Platelet-Activating Factor-Induced Inflammation in Obesity: A Two-Sided Coin of Protection and Risk
Abstract
1. Introduction
2. Animal Studies and In Vitro Experiments
3. Human Studies
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGMO | alkylglycerol monooxygenase |
| Akt | protein kinase B |
| AT | adipose tissue |
| ATMs | adipose tissue macrophages |
| BAT | brown adipose tissue |
| BMI | body mass index |
| C/EBP-α | CCAAT/enhancer-binding protein-α |
| cPLA2 | cytoplasmic phospholipase A2 |
| HFD | high-fat diet |
| IL-6 | interleukin-6 |
| JAK | Janus kinase |
| LPC | lyso-phosphatidylcholine |
| LpPLA2 | lipoprotein-associated phospholipase A2 |
| Lyso-PAF AT | acetyl-CoA:lyso–platelet-activating factor acetyltransferases |
| PAF | platelet-activating factor |
| PAF-AHs | PAF-specific acetylhydrolases |
| PAF-CPT | 1-alkyl-2-acetyl-sn-glycerol cholinephosphotransferase |
| PAFR | PAF G-protein coupled receptor |
| PAFR-KO | PAFR-knockout |
| PI3K | phosphatidylinositol-3-kinase |
| PLAL | PAF-like activity lipids |
| PPARα | proliferator-activated receptor alpha |
| PPARγ | proliferator-activated receptor gamma |
| STAT3 | Signal transducer and activator of transcription 3 |
| SVC | stromal vascular cells |
| TAG | triacylglycerols |
| TNF-α | tumor necrosis factor-α |
| UCP1 | uncoupling protein-1 |
| WAT | white adipose tissue |
| WT | wild-type |
References
- WHO: Obesity Website. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 25 January 2025).
- Corvera, S.; Gealekman, O. Adipose tissue angiogenesis: Impact on obesity and type-2 diabetes. Biochim. Biophys. Acta 2014, 1842, 463–472. [Google Scholar] [CrossRef]
- Soták, M.; Clark, M.; Suur, B.E.; Börgeson, E. Inflammation and resolution in obesity. Nat. Rev. Endocrinol. 2025, 21, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Wollam, J.; Olefsky, J.M. An Integrated View of Immunometabolism. Cell 2018, 172, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Wernstedt Asterholm, I.; Tao, C.; Morley, T.S.; Wang, Q.A.; Delgado-Lopez, F.; Wang, Z.V.; Scherer, P.E. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014, 20, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; McGuinness, O.P. Inflammation during obesity is not all bad: Evidence from animal and human studies. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E466–E477. [Google Scholar] [CrossRef]
- Demopoulos, C.A.; Pinckard, R.N.; Hanahan, D.J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J. Biol. Chem. 1979, 254, 9355–9358. [Google Scholar]
- Kulikov, V.I.; Muzya, G.I. Ether lipids and platelet-activating factor: Evolution and cellular function. Biochemistry 1997, 62, 1103–1108. [Google Scholar]
- Lordan, R.; Tsoupras, A.; Zabetakis, I.; Demopoulos, C.A. Forty Years Since the Structural Elucidation of Platelet-Activating Factor (PAF): Historical, Current, and Future Research Perspectives. Molecules 2019, 24, 4414. [Google Scholar] [CrossRef]
- Snyder, F. CDP-choline:alkylacetylglycerol cholinephosphotransferase catalyzes the final step in the de novo synthesis of platelet-activating factor. Biochim. Biophys. Acta 1997, 1348, 111–116. [Google Scholar] [CrossRef]
- Harayama, T.; Shindou, H.; Ogasawara, R.; Suwabe, A.; Shimizu, T. Identification of a novel noninflammatory biosynthetic pathway of platelet-activating factor. J. Biol. Chem. 2008, 283, 11097–11106. [Google Scholar] [CrossRef]
- Marathe, G.K.; Davies, S.S.; Harrison, K.A.; Silva, A.R.; Murphy, R.C.; Castro-Faria-Neto, H.; Prescott, S.M.; Zimmerman, G.A.; McIntyre, T.M. Inflammatory platelet-activating factor-like phospholipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. J. Biol. Chem. 1999, 274, 28395–28404. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Stafforini, D.M. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J. Lipid Res. 2012, 53, 1767–1782. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Guo, H.; Tam, J.W.; Johnson, B.M.; Brickey, W.J.; New, J.S.; Lenox, A.; Shi, H.; Golenbock, D.T.; Koller, B.H.; et al. Platelet-activating factor (PAF) mediates NLRP3-NEK7 inflammasome induction independently of PAFR. J. Exp. Med. 2019, 216, 2838–2853. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Shimizu, T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog. Lipid Res. 2000, 39, 41–82. [Google Scholar] [CrossRef]
- Upton, J.E.M.; Grunebaum, E.; Sussman, G.; Vadas, P. Platelet Activating Factor (PAF): A Mediator of Inflammation. Biofactors 2022, 48, 1189–1202. [Google Scholar] [CrossRef]
- Koga, M.M.; Bizzarro, B.; Sá-Nunes, A.; Rios, F.J.; Jancar, S. Activation of PAF-receptor induces regulatory dendritic cells through PGE2 and IL-10. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 319–326. [Google Scholar] [CrossRef]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Farooqui, T.; Horrocks, L.A. Roles of Platelet-Activating Factor in Brain. In Metabolism and Functions of Bioactive Ether Lipids in the Brain; Springer: New York, NY, USA, 2008; pp. 171–195. [Google Scholar]
- Sakellariou, M.; Drakakis, P.; Antonopoulou, S.; Anagnostou, E.; Loutradis, D.; Patargias, T. Intravenous infusion of PAF affects ovulation, fertilization and preimplantation embryonic development in nzb x nzw f1 hybrid mice. Prostaglandins Other Lipid Mediat. 2008, 85, 125–133. [Google Scholar] [CrossRef]
- O’Neill, C. The role of paf in embryo physiology. Hum. Reprod. Update 2005, 11, 215–228. [Google Scholar] [CrossRef]
- Yu, H.; Dilbaz, S.; Coßmann, J.; Hoang, A.C.; Diedrich, V.; Herwig, A.; Harauma, A.; Hoshi, Y.; Moriguchi, T.; Landgraf, K.; et al. Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J. Clin. Investig. 2019, 129, 2485–2499. [Google Scholar] [CrossRef]
- Sugatani, J.; Sadamitsu, S.; Yamaguchi, M.; Yamazaki, Y.; Higa, R.; Hattori, Y.; Uchida, T.; Ikari, A.; Sugiyama, W.; Watanabe, T.; et al. Antiobese function of platelet-activating factor: Increased adiposity in platelet-activating factor receptor-deficient mice with age. FASEB J. 2014, 28, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Garcia, Z.; Oliveira, M.C.; Lima, R.L.; Soriani, F.M.; Cisalpino, D.; Botion, L.M.; Teixeira, M.M.; Souza, D.G.; Ferreira, A.V. Lack of platelet-activating factor receptor protects mice against diet-induced adipose inflammation and insulin-resistance despite fat pad expansion. Obesity 2014, 22, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, L.R.; Koga, M.M.; Quaresma, P.G.; Ishizuka, E.K.; Montes, M.B.A.; Prada, P.O.; Saad, M.J.; Jancar, S.; Rios, F.J. PAFR in adipose tissue macrophages is associated with anti-inflammatory phenotype and metabolic homoeostasis. Clin. Sci. 2016, 130, 601–612. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Matsui, M.; Higa, R.; Yamazaki, Y.; Ikari, A.; Miyake, M.; Miwa, M.; Ishii, S.; Sugatani, J.; Shimizu, T. A platelet-activating factor (PAF) receptor deficiency exacerbates diet-induced obesity but PAF/PAF receptor signaling does not contribute to the development of obesity-induced chronic inflammation. Biochem. Pharmacol. 2015, 93, 482–495. [Google Scholar] [CrossRef]
- Lacerda, D.R.; Soares, D.D.; Costa, K.A.; Nunes-Silva, A.; Rodrigues, D.F.; Sabino, J.L.; Silveira, A.L.M.; Pinho, V.; Vieira, É.L.M.; Menezes, G.B.; et al. Mechanisms underlying fat pad remodeling induced by fasting: Role of PAF receptor. Nutrition 2020, 71, 110616. [Google Scholar] [CrossRef]
- Costa, K.A.; Lacerda, D.R.; Silveira, A.L.M.; Martins, L.B.; Oliveira, M.C.; Rezende, B.M.; Menezes-Garcia, Z.; Mügge, F.L.B.; Silva, A.M.; Teixeira, M.M.; et al. PAF signaling plays a role in obesity-induced adipose tissue remodeling. Int. J. Obes. 2022, 46, 68–76. [Google Scholar] [CrossRef]
- Kosteli, A.; Sugaru, E.; Haemmerle, G.; Martin, J.F.; Lei, J.; Zechner, R.; Ferrante, A.W., Jr. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Investig. 2010, 120, 3466–3479. [Google Scholar] [CrossRef]
- Kang, S.; Bennett, C.N.; Gerin, I.; Rapp, L.A.; Hankenson, K.D.; Macdougald, O.A. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2007, 282, 14515–14524. [Google Scholar] [CrossRef]
- Liao, Y.; Badmann, S.; Kraus, F.; Topalov, N.E.; Mayr, D.; Kolben, T.; Hester, A.; Beyer, S.; Mahner, S.; Jeschke, U.; et al. PLA2G7/PAF-AH as Potential Negative Regulator of the Wnt Signaling Pathway Mediates Protective Effects in BRCA1 Mutant Breast Cancer. Int. J. Mol. Sci. 2023, 24, 882. [Google Scholar] [CrossRef]
- Sarantopoulos, C.N.; Banyard, D.A.; Ziegler, M.E.; Sun, B.; Shaterian, A.; Widgerow, A.D. Elucidating the Preadipocyte and Its Role in Adipocyte Formation: A Comprehensive Review. Stem Cell Rev. Rep. 2018, 14, 27–42. [Google Scholar] [CrossRef]
- Watschinger, K.; Werner, E.R. Alkylglycerol monooxygenase. IUBMB Life 2013, 65, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Tokuoka, S.M.; Kita, Y.; Shindou, H.; Shimizu, T. Alkylglycerol monooxygenase as a potential modulator for PAF synthesis in macrophages. Biochem. Biophys. Res. Commun. 2013, 436, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Sailer, S.; Lackner, K.; Pras-Raves, M.L.; Wever, E.J.M.; van Klinken, J.B.; Dane, A.D.; Geley, S.; Koch, J.; Golderer, G.; Werner-Felmayer, G.; et al. Adaptations of the 3T3-L1 adipocyte lipidome to defective ether lipid catabolism upon Agmo knockdown. J. Lipid Res. 2022, 63, 100222. [Google Scholar] [CrossRef] [PubMed]
- Watschinger, K.; Keller, M.A.; McNeill, E.; Alam, M.T.; Lai, S.; Sailer, S.; Rauch, V.; Patel, J.; Hermetter, A.; Golderer, G.; et al. Tetrahydrobiopterin and alkylglycerol monooxygenase substantially alter the murine macrophage lipidome. Proc. Natl. Acad. Sci. USA 2015, 112, 2431–2436. [Google Scholar] [CrossRef]
- Bartz, R.; Li, W.-H.; Venables, B.; Zehmer, J.K.; Roth, M.R.; Welti, R.; Anderson, R.G.W.; Liu, P.; Chapman, K.D. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res. 2007, 48, 837–847. [Google Scholar] [CrossRef]
- Cox, C.P.; Linden, J.; Said, S.I. VIP elevates platelet cyclic AMP (cAMP) levels and inhibits in vitro platelet activation induced by platelet-activating factor (PAF). Peptides 1984, 5, 325–328. [Google Scholar] [CrossRef]
- Fradin, A.; Rothhut, B.; Poincelot-Canton, B.; Errasfa, M.; Russo-Marie, F. Inhibition of eicosanoid and PAF formation by dexamethasone in rat inflammatory polymorphonuclear neutrophils may implicate lipocortin ’s’. Biochim. Biophys. Acta 1988, 963, 248–257. [Google Scholar] [CrossRef]
- Cheng, L.; Han, X.; Shi, Y. A regulatory role of LPCAT1 in the synthesis of inflammatory lipids, PAF and LPC, in the retina of diabetic mice. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1276–E1282. [Google Scholar] [CrossRef]
- Spadaro, O.; Youm, Y.; Shchukina, I.; Ryu, S.; Sidorov, S.; Ravussin, A.; Nguyen, K.; Aladyeva, E.; Predeus, A.N.; Smith, S.R.; et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science 2022, 375, 671–677. [Google Scholar] [CrossRef]
- Tzotzas, T.; Filippatos, T.D.; Triantos, A.; Bruckert, E.; Tselepis, A.D.; Kiortsis, D.N. Effects of a low-calorie diet associated with weight loss on lipoprotein-associated phospholipase A2 (Lp-PLA2) activity in healthy obese women. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 477–482. [Google Scholar] [CrossRef]
- Ntzouvani, A.; Giannopoulou, E.; Fragopoulou, E.; Nomikos, T.; Antonopoulou, S. Energy Intake and Plasma Adiponectin as Potential Determinants of Lipoprotein-Associated Phospholipase A2 Activity: A Cross-Sectional Study. Lipids 2019, 54, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Nomikos, T.; Fragopoulou, E.; Stamatakis, G.; Panagiotakos, D.B.; Antonopoulou, S. PAF and its metabolic enzymes in healthy volunteers: Interrelations and correlations with basic characteristics. Prostaglandins Other Lipid Mediat. 2012, 97, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Seyfarth, J.; Reinehr, T.; Hoyer, A.; Reinauer, C.; Bächle, C.; Karges, B.; Mayatepek, E.; Roden, M.; Hofer, S.E.; Wiegand, S.; et al. Lipoprotein-associated phospholipase A2 activity in obese adolescents with and without type 2 diabetes. J. Inherit. Metab. Dis. 2018, 41, 73–79. [Google Scholar] [CrossRef] [PubMed]
- English, C.J.; Lohning, A.E.; Mayr, H.L.; Jones, M.; Reidlinger, D.P. Interrelationships among platelet-activating factor and lipoprotein-associated phospholipase A2 activity and traditional cardiovascular risk factors. BioFactors 2023, 49, 457–471. [Google Scholar] [CrossRef]
- Detopoulou, P.; Nomikos, T.; Fragopoulou, E.; Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C.; Antonopoulou, S. Lipoprotein-associated phospholipase A2 (Lp-PLA2) activity, platelet-activating factor acetylhydrolase (PAF-AH) in leukocytes and body composition in healthy adults. Lipids Health Dis. 2009, 8, 19. [Google Scholar] [CrossRef]
- Mutoh, H.; Ishii, S.; Izumi, T.; Kato, S.; Shimizu, T. Platelet-activating factor (PAF) positively auto-regulates the expression of human PAF receptor transcript 1 (leukocyte-type) through NF-kappa B. Biochem. Biophys. Res. Commun. 1994, 205, 1137–1142. [Google Scholar] [CrossRef]
- Pietiläinen, K.H.; Sysi-Aho, M.; Rissanen, A.; Seppänen-Laakso, T.; Yki-Järvinen, H.; Kaprio, J.; Oresic, M. Acquired Obesity Is Associated with Changes in the Serum Lipidomic Profile Independent of Genetic Effects—A Monozygotic Twin Study. PLoS ONE 2007, 2, e218. [Google Scholar] [CrossRef]
- Stafforini, D.M. Chapter Six—Plasma PAF-AH (PLA2G7): Biochemical Properties, Association with LDLs and HDLs, and Regulation of Expression. In The Enzymes; Inoue, K., Stafforini, D.M., Tamanoi, F., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 38, pp. 71–93. [Google Scholar] [CrossRef]
- Hourton, D.; Delerive, P.; Stankova, J.; Staels, B.; Chapman, M.J.; Ninio, E. Oxidized low-density lipoprotein and peroxisome-proliferator-activated receptor alpha down-regulate platelet-activating-factor receptor expression in human macrophages. Biochem. J. 2001, 354, 225–232. [Google Scholar] [CrossRef]
- Howard, K.M.; Abdel-al, M.; Ditmyer, M.; Patel, N. Lipopolysaccharide and platelet-activating factor stimulate expression of platelet-activating factor acetylhydrolase via distinct signaling pathways. Inflamm. Res. 2011, 60, 735–744. [Google Scholar] [CrossRef]
- Boutens, L.; Stienstra, R. Adipose tissue macrophages: Going off track during obesity. Diabetologia 2016, 59, 879–894. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Mimura, K.; Yukawa, S.; Mori, Y.; Okada, K.; Mune, M.; Nishikawa, O.; Hibino, A.; Sonobe, M.; Goto, T.; Nomoto, H. Effect of platelet-activating factor on lipoprotein lipase and blood lipids. Lipids 1991, 26, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.D.; Ilic, V.; Williamson, D.H. Effects of platelet-activating factor on lipid metabolism in rats in vivo. Origin of the hypertriglyceridaemia. Biochem. J. 1991, 280, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Dalmaso, B.; Silva-Junior, I.A.d.; Jancar, S.; Del Debbio, C.B. Platelet-Activating Factor Receptor (PAFR) Regulates Retinal Progenitor/Stem Cells Profile in Ciliary Epithelium Cells. Int. J. Mol. Sci. 2024, 25, 3084. [Google Scholar] [CrossRef]
- Dahal, A.; Hong, Y.; Mathew, J.S.; Geber, A.; Eckl, S.; Renner, S.; Sailer, C.J.; Ryan, A.T.; Mir, S.; Lim, K.; et al. Platelet-activating factor (PAF) promotes immunosuppressive neutrophil differentiation within tumors. Proc. Natl. Acad. Sci. USA 2024, 121, e2406748121. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Ibe, B.O.; Portugal, A.M.; Usha, R.J. Metabolism of platelet activating factor by intrapulmonary vascular smooth muscle cells. Effect of oxygen on phospholipase A2 protein expression and activities of acetyl-CoA acetyltransferase and cholinephosphotransferase. Mol. Genet. Metab. 2002, 77, 237–248. [Google Scholar] [CrossRef]
- Keely, S.; Glover, L.E.; Weissmueller, T.; MacManus, C.F.; Fillon, S.; Fennimore, B.; Colgan, S.P. Hypoxia-inducible factor-dependent regulation of platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia. Mol. Biol. Cell 2010, 21, 538–546. [Google Scholar] [CrossRef]
- Gountopoulou, A.; Leondaritis, G.; Galanopoulou, D.; Mavri-Vavayanni, M. TNFalpha is a potent inducer of platelet-activating factor synthesis in adipocytes but not in preadipocytes. Differential regulation by PI3K. Cytokine 2008, 41, 174–181. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Iatrou, C.; Antonopoulou, S.; Ruan, X.Z.; Fernando, R.L.; Powis, S.H.; Moorhead, J.F.; Varghese, Z. Platelet-activating factor (PAF) increase intracellular lipid accumulation by increasing both LDL and scavenger receptors in human mesangial cells. J. Lab. Clin. Med. 2006, 147, 281–289. [Google Scholar] [CrossRef]
- Bondareva, O.; Rodríguez-Aguilera, J.R.; Oliveira, F.; Liao, L.; Rose, A.; Gupta, A.; Singh, K.; Geier, F.; Schuster, J.; Boeckel, J.-N.; et al. Single-cell profiling of vascular endothelial cells reveals progressive organ-specific vulnerabilities during obesity. Nat. Metab. 2022, 4, 1591–1610. [Google Scholar] [CrossRef]
- Kudoloa, G.B.; Koopmans, S.J.; Haywood, J.R.; DeFronzo, R.A. Chronic hyperinsulinernia inhibits platelet-activating factor (PAF) biosynthesis in the rat kidney. J. Lipid Mediat. Cell Signal. 1997, 16, 23–37. [Google Scholar] [CrossRef]
- Mahmoud, A.M. An Overview of Epigenetics in Obesity: The Role of Lifestyle and Therapeutic Interventions. Int. J. Mol. Sci. 2022, 23, 1341. [Google Scholar] [CrossRef] [PubMed]
- Hinte, L.C.; Castellano-Castillo, D.; Ghosh, A.; Melrose, K.; Gasser, E.; Noé, F.; Massier, L.; Dong, H.; Sun, W.; Hoffmann, A.; et al. Adipose tissue retains an epigenetic memory of obesity after weight loss. Nature 2024, 636, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Andriessen, E.M.M.A.; Hata, M.; Diaz-Marin, R.; Fournier, F.; Crespo-Garcia, S.; Blot, G.; Juneau, R.; Pilon, F.; Dejda, A.; et al. Past history of obesity triggers persistent epigenetic changes in innate immunity and exacerbates neuroinflammation. Science 2023, 379, 45–62. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bae, J.U.; Lee, S.J.; Park, S.Y.; Kim, C.D. SIRT1 attenuates PAF-induced MMP-2 production via down-regulation of PAF receptor expression in vascular smooth muscle cells. Vasc. Pharmacol. 2015, 72, 35–42. [Google Scholar] [CrossRef]
- Damiani, E.; Puebla-Osorio, N.; Gorbea, E.; Ullrich, S.E. Platelet-Activating Factor Induces Epigenetic Modifications in Human Mast Cells. J. Investig. Dermatol. 2015, 135, 3034–3040. [Google Scholar] [CrossRef]
- Damiani, E.; Puebla-Osorio, N.; Lege, B.; Liu, J.; Neelapu, S.S.; Ullrich, S.E. Platelet activating factor-induced expression of p21 is correlated with histone acetylation. Sci. Rep. 2017, 7, 41959. [Google Scholar] [CrossRef]
- Yan, F.; Wufuer, D.; Ding, J.; Wang, J. MicroRNA miR-146a-5p inhibits the inflammatory response and injury of airway epithelial cells via targeting TNF receptor-associated factor 6. Bioengineered 2021, 12, 1916–1926. [Google Scholar] [CrossRef]
- Petsini, F.; Detopoulou, M.; Choleva, M.; Kostakis, I.K.; Fragopoulou, E.; Antonopoulou, S. Exploring the Effect of Resveratrol, Tyrosol, and Their Derivatives on Platelet-Activating Factor Biosynthesis in U937 Cells. Molecules 2024, 29, 5419. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Nomikos, T.; Karantonis, H.C.; Apostolakis, C.; Pliakis, E.; Samiotaki, M.; Panayotou, G.; Antonopoulou, S. Biological Activity of Acetylated Phenolic Compounds. J. Agric. Food Chem. 2007, 55, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Wang, Y.; Zhu, P.; Wei, Z.; Wang, S.; Tao, L.; Liu, Z.; Wu, H.; Sheng, X.; et al. Suppressive role of diallyl trisulfide in the activated platelet-mediated hematogenous metastasis of MDA-MB-231 human breast cancer cells. Int. J. Mol. Med. 2017, 39, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.H.; Shin, J.I.; Mo, S.J.; Yun, S.J.; Kim, S.H.; Rhee, Y.H. Antiplatelet activity of L-sulforaphane by regulation of platelet activation factors, glycoprotein IIb/IIIa and thromboxane A2. Blood Coagul. Fibrinolysis 2013, 24, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Vlachogianni, I.C.; Fragopoulou, E.; Stamatakis, G.M.; Kostakis, I.K.; Antonopoulou, S. Platelet Activating Factor (PAF) biosynthesis is inhibited by phenolic compounds in U-937 cells under inflammatory conditions. Prostaglandins Other Lipid Mediat. 2015, 121, 176–183. [Google Scholar] [CrossRef]
- Detopoulou, P.; Fragopoulou, E.; Nomikos, T.; Yannakoulia, M.; Stamatakis, G.; Panagiotakos, D.B.; Antonopoulou, S. The relation of diet with PAF and its metabolic enzymes in healthy volunteers. Eur. J. Nutr. 2015, 54, 25–34. [Google Scholar] [CrossRef]
- Nomikos, T.; Fragopoulou, E.; Antonopoulou, S.; Panagiotakos, D.B. Mediterranean diet and platelet-activating factor; a systematic review. Clin. Biochem. 2018, 60, 1–10. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Demopoulos, C.A. Protective Effect of Olive Oil Microconstituents in Atherosclerosis: Emphasis on PAF Implicated Atherosclerosis Theory. Biomolecules 2023, 13, 700. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Pacheco, L.S.; Tessier, A.-J.; Li, Y.; Willett, W.C.; Sun, Q.; Salas-Salvadó, J.; Martínez-González, M.A.; Stampfer, M.J.; Hu, F.B. Changes in olive oil consumption and long-term body weight changes in three U.S. prospective cohort studies. Am. J. Clin. Nutr. 2025. [Google Scholar] [CrossRef]
- Dong, S.-Y.; Li, W.; Wang, L.-F.; Lin, Z.-M.; Chen, M.-J.; Li, T. The effect of exercise on platelet-activating factor metabolism in the livers of rats fed high-fat diet. Acta Physiol. Sin. 2024, 76, 537–546. [Google Scholar] [CrossRef]
- Grypioti, A.D.; Kostopanagiotou, G.; Demopoulos, C.A.; Roussos, A.; Mykoniatis, M. Platelet activating factor (PAF) antagonism with ginkgolide B protects the liver against acute injury. importance of controlling the receptor of PAF. Dig. Dis. Sci. 2008, 53, 1054–1062. [Google Scholar] [CrossRef]
- Didamoony, M.A.; Atwa, A.M.; Ahmed, L.A. A novel mechanistic approach for the anti-fibrotic potential of rupatadine in rat liver via amendment of PAF/NF-ĸB p65/TGF-β1 and hedgehog/HIF-1α/VEGF trajectories. Inflammopharmacology 2023, 31, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Didamoony, M.A.; Atwa, A.M.; Ahmed, L.A. Modulatory effect of rupatadine on mesenchymal stem cell-derived exosomes in hepatic fibrosis in rats: A potential role for miR-200a. Life Sci. 2023, 324, 121710. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Demopoulos, C.A.; Antonopoulou, S. Micronutrients, Phytochemicals and Mediterranean Diet: A Potential Protective Role against COVID-19 through Modulation of PAF Actions and Metabolism. Nutrients 2021, 13, 462. [Google Scholar] [CrossRef] [PubMed]
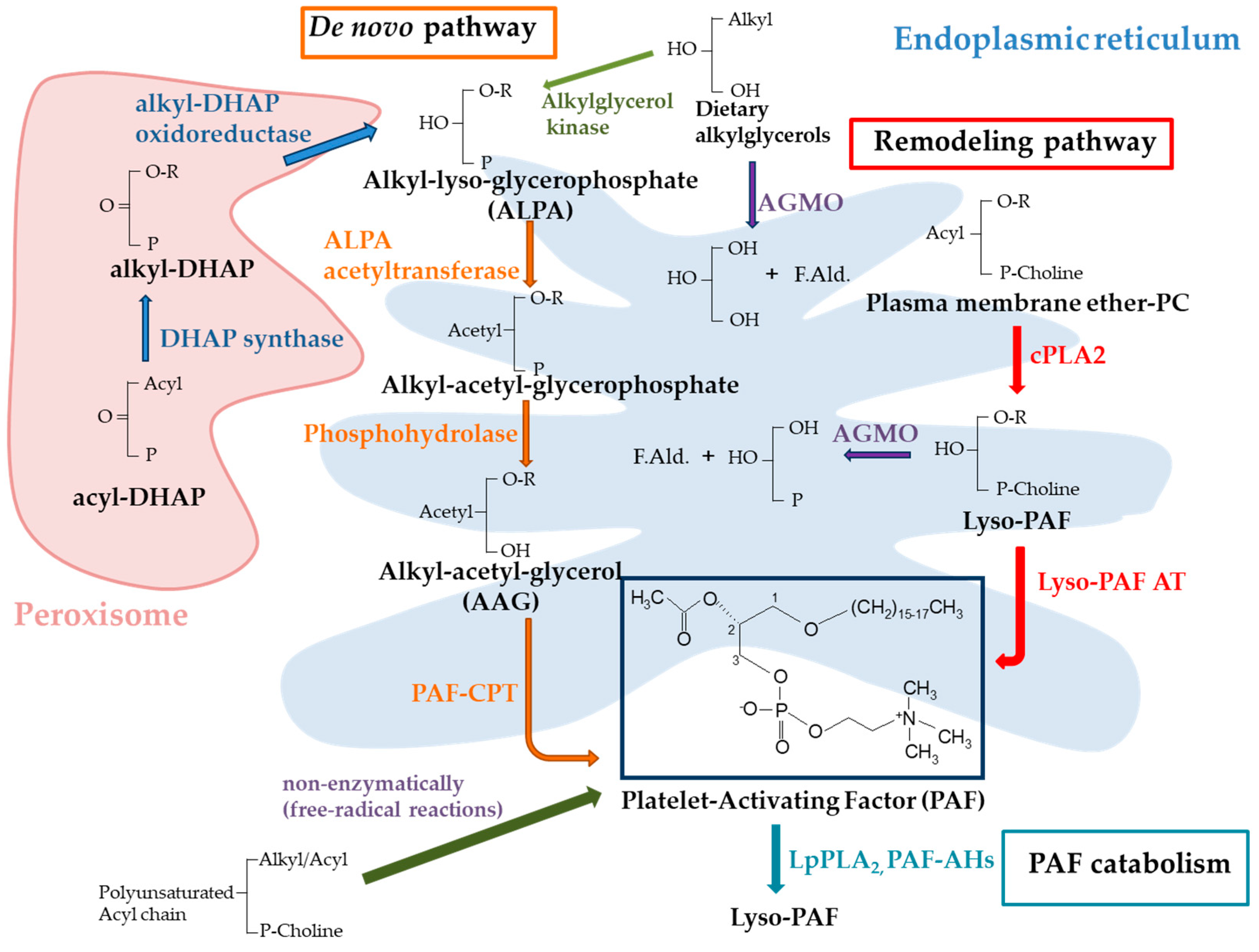
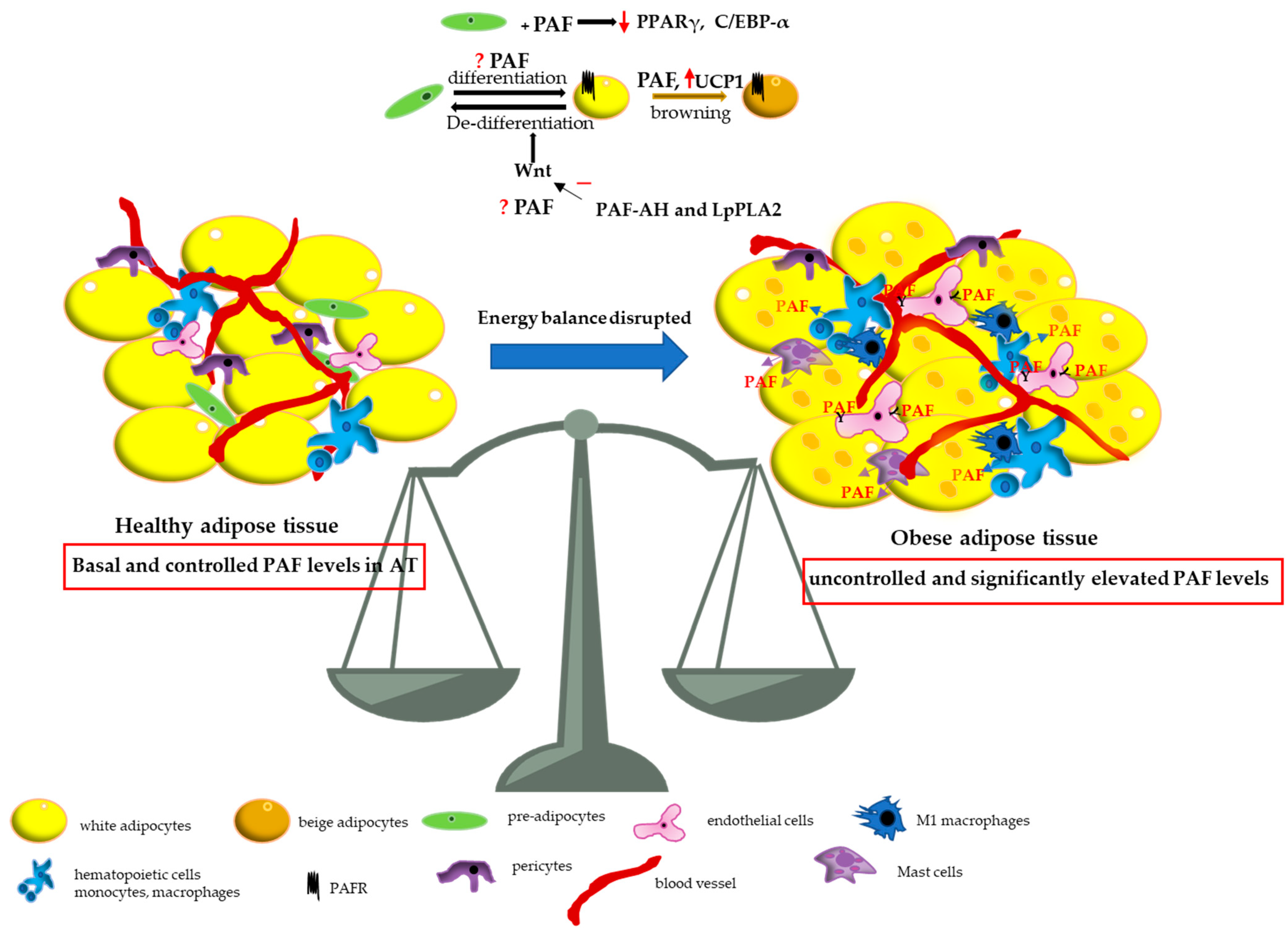
| Experimental Model | Study Design | Main Findings | Ref. |
|---|---|---|---|
| PAFR-KO and WT male mice | mice were fed CD for up to 36 w single injection of the β3-AR agonist, CL-316,243 (25 μg/kg) | PAFR-KO vs. WT ↑ body weight gain ↑ epididymal WAD at 12 w ↑ liver weight at 36 w ↑ TNFα, IL-6, CCL3 mRNA expression in the epididymal WAT at > 24 w ↓ UCP1 and UCP3 mRNA in the BAT and UCP2 mRNA in the WAT ↓ thermogenesis ↑ LPCAT2 and cPLA2α mRNAs in BAT | [23] |
| PAFR-KO and WT male mice | mice were fed a (i) CD or (ii) HCD for 8 w | PAFR-KO HCD vs. WT HCD ↑ body weight ↑ VAI ↑ lipogenesis ↑ PPARγ ↓ resistin ↑ leptin ↓ protein levels of TNFα, IL-6, IL-1β, IL-10, CCL3, and CCL5 ↓ HSL mRNA expression in AT | [24] |
| PAFR-KO and WT BALB/c mice | mice were fed a (i) CD or (ii) HFD for 12 w | PAFR-KO vs. WT ↑ IL12/IL10 in epididymal ATM ↑ insulin resistance PAFR-KO HFD vs. WT HFD ↑ body weight gain ↑ epididymal fat ↑ liver weight ↑ insulin resistance | [25] |
| PAFR-KO and WT male mice | mice were fed a (i) CD or (ii) HFD for 12 w | PAFR highly expressed in adipocytes and SVC of epididymal WAT PAFR weakly expressed in preadipocytes cPLA2a and LPCAT2 highly expressed in BAT and WAT PAFR-KO HFD vs. WT HFD ↑ body weight gain ↑ epididymal WAT ↑ TNFα mRNA ↑ CD11c-positive macrophages into epididymal WAT ↑ fasting serum glucose | [26] |
| PAFR-KO and WT BALB/c mice | mice were fed a HCD and subjected to 24 h fasting | PAFR-KO fasting vs. WT fasting ↓ decrease in epididymal AT ↓ IL-6, TNFα, IL-1β, IL-10, and TGF-β in AT | [27] |
| PAFR-KO and WT C57BL/6 male mice | mice subjected to BM transplantation and fed a CD or HCD for 8 w (i) PAFR-KO -BM→PAFR-KO; (ii) WT-BM→WT (iii) PAFR-KO-BM→WT, and (iv) WT-BM → PAFR-KO | PAFR-KO-BM → PAFR-KO HCD vs. WT-BM → WT HCD ↓ O2 consumption PAFR-KO-BM→PAFR-KO HCD, WT-BM→PAFR-KO HCD vs. WT-BM → WT HCD ↑ leptin ↓ TNFα, IL-6 WT-BM → PAFR-KO CD vs. WT-BM → WT CD ↑ serum glucose ↑ insulin PAFR-KO-BM → PAFR-KO, WT-BM→PAFR-KO vs. WT-BM → WT ↓ rolling of leukocytes in AT | [28] |
| Study Population | Study Intervention | Measurements | Main Findings | Ref. |
|---|---|---|---|---|
| 43 obese subjects (both sexes, BMI 46.7 ± 6.9 kg/m2) | - | anthropometric data Gly, Ins, HOMA-IR, TC, TAG, HDL- and LDL-chol, Apo A1 and B IL-6, TNFα, IL-10, adip: omental AT gene expression | PAFR expression in omental WAT negatively correlated with BW, BMI, and FM | [28] |
| 28 obese women (BMI 38.0 ± 4.9 kg/m2) | low-calorie diet for 16 w | anthropometric data Gly, Ins, HOMA-IR, TC, TAG, HDL- and LDL-chol, Apo A1 and B Lp-PLA2 activity LDL phenotype | ↓ Lp-PLA2 activity at 16 w changes in Lp-PLA2 activity were correlated only with the changes in VLDL-chol | [42] |
| 52 men and 48 age- and BMI-matched women | - | anthropometric data DXA measurements Gly, TC, TAG, HDL- and LDL-chol, CRP lyso-PAF-AT, PAF-CPT, PAF-AH activities in leukocytes and Lp-PLA2 in plasma Blood PAF levels | Lp-PLA2 activity positively correlated with TAG, TC, LDL-chol in men and women PAF-AH in leukocytes positively correlated with CRP in men and women upper and total adiposity measures positively associated with Lp-PLA2 activity in men WC and W/H negatively correlated with PAF levels | [44,47] |
| 14 MZ twin pairs, (8 male and 6 female pairs) one co-twin with BMI 25 kg/m2 the other with BMI 30 kg/m2 | - | DXA measurements, MRI Gly, Ins, Ins sensitivity, TC, TAG, HDL- and LDL-chol, leptin, adip, CRP Lipidomics’ analysis | obese vs. non-obese co-twins ↑ LPCs ↓ ether PL ether PL negatively correlated with subcutaneous obesity and positively with insulin sensitivity | [49] |
| 65 obese with T2D and 72 obese subjects without T2D | - | anthropometric data TC, HDL- and LDL-chol, HbA1c, leptin, adip Lp-PLA2 activity | obese with T2D vs. obese without T2D ↓ Lp-PLA2 activity Lp-PLA2 activity positively associated with BMI and negatively associated with leptin and adip | [45] |
| 284 subjects (both sexes) | - | anthropometric data Gly, Ins, TC, TAG, HDL- and LDL-chol, UA, creatinine, SGOT, SGPT, γ-GT, IL-6, adip Lp-PLA2 activity | Lp-PLA2 activity negatively correlated with HDL-chol, SGOT, SGPT, and adip total energy intake positively associated with Lp-PLA2 activity | [43] |
| 238 healthy subjects | (i) 2y CR by about 14% (ii) ad libitum control group | anthropometric data TDEE, AT gene expression thymic function, fat composition of thymus, sjTRECs | 2y CR ↑thymic mass ↓FM ↑ mitochondrial biogenesis, PPAR-α–FAO ↓ expression of the gene encoding Lp-PLA2 in AT | [41] |
| 100 subjects with high and low risk of CVD | - | anthropometric data TC, LDL-, HDL- and non-HDL-chol, TAG, Lp-PLA2 activity | Lp-PLA2 positively correlated with LDL- and non-HDL-chol medium positive correlation between Lp-PLA2 and absolute CVD risk score | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonopoulou, S. Platelet-Activating Factor-Induced Inflammation in Obesity: A Two-Sided Coin of Protection and Risk. Cells 2025, 14, 471. https://doi.org/10.3390/cells14070471
Antonopoulou S. Platelet-Activating Factor-Induced Inflammation in Obesity: A Two-Sided Coin of Protection and Risk. Cells. 2025; 14(7):471. https://doi.org/10.3390/cells14070471
Chicago/Turabian StyleAntonopoulou, Smaragdi. 2025. "Platelet-Activating Factor-Induced Inflammation in Obesity: A Two-Sided Coin of Protection and Risk" Cells 14, no. 7: 471. https://doi.org/10.3390/cells14070471
APA StyleAntonopoulou, S. (2025). Platelet-Activating Factor-Induced Inflammation in Obesity: A Two-Sided Coin of Protection and Risk. Cells, 14(7), 471. https://doi.org/10.3390/cells14070471






