Exploring Sertoli Cells’ Innate Bulwark Role Against Infections: In Vitro Performances on Candida tropicalis Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of MP and AmB-MP
2.2. MP and AmB-MP Size, Morphology and Quantification
2.3. In Vitro Drug Release
2.4. Porcine Prepubertal SCs Isolation
2.5. Uptake Process Evaluation
2.6. Biofilm Formation Protocol
2.7. Dose–Response Evaluation
2.8. Experimental Design
- Control: unexposed SCs
- SCs + C. tropicalis
- SCs + AmB-MP
- SCs + AmB-MP SCs + C. tropicalis
- SCs + MP
- SCs + MP + C. tropicalis
2.9. RT-PCR Analysis
2.10. Western Blot (WB) Analysis
2.11. Statistical Analysis
2.12. Ethics Approval
3. Results
3.1. MP and AmB-MP Characterization
3.1.1. Size and Morphology
3.1.2. AmB Analysis in Formulation and In Vitro Drug Release
3.2. Uptake Process Evaluation and SC Viability
3.3. SCs Anti-Infective Action
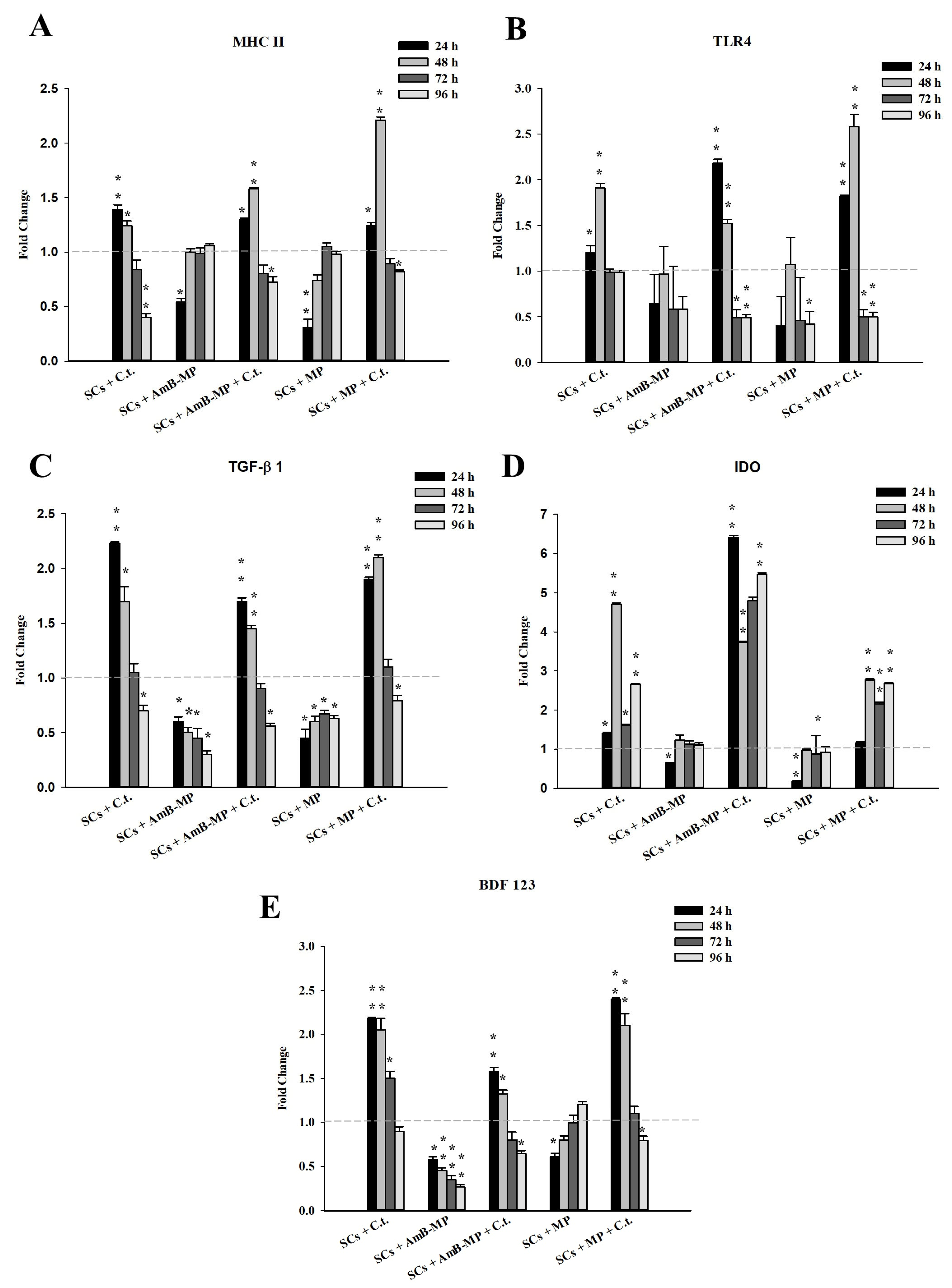
3.4. SCs Innate Immunity Response
3.5. MAPK, AKT, and NF-kB Signal Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pierie, F.; Serafini, S.; Rossi, L.; Magnani, M. Cell-base drag delivery. Adv. Drug Deliv. Rev. 2008, 60, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Baranova, E.V.; Gendelman, H.E.; Kabanov, A.V. Cell-mediated drug delivery. Expert Opin. Drug Deliv. 2011, 8, 415–443. [Google Scholar] [CrossRef]
- Shao, J.; DeHaven, J.; Lamm, D.; Weissman, D.N.; Malanga, C.J.; Rojana Akul, Y.; Ma, J.K. A cell-based drug delivery system for lung targeting: I. Preparation and pharmacokinetics. Drug Deliv. 2001, 8, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; DeHaven, J.; Lamm, D.; Weissman, D.N.; Malanga, C.J.; Rojana Akul, Y.; Ma, J.K. Cell-based drug delivery system for lung targeting: II. Therapeutic activities on B16-F10 melanoma in mouse lungs. Drug Deliv. 2001, 8, 71–76. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Bluestone, J.A.; Lim, W.A. Cell-base therapeutics: The next pillar of medicine. Sci. Transl. Med. 2013, 5, 179ps7. [Google Scholar] [CrossRef]
- Yoo, J.W.; Irvine, D.J.; Discher, D.E.; Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521–535. [Google Scholar] [CrossRef]
- Biagiotti, S.; Paoletti, M.F.; Fraternal, A.; Rossi, L.; Magnani, M. Drug delivery by red blood cells. IUBMB Life 2011, 63, 621–631. [Google Scholar] [CrossRef]
- Raville, S.; Chandu, B.R.; Nama, S.; Nagaveni, B. Erythrocytes as carrier for drugs, enzymes and peptides. J. Appl. Pharm. Sci. 2012, 2, 166–176. [Google Scholar] [CrossRef]
- Muzykantov, V.R. Drug delivery by red blood cells: Vascular carriers designed by Mother Nature. Exper. Opin. Drug Deliv. 2010, 7, 403–427. [Google Scholar] [CrossRef]
- Gamaledin, H.I.; Mohamed, F.I.; Fars, K.A.; Alsarra, I.A. Application and safety of erythrocytes as a novel drug delivery system. Asian J. Biochem. 2011, 6, 309–321. [Google Scholar] [CrossRef]
- Dawson, L.; Bateman-House, A.S.; MuellerAgnew, D.; Bok, H.; Brock, D.W.; Chakravarti, A.; Greene, M.; King, P.A.; O’Brien, S.J.; Sachs, D.H.; et al. Safety issues in cell-based intervent ion trials. Fertil. Steril. 2003, 80, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K. Sertoli Cell Biology; Griswold, M.D., Ed.; Elsevier Academic Press: San Diego, CA, USA, 2005; Volume 1. [Google Scholar]
- Simoni, M.; Gromoll, J.R.; Nieschlag, E. The follicle-stimulating hormone receptor: Biochemistry, molecular biology, physiology, and pathophysiology. Endocr. Rev. 1997, 18, 739–773. [Google Scholar] [CrossRef] [PubMed]
- Makanji, Y.; Harrison, C.A.; Robertson, D.M. Feedback regulation by inhibins A and B of the pituitary secretion of follicle-stimulating hormone. In Vitamins and, Hormones; Litwack, G., Ed.; Academic Press: Burlington, NJ, USA, 2011; Volume 85, pp. 299–321. [Google Scholar]
- Sun, B.; Qi, N.; Shang, T.; Wu, H.; Deng, T.; Han, D. Sertoli cell-initiated testicular innate immune response through toll-like receptor-3 activation is negatively regulated by Tyro3, Axl, and Mer receptors. Endocrinology 2010, 151, 2886–2897. [Google Scholar] [CrossRef] [PubMed]
- Mital, P.; Kaur, G.; Dufour, J.M. Immunoprotective Sertoli cells: Making allogeneic and xenogeneic transplantation feasible. Reproduction 2010, 139, 495–504. [Google Scholar] [CrossRef]
- Luca, G.; Fallarino, F.; Calvitti, M.; Mancuso, F.; Nastruzzi, C.; Arato, I.; Falabella, G.; Grohmann, U.; Becchetti, E.; Puccetti, P.; et al. Xenograft of Microencapsulated Sertoli Cells Reverses T1DM in NOD Mice by Inducing Neogenesis of β-Cells. Transplantation 2010, 90, 1352–1357. [Google Scholar] [CrossRef]
- Luca, G.; Arato, I.; Mancuso, F.; Calvitti, M.; Falabella, G.; Murdolo, G.; Basta, G.; Cameron, D.F.; Hansen, B.C.; Fallarino, F.; et al. Xenograft of microencapsulated Sertoli cells restores glucose homeostasis in db/db mice with spontaneous diabetes mellitus. Xenotransplantation 2016, 23, 429–439. [Google Scholar] [CrossRef]
- Luca, G.; Calafiore, R.; Basta, G.; Ricci, M.; Calvitti, M.; Neri, L.; Nastruzzi, C.; Becchetti, E.; Capitani, S.; Brunetti, P.; et al. Improved function of rat islets upon comicroencapsulation with Sertoli cells in alginate/poly-l-ornithine. AAPS Pharm. Sci. Technol. 2001, 2, 48–54. [Google Scholar] [CrossRef]
- Korbutt, G.S.; Elliott, J.F.; Rajotte, R.V. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long term graft survival without systemic immunosuppression. Diabetes 1997, 46, 317–322. [Google Scholar] [CrossRef]
- Willing, A.E.; Cameron, D.F.; Sanberg, P.R. Sertoli cell transplants: Their use in the treatment of neurodegenerative disease. Mol. Med. Today 1998, 4, 471–477. [Google Scholar] [CrossRef]
- Sanberg, P.R.; Borlongan, C.V.; Saporta, S.; Cameron, D.F. Testis-derived Sertoli cells survive and provide localized immunoprotection for xenografts in rat brain. Nat. Biotechnol. 1996, 14, 1692–1695. [Google Scholar] [CrossRef]
- Turek, P.J.; Malkowicz, S.B.; Tomaszewski, J.E.; Wein, A.J.; Peehl, D. The role of the Sertoli cell in active immunosuppression in the human testis. Br. J. Urol. 1996, 77, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Emerich, D.F.; Hemendinger, R.; Halberstadt, C.R. The testicular-derived Sertoli cell: Cellular immuno science to enable transplantation. Cell Transpl. 2003, 12, 335–349. [Google Scholar] [CrossRef]
- Kumar, A.; Glaum, M.; El-Badri, N.; Mohapatra, S.; Haller, E.; Park, S.; Patrick, L.; Nattkemper, L.; Vo, D.; Cameron, D.F. Initial observation of cells mediated drug delivery to the deep lung. Cell Transpl. 2011, 20, 609–618. [Google Scholar] [CrossRef]
- Nagaosa, K.; Nakashima, C.; Kishimoto, A.; Nakanishi, Y. Immune response to bacteria in seminiferous epithelium. Reproduction 2009, 137, 879–888. [Google Scholar] [CrossRef]
- Sang, Y.; Ortega, M.T.; Blecha, F.; Prakash, O.; Melgarejo, T. Molecular cloning and characterization of three b-defensins from canine testes. Infect. Immun. 2005, 73, 2611–2620. [Google Scholar] [CrossRef]
- Grandjean, V.; Vincent, S.; Martin, L.; Rassoulzadegan, M.; Cuzin, F. Antimicrobial protection of the mouse testis: Synthesis of defensins of the cryptdin family. Biol. Reprod. 1997, 57, 1115–1122. [Google Scholar] [CrossRef]
- Zetterström, C.K.; Strand, M.L.; Söder, O. The high mobility group box chromosomal protein 1 is expressed in the human and rat testis where it may function as an antibacterial factor. Hum. Reprod. 2006, 21, 2801–2809. [Google Scholar] [CrossRef]
- Doyle, T.J.; Kaur, G.; Putrevu, S.M.; Dyson, E.L.; Dyson, M.; McCunniff, W.T.; Pasham, M.R.; Kim, K.H.; Dufour, J.M. Immuno-protective properties of primary Sertoli cells in mice: Potential functional pathways that confer immune privilege. Biol. Reprod. 2012, 86, 1–14. [Google Scholar] [CrossRef]
- Giovagnoli, S.; Mancuso, F.; Vannini, S.; Calvitti, M.; Piroddi, M.; Pietrella, D.; Arato, I.; Falabella, G.; Galli, F.; Moretti, M.; et al. Microparticle-loaded neonatal porcine Sertoli cells for cell-based therapeutic and drug delivery system. J. Controlled. Release 2014, 192, 249–261. [Google Scholar] [CrossRef]
- Arato, I.; Milardi, D.; Giovagnoli, S.; Grande, G.; Bellucci, C.; Lilli, C.; Bartoli, S.; Corneli, S.; Mazzone, P.; Calvitti, M.; et al. In “Vitro” LPS-stimulated Sertoli cells pre-loaded with microparticles: Intracellular activation pathways. Front. Endocrinol. 2021, 11, 611932. [Google Scholar] [CrossRef]
- Corte, L.; Roscini, L.; Colabella, C.; Tascini, C.; Leonildi, A.; Sozio, E.; Menichetti, F.; Merelli, M.; Scarparo, C.; Meyer, W.; et al. Exploring ecological modelling to investigate factors governing the colonization success in nosocomial environment of Candida albicans and other pathogenic yeasts. Sci. Rep. 2016, 6, 26860. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Alhede, M.; Alhede, M.; Eickhardt-Sørensen, S.R.; Moser, C.; Kühl, M.; Jensen, P.Ø.; Høiby, N. The In Vivo biofilm. Trends Microbiol. 2013, 21, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Li, W.S.; Chen, Y.C.; Kuo, S.F.; Chen, F.J.; Lee, C.H. The Impact of Biofilm Formation on the Persistence of Candidemia. Front. Microbiol. 2018, 9, 1196. [Google Scholar] [CrossRef]
- Tascini, C.; Sozio, E.; Corte, L.; Sbrana, F.; Scarparo, C.; Ripoli, A.; Bertolino, G.; Merelli, M.; Tagliaferri, E.; Corcione, A.; et al. The role of biofilm forming on mortality in patients with candidemia: A study derived from real world data. Infect. Dis. 2018, 50, 214–219. [Google Scholar] [CrossRef]
- Corte, L.; Casagrande Pierantoni, D.; Tascini, C.; Roscini, L.; Cardinali, G. Biofilm Specific Activity: A Measure to Quantify Microbial Biofilm. Microorganisms 2019, 7, 73. [Google Scholar] [CrossRef]
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef]
- Forastiero, A.; Mesa-Arango, A.C.; Alastruey-Izquierdo, A.; Alcazar-Fuoli, L.; Bernal-Martinez, L.; Pelaez, T.; Lopez, J.F.; Grimalt, J.O.; Gomez-Lopez, A.; Cuesta, I.; et al. Candida tropicalis Antifungal Cross-Resistance Is Related to Different Azole Target (Erg11p) Modifications. Antimicrob. Agents Chemother. 2013, 57, 4769–4781. [Google Scholar] [CrossRef]
- Palazzo, F.; Giovagnoli, S.; Schoubben, A.; Blasi, P.; Rossi, C.; Ricci, M. Development of a spray-drying method for the formulation of respirable microparticles containing ofloxacin–palladium complex. Int. J. Pharm. 2013, 440, 273–282. [Google Scholar] [CrossRef]
- Giovagnoli, S.; Palazzo, F.; Di Michele, A.; Schoubben, A.; Blasi, P.; Ricci, M. The influence of feedstock and process variables on the encapsulation of drug suspensions by spray-drying in fast drying regime: The case of novel antitubercular drug–palladium complex containing polymeric microparticles. J. Pharm. Sci. 2014, 103, 1255–1268. [Google Scholar] [CrossRef]
- Cannarella, R.; Mancuso, F.; Condorelli, R.A.; Arato, I.; Mongioì, L.M.; Giacone, F.; Lilli, C.; Bellucci, C.; La Vignera, S.; Calafiore, R.; et al. Effects of GH and IGF1 on Basal and FSH-Modulated Porcine Sertoli Cells In-Vitro. J. Clin. Med. 2019, 8, 811. [Google Scholar] [CrossRef] [PubMed]
- Arato, I.; Luca, G.; Mancuso, F.; Bellucci, C.; Lilli, C.; Calvitti, M.; Eugeni, E.; Gaggia, F.; Baroni, T.; Mancuso, F.; et al. An in vitro prototype of a porcine biomimetictestis−like cell culture system: A novel tool for the study of reassembled Sertoli and Leydig cells. Asian J. Androl. 2018, 20, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Arato, I.; Grande, G.; Barrachina, F.; Bellucci, C.; Lilli, C.; Jodar, M.; Aglietti, M.C.; Mancini, F.; Vincenzoni, F.; Pontecorvi, A.; et al. “In Vitro” Effect of Different Follicle-Stimulating Hormone Preparations on Sertoli Cells: Toward a Personalized Treatment for Male Infertility. Front. Endocrinol. 2020, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Arato, I.; Ceccarelli, V.; Mancuso, F.; Bellucci, C.; Lilli, C.; Ferolla, P.; Perruccio, K.; D’Arpino, A.; Aglietti, M.C.; Calafiore, R.; et al. Effect of EPA on Neonatal Pig Sertoli Cells “In Vitro”: A Possible Treatment to Help Maintain Fertility in Pre-Pubertal Boys Undergoing Treatment with Gonado-Toxic Therapies. Front. Endocrinol. 2021, 12, 694796. [Google Scholar] [CrossRef]
- Cannarella, R.; Arato, I.; Condorelli, R.A.; Luca, G.; Barbagallo, F.; Alamo, A.; Bellucci, C.; Lilli, C.; La Vignera, S.; Calafiore, R.; et al. The IGF1 Receptor Is Involved in Follicle-Stimulating Hormone Signaling in Porcine Neonatal Sertoli Cells. J. Clin. Med. 2019, 8, 577. [Google Scholar] [CrossRef]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L., Jr.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- Luca, G.; Mancuso, F.; Calvitti, M.; Arato, I.; Falabella, G.; Bufalari, A.; De Monte, V.; Tresoldi, E.; Nastruzzi, C.; Basta, G.; et al. Long-term stability, functional competence, and safety of microencapsulated specific pathogen-free neonatal porcine Sertoli cells: A potential product for cell transplant therapy. Xenotransplantation 2015, 22, 273–283. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mancuso, F.; Arato, I.; Lilli, C.; Bellucci, C.; Bodo, M.; Calvitti, M.; Aglietti, M.C.; dell’Omo, M.; Nastruzzi, C.; Calafiore, R.; et al. Acute effects of lead on porcine neonatal Sertoli cells In Vitro. Toxicol Vitr. 2018, 48, 45–52. [Google Scholar] [CrossRef]
- Yi, Y.; Lin, G.; Chen, S.; Liu, J.; Zhang, H.; Mi, P. Polyester micelles for drug delivery and cancer theranostics: Current achievements, progresses and future perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 218–232. [Google Scholar] [CrossRef]
- Mohamed, F.; Van der Walle, C.F. Engineering biodegradable polyester particles with specific drug targeting and drug release properties. J. Pharm. Sci. 2008, 97, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Van der Meer, J.W.; Kullberg, B.J. Both TLR2 and TLR4 are involved in the recognition of Candida albicans. Reply to “TLR2, but not TLR4, triggers cytokine production by murine cells in response to Candida albicans yeasts and hyphae” by Gil and Gozalbo, Microbes and Infection 8 (2006) 2823–2824. Microbes Infect. 2006, 8, 2821–2822, author reply 2823–2824. [Google Scholar] [CrossRef] [PubMed]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2004, 4, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Puccetti, P. Protective tolerance to fungi: The role of IL-10 and tryptophan catabolism. Trends Microbiol. 2006, 14, 183–189. [Google Scholar] [CrossRef]
- Hori, S.; Carvalho, T.L.; Demengeot, J. CD25+CD4+ regulatory T cells suppress CD4+T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 2002, 32, 1282–1291. [Google Scholar] [CrossRef]
- Montagnoli, C.; Bozza, S.; Bacci, A.; Gaziano, R.; Mosci, P.; Morschhauser, J.; Pitzurra, L.; Kopf, M.; Cutler, J.; Romani, L. A role for antibodies in the generation of memory antifungal immunity. Eur. J. Immunol 2003, 33, 1193–1204. [Google Scholar] [CrossRef]
- Montagnoli, C.; Fallarino, F.; Gaziano, R.; Bozza, S.; Bellocchio, S.; Zelante, T.; Kurup, W.P.; Pitzurra, L.; Puccetti, P.; Romani, L. Immunity and tolerance to Aspergillus involve functionally distinct regulatory T cells and tryptophan catabolism. J. Immunol. 2006, 176, 1712–1723. [Google Scholar] [CrossRef]
- McKinley, L.; Logar, A.J.; McAllister, F.; Zheng, M.; Steele, C.; Kolls, J.K. Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of Pneumocystis Pneumonia. J. Immunol. 2006, 177, 6215–6226. [Google Scholar] [CrossRef]
- Cavassani, K.A.; Campanelli, A.P.; Moreira, A.P.; Vancim, J.O.; Vitali, L.H.; Mamede, R.C.; Martinez, R.; Silva, J.S. Systemic and local characterization of regulatory T cells in a chronic fungal infection in humans. J. Immunol. 2006, 177, 5811–5818. [Google Scholar] [CrossRef]
- De Luca, A.; Montagnoli, C.; Zelante, T.; Bonifazi, P.; Bozza, S.; Moretti, S.; D’Angelo, C.; Vacca, C.; Boon, L.; Bistoni, F.; et al. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J. Immunol. 2007, 179, 5999–6008. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Puccetti, P.; Grohmann, U. IDO and regulatory T cells: A role for reverse signalling and non-canonical NF-kappaB activation. Nat. Rev. Immunol. 2007, 7, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.A.; Wong, R.; Hackl, S.I.; Moua, O.; Gill, R.G.; Wiseman, A.; Davidson, H.W.; Hutton, J.C. Induction of indoleamine 2,3-dioxygenase by interferon-gamma in human islets. Diabetes 2007, 56, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, U.; Fallarino, F.; Bianchi, R.; Orabona, C.; Vacca, C.; Fioretti, M.C.; Puccetti, P. A defect in tryptophan catabolism impairs tolerance in nonobese diabetic mice. J. Exp. Med. 2013, 198, 153–160. [Google Scholar] [CrossRef]
- Fallarino, F.; Luca, G.; Calvitti, M.; Mancuso, F.; Nastruzzi, C.; Fioretti, M.C.; Grohmann, U.; Becchetti, E.; Burgevin, A.; Kratzer, R.; et al. Therapy of experimental type 1 diabetes by isolated Sertoli cell xenografts alone. J. Exp. Med. 2009, 206, 2511–2526. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Tian, X.; Shao, H.; Wu, X.; Wang, Q.; Su, X.; Shi, Y. Activation of NF-κB and respiratory burst following Aspergillus fumigatus stimulation of macrophages. Immunobiology 2014, 219, 25–36. [Google Scholar] [CrossRef]
- Moyes, D.L.; Shen, C.; Murciano, C.; Runglall, M.; Richardson, J.P.; Arno, M.; Aldecoa-Otalora, E.; Naglik, J.R. Protection against epithelial damage during Candida albicans infection is mediated by PI3K/Akt and Mammalian Target of Rapamycin Signaling. J. Infect. Dis. 2014, 209, 1816–1826. [Google Scholar] [CrossRef]
- Bruder, J.T.; Kovesdi, I. Adenovirus Infection Stimulates the Raf/MAPK Signaling Pathway and Induces Interleukin-8 Expression. J. Virol. 1997, 71, 398–404. [Google Scholar] [CrossRef]
- Kishi-Kaboshi, M.; Okada, K.; Kurimoto, L.; Murakami, S.; Umezawa, T.; Shibuya, N.; Yamane, H.; Miya, A.; Takatsuji, H.; Takahashi, A.; et al. A rice fungal MAMP-responsive MAPK cascade regulates metabolic flow to antimicrobial metabolite synthesis. Plant J. 2010, 63, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Rahman, D.; Mistry, M.; Thavaraj, S.; Challacombe, S.J.; Naglik, J.R. Murine model of concurrent oral and vaginal Candida albicans colonization to study epithelial host-pathogen interactions. Microbes Infect. 2007, 9, 615–622. [Google Scholar] [CrossRef]
- Osada, Y.; Sunatani, T.; Kim, I.S.; Nakanishi, Y.; Shiratsuchi, A. Signalling Pathway Involving GULP, MAPK and Rac1 for SR-BI-Induced Phagocytosis of Apoptotic Cells. J. Biochem. 2009, 145, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Moyes, D.L.; Runglall, M.; Murciano, C.; Shen, C.; Nayar, D.; Thavaraj, S.; Kohli, A.; Islam, A.; Mora-Monte, H.; Challacombe, S.J.; et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 2010, 8, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, M.J.; Reuss, L.E. Techniques, In Biological Electron Microscopy; Springer: Boston, MA, USA, 2003. [Google Scholar] [CrossRef]
- Ceccarelli, V.; Ronchetti, S.; Marchetti, M.C.; Calvitti, M.; Riccardi, C.; Grignani, F.; Vecchini, A. Molecular mechanisms underlying eicosapentaenoic acid inhibition of HDAC1 and DNMT expression and activity in carcinoma cells. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194481. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. Methods Mol. Biol. 2017, 1601, 1–17. [Google Scholar] [CrossRef]
- Fai, P.B.; Grant, A. A rapid resazurin bioassay for assessing the toxicity of fungicides. Chemosphere 2009, 74, 1165–1170. [Google Scholar] [CrossRef]








| Gene | Forward | Reverse | |
|---|---|---|---|
| β-actin | ATGGTGGGTATGGGTCAGAA | CTTCTCCATGTCGTCCCAGT | 56 °C |
| MHCII | GACCAGATGAGGTTATTGG | GGTCCTGTAGTTGTGTCT | 56 °C |
| IDO | ATGAAGGCGTTTGGGACACC | GAGGAATCCAGCAGCAGAGC | 56 °C |
| TGF1β | GCCCTGGACACCAACTATTGC | GCTGCACTTGCAGGAGCGCAC | 56 °C |
| TLR-4 | CTTCACTACAGAGACTTCA | ACAATAACCTTCCGACTT | 56 °C |
| BDF123 | GAGTGCGTTGGGAAGATG | TCGGTATGTACTTGGGATGT | 56 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arato, I.; Giovagnoli, S.; Roscini, L.; Calvitti, M.; Bellucci, C.; Lilli, C.; Eugeni, E.; Brancorsini, S.; Cardinali, G.; Luca, G.; et al. Exploring Sertoli Cells’ Innate Bulwark Role Against Infections: In Vitro Performances on Candida tropicalis Biofilms. Cells 2025, 14, 495. https://doi.org/10.3390/cells14070495
Arato I, Giovagnoli S, Roscini L, Calvitti M, Bellucci C, Lilli C, Eugeni E, Brancorsini S, Cardinali G, Luca G, et al. Exploring Sertoli Cells’ Innate Bulwark Role Against Infections: In Vitro Performances on Candida tropicalis Biofilms. Cells. 2025; 14(7):495. https://doi.org/10.3390/cells14070495
Chicago/Turabian StyleArato, Iva, Stefano Giovagnoli, Luca Roscini, Mario Calvitti, Catia Bellucci, Cinzia Lilli, Elena Eugeni, Stefano Brancorsini, Gianluigi Cardinali, Giovanni Luca, and et al. 2025. "Exploring Sertoli Cells’ Innate Bulwark Role Against Infections: In Vitro Performances on Candida tropicalis Biofilms" Cells 14, no. 7: 495. https://doi.org/10.3390/cells14070495
APA StyleArato, I., Giovagnoli, S., Roscini, L., Calvitti, M., Bellucci, C., Lilli, C., Eugeni, E., Brancorsini, S., Cardinali, G., Luca, G., & Mancuso, F. (2025). Exploring Sertoli Cells’ Innate Bulwark Role Against Infections: In Vitro Performances on Candida tropicalis Biofilms. Cells, 14(7), 495. https://doi.org/10.3390/cells14070495










