SR-FTIR Biomolecular Characterization of the Hippocampus: The Role of Tenascin C in Adult Murine Neurogenesis in the Subgranular Zone
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Paradigm for Enriched Environment
2.3. Synchrotron-Based FTIR Spectroscopy and Imaging
2.3.1. Tissue Preparation
2.3.2. SR-FTIR Spectroscopy
2.3.3. Data Analysis
Correlation Analysis
Hierarchical Cluster Analysis
Principal Component Analysis
Linear Discriminant Analysis
Random Forest
Analysis of Integrated Band Area
2.4. Immunofluorescence
2.4.1. Tissue Preparation and Immunostaining
2.4.2. Image Acquisition
2.5. Statistics
3. Results
3.1. Correlation Analysis of FTIR Spectral Data Between Hippocampal Layers
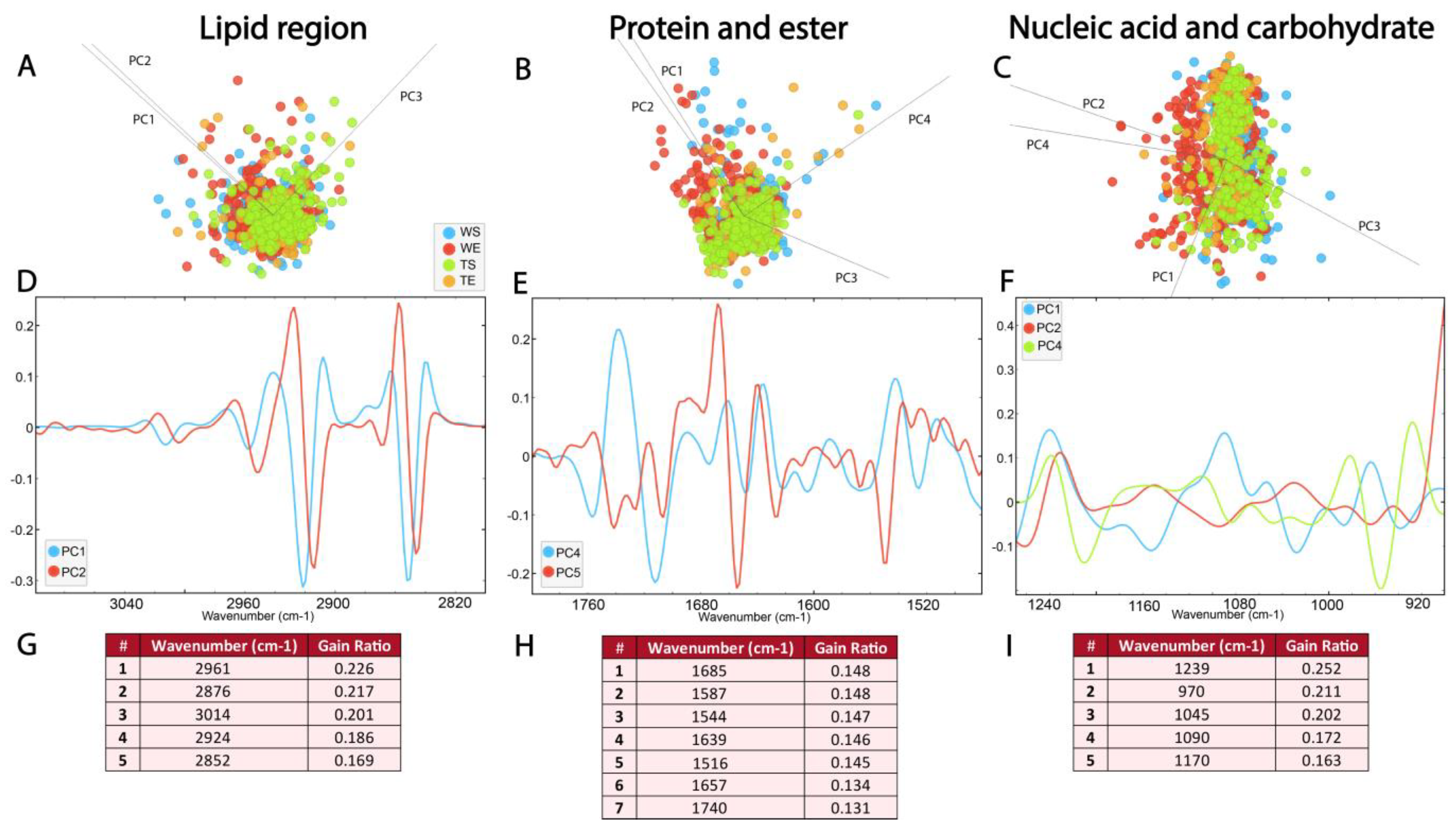
3.2. Similarity of Hippocampal Layers
3.3. Biomolecular Characterization of Hippocampal Layers
3.4. Integrated Band Area Analysis of the Hippocampal Biomolecular Content
3.5. Tenascin C in the Adult SGZ and the Effect of Enriched Environment
3.6. Comparison of the Biomolecular Composition of the SGZ in Different Experimental Conditions and the Role of TnC
3.7. Biomolecular Profile of the SGZ Depends on the Environmental Conditions and Expression of TnC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DG | Dentate gyrus |
| FTIR | Fourier transform infrared |
| SR | Synchrotron radiation |
| SGZ | Subgranular zone |
| TnC | Tenascin C |
| EE | Enriched environment |
| SC | Standard condition |
| GZ | Granular zone |
| TnC KO | Tenascin-C-deficient |
| HCA | Hierarchical cluster analysis |
| PCA | Principal component analysis |
| PC | Principal component |
| LDA | Linear discriminant analysis |
| RF | Random forest |
| WS | Wild type mice housed in standard conditions |
| WE | Wild type mice house in enriched environment |
| TS | TnC-deficient mice housed in standard conditions |
| TE | TnC-deficient mice housed in enriched environment |
References
- Flavell, S.W.; Greenberg, M.E. Signaling Mechanisms Linking Neuronal Activity to Gene Expression and Plasticity of the Nervous System. Annu. Rev. Neurosci. 2008, 31, 563–590. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the Adult Hippocampus. Cold Spring Harb. Perspect. Biol. 2015, 7, a018812. [Google Scholar] [CrossRef]
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef] [PubMed]
- A Alkadhi, K. Cellular and Molecular Differences Between Area CA1 and the Dentate Gyrus of the Hippocampus. Mol. Neurobiol. 2019, 56, 6566–6580. [Google Scholar] [CrossRef] [PubMed]
- Hörtnagl, H.; Berger, M.; Sperk, G.; Pifl, C. Regional heterogeneity in the distribution of neurotransmitter markers in the rat hippocampus. Neuroscience 1991, 45, 261–272. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Petibois, C.; Piccinini, M.; Guidi, M.C.; Marcelli, A. Facing the challenge of biosample imaging by FTIR with a synchrotron radiation source. J. Synchrotron Radiat. 2009, 17, 1–11. [Google Scholar] [CrossRef]
- Kastyak-Ibrahim, M.; Nasse, M.; Rak, M.; Hirschmugl, C.; Del Bigio, M.; Albensi, B.; Gough, K. Biochemical label-free tissue imaging with subcellular-resolution synchrotron FTIR with focal plane array detector. NeuroImage 2011, 60, 376–383. [Google Scholar] [CrossRef]
- Dudała, J.; Janeczko, K.; Setkowicz, Z.; Eichert, D.; Chwiej, J. The use of SR-FTIR microspectroscopy for a preliminary biochemical study of the rat hippocampal formation tissue in case of pilocarpine induced epilepsy and neutroprotection with FK-506. Nukleonika 2012, 57, 615–619. [Google Scholar]
- Ustaoglu, S.G.; Ali, M.H.M.; Rakib, F.; Blezer, E.L.A.; Van Heijningen, C.L.; Dijkhuizen, R.M.; Severcan, F. Biomolecular changes and subsequent time-dependent recovery in hippocampal tissue after experimental mild traumatic brain injury. Sci. Rep. 2021, 11, 12468. [Google Scholar] [CrossRef]
- van Praag, H.; Schinder, A.F.; Christie, B.R.; Toni, N.; Palmer, T.D.; Gage, F.H. Functional neurogenesis in the adult hippocampus. Nature 2002, 415, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Kjell, J.; Fischer-Sternjak, J.; Thompson, A.J.; Friess, C.; Sticco, M.J.; Salinas, F.; Cox, J.; Martinelli, D.C.; Ninkovic, J.; Franze, K.; et al. Defining the Adult Neural Stem Cell Niche Proteome Identifies Key Regulators of Adult Neurogenesis. Cell Stem Cell 2020, 26, 277–293.e8. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a glance. J. Cell Sci. 2016, 129, 4321–4327. [Google Scholar] [CrossRef]
- Giblin, S.P.; Midwood, K.S. Tenascin-C: Form versus function. Cell Adhes. Migr. 2014, 9, 48–82. [Google Scholar] [CrossRef]
- Tucić, M.; Stamenković, V.; Andjus, P. The Extracellular Matrix Glycoprotein Tenascin C and Adult Neurogenesis. Front. Cell Dev. Biol. 2021, 9, 674199. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Hussenet, T.; Langlois, B.; Orend, G. Advances in tenascin-C biology. Cell. Mol. Life Sci. 2011, 68, 3175–3199. [Google Scholar] [CrossRef]
- Garcion, E.; Faissner, A.; Ffrench-Constant, C. Knockout mice reveal a contribution of the extracellular matrix molecule tenascin-C to neural precursor proliferation and migration. Development 2001, 128, 2485–2496. [Google Scholar] [CrossRef]
- Garwood, J.; Garcion, E.; Dobbertin, A.; Heck, N.; Calco, V.; Ffrench-Constant, C.; Faissner, A. The extracellular matrix glycoprotein Tenascin-C is expressed by oligodendrocyte precursor cells and required for the regulation of maturation rate, survival and responsiveness to platelet-derived growth factor. Eur. J. Neurosci. 2004, 20, 2524–2540. [Google Scholar] [CrossRef]
- Garcion, E.; Halilagic, A.; Faissner, A.; Ffrench-Constant, C. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development 2004, 131, 3423–3432. [Google Scholar] [CrossRef]
- Cope, E.C.; Gould, E. Adult Neurogenesis, Glia, and the Extracellular Matrix. Cell Stem Cell 2019, 24, 690–705. [Google Scholar] [CrossRef]
- Yamada, J.; Nadanaka, S.; Kitagawa, H.; Takeuchi, K.; Jinno, S. Increased Synthesis of Chondroitin Sulfate Proteoglycan Promotes Adult Hippocampal Neurogenesis in Response to Enriched Environment. J. Neurosci. 2018, 38, 8496–8513. [Google Scholar] [CrossRef] [PubMed]
- Komitova, M.; Mattsson, B.; Johansson, B.B.; Eriksson, P.S. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke 2005, 36, 1278–1282. [Google Scholar] [CrossRef]
- Brown, J.; Cooper-Kuhn, C.M.; Kempermann, G.; Van Praag, H.; Winkler, J.; Gage, F.H.; Kuhn, H.G. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 2003, 17, 2042–2046. [Google Scholar] [CrossRef]
- van Praag, H.; Shubert, T.; Zhao, C.; Gage, F.H. Exercise Enhances Learning and Hippocampal Neurogenesis in Aged Mice. J. Neurosci. 2005, 25, 8680–8685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and Functional Implications of Adult Neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, Q.; Guan, X.; Zhang, X.; Zhang, Y.; Cao, J.; Li, X. Effects of enriched environment on depression and anxiety-like behavior induced by early life stress: A comparison between different periods. Behav. Brain Res. 2021, 411, 113389. [Google Scholar] [CrossRef]
- Chatzi, C.; Schnell, E.; Westbrook, G.L.; States, U. Localized hypoxia within the subgranular zone determines the early survival of newborn hippocampal granule cells. eLife 2015, 4, e08722. [Google Scholar] [CrossRef]
- Cembrowski, M.S.; Wang, L.; Sugino, K.; Shields, B.C.; Spruston, N.; States, U. Hipposeq: A comprehensive RNA-seq database of gene expression in hippocampal principal neurons. eLife 2016, 5, e14997. [Google Scholar] [CrossRef]
- Chwiej, J.G.; Ciesielka, S.W.; Skoczen, A.K.; Janeczko, K.J.; Sandt, C.; Planeta, K.L.; Setkowicz, Z.K. Biochemical Changes Indicate Developmental Stage in the Hippocampal Formation. ACS Chem. Neurosci. 2018, 10, 628–635. [Google Scholar] [CrossRef]
- Arellano, J.I.; Duque, A.; Rakic, P. A coming-of-age story: Adult neurogenesis or adolescent neurogenesis in rodents? Front. Neurosci. 2024, 18, 1383728. [Google Scholar] [CrossRef]
- Dučić, T.; Sanchez-Mata, A.; Castillo-Sanchez, J.; Algarra, M.; Gonzalez-Munoz, E. Monitoring oocyte-based human pluripotency acquisition using synchrotron-based FTIR microspectroscopy reveals specific biomolecular trajectories. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 297, 122713. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, L.; Putignano, E.; Sale, A.; Viegi, A.; Berardi, N.; Maffei, L. Acceleration of visual system development by environmental enrichment. J. Neurosci. 2004, 24, 4840–4848. [Google Scholar] [CrossRef]
- de Magalhães, C.R.; Carrilho, R.; Schrama, D.; Cerqueira, M.; da Costa, A.M.R.; Rodrigues, P.M. Mid-infrared spectroscopic screening of metabolic alterations in stress-exposed gilthead seabream (Sparus aurata). Sci. Rep. 2020, 10, 16343. [Google Scholar] [CrossRef]
- Evers, M.R.; Salmen, B.; Bukalo, O.; Rollenhagen, A.; Bösl, M.R.; Morellini, F.; Bartsch, U.; Dityatev, A.; Schachner, M. Impairment of L-type Ca2+ Channel-Dependent Forms of Hippocampal Synaptic Plasticity in Mice Deficient in the Extracellular Matrix Glycoprotein Tenascin-C. J. Neurosci. 2002, 22, 7177–7194. [Google Scholar] [CrossRef] [PubMed]
- Crase, S.; Hall, B.; Thennadil, S.N. Cluster Analysis for IR and NIR Spectroscopy: Current Practices to Future Perspectives. Comput. Mater. Contin. 2021, 69, 1945–1965. [Google Scholar] [CrossRef]
- Koehler, A.; Scroferneker, M.L.; de Souza, N.M.P.; de Moraes, P.C.; Pereira, B.A.S.; Cavalcante, R.d.S.; Mendes, R.P.; Corbellini, V.A. Rapid Classification of Serum from Patients with Paracoccidioidomycosis Using Infrared Spectroscopy, Univariate Statistics, and Linear Discriminant Analysis (LDA). J. Fungi 2024, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.O.M.; Saad, A.S.I.; Tadano, S.; Takeda, Y.; Konaka, T.; Yamasaki, Y.; Tahir, I.S.A.; Tsujimoto, H.; Akashi, K. Chemical Fingerprinting of Heat Stress Responses in the Leaves of Common Wheat by Fourier Transform Infrared Spectroscopy. Int. J. Mol. Sci. 2022, 23, 2842. [Google Scholar] [CrossRef]
- Pereira, D.C.; Pupin, B.; Borma, L.d.S. Influence of sample preparation methods on FTIR spectra for taxonomic identification of tropical trees in the Atlantic forest. Heliyon 2024, 10, e27232. [Google Scholar] [CrossRef]
- Chen, L.; Holman, H.-Y.N.; Hao, Z.; Bechtel, H.A.; Martin, M.C.; Wu, C.; Chu, S. Synchrotron Infrared Measurements of Protein Phosphorylation in Living Single PC12 Cells during Neuronal Differentiation. Anal. Chem. 2012, 84, 4118–4125. [Google Scholar] [CrossRef]
- Toplak, M.; Read, S.T.; Sandt, C.; Borondics, F. Quasar: Easy Machine Learning for Biospectroscopy. Cells 2021, 10, 2300. [Google Scholar] [CrossRef]
- Chwiej, J.; Dulinska, J.; Janeczko, K.; Dumas, P.; Eichert, D.; Dudala, J.; Setkowicz, Z. Synchrotron FTIR micro-spectroscopy study of the rat hippocampal formation after pilocarpine-evoked seizures. J. Chem. Neuroanat. 2010, 40, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Ferhat, L.; Louis, N.C.A.; Jorquera, I.; Niquet, J.; Khrestchatisky, M.; Ben-Ari, Y.; Represa, A. Transient increase of tenascin-C in immature hippocampus: Astroglial and neuronal expression. J. Neurocytol. 1996, 25, 53–66. [Google Scholar] [CrossRef]
- Cakmak-Arslan, G.; Kaya, Y.; Mamuk, S.; Akarsu, E.S.; Severcan, F. The investigation of the molecular changes duringlipopolysaccharide-induced systemic inflammation on rat hippocampus by usingFTIRspectroscopy. J. Biophotonics 2024, 17, e202300541. [Google Scholar] [CrossRef]
- Einenkel, A.; Salameh, A. Selective vulnerability of hippocampal CA1 and CA3 pyramidal cells: What are possible pathomechanisms and should more attention be paid to the CA3 region in future studies? J. Neurosci. Res. 2023, 102, e25276. [Google Scholar] [CrossRef] [PubMed]
- Andjus, P.; Stamenković, S.; Dučić, T. Synchrotron radiation-based FTIR spectro-microscopy of the brainstem of the hSOD1 G93A rat model of amyotrophic lateral sclerosis. Eur. Biophys. J. 2019, 48, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Seri, B.; García-Verdugo, J.M.; Collado-Morente, L.; McEwen, B.S.; Alvarez-Buylla, A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J. Comp. Neurol. 2004, 478, 359–378. [Google Scholar] [CrossRef]
- McNair, K.; Broad, J.; Riedel, G.; Davies, C.; Cobb, S. Global changes in the hippocampal proteome following exposure to an enriched environment. Neuroscience 2007, 145, 413–422. [Google Scholar] [CrossRef]
- Carulli, D.; Foscarin, S.; Rossi, F. Activity-Dependent Plasticity and Gene Expression Modifications in the Adult CNS. Front. Mol. Neurosci. 2011, 4, 15205. [Google Scholar] [CrossRef]
- Nakic, M.; Manahan-Vaughan, D.; Reymann, K.G.; Schachner, M. Long-term potentiationin vivo increases rat hippocampal tenascin-C expression. J. Neurobiol. 1998, 37, 393–404. [Google Scholar] [CrossRef]
- Shin, J.; Berg, D.A.; Zhu, Y.; Shin, J.Y.; Song, J.; Bonaguidi, M.A.; Enikolopov, G.; Nauen, D.W.; Christian, K.M.; Ming, G.-L.; et al. Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell Stem Cell 2015, 17, 360–372. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Stamenkovic, S.; Jaworski, T.; Gawlak, M.; Jovanovic, M.; Jakovcevski, I.; Wilczynski, G.M.; Kaczmarek, L.; Schachner, M.; Radenovic, L.; et al. The extracellular matrix glycoprotein tenascin-C and matrix metalloproteinases modify cerebellar structural plasticity by exposure to an enriched environment. Anat. Embryol. 2016, 222, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Leger, M.; Paizanis, E.; Dzahini, K.; Quiedeville, A.; Bouet, V.; Cassel, J.-C.; Freret, T.; Schumann-Bard, P.; Boulouard, M. Environmental Enrichment Duration Differentially Affects Behavior and Neuroplasticity in Adult Mice. Cereb. Cortex 2014, 25, 4048–4061. [Google Scholar] [CrossRef] [PubMed]
- Josifovska, N.; Andjelic, S.; Lytvynchuk, L.; Lumi, X.; Dučić, T.; Petrovski, G. Biomacromolecular Profile in Human Primary Retinal Pigment Epithelial Cells—A Study of Oxidative Stress and Autophagy by Synchrotron-Based FTIR Microspectroscopy. Biomedicines 2023, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Saccaro, L.F.; Delavari, F.; Van De Ville, D.; Piguet, C. Hippocampal temporal dynamics and spatial heterogeneity unveil vulnerability markers in the offspring of bipolar patients. Bipolar Disord. 2024, 27, 17–27. [Google Scholar] [CrossRef]
- Maffezzini, C.; Calvo-Garrido, J.; Wredenberg, A.; Freyer, C. Metabolic regulation of neurodifferentiation in the adult brain. Cell. Mol. Life Sci. 2020, 77, 2483–2496. [Google Scholar] [CrossRef]
- Kappler, J.; Baader, S.L.; Franken, S.; Pesheva, P.; Schilling, K.; Rauch, U.; Gieselmann, V. Tenascins Are Associated with Lipid Rafts Isolated from Mouse Brain. Biochem. Biophys. Res. Commun. 2002, 294, 742–747. [Google Scholar] [CrossRef]
- Czopka, T.; von Holst, A.; Ffrench-Constant, C.; Faissner, A. Regulatory mechanisms that mediate tenascin c-dependent inhibition of oligodendrocyte precursor differentiation. J. Neurosci. 2010, 30, 12310–12322. [Google Scholar] [CrossRef]
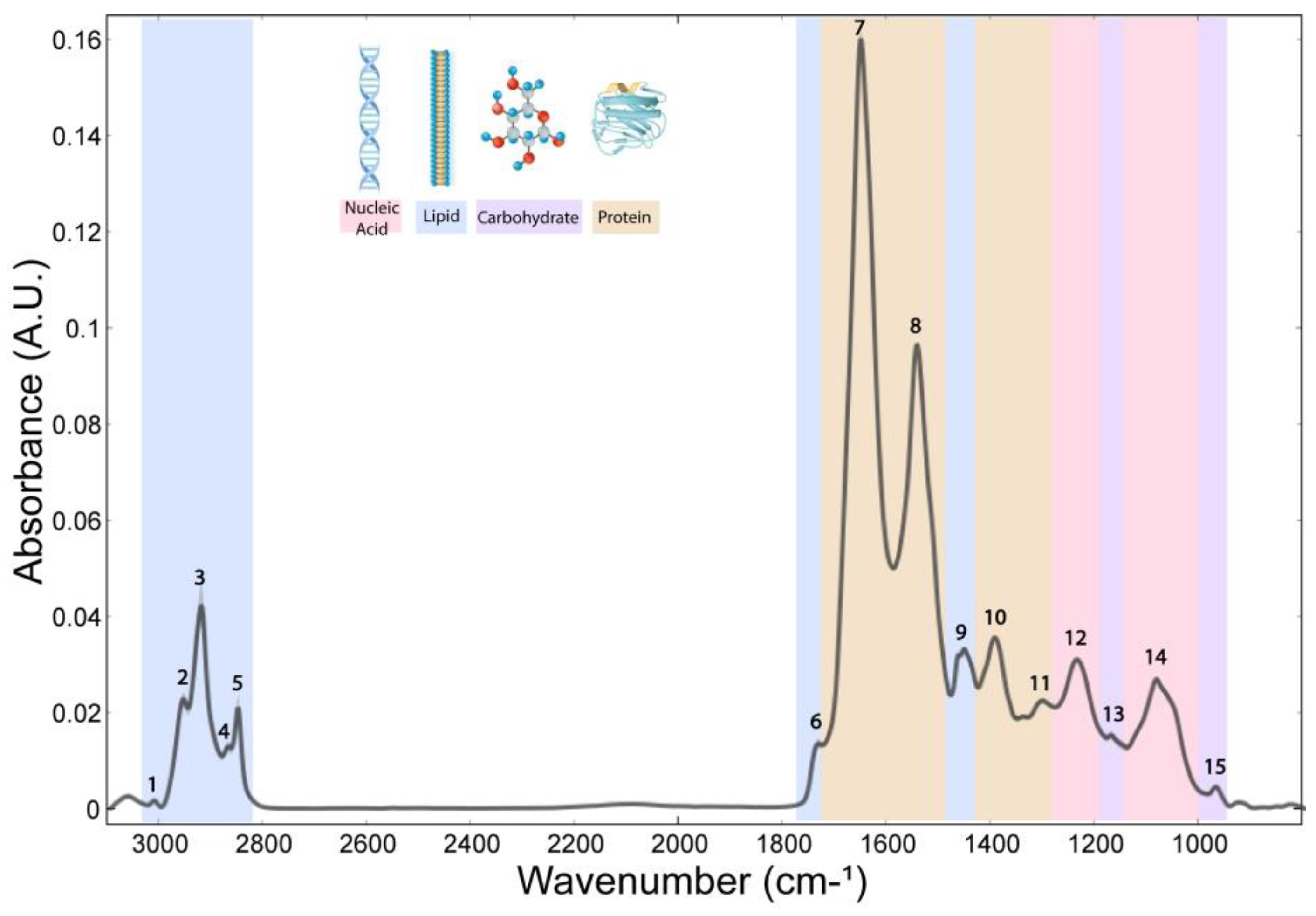





| Band | Wavenumber (cm−1) | Vibrational Modes and Functional Groups | Main Biochemical Compounds | Other Biochemical Compounds | References |
|---|---|---|---|---|---|
| 1 | 3012 | νC=C | Unsaturated fatty acids | Aromatics | [30,41] |
| 2 | 2957 | νasCH3 | Saturated lipids | Proteins, carbohydrates, nucleic acids | [11,41] |
| 3 | 2924 | νasCH2 | Saturated lipids | Proteins, carbohydrates, nucleic acids | [11,41] |
| 4 | 2872 | νsCH3 | Saturated lipids | [41] | |
| 5 | 2852 | νsCH2 | Saturated lipids | [9,41] | |
| 6 | 1739 | νC=O | Triglycerides, cholesterol esters | Lipids, phospholipids | [9,30] |
| 7 | 1657 | Amide I | Proteins | Unsaturated fatty acids | [30,41] |
| 8 | 1544 | Amide II | Proteins | Aromatics | [41] |
| 9 | 1468 | δCH2 | Lipids | Proteins | [6] |
| 10 | 1393 | nsCOO− | Amino acids and fatty acids | Other carboxylates | [41] |
| 11 | 1304 | Amide III | Proteins | [41] | |
| 12 | 1241 | νasPO2− | Nucleic acids | Phospholipids | [30] |
| 13 | 1171 | νasCO-O-C | Carbohydrates | Proteins | [6] |
| 14 | 1086 | νsPO2− | Nucleic acids | Phospholipids | [30] |
| 15 | 967 | C-O deoxyribose, C-C DNA | Carbohydrates | Nucleic acid | [6] |
| (A) | ||||
| C=C | ||||
| Mean rank difference | Signifficance | p-value | Z score | |
| GZ vs. SGZ | −65.4 | ** | 0.006 | 3.31 |
| GZ vs. CA1 | −93.7 | *** | <0.001 | 5.12 |
| GZ vs. CA3 | −154 | *** | <0.001 | 8.02 |
| SGZ vs. CA1 | −28.3 | ns | 0.961 | 1.4 |
| SGZ vs. CA3 | −88.9 | *** | <0.001 | 4.23 |
| CA1 vs. CA3 | −60.6 | * | 0.012 | 3.08 |
| (B) | ||||
| C=O | ||||
| Mean rank difference | Signifficance | p-value | Z score | |
| GZ vs. SGZ | −53 | * | 0.044 | 2.68 |
| GZ vs. CA1 | −90.8 | *** | <0.001 | 4.96 |
| GZ vs. CA3 | −162 | *** | <0.001 | 8.44 |
| SGZ vs. CA1 | −37.8 | ns | 0.365 | 1.87 |
| SGZ vs. CA3 | −109 | *** | <0.001 | 5.2 |
| CA1 vs. CA3 | −71.6 | ** | 0.002 | 3.64 |
| (C) | ||||
| νas PO2− | ||||
| Mean rank difference | Signifficance | p-value | Z score | |
| GZ vs. SGZ | −45.1 | ns | 0.134 | 2.29 |
| GZ vs. CA1 | −27.3 | ns | 0.816 | 1.49 |
| GZ vs. CA3 | −70.6 | ** | 0.001 | 3.67 |
| SGZ vs. CA1 | 17.9 | ns | >0.999 | 0.886 |
| SGZ vs. CA3 | −25.5 | ns | >0.999 | 1.21 |
| CA1 vs. CA3 | −43.4 | ns | 0.164 | 2.21 |
| (D) | ||||
| C-O deoxyribose | ||||
| Mean rank difference | Signifficance | p-value | Z score | |
| GZ vs. SGZ | −6.7 | ns | >0.999 | 0.339 |
| GZ vs. CA1 | 163 | *** | <0.001 | 8.94 |
| GZ vs. CA3 | 123 | *** | <0.001 | 6.37 |
| SGZ vs. CA1 | 170 | *** | <0.001 | 8.44 |
| SGZ vs. CA3 | 129 | *** | <0.001 | 6.15 |
| CA1 vs. CA3 | −40.8 | ns | 0.227 | 2.08 |
| (E) | ||||
| νs PO2− | ||||
| Mean rank difference | Signifficance | p-value | Z score | |
| GZ vs. SGZ | −50.5 | ns | 0.063 | 2.56 |
| GZ vs. CA1 | −24.9 | ns | >0.999 | 1.36 |
| GZ vs. CA3 | −109 | *** | <0.001 | 5.68 |
| SGZ vs. CA1 | 25.6 | ns | >0.999 | 1.27 |
| SGZ vs. CA3 | −58.8 | * | 0.031 | 2.8 |
| CA1 vs. CA3 | −84.4 | *** | <0.001 | 4.29 |
| (F) | ||||
| νs PO2− POSITION | ||||
| Mean rank difference | Signifficance | p-value | Z score | |
| GZ vs. SGZ | 73.1 | *** | <0.001 | 5.06 |
| GZ vs. CA1 | 92.8 | *** | <0.001 | 6.83 |
| GZ vs. CA3 | 80.4 | *** | <0.001 | 5.38 |
| SGZ vs. CA1 | 19.8 | ns | 0.959 | 1.41 |
| SGZ vs. CA3 | 7.32 | ns | >0.999 | 0.475 |
| CA1 vs. CA3 | −12.5 | ns | >0.999 | 0.853 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korenić, M.; Korenić, A.; Stamenković, V.; Dučić, T.; Andjus, P. SR-FTIR Biomolecular Characterization of the Hippocampus: The Role of Tenascin C in Adult Murine Neurogenesis in the Subgranular Zone. Cells 2025, 14, 435. https://doi.org/10.3390/cells14060435
Korenić M, Korenić A, Stamenković V, Dučić T, Andjus P. SR-FTIR Biomolecular Characterization of the Hippocampus: The Role of Tenascin C in Adult Murine Neurogenesis in the Subgranular Zone. Cells. 2025; 14(6):435. https://doi.org/10.3390/cells14060435
Chicago/Turabian StyleKorenić, Milena, Andrej Korenić, Vera Stamenković, Tanja Dučić, and Pavle Andjus. 2025. "SR-FTIR Biomolecular Characterization of the Hippocampus: The Role of Tenascin C in Adult Murine Neurogenesis in the Subgranular Zone" Cells 14, no. 6: 435. https://doi.org/10.3390/cells14060435
APA StyleKorenić, M., Korenić, A., Stamenković, V., Dučić, T., & Andjus, P. (2025). SR-FTIR Biomolecular Characterization of the Hippocampus: The Role of Tenascin C in Adult Murine Neurogenesis in the Subgranular Zone. Cells, 14(6), 435. https://doi.org/10.3390/cells14060435







