L.U.; Wang, X. Overexpression of short TRPM8 variant alpha promotes cell migration and invasion, and decreases starvation-induced apoptosis in prostate cancer LNCaP cells. Oncol. Lett. 2015, 10, 1378–1384. [Google Scholar] [PubMed Central]
- Thomas, P. Membrane androgen receptors unrelated to nuclear steroid receptors. Endocrinology 2019, 160, 772–781. [Google Scholar]
- Ku, S.Y.; Rosario, S.; Wang, Y.; Mu, P.; Seshadri, M.; Goodrich, Z.W.; Goodrich, M.M.; Labbe, D.P.; Gomez, E.C.; Wang, J.; et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017, 355, 78–83. [Google Scholar] [PubMed Central]
- Yao, Y.; Gu, X.; Xu, X.; Ge, S.; Jia, R. Novel insights into RB1 mutation. Cancer Lett. 2022, 547, 215870. [Google Scholar]
- Jiang, T.; Guo, J.; Hu, Z.; Zhao, M.; Gu, Z.; Miao, S. Identification of potential prostate cancer-related pseudogenes based on competitive endogenous RNA network hypothesis. Med. Sci. Monit. 2018, 24, 4213–4239. [Google Scholar] [PubMed Central]
- Xu, N.; Wu, Y.; Yin, H.; Xue, X.; Gou, X. Molecular network-based identification of competing endogenous RNAs and mRNA signatures that predict survival in prostate cancer. J. Transl. Med. 2018, 16, 1–15. [Google Scholar] [PubMed Central]
- Zhang, X.; Xie, K.; Zhou, H.; Wu, Y.; Li, C.; Liu, Y.; Liu, Z.; Xu, Q.; Liu, S.; Xiao, D.; et al. Role of non-coding RNAs and RNA modifiers in cancer therapy resistance. Mol. Cancer 2020, 19, 47. [Google Scholar] [PubMed Central]
- Hashemi, M.; Hasani, S.; Hajimazdarany, S.; Mirmazloomi, S.R.; Makvandy, S.; Zabihi, A.; Goldoost, Y.; Gholinia, N.; Kakavand, A.; Tavakolpournegari, A.; et al. Non-coding RNAs targeting notch signaling pathway in cancer: From proliferation to cancer therapy resistance. Int. J. Biol. Macromol. 2022, 222 Pt A, 1151–1167. [Google Scholar]
- Turgambayeva, A.; Duisekova, S.; Tashenova, G.; Tulebayeva, A.; Kapanova, G.; Akhenbekova, A.; Farooqi, A.A. Role of TRP channels in carcinogenesis and metastasis: Pathophysiology and regulation by non-coding RNAs. Non-coding RNA Res. 2023, 9, 359–366. [Google Scholar] [PubMed Central]

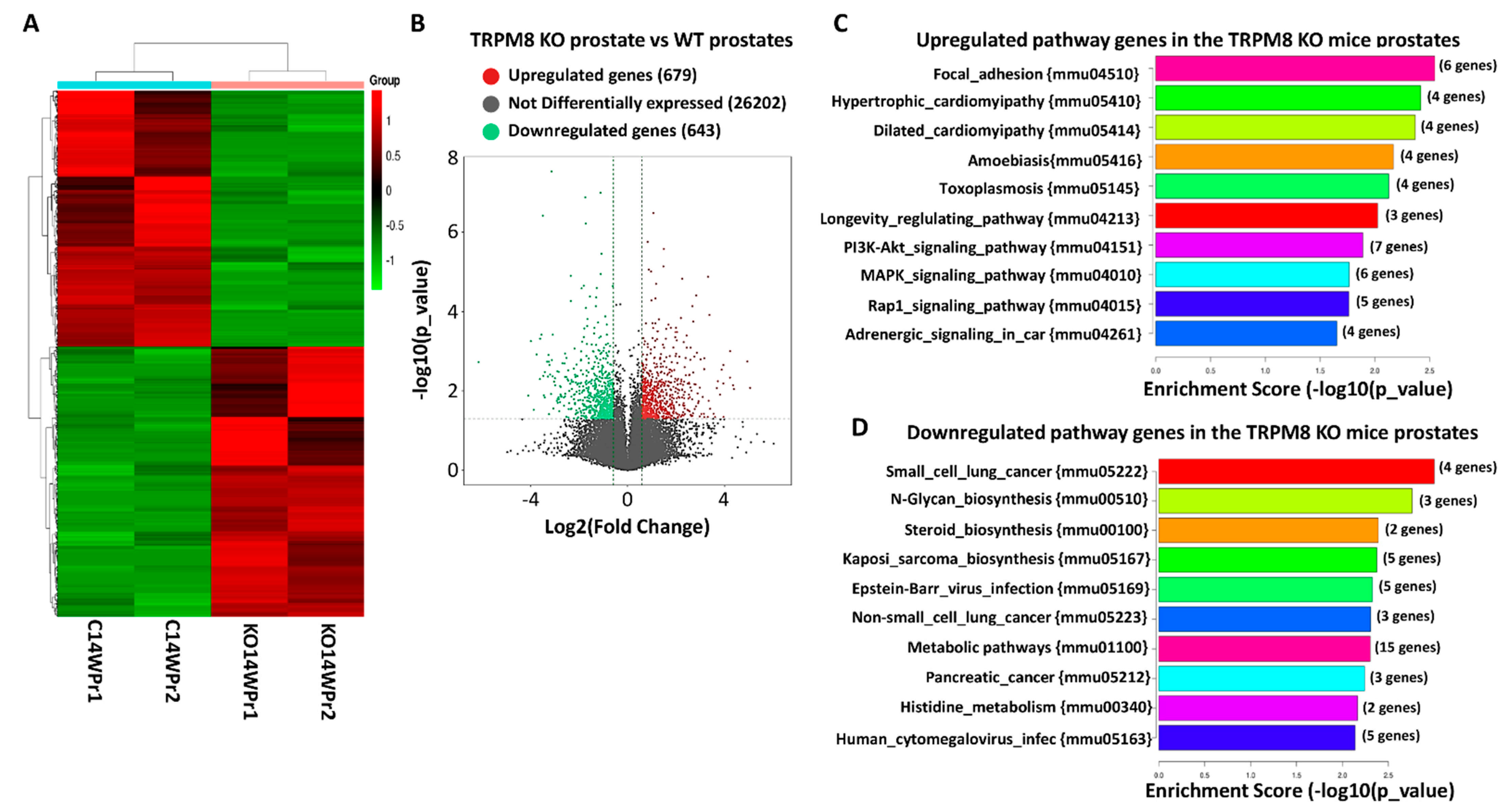
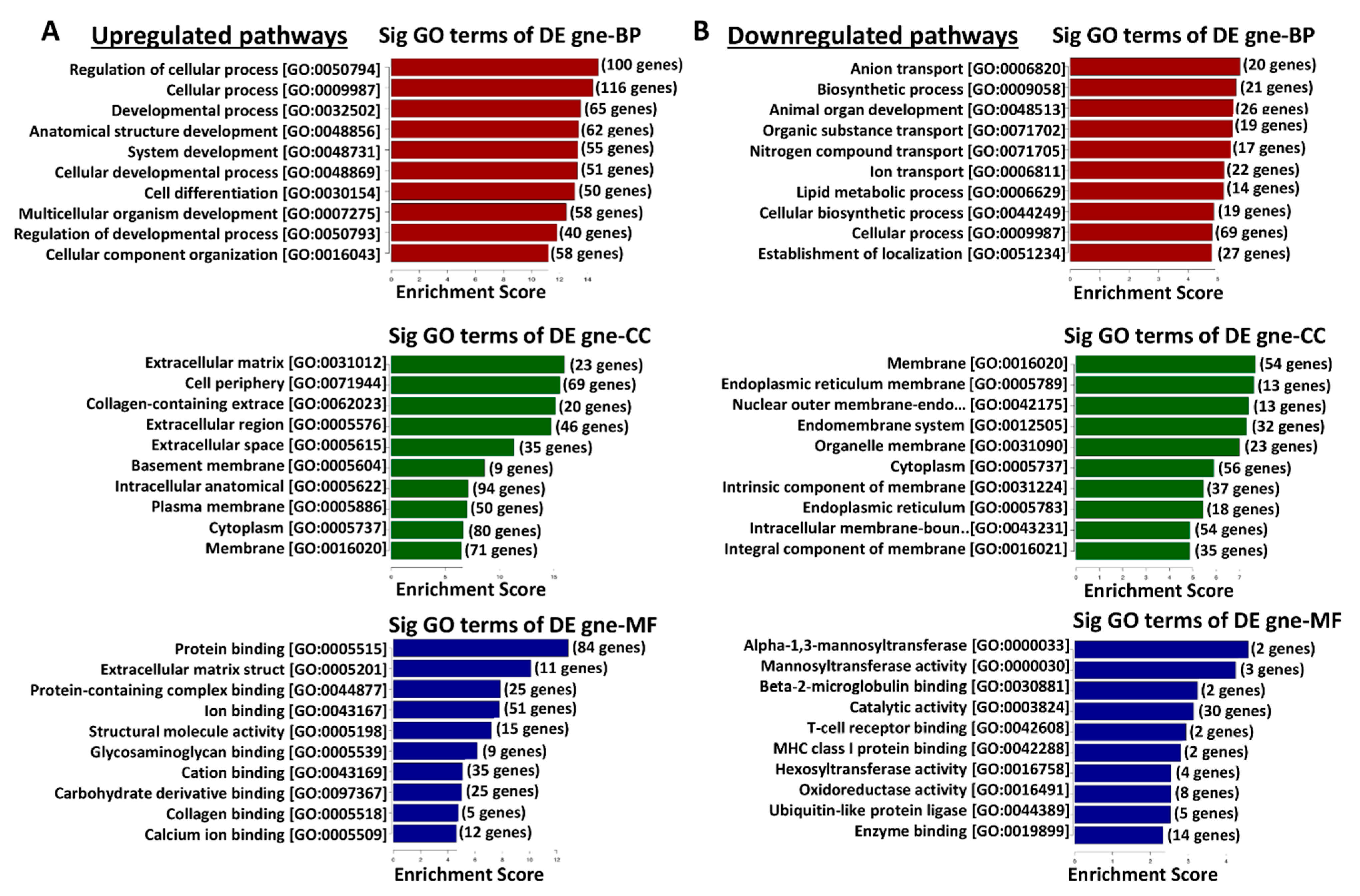
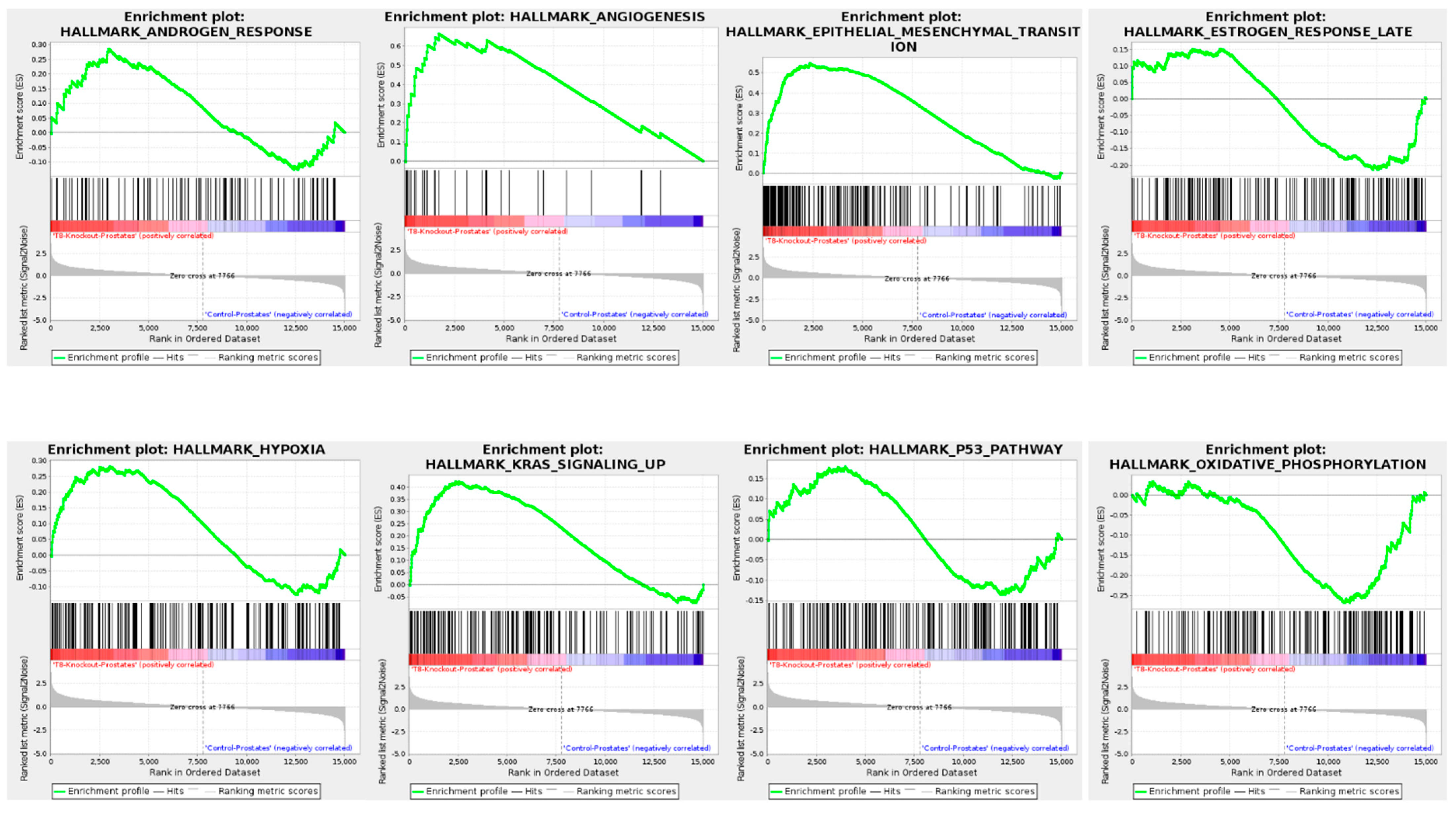



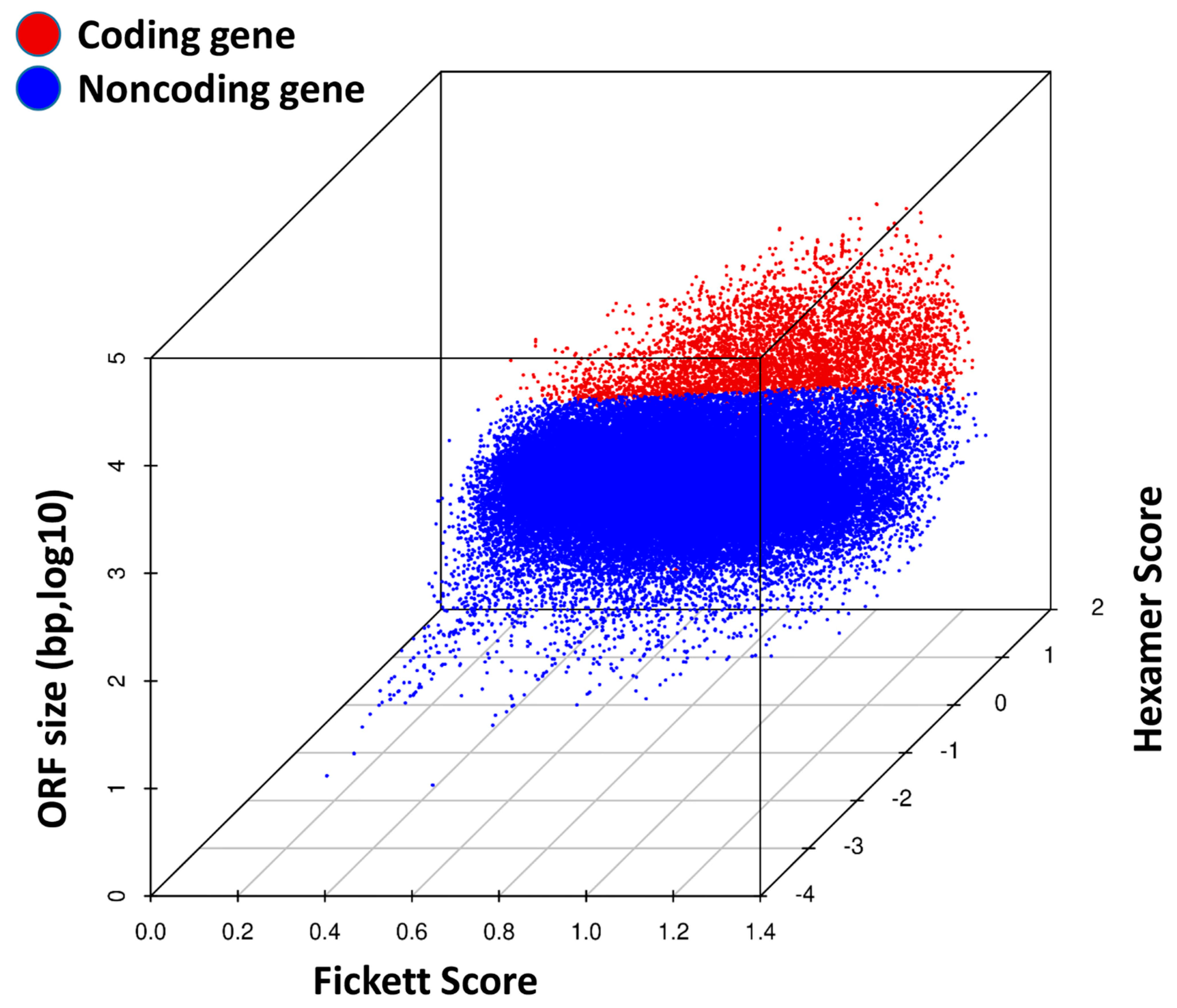
| Pathway ID | Definition | Fisher p-Value | Genes |
|---|---|---|---|
| mmu04510 | Focal_adhesion | 0.00283935 | Col4a5//Flnc//Igf1//Lama2//Lamb1//Pdgfra |
| mmu05410 | Hypertrophic_cardiomyopathy | 0.003817752 | Igf1//Lama2//Myl3//Tpm2 |
| mmu05414 | Dilated_cardiomyopathy | 0.004288076 | Igf1//Lama2//Myl3//Tpm2 |
| mmu05146 | Amoebiasis | 0.00677927 | Col4a5//Gna15//Lama2//Lamb1 |
| mmu05145 | Toxoplasmosis | 0.00746598 | Hspa1a//Hspa1b//Lama2//Lamb1 |
| mmu04213 | Longevity_regulating_pathway-multiple_species | 0.009447223 | Hspa1a//Hspa1b//Igf1 |
| mmu04151 | PI3K-Akt_signaling_pathway | 0.012866392 | Angpt1//Col4a5//Igf1//Lama2//Lamb1//Lpar1//Pdgfra |
| mmu04010 | MAPK_signaling_pathway | 0.017163356 | Angpt1//Flnc//Hspa1a//Hspa1b//Igf1//Pdgfra |
| mmu04015 | Rap1_signaling_pathway | 0.017293189 | Angpt1//Igf1//Lpar1//Pdgfra//Rapgef4 |
| mmu04261 | Adrenergic_signaling_in_cardiomyocytes | 0.022211878 | Myl3//Rapgef4//Scn7a//Tpm2 |
| mmu04512 | ECM-receptor_interaction | 0.024093049 | Col4a5//Lama2//Lamb1 |
| mmu04610 | Complement_and_coagulation_cascades | 0.027795597 | C7//Cfh//Pros1 |
| mmu05222 | Small_cell_lung_cancer | 0.027795597 | Col4a5//Lama2//Lamb1 |
| mmu04061 | Viral_protein_interaction_with_cytokine_and_cytokine_receptor | 0.029357007 | Ccl21a//Ccl21b//Ppbp |
| mmu05200 | Pathways_in_cancer | 0.036273337 | Apc//Col4a5//Igf1//Lama2//Lamb1//Lpar1//Pdgfra//Pmaip1 |
| mmu04064 | NF-kappa_B_signaling_pathway | 0.03784812 | Bcl2a1d//Ccl21a//Ccl21b |
| mmu04974 | Protein_digestion_and_absorption | 0.040615603 | Col14a1//Col4a5//Col8a1 |
| mmu04066 | HIF-1_signaling_pathway | 0.046450354 | Angpt1//Eno1b//Igf1 |
| Pathway ID | Definition | Fisher p-Value | Genes |
|---|---|---|---|
| mmu05222 | Small_cell_lung_cancer | 0.001001771 | Akt2//Casp9//Cks2//E2f1 |
| mmu00510 | N-Glycan_biosynthesis | 0.001747349 | Alg3//Alg8//Dpm1 |
| mmu00100 | Steroid_biosynthesis | 0.004082726 | Hsd17b7//Tm7sf2 |
| mmu05167 | Kaposi_sarcoma-associated_herpesvirus_infection | 0.004227729 | Akt2//Casp9//E2f1//H2-K1//H2-T23 |
| mmu05169 | Epstein-Barr_virus_infection | 0.004729153 | Akt2//Casp9//E2f1//H2-K1//H2-T23 |
| mmu05223 | Non-small_cell_lung_cancer | 0.004939961 | Akt2//Casp9//E2f1 |
| mmu01100 | Metabolic_pathways | 0.004987554 | Acyp1//Aldh3b2//Alg3//Alg8//Amdhd1//Arg1//Cbs//Cmpk2//Cyp4a12b//Dpm1//Hsd17b7//Lipt1//Pigp//Sqor//Tm7sf2 |
| mmu05212 | Pancreatic_cancer | 0.005745739 | Akt2//Casp9//E2f1 |
| mmu00340 | Histidine_metabolism | 0.006856938 | Aldh3b2//Amdhd1 |
| mmu05163 | Human_cytomegalovirus_infection | 0.007282135 | Akt2//Casp9//E2f1//H2-K1//H2-T23 |
| mmu05416 | Viral_myocarditis | 0.008351367 | Casp9//H2-K1//H2-T23 |
| mmu04218 | Cellular_senescence | 0.011345937 | Akt2//E2f1//H2-K1//H2-T23 |
| Gene | Direction | Molecular Function | Implication in Cancer |
|---|---|---|---|
| TLR13 | Upregulated | Immune signaling | Enhances inflammatory response, tumor microenvironment modification |
| CXCL1 | Upregulated | Chemokine activity | Promotes inflammation and attracts immune cells, supports metastasis |
| SERPINE1 | Upregulated | Extracellular matrix remodeling | Involved in invasion and metastasis through ECM interactions |
| FAP | Upregulated | ECM remodeling | Supports tumor invasion and cell migration |
| VEGFD | Upregulated | Angiogenesis | Promotes blood vessel formation, facilitating tumor growth |
| COL8A1 | Upregulated | Structural constituent of ECM | Aids in ECM structure, supporting metastasis |
| CYB5R3 | Downregulated | Redox balance | Impaired cellular metabolism and oxidative stress response |
| CASP9 | Downregulated | Apoptosis initiation | Reduced apoptosis, leading to unchecked cell survival |
| E2F1 | Downregulated | Cell cycle regulation | Impaired cell cycle control, promoting proliferation |
| Aldh3b2 | Downregulated | Metabolism | Altered cellular energy metabolism |
| Hsd17β7 | Downregulated | Steroid biosynthesis | Alters hormonal balance, may support tumorigenesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asuthkar, S.; Bayen, S.; Saldes, E.B.; Tom, B.; Velpula, J.; Siddharth, S.; Koeltzow, T.E.; Vander Griend, D.J. The Impact of TRPM8 on Prostate Cancer Transcriptomic Dynamics. Cells 2025, 14, 501. https://doi.org/10.3390/cells14070501
Asuthkar S, Bayen S, Saldes EB, Tom B, Velpula J, Siddharth S, Koeltzow TE, Vander Griend DJ. The Impact of TRPM8 on Prostate Cancer Transcriptomic Dynamics. Cells. 2025; 14(7):501. https://doi.org/10.3390/cells14070501
Chicago/Turabian StyleAsuthkar, Swapna, Susovon Bayen, Erick B. Saldes, Benny Tom, Jai Velpula, Sarangi Siddharth, Timothy E. Koeltzow, and Donald J. Vander Griend. 2025. "The Impact of TRPM8 on Prostate Cancer Transcriptomic Dynamics" Cells 14, no. 7: 501. https://doi.org/10.3390/cells14070501
APA StyleAsuthkar, S., Bayen, S., Saldes, E. B., Tom, B., Velpula, J., Siddharth, S., Koeltzow, T. E., & Vander Griend, D. J. (2025). The Impact of TRPM8 on Prostate Cancer Transcriptomic Dynamics. Cells, 14(7), 501. https://doi.org/10.3390/cells14070501






