Diallyl Trisulfide Attenuates Ischemia-Reperfusion-Induced ER Stress and Kidney Dysfunction in Aged Female Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Groups and DATS Treatment
2.2. Rationale for the Chosen Age Range
2.3. Ischemia-Reperfusion Injury (IRI)
2.4. Antibodies and Reagents
2.5. H2S Measurement
2.6. Antibody Authentication
2.7. Western Blotting
2.8. Gene Expression
2.9. Immunohistochemistry
2.10. Renal Cortical Blood Flow
2.11. Renal Microvasculature
2.12. Conventional Ultrasonography
2.13. Renal Function
2.14. Statistical Analysis and Blinding
3. Results
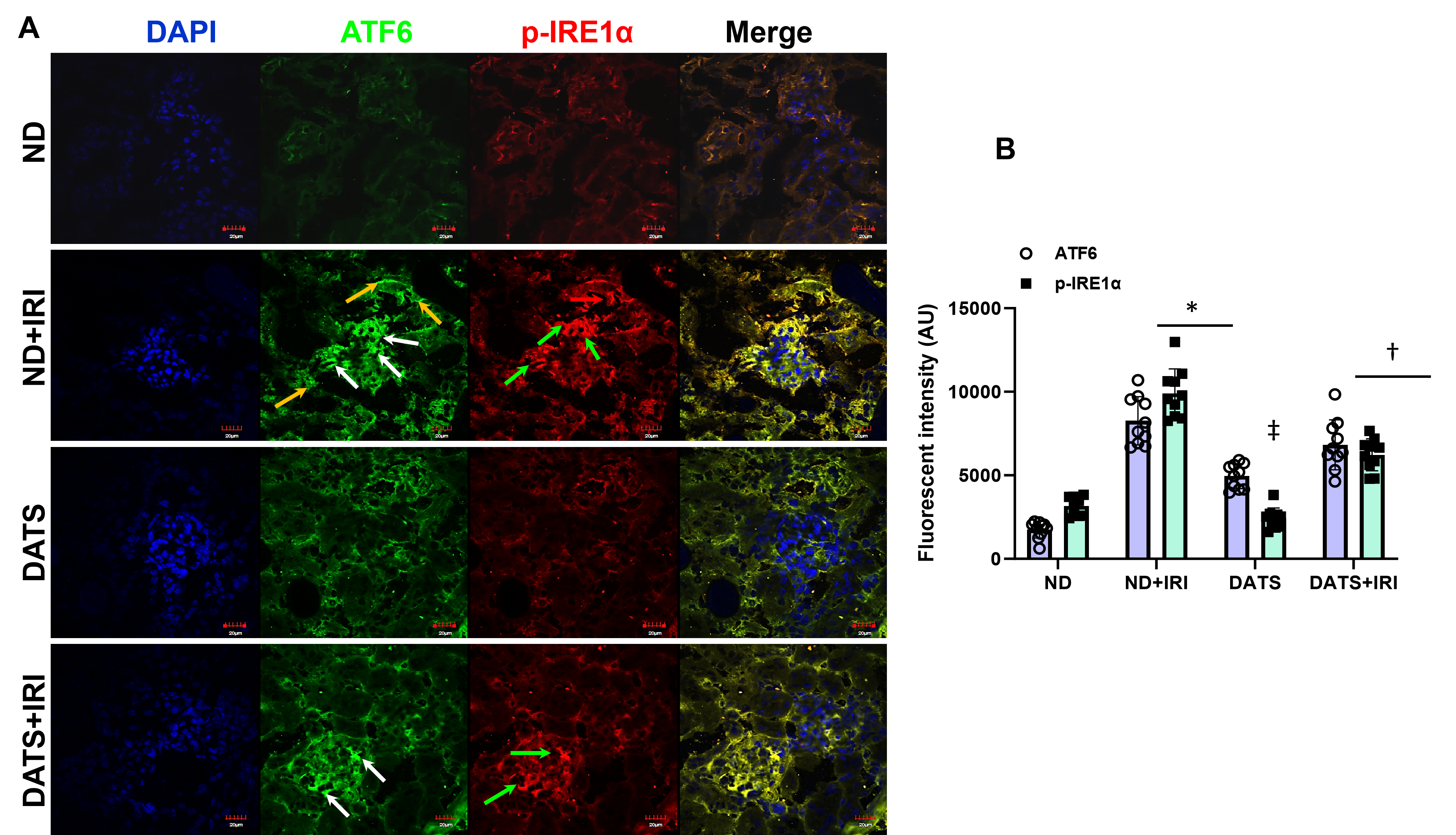
3.1. DATS Attenuated IRI-Induced ER Stress in the Aged Kidney
3.2. DATS Enhanced Renal Angiogenic Factors Following IRI
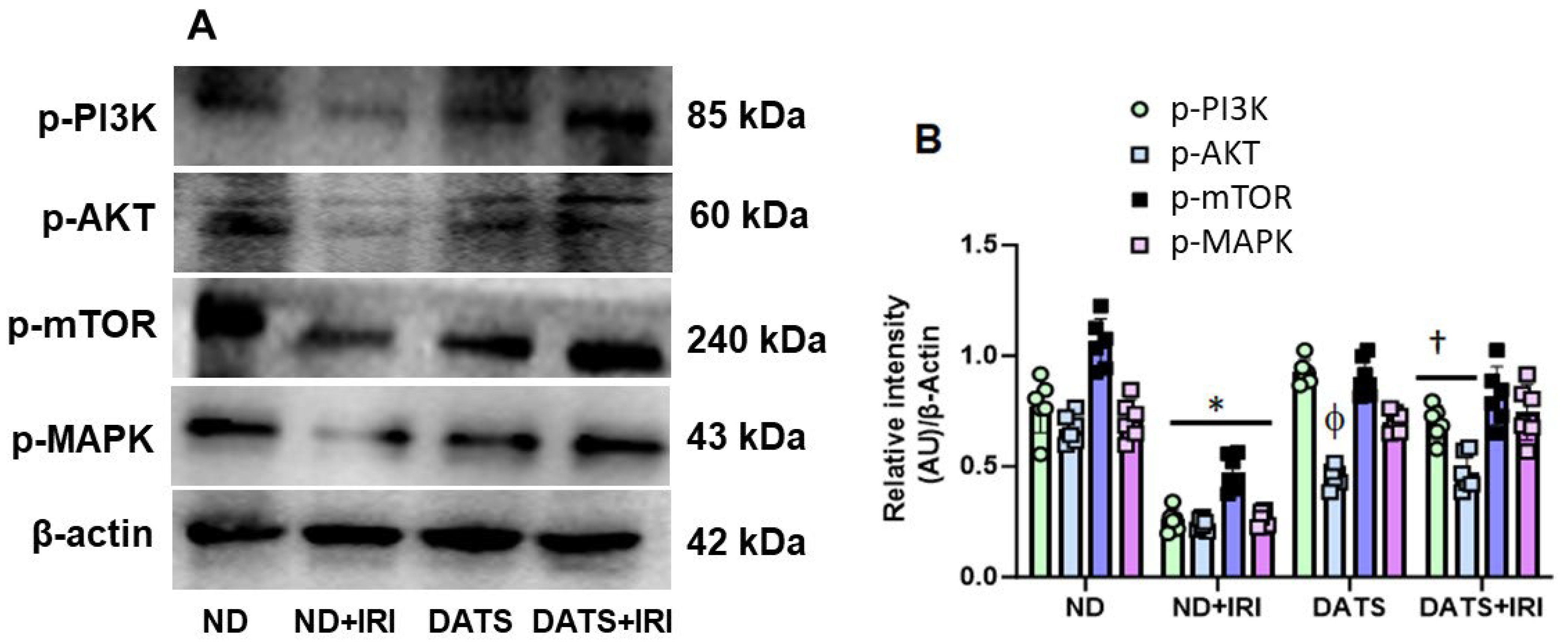
3.3. DATS Activated PI3K/AKT/mTOR Pathway to Induce Angiogenesis in Renal IRI
3.4. DATS Attenuated Renal Inflammation and Injury in the Aged Kidney IRI
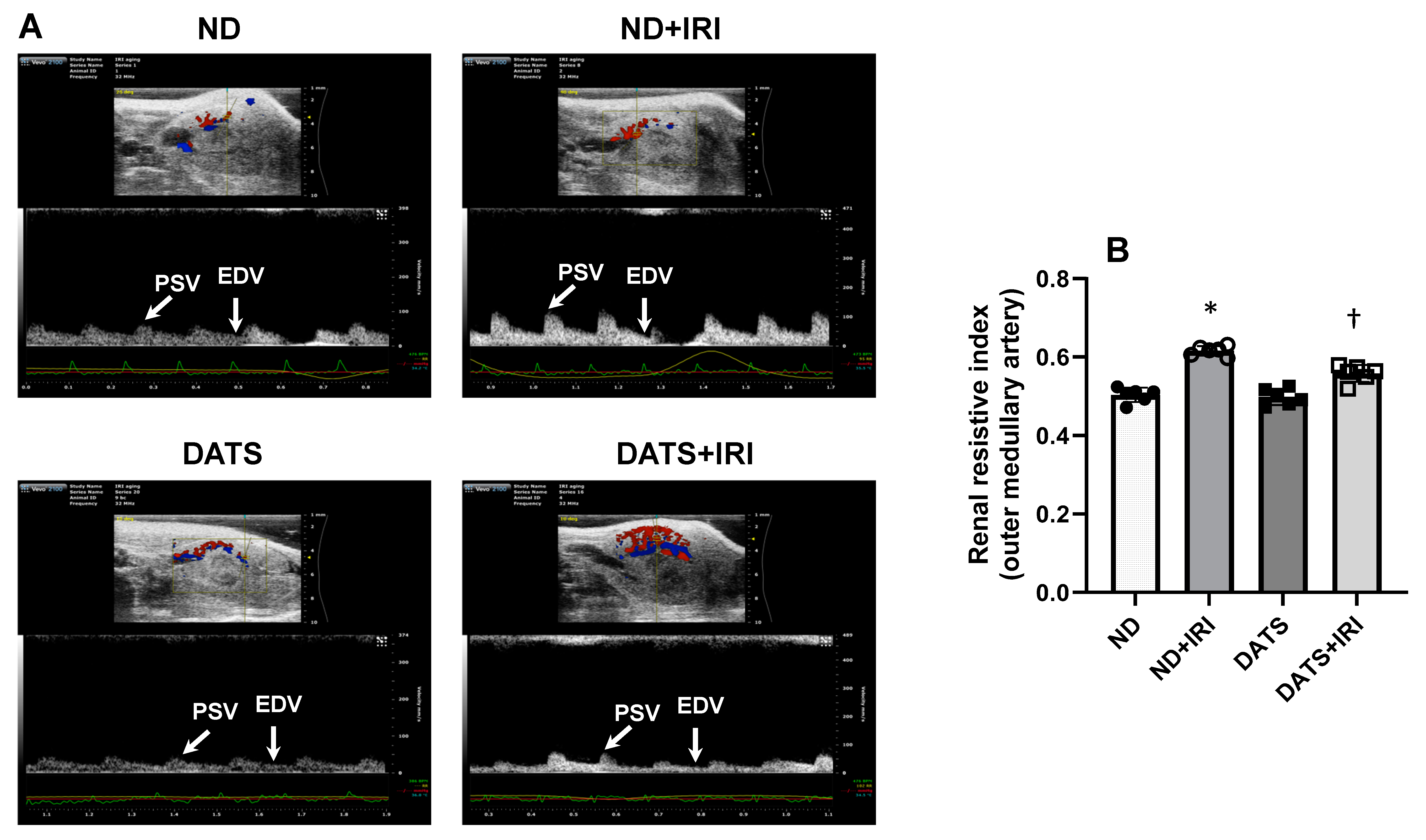
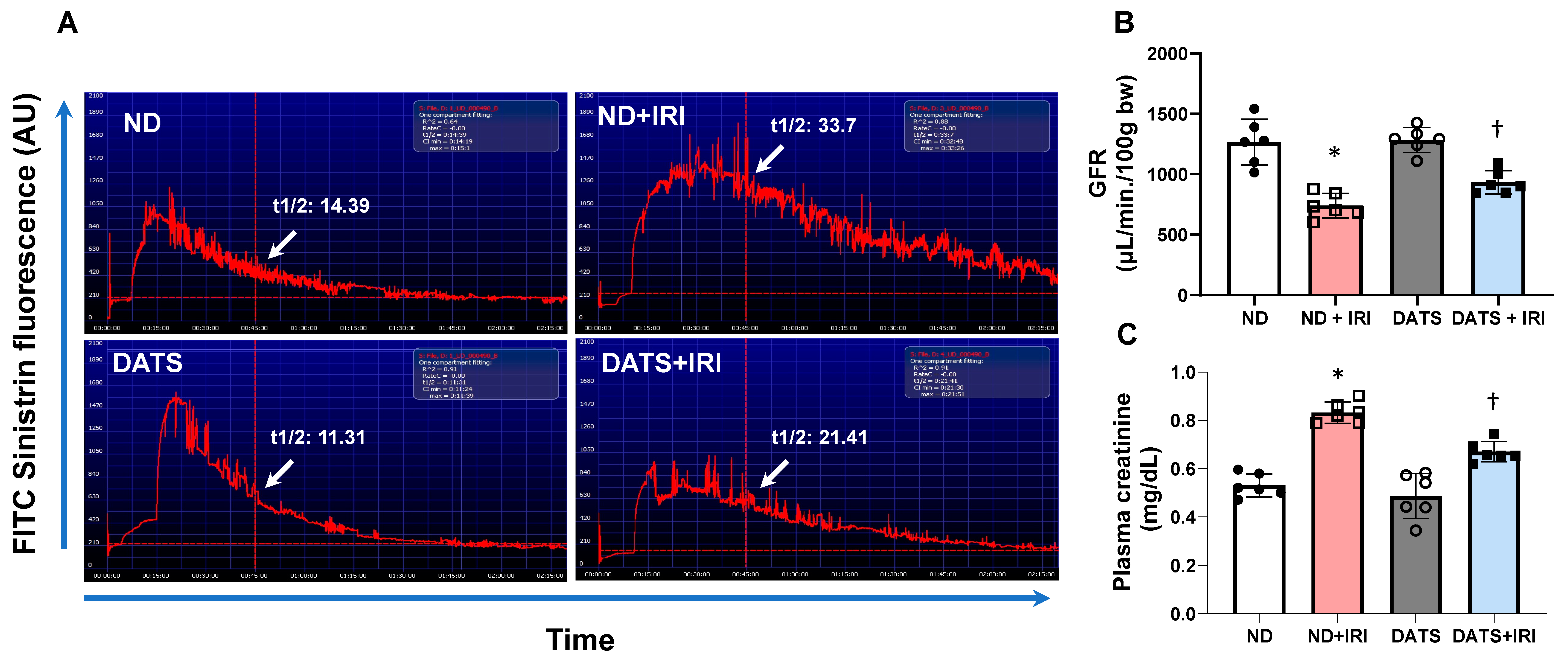
3.5. DATS Rescued Renal Blood Flow and Reduced Renovascular Resistance to the IRI Kidney
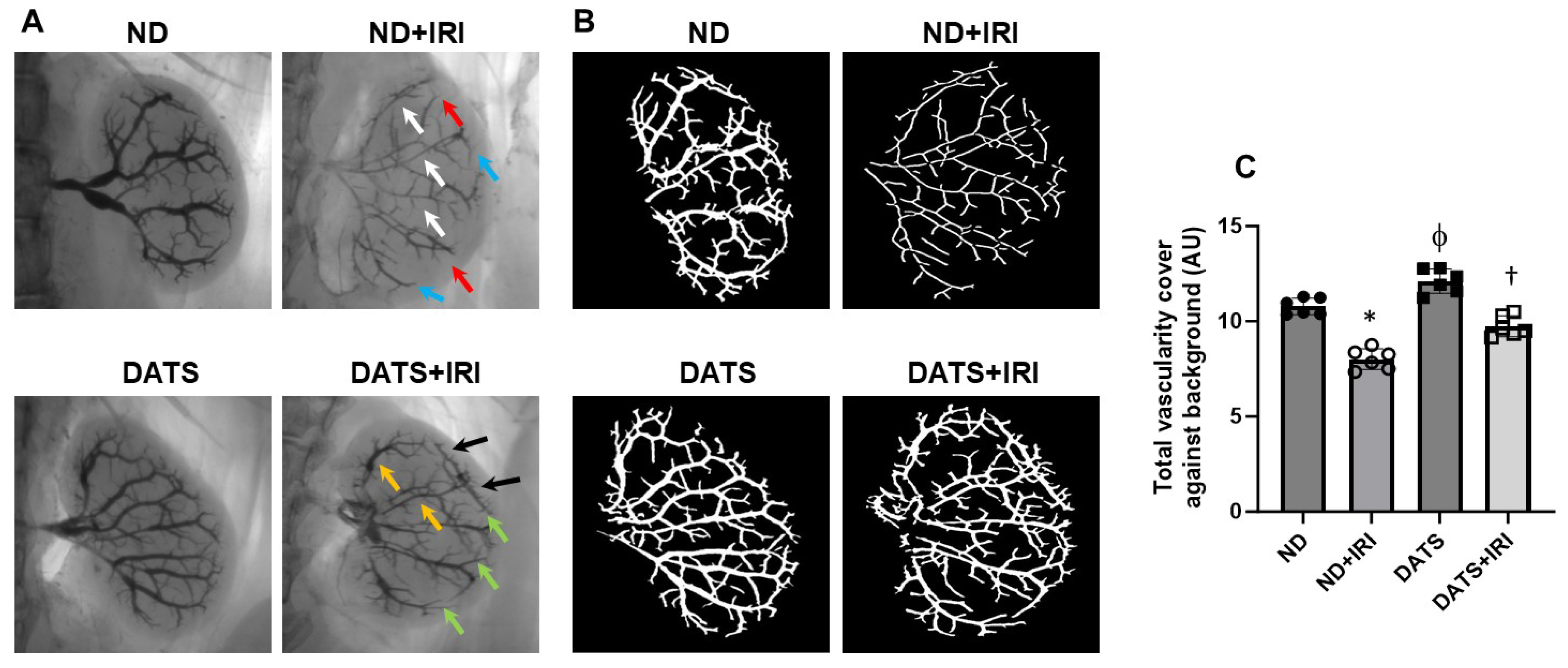
3.6. DATS Ameliorated Renal Microvasculature and Function in the IRI Kidney
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef]
- Tammaro, A.; Kers, J.; Scantlebery, A.M.L.; Florquin, S. Metabolic Flexibility and Innate Immunity in Renal Ischemia Reperfusion Injury: The Fine Balance Between Adaptive Repair and Tissue Degeneration. Front. Immunol. 2020, 11, 1346. [Google Scholar] [CrossRef]
- Thadhani, R.; Pascual, M.; Bonventre, J.V. Acute renal failure. N. Engl. J. Med. 1996, 334, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Shu, S.; Guo, C.; Tang, C.; Dong, Z. Endoplasmic reticulum stress in ischemic and nephrotoxic acute kidney injury. Ann. Med. 2018, 50, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Yoshida, H. Endoplasmic reticulum stress in kidney function and disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.R.; Kim, J.I.; Han, S.J.; Lee, T.J.; Park, K.M. C/EBP homologous protein (CHOP) gene deficiency attenuates renal ischemia/reperfusion injury in mice. Biochim. Biophys. Acta 2015, 1852, 1895–1901. [Google Scholar] [CrossRef]
- Battson, M.L.; Lee, D.M.; Gentile, C.L. Endoplasmic reticulum stress and the development of endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H355–H367. [Google Scholar] [CrossRef]
- Tam, A.B.; Mercado, E.L.; Hoffmann, A.; Niwa, M. ER stress activates NF-kappaB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS ONE 2012, 7, e45078. [Google Scholar] [CrossRef]
- Shu, S.; Zhu, J.; Liu, Z.; Tang, C.; Cai, J.; Dong, Z. Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. Ebiomedicine 2018, 37, 269–280. [Google Scholar] [CrossRef]
- Porter, A.W.; Brodsky, J.L.; Buck, T.M. Emerging links between endoplasmic reticulum stress responses and acute kidney injury. Am. J. Physiol. Cell Physiol. 2022, 323, C1697–C1703. [Google Scholar] [CrossRef]
- Wu, D.; Huang, L.F.; Chen, X.C.; Huang, X.R.; Li, H.Y.; An, N.; Tang, J.X.; Liu, H.F.; Yang, C. Research progress on endoplasmic reticulum homeostasis in kidney diseases. Cell Death Dis. 2023, 14, 473. [Google Scholar] [CrossRef]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef] [PubMed]
- Horbelt, M.; Lee, S.Y.; Mang, H.E.; Knipe, N.L.; Sado, Y.; Kribben, A.; Sutton, T.A. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am. J. Physiol. Ren. Physiol. 2007, 293, F688–F695. [Google Scholar] [CrossRef]
- Basile, D.P.; Fredrich, K.; Chelladurai, B.; Leonard, E.C.; Parrish, A.R. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am. J. Physiol. Ren. Physiol. 2008, 294, F928–F936. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Hong, S.M.; Sutton, T.A.; Temm, C.J. Preservation of peritubular capillary endothelial integrity and increasing pericytes may be critical to recovery from postischemic acute kidney injury. Am. J. Physiol. Ren. Physiol. 2008, 295, F351–F359. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Kwiatkowski, S.; Dziedziejko, V.; Tomasiewicz, I.; Domanski, L. Renal Microcirculation Injury as the Main Cause of Ischemic Acute Kidney Injury Development. Biology 2023, 12, 327. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Basile, D.P. The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int. 2007, 72, 151–156. [Google Scholar] [CrossRef]
- Maringer, K.; Sims-Lucas, S. The multifaceted role of the renal microvasculature during acute kidney injury. Pediatr. Nephrol. 2016, 31, 1231–1240. [Google Scholar] [CrossRef][Green Version]
- Dimke, H.; Sparks, M.A.; Thomson, B.R.; Frische, S.; Coffman, T.M.; Quaggin, S.E. Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. J. Am. Soc. Nephrol. 2015, 26, 1027–1038. [Google Scholar] [CrossRef]
- Dong, Y.; Fernandes, C.; Liu, Y.; Wu, Y.; Wu, H.; Brophy, M.L.; Deng, L.; Song, K.; Wen, A.; Wong, S.; et al. Role of endoplasmic reticulum stress signalling in diabetic endothelial dysfunction and atherosclerosis. Diabetes Vasc. Dis. Res. 2017, 14, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Kassan, M.; Galan, M.; Partyka, M.; Saifudeen, Z.; Henrion, D.; Trebak, M.; Matrougui, K. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1652–1661. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, Y.; Adachi, M.T.; Oshiro, S.; Aso, T.; Kaufman, R.J.; Kitajima, S. Homocysteine induces programmed cell death in human vascular endothelial cells through activation of the unfolded protein response. J. Biol. Chem. 2001, 276, 35867–35874. [Google Scholar] [CrossRef]
- Sanson, M.; Auge, N.; Vindis, C.; Muller, C.; Bando, Y.; Thiers, J.C.; Marachet, M.A.; Zarkovic, K.; Sawa, Y.; Salvayre, R.; et al. Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: Prevention by oxygen-regulated protein 150 expression. Circ. Res. 2009, 104, 328–336. [Google Scholar] [CrossRef]
- Lenna, S.; Han, R.; Trojanowska, M. Endoplasmic reticulum stress and endothelial dysfunction. IUBMB Life 2014, 66, 530–537. [Google Scholar] [CrossRef]
- Pushpakumar, S.; Kundu, S.; Weber, G.; Sen, U. Exogenous hydrogen sulfide and miR-21 antagonism attenuates macrophage-mediated inflammation in ischemia reperfusion injury of the aged kidney. Geroscience 2021, 43, 1349–1367. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Luo, J.; Wu, Z.; Xiao, T.; Zeng, O.; Li, L.; Li, Y.; Yang, J. Hydrogen sulfide exhibits cardioprotective effects by decreasing endoplasmic reticulum stress in a diabetic cardiomyopathy rat model. Mol. Med. Rep. 2016, 14, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, M.; Wang, Y.; Lu, H.; Deng, J.; Yan, X. Hydrogen sulfide preconditioning protects against myocardial ischemia/reperfusion injury in rats through inhibition of endo/sarcoplasmic reticulum stress. Int. J. Clin. Exp. Pathol. 2015, 8, 7740–7751. [Google Scholar]
- Zhong, H.; Yu, H.; Chen, J.; Sun, J.; Guo, L.; Huang, P.; Zhong, Y. Hydrogen Sulfide and Endoplasmic Reticulum Stress: A Potential Therapeutic Target for Central Nervous System Degeneration Diseases. Front. Pharmacol. 2020, 11, 702. [Google Scholar] [CrossRef]
- Zou, W.; Yuan, J.; Tang, Z.J.; Wei, H.J.; Zhu, W.W.; Zhang, P.; Gu, H.F.; Wang, C.Y.; Tang, X.Q. Hydrogen sulfide ameliorates cognitive dysfunction in streptozotocin-induced diabetic rats: Involving suppression in hippocampal endoplasmic reticulum stress. Oncotarget 2017, 8, 64203–64216. [Google Scholar] [CrossRef]
- Yang, R.; Teng, X.; Li, H.; Xue, H.M.; Guo, Q.; Xiao, L.; Wu, Y.M. Hydrogen Sulfide Improves Vascular Calcification in Rats by Inhibiting Endoplasmic Reticulum Stress. Oxid. Med. Cell. Longev. 2016, 2016, 9095242. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Z.; Miao, Y.; Wei, H.; Peng, J.; Zhou, Y. Diallyl Trisulfide Promotes Placental Angiogenesis by Regulating Lipid Metabolism and Alleviating Inflammatory Responses in Obese Pregnant Mice. Nutrients 2022, 14, 2230. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.T.; Stan, S.D. Dietary Bioactive Diallyl Trisulfide in Cancer Prevention and Treatment. Int. J. Mol. Sci. 2017, 18, 1645. [Google Scholar] [CrossRef]
- Disbrow, E.; Stokes, K.Y.; Ledbetter, C.; Patterson, J.; Kelley, R.; Pardue, S.; Reekes, T.; Larmeu, L.; Batra, V.; Yuan, S.; et al. Plasma hydrogen sulfide: A biomarker of Alzheimer’s disease and related dementias. Alzheimers Dement. 2021, 17, 1391–1402. [Google Scholar] [CrossRef]
- Guzel, C.; Yesiltas, S.; Daskaya, H.; Uysal, H.; Sumer, I.; Turkay, M. The effect of gender on acute kidney injury developing in the intensive care unit. Hippokratia 2019, 23, 126–130. [Google Scholar]
- Sawhney, S.; Bell, S.; Black, C.; Christiansen, C.F.; Heide-Jorgensen, U.; Jensen, S.K.; Ronksley, P.E.; Tan, Z.; Tonelli, M.; Walker, H.; et al. Harmonization of epidemiology of acute kidney injury and acute kidney disease produces comparable findings across four geographic populations. Kidney Int. 2022, 101, 1271–1281. [Google Scholar] [CrossRef]
- Kim, J.; Cannon, B.A.; Freeman, L.E.; Tan, S.; Knych, H.K.; Kendall, L.V. High-dose Meloxicam Provides Improved Analgesia in Female CD1 Mice: A Pharmacokinetic and Efficacy Study. J. Am. Assoc. Lab. Anim. Sci. 2023, 62, 74–80. [Google Scholar] [CrossRef]
- Kendall, L.V.; Bailey, A.L.; Singh, B.; McGee, W. Toxic Effects of High-dose Meloxicam and Carprofen on Female CD1 Mice. J. Am. Assoc. Lab. Anim. Sci. 2022, 61, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Pushpakumar, S.; Sen, U. MMP-9- and NMDA receptor-mediated mechanism of diabetic renovascular remodeling and kidney dysfunction: Hydrogen sulfide is a key modulator. Nitric Oxide Biol. Chem. Off. J. Nitric Oxide Soc. 2015, 46, 172–185. [Google Scholar] [CrossRef]
- Pushpakumar, S.; Ren, L.; Juin, S.K.; Majumder, S.; Kulkarni, R.; Sen, U. Methylation-dependent antioxidant-redox imbalance regulates hypertensive kidney injury in aging. Redox Biol. 2020, 37, 101754. [Google Scholar] [CrossRef]
- Pushpakumar, S.; Kundu, S.; Pryor, T.; Givvimani, S.; Lederer, E.; Tyagi, S.C.; Sen, U. Angiotensin-II induced hypertension and renovascular remodelling in tissue inhibitor of metalloproteinase 2 knockout mice. J. Hypertens. 2013, 31, 2270–2281, discussion 2281. [Google Scholar] [CrossRef] [PubMed]
- Pushpakumar, S.; Ren, L.; Kundu, S.; Gamon, A.; Tyagi, S.C.; Sen, U. Toll-like Receptor 4 Deficiency Reduces Oxidative Stress and Macrophage Mediated Inflammation in Hypertensive Kidney. Sci. Rep. 2017, 7, 6349. [Google Scholar] [CrossRef] [PubMed]
- Schock-Kusch, D.; Sadick, M.; Henninger, N.; Kraenzlin, B.; Claus, G.; Kloetzer, H.M.; Weiss, C.; Pill, J.; Gretz, N. Transcutaneous measurement of glomerular filtration rate using FITC-sinistrin in rats. Nephrol. Dial. Transplant. 2009, 24, 2997–3001. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, B.F.; Flyvbjerg, A.; De Vriese, A.S. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004, 65, 2003–2017. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef]
- Song, Y.Y.; Liang, D.; Liu, D.K.; Lin, L.; Zhang, L.; Yang, W.Q. The role of the ERK signaling pathway in promoting angiogenesis for treating ischemic diseases. Front. Cell Dev. Biol. 2023, 11, 1164166. [Google Scholar] [CrossRef]
- Hosszu, A.; Fekete, A.; Szabo, A.J. Sex differences in renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2020, 319, F149–F154. [Google Scholar] [CrossRef]
- Kang, K.P.; Lee, J.E.; Lee, A.S.; Jung, Y.J.; Kim, D.; Lee, S.; Hwang, H.P.; Kim, W.; Park, S.K. Effect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in mice. Mol. Med. Rep. 2014, 9, 2061–2068. [Google Scholar] [CrossRef]
- Gewin, L.S. Transforming Growth Factor-beta in the Acute Kidney Injury to Chronic Kidney Disease Transition. Nephron 2019, 143, 154–157. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Ge, J. A cardioprotective insight of the cystathionine gamma-lyase/hydrogen sulfide pathway. Int. J. Cardiol. Heart Vasc. 2015, 7, 51–57. [Google Scholar] [CrossRef]
- Gupta, R.; Sahu, M.; Tripathi, R.; Ambasta, R.K.; Kumar, P. Protein S-sulfhydration: Unraveling the prospective of hydrogen sulfide in the brain, vasculature and neurological manifestations. Ageing Res. Rev. 2022, 76, 101579. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xu, W.; Chen, Z.; Cui, C.; Fan, X.; Cai, J.; Gong, Y.; Geng, B. Hydrogen sulphide reduces hyperhomocysteinaemia-induced endothelial ER stress by sulfhydrating protein disulphide isomerase to attenuate atherosclerosis. J. Cell Mol. Med. 2021, 25, 3437–3448. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, Q.; Chen, Y.; Li, Y.; Wang, H. Exogenous hydrogen sulfide improves non-alcoholic fatty liver disease by inhibiting endoplasmic reticulum stress/NLRP3 inflammasome pathway. Mol. Cell Biochem. 2025. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, X.; Wang, P.; Yu, Y.; Wang, J.; Jiang, Q.; Xiao, J. Angiotensin 1 peptide-conjugated CdSe/ZnS quantum dots for cardiac-specific hydrogen sulfide targeted therapy in myocardial ischemia-reperfusion injury. Front. Pharmacol. 2024, 15, 1435282. [Google Scholar] [CrossRef]
- Basile, D.P.; Yoder, M.C. Renal endothelial dysfunction in acute kidney ischemia reperfusion injury. Cardiovasc. Hematol. Disord. Drug Targets 2014, 14, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Krothapalli, S.; Chen, Y.M. Targeting Endoplasmic Reticulum for Novel Therapeutics and Monitoring in Acute Kidney Injury. Nephron 2023, 147, 21–24. [Google Scholar] [CrossRef]
- Gallazzini, M.; Pallet, N. Endoplasmic reticulum stress and kidney dysfunction. Biol. Cell 2018, 110, 205–216. [Google Scholar] [CrossRef]
- Ricciardi, C.A.; Gnudi, L. The endoplasmic reticulum stress and the unfolded protein response in kidney disease: Implications for vascular growth factors. J. Cell. Mol. Med. 2020, 24, 12910–12919. [Google Scholar] [CrossRef]
- Hodeify, R.; Megyesi, J.; Tarcsafalvi, A.; Mustafa, H.I.; Hti Lar Seng, N.S.; Price, P.M. Gender differences control the susceptibility to ER stress-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2013, 304, F875–F882. [Google Scholar] [CrossRef]
- Cai, Y.R.; Hu, C.H. Computational Study of H(2)S Release in Reactions of Diallyl Polysulfides with Thiols. J. Phys. Chem. B 2017, 121, 6359–6366. [Google Scholar] [CrossRef]
- Liang, D.; Wu, H.; Wong, M.W.; Huang, D. Diallyl Trisulfide Is a Fast H2S Donor, but Diallyl Disulfide Is a Slow One: The Reaction Pathways and Intermediates of Glutathione with Polysulfides. Org. Lett. 2015, 17, 4196–4199. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, N.; Fougeray, S.; Beaune, P.; Thervet, E.; Pallet, N. The unfolded protein response regulates an angiogenic response by the kidney epithelium during ischemic stress. J. Biol. Chem. 2012, 287, 14557–14568. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, J.; Chen, Y.; Wang, J.J.; Ratan, R.; Zhang, S.X. Activation of endoplasmic reticulum stress by hyperglycemia is essential for Muller cell-derived inflammatory cytokine production in diabetes. Diabetes 2012, 61, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Binet, F.; Mawambo, G.; Sitaras, N.; Tetreault, N.; Lapalme, E.; Favret, S.; Cerani, A.; Leboeuf, D.; Tremblay, S.; Rezende, F.; et al. Neuronal ER stress impedes myeloid-cell-induced vascular regeneration through IRE1alpha degradation of netrin-1. Cell Metab. 2013, 17, 353–371. [Google Scholar] [CrossRef] [PubMed]
- Loinard, C.; Zouggari, Y.; Rueda, P.; Ramkhelawon, B.; Cochain, C.; Vilar, J.; Recalde, A.; Richart, A.; Charue, D.; Duriez, M.; et al. C/EBP homologous protein-10 (CHOP-10) limits postnatal neovascularization through control of endothelial nitric oxide synthase gene expression. Circulation 2012, 125, 1014–1026. [Google Scholar] [CrossRef]
- Mellor, P.; Deibert, L.; Calvert, B.; Bonham, K.; Carlsen, S.A.; Anderson, D.H. CREB3L1 is a metastasis suppressor that represses expression of genes regulating metastasis, invasion, and angiogenesis. Mol. Cell. Biol. 2013, 33, 4985–4995. [Google Scholar] [CrossRef]
- Todd, D.J.; Lee, A.H.; Glimcher, L.H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 2008, 8, 663–674. [Google Scholar] [CrossRef]
- Wang, H.; Shi, X.; Qiu, M.; Lv, S.; Liu, H. Hydrogen Sulfide Plays an Important Protective Role through Influencing Endoplasmic Reticulum Stress in Diseases. Int. J. Biol. Sci. 2020, 16, 264–271. [Google Scholar] [CrossRef]
- Szabo, C.; Papapetropoulos, A. Hydrogen sulphide and angiogenesis: Mechanisms and applications. Br. J. Pharmacol. 2011, 164, 853–865. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Jing, M.R.; Cai, C.B.; Zhu, S.G.; Zhang, C.J.; Wang, Q.M.; Zhai, Y.K.; Ji, X.Y.; Wu, D.D. Role of hydrogen sulphide in physiological and pathological angiogenesis. Cell Prolif. 2023, 56, e13374. [Google Scholar] [CrossRef]
- Kimura, H. Production and physiological effects of hydrogen sulfide. Antioxid. Redox Signal. 2014, 20, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H(2)S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Feng, J.; Lu, X.; Li, H.; Wang, S. The roles of hydrogen sulfide in renal physiology and disease states. Ren. Fail. 2022, 44, 1289–1308. [Google Scholar] [CrossRef]
- Johansen, D.; Ytrehus, K.; Baxter, G.F. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury--Evidence for a role of K ATP channels. Basic Res. Cardiol. 2006, 101, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Lobb, I.; Zhu, J.; Liu, W.; Haig, A.; Lan, Z.; Sener, A. Hydrogen sulfide treatment ameliorates long-term renal dysfunction resulting from prolonged warm renal ischemia-reperfusion injury. Can. Urol. Assoc. J. 2014, 8, E413–418. [Google Scholar] [CrossRef] [PubMed]
- Sekijima, M.; Sahara, H.; Miki, K.; Villani, V.; Ariyoshi, Y.; Iwanaga, T.; Tomita, Y.; Yamada, K. Hydrogen sulfide prevents renal ischemia-reperfusion injury in CLAWN miniature swine. J. Surg. Res. 2017, 219, 165–172. [Google Scholar] [CrossRef]
- Andrey, M.n.; Natalia, Z.; Alexandr, O.; Iryna, P. P1 Gender dimorphism of hydrogen sulfide production and physiological effects in cardiovascular system. Nitric Oxide Biol. Chem. Off. J. Nitric Oxide Soc. 2014, 39, S16. [Google Scholar] [CrossRef]
- Rajpal, S.; Katikaneni, P.; Deshotels, M.; Pardue, S.; Glawe, J.; Shen, X.; Akkus, N.; Modi, K.; Bhandari, R.; Dominic, P.; et al. Total sulfane sulfur bioavailability reflects ethnic and gender disparities in cardiovascular disease. Redox Biol. 2018, 15, 480–489. [Google Scholar] [CrossRef]
- Tanaka, R.; Tsutsui, H.; Ohkita, M.; Takaoka, M.; Yukimura, T.; Matsumura, Y. Sex differences in ischemia/reperfusion-induced acute kidney injury are dependent on the renal sympathetic nervous system. Eur. J. Pharmacol. 2013, 714, 397–404. [Google Scholar] [CrossRef]
- Privratsky, J.R.; Fuller, M.; Raghunathan, K.; Ohnuma, T.; Bartz, R.R.; Schroeder, R.; Price, T.M.; Martinez, M.R.; Sigurdsson, M.I.; Mathis, M.R.; et al. Postoperative Acute Kidney Injury by Age and Sex: A Retrospective Cohort Association Study. Anesthesiology 2023, 138, 184–194. [Google Scholar] [CrossRef]
- Hamid, S.M.; Citir, M.; Terzi, E.M.; Cimen, I.; Yildirim, Z.; Dogan, A.E.; Kocaturk, B.; Onat, U.I.; Arditi, M.; Weber, C.; et al. Inositol-requiring enzyme-1 regulates phosphoinositide signaling lipids and macrophage growth. EMBO Rep. 2020, 21, e51462. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Kaczmarek, A.; Krysko, O.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. ER stress-induced inflammation: Does it aid or impede disease progression? Trends Mol. Med. 2012, 18, 589–598. [Google Scholar] [CrossRef]
- Chen, J.; Gao, J.; Sun, W.; Li, L.; Wang, Y.; Bai, S.; Li, X.; Wang, R.; Wu, L.; Li, H.; et al. Involvement of exogenous H2S in recovery of cardioprotection from ischemic post-conditioning via increase of autophagy in the aged hearts. Int. J. Cardiol. 2016, 220, 681–692. [Google Scholar] [CrossRef]
- Rozpedek, W.; Pytel, D.; Mucha, B.; Leszczynska, H.; Diehl, J.A.; Majsterek, I. The Role of the PERK/eIF2alpha/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef]
- Shu, S.; Wang, Y.; Zheng, M.; Liu, Z.; Cai, J.; Tang, C.; Dong, Z. Hypoxia and Hypoxia-Inducible Factors in Kidney Injury and Repair. Cells 2019, 8, 207. [Google Scholar] [CrossRef]
- Lemus-Varela, M.L.; Flores-Soto, M.E.; Cervantes-Munguia, R.; Torres-Mendoza, B.M.; Gudino-Cabrera, G.; Chaparro-Huerta, V.; Ortuno-Sahagun, D.; Beas-Zarate, C. Expression of HIF-1 alpha, VEGF and EPO in peripheral blood from patients with two cardiac abnormalities associated with hypoxia. Clin. Biochem. 2010, 43, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Miguelez, P.; Lima-Cabello, E.; Martinez-Florez, S.; Almar, M.; Cuevas, M.J.; Gonzalez-Gallego, J. Hypoxia-inducible factor-1 modulates the expression of vascular endothelial growth factor and endothelial nitric oxide synthase induced by eccentric exercise. J Appl Physiol (1985) 2015, 118, 1075–1083. [Google Scholar] [CrossRef]
- Mao, H.; Jiang, C.; Xu, L.; Chen, D.; Liu, H.; Xu, Y.; Ma, K.; Wang, M. Ginsenoside protects against AKI via activation of HIF-1alpha and VEGF-A in the kidney-brain axis. Int. J. Mol. Med. 2020, 45, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.; Balsari, A.; Giallongo, T.; Ottolenghi, S.; Di Giulio, A.M.; Samaja, M.; Carelli, S. Erythropoietin as a Neuroprotective Molecule: An Overview of Its Therapeutic Potential in Neurodegenerative Diseases. ASN Neuro 2019, 11, 1–18. [Google Scholar] [CrossRef]
- Correa-Costa, M.; Gallo, D.; Csizmadia, E.; Gomperts, E.; Lieberum, J.L.; Hauser, C.J.; Ji, X.; Wang, B.; Camara, N.O.S.; Robson, S.C.; et al. Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc. Natl. Acad. Sci. USA 2018, 115, E2302–E2310. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, T.; Sharma, A.K.; Kaur, J.; Yadav, H.N.; Pathak, D.; Singh, A.P. Fenofibrate attenuates ischemia reperfusion-induced acute kidney injury and associated liver dysfunction in rats. Drug Dev. Res. 2021, 82, 412–421. [Google Scholar] [CrossRef]
- Tanaka, T.; Nangaku, M. Angiogenesis and hypoxia in the kidney. Nat. Rev. Nephrol. 2013, 9, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Nakada, M.; Kita, D.; Watanabe, T.; Hayashi, Y.; Teng, L.; Pyko, I.V.; Hamada, J. Aberrant signaling pathways in glioma. Cancers 2011, 3, 3242–3278. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhang, Z.; Chen, C.; Liu, Y.; Yuan, D.; Hei, Z.; Luo, G. PI3K/AKT activation attenuates acute kidney injury following liver transplantation by inducing FoxO3a nuclear export and deacetylation. Life Sci. 2021, 272, 119119. [Google Scholar] [CrossRef]
- Sang, Z.; Dong, S.; Zhang, P.; Wei, Y. miR-214 ameliorates sepsis-induced acute kidney injury via PTEN/AKT/mTOR-regulated autophagy. Mol. Med. Rep. 2021, 24, 683. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Chen, Y.H.; Chen, N.; Wang, L.J.; Chen, D.X.; Weng, H.L.; Dooley, S.; Ding, H.G. Hydrogen sulfide promotes autophagy of hepatocellular carcinoma cells through the PI3K/Akt/mTOR signaling pathway. Cell Death Dis. 2017, 8, e2688. [Google Scholar] [CrossRef]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef]
- Chiba, T.; Oda, A.; Zhang, Y.; Pfister, K.E.; Bons, J.; Bharathi, S.S.; Kinoshita, A.; Zhang, B.B.; Richert, A.C.; Schilling, B.; et al. Loss of long-chain acyl-CoA dehydrogenase protects against acute kidney injury. JCI Insight 2025, in press . [CrossRef]
- Piko, N.; Bevc, S.; Hojs, R.; Ekart, R. The Role of Oxidative Stress in Kidney Injury. Antioxidants 2023, 12, 1772. [Google Scholar] [CrossRef]
- Xie, C.; Zhou, X.; Chen, W.; Ren, D.; Li, X.; Jiang, R.; Zhong, C.; Zhu, J. Diallyl trisulfide induces pyroptosis and impairs lung CSC-like properties by activating the ROS/Caspase 1 signaling pathway. Chem. Biol. Interact. 2024, 397, 111083. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cheng, X.; Xu, S.; Zhang, L.; Pan, J.; Yu, H.; Bao, J.; Lu, R. Diallyl trisulfide induces G2/M cell-cycle arrest and apoptosis in anaplastic thyroid carcinoma 8505C cells. Food Funct 2019, 10, 7253–7261. [Google Scholar] [CrossRef]
- Majumder, S.; Pushpakumar, S.; Juin, S.K.; Jala, V.R.; Sen, U. Toll-like receptor 4 mutation protects the kidney from Ang-II-induced hypertensive injury. Pharmacol. Res. 2022, 175, 106030. [Google Scholar] [CrossRef] [PubMed]
- Kanda, J.; Mori, K.; Kawabata, H.; Kuwabara, T.; Mori, K.P.; Imamaki, H.; Kasahara, M.; Yokoi, H.; Mizumoto, C.; Thoennissen, N.H.; et al. An AKI biomarker lipocalin 2 in the blood derives from the kidney in renal injury but from neutrophils in normal and infected conditions. Clin. Exp. Nephrol. 2015, 19, 99–106. [Google Scholar] [CrossRef]
- Zhang, J.; Rudemiller, N.P.; Patel, M.B.; Wei, Q.; Karlovich, N.S.; Jeffs, A.D.; Wu, M.; Sparks, M.A.; Privratsky, J.R.; Herrera, M.; et al. Competing Actions of Type 1 Angiotensin II Receptors Expressed on T Lymphocytes and Kidney Epithelium during Cisplatin-Induced AKI. J. Am. Soc. Nephrol. 2016, 27, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Shou, S.; Jin, H. The role of IL-17 in acute kidney injury. Int. Immunopharmacol. 2023, 119, 110307. [Google Scholar] [CrossRef]
- Anders, H.J. Of Inflammasomes and Alarmins: IL-1beta and IL-1alpha in Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 2564–2575. [Google Scholar] [CrossRef]
- Gentle, M.E.; Shi, S.; Daehn, I.; Zhang, T.; Qi, H.; Yu, L.; D’Agati, V.D.; Schlondorff, D.O.; Bottinger, E.P. Epithelial cell TGFbeta signaling induces acute tubular injury and interstitial inflammation. J. Am. Soc. Nephrol. 2013, 24, 787–799. [Google Scholar] [CrossRef]
- Hu, Z.; Zhan, J.; Pei, G.; Zeng, R. Depletion of macrophages with clodronate liposomes partially attenuates renal fibrosis on AKI-CKD transition. Ren. Fail. 2023, 45, 2149412. [Google Scholar] [CrossRef]
- Johnson, F.L.; Patel, N.S.A.; Purvis, G.S.D.; Chiazza, F.; Chen, J.; Sordi, R.; Hache, G.; Merezhko, V.V.; Collino, M.; Yaqoob, M.M.; et al. Inhibition of IkappaB Kinase at 24 Hours After Acute Kidney Injury Improves Recovery of Renal Function and Attenuates Fibrosis. J. Am. Heart Assoc. 2017, 6, e005092. [Google Scholar] [CrossRef]
- Ruiz, S.; Vardon-Bounes, F.; Virtos, M.; Seguin, T.; Crognier, L.; Rouget, A.; Georges, B.; Conil, J.M.; Minville, V. Influence of arterial blood gases on the renal arterial resistive index in intensive care unit. J. Transl. Med. 2023, 21, 541. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Bilska-Wilkosz, A.; Gorny, M.; Sokolowska-Jezewicz, M.; Kowalczyk-Pachel, D. The Effects of Different Garlic-Derived Allyl Sulfides on Anaerobic Sulfur Metabolism in the Mouse Kidney. Antioxidants 2016, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kwiecien, I.; Chwatko, G.; Sokolowska-Jezewicz, M.; Kowalczyk-Pachel, D.; Rokita, H. The effects of garlic-derived sulfur compounds on cell proliferation, caspase 3 activity, thiol levels and anaerobic sulfur metabolism in human hepatoblastoma HepG2 cells. Cell Biochem. Funct. 2012, 30, 198–204. [Google Scholar] [CrossRef] [PubMed]





| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| GRP78 | TTCAGCCAATTATCAGCAAACTCT | TTTTCTGATGTATCCTCTTCACCAGT |
| GRP94 | AAGAATGAAGGAAAAACAGGACAAAA | CAAATGGAGAAGATTCCGCC |
| ATF4 | GGGTTCTGTCTTCCACTCCA | AAGCAGCAGAGTCAGGCTTTC |
| CHOP | CCACCACACCTGAAAGCAGAA | AGGTGAAAGGCAGGGACTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pushpakumar, S.; Juin, S.K.; Almarshood, H.; Gondim, D.D.; Ouseph, R.; Sen, U. Diallyl Trisulfide Attenuates Ischemia-Reperfusion-Induced ER Stress and Kidney Dysfunction in Aged Female Mice. Cells 2025, 14, 420. https://doi.org/10.3390/cells14060420
Pushpakumar S, Juin SK, Almarshood H, Gondim DD, Ouseph R, Sen U. Diallyl Trisulfide Attenuates Ischemia-Reperfusion-Induced ER Stress and Kidney Dysfunction in Aged Female Mice. Cells. 2025; 14(6):420. https://doi.org/10.3390/cells14060420
Chicago/Turabian StylePushpakumar, Sathnur, Subir Kumar Juin, Hebah Almarshood, Dibson Dibe Gondim, Rosemary Ouseph, and Utpal Sen. 2025. "Diallyl Trisulfide Attenuates Ischemia-Reperfusion-Induced ER Stress and Kidney Dysfunction in Aged Female Mice" Cells 14, no. 6: 420. https://doi.org/10.3390/cells14060420
APA StylePushpakumar, S., Juin, S. K., Almarshood, H., Gondim, D. D., Ouseph, R., & Sen, U. (2025). Diallyl Trisulfide Attenuates Ischemia-Reperfusion-Induced ER Stress and Kidney Dysfunction in Aged Female Mice. Cells, 14(6), 420. https://doi.org/10.3390/cells14060420






