Effects of Space Flight on Inflammasome Activation in the Brain of Mice
Abstract
1. Introduction
2. Materials and Methods
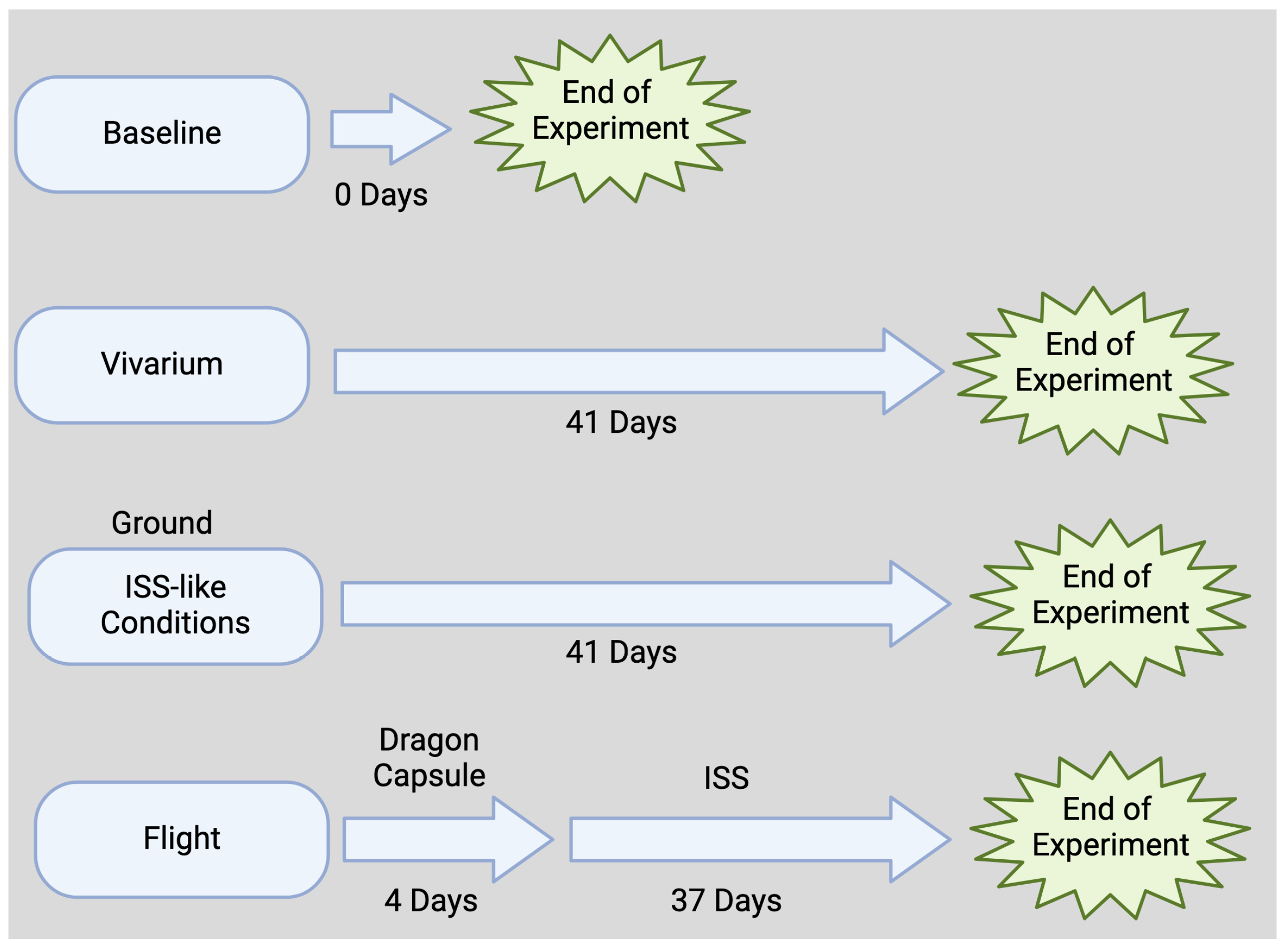
2.1. Flight Animals
2.2. Ground Control Animals
2.3. Vivarium and Baseline Animals
2.4. Brain Dissection
2.5. Immunoblotting
2.6. Electrochemiluminescence Immunoassay (ECLIA)
2.7. Statistical Analyses
3. Results
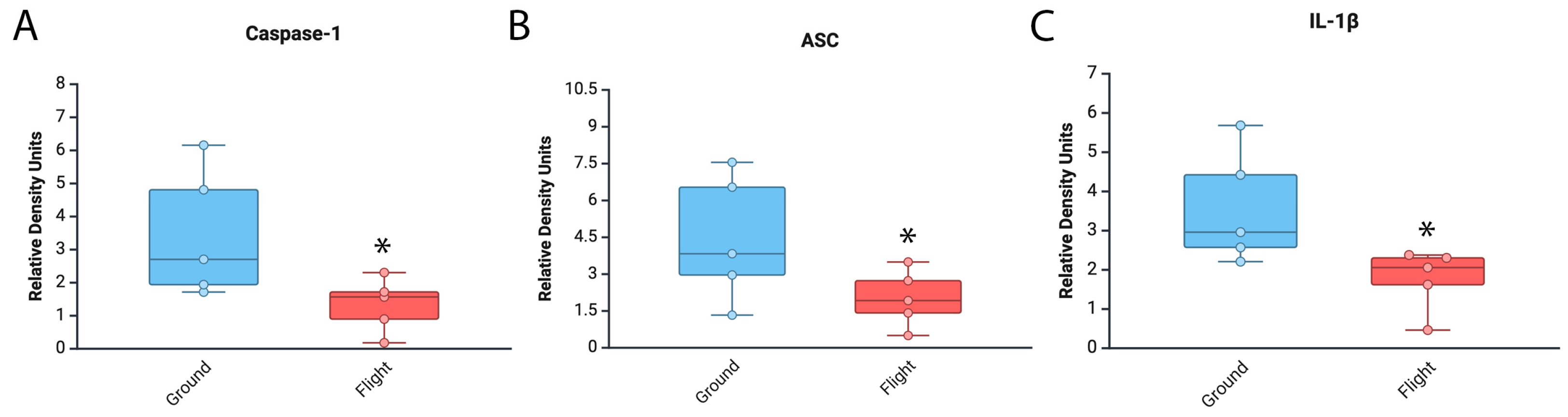
3.1. Levels of Inflammasome Signaling Molecules Are Decreased in the Brain of Mice That Experienced Spaceflight
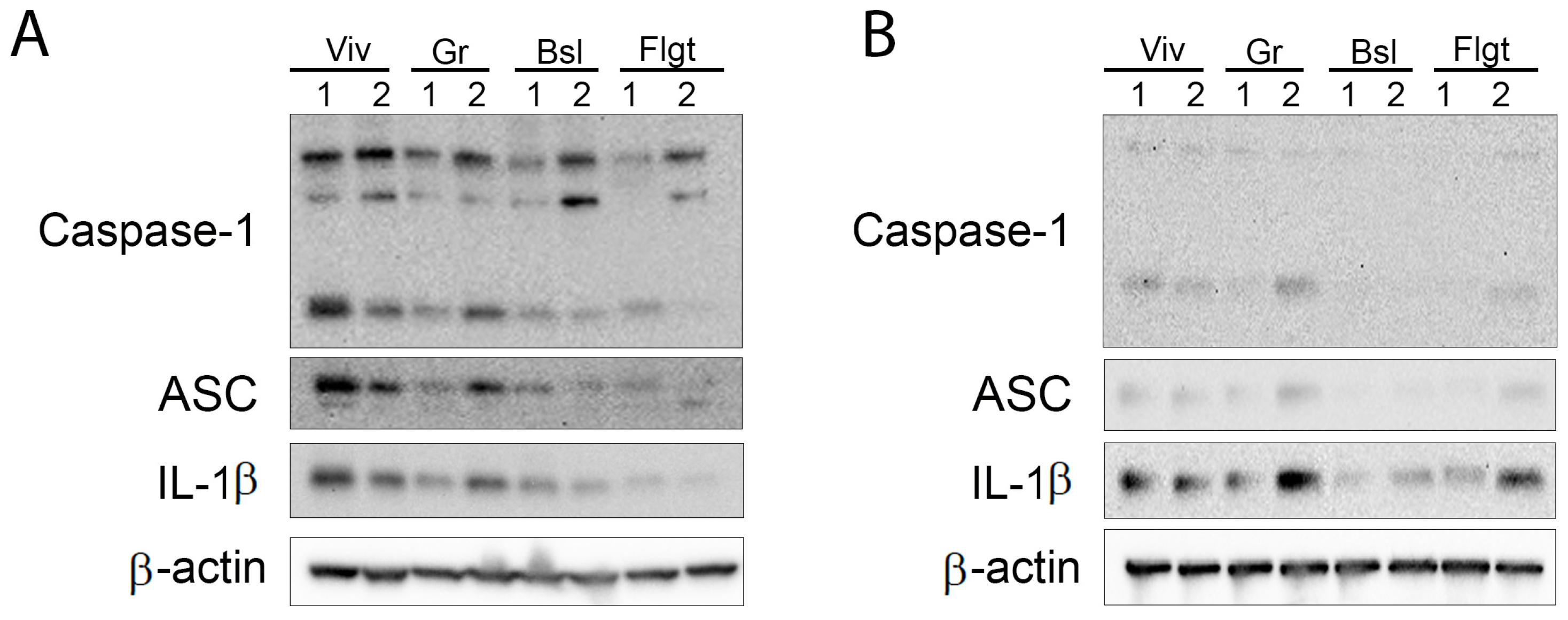
3.2. Inflammasome Proteins Are Decreased in the Cortex and Hippocampus of Mice After Spaceflight
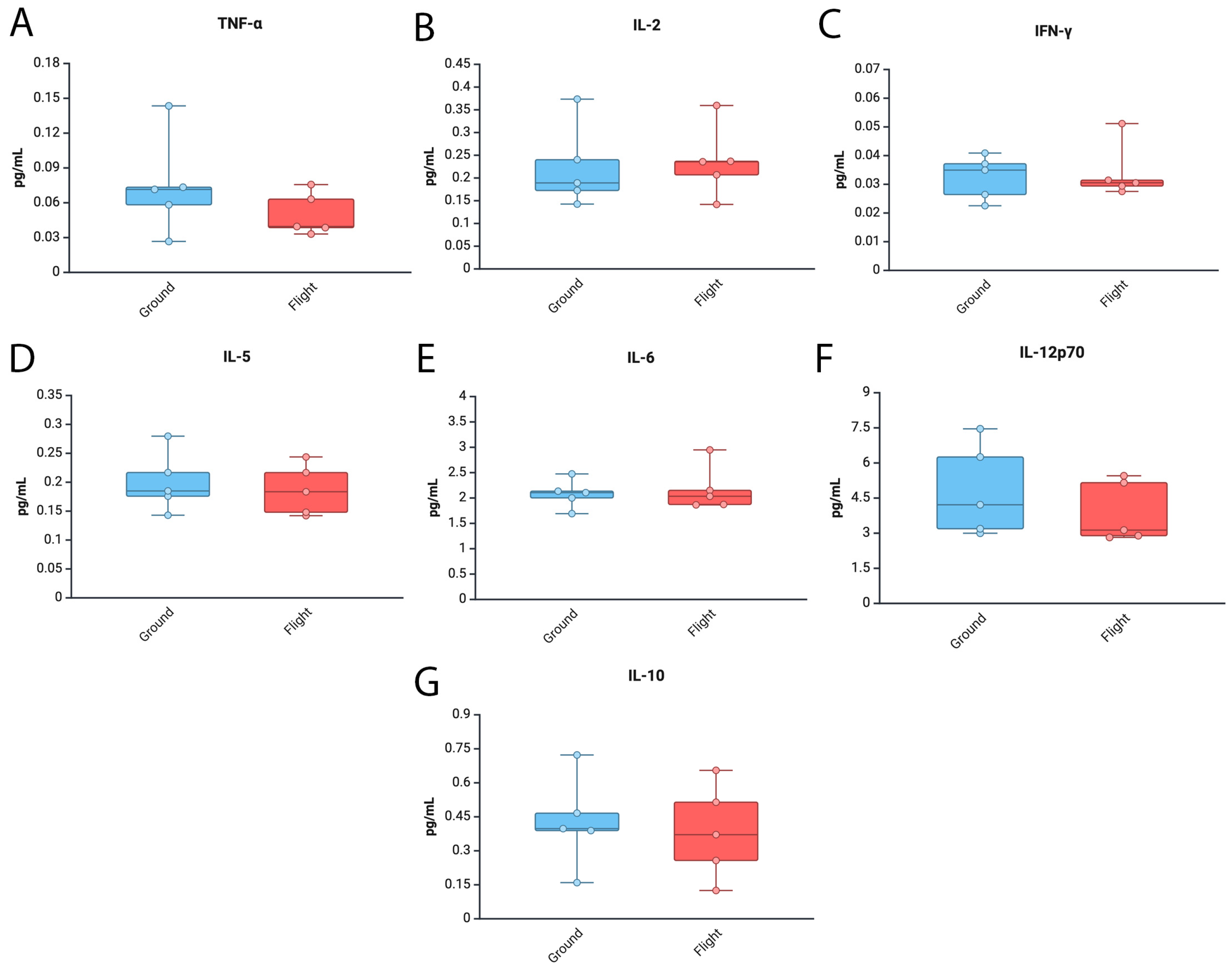
3.3. Levels of Tnf-α and Il12p70 Are Decreased in the Brain of Mice After Spaceflight
3.4. Inflammatory Cytokine Levels in the Cortex and Hippocampus of Mice After Spaceflight
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchheim, J.I.; Matzel, S.; Rykova, M.; Vassilieva, G.; Ponomarev, S.; Nichiporuk, I.; Horl, M.; Moser, D.; Biere, K.; Feuerecker, M.; et al. Stress Related Shift Toward Inflammaging in Cosmonauts After Long-Duration Space Flight. Front. Physiol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Carpo, N.; Tran, V.; Biancotti, J.C.; Cepeda, C.; Espinosa-Jeffrey, A. Space Flight Enhances Stress Pathways in Human Neural Stem Cells. Biomolecules 2024, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Kvetnansky, R.; Ksinantova, L.; Koska, J.; Noskov, V.B.; Vigas, M.; Grigoriev, A.I.; Macho, L. Effect of space flight and head-down bedrest on neuroendocrine response to metabolic stress in physically trained subjects. J. Gravitational Physiol. 2004, 11, P57–P60. [Google Scholar]
- Macho, L.; Koska, J.; Ksinantova, L.; Pacak, K.; Hoff, T.; Noskov, V.B.; Grigoriev, A.I.; Vigas, M.; Kvetnansky, R. The response of endocrine system to stress loads during space flight in human subject. Adv. Space Res. 2003, 31, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.M.; Corsetto, P.A.; Montorfano, G.; Milani, S.; Zava, S.; Tavella, S.; Cancedda, R.; Berra, B. Effects of long-term space flight on erythrocytes and oxidative stress of rodents. PLoS ONE 2012, 7, e32361. [Google Scholar] [CrossRef]
- Sonnenfeld, G. Space flight, microgravity, stress, and immune responses. Adv. Space Res. 1999, 23, 1945–1953. [Google Scholar] [CrossRef]
- Stein, T.P. Space flight and oxidative stress. Nutrition 2002, 18, 867–871. [Google Scholar] [CrossRef]
- Verhaar, A.P.; Hoekstra, E.; Tjon, A.S.; Utomo, W.K.; Deuring, J.J.; Bakker, E.R.; Muncan, V.; Peppelenbosch, M.P. Dichotomal effect of space flight-associated microgravity on stress-activated protein kinases in innate immunity. Sci. Rep. 2014, 4, 5468. [Google Scholar] [CrossRef]
- Afshinnekoo, E.; Scott, R.T.; MacKay, M.J.; Pariset, E.; Cekanaviciute, E.; Barker, R.; Gilroy, S.; Hassane, D.; Smith, S.M.; Zwart, S.R.; et al. Fundamental Biological Features of Spaceflight: Advancing the Field to Enable Deep-Space Exploration. Cell 2020, 183, 1162–1184. [Google Scholar] [CrossRef]
- Chapes, S.K.; Woods, K.M.; Armstrong, J.W. Ground-Based experiments complement microgravity flight opportunities in the investigation of the effects of space flight on the immune response: Is protein kinase C gravity sensitive? Trans. Kans. Acad. Sci. 1993, 96, 74–79. [Google Scholar] [CrossRef]
- Crucian, B.E.; Chouker, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune System Dysregulation During Spaceflight: Potential Countermeasures for Deep Space Exploration Missions. Front. Immunol. 2018, 9, 1437. [Google Scholar] [CrossRef] [PubMed]
- Marcos, A.; Nova, E.; Montero, A. Changes in the immune system are conditioned by nutrition. Eur. J. Clin. Nutr. 2003, 57 (Suppl. S1), S66–S69. [Google Scholar] [CrossRef] [PubMed]
- Sephton, S.; Spiegel, D. Circadian disruption in cancer: A neuroendocrine-immune pathway from stress to disease? Brain Behav. Immun. 2003, 17, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Fornace, A.J., Jr. Countermeasure development against space radiation-induced gastrointestinal carcinogenesis: Current and future perspectives. Life Sci. Space Res. 2022, 35, 53–59. [Google Scholar] [CrossRef]
- Ruff, G.E. Experimental studies of stress in space flight. Am. J. Psychiatry 1959, 115, 1109–1110. [Google Scholar]
- Tigranyan, R.A.; Macho, L.; Kvetnansky, R.; Nemeth, S.; Kalita, N.F. Stress in space flight: Metabolic aspects. Physiologist 1980, 23, S45–S50. [Google Scholar]
- Cogoli, A. The effect of space flight on human cellular immunity. Environ. Med. 1993, 37, 107–116. [Google Scholar]
- Mandel, A.D.; Balish, E. Effect of space flight on cell-mediated immunity. Aviat. Space Environ. Med. 1977, 48, 1051–1057. [Google Scholar]
- Sonnenfeld, G.; Miller, E.S. Space flight and humoral and cellular immunity of animals. Physiologist 1993, 36, S68–S70. [Google Scholar]
- de Rivero Vaccari, J.P.; Dietrich, W.D.; Keane, R.W. Therapeutics targeting the inflammasome after central nervous system injury. Transl. Res. 2016, 167, 35–45. [Google Scholar] [CrossRef]
- Lang, Y.; Chu, F.; Shen, D.; Zhang, W.; Zheng, C.; Zhu, J.; Cui, L. Role of Inflammasomes in Neuroimmune and Neurodegenerative Diseases: A Systematic Review. Mediat. Inflamm. 2018, 2018, 1549549. [Google Scholar] [CrossRef]
- Patel, S. Inflammasomes, the cardinal pathology mediators are activated by pathogens, allergens and mutagens: A critical review with focus on NLRP3. Biomed. Pharmacother. 2017, 92, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Singhal, G.; Jaehne, E.J.; Corrigan, F.; Toben, C.; Baune, B.T. Inflammasomes in neuroinflammation and changes in brain function: A focused review. Front. Neurosci. 2014, 8, 315. [Google Scholar] [CrossRef]
- Yang, C.A.; Chiang, B.L. Inflammasomes and human autoimmunity: A comprehensive review. J. Autoimmun. 2015, 61, 1–8. [Google Scholar] [CrossRef]
- de Rivero Vaccari, J.P.; Dietrich, W.D.; Keane, R.W. Activation and regulation of cellular inflammasomes: Gaps in our knowledge for central nervous system injury. J. Cereb. Blood Flow Metab. 2014, 34, 369–375. [Google Scholar] [CrossRef]
- Brint, A.; Greene, S.; Fennig-Victor, A.R.; Wang, S. Multiple sclerosis: The NLRP3 inflammasome, gasdermin D, and therapeutics. Ann. Transl. Med. 2024, 12, 62. [Google Scholar] [CrossRef]
- Fan, H.; Fu, Q.; Du, G.; Qin, L.; Shi, X.; Wang, D.; Yang, Y. Microglial Mayhem NLRP3 Inflammasome’s Role in Multiple Sclerosis Pathology. CNS Neurosci. Ther. 2024, 30, e70135. [Google Scholar] [CrossRef]
- Malhotra, S.; Hurtado-Navarro, L.; Pappolla, A.; Villar, L.M.M.; Rio, J.; Montalban, X.; Pelegrin, P.; Comabella, M. Increased NLRP3 Inflammasome Activation and Pyroptosis in Patients With Multiple Sclerosis With Fingolimod Treatment Failure. Neurol. Neuroimmunol. Neuroinflammation 2023, 10, e200100. [Google Scholar] [CrossRef]
- Otalora-Alcaraz, A.; Reilly, T.; Oro-Nolla, M.; Sun, M.C.; Costelloe, L.; Kearney, H.; Patra, P.H.; Downer, E.J. The NLRP3 inflammasome: A central player in multiple sclerosis. Biochem. Pharmacol. 2025, 232, 116667. [Google Scholar] [CrossRef]
- Shadab, A.; Abbasi-Kolli, M.; Saharkhiz, M.; Ahadi, S.H.; Shokouhi, B.; Nahand, J.S. The interplay between mitochondrial dysfunction and NLRP3 inflammasome in multiple sclerosis: Therapeutic implications and animal model studies. Biomed. Pharmacother. 2024, 175, 116673. [Google Scholar] [CrossRef] [PubMed]
- Cabrera Ranaldi, E.; Nuytemans, K.; Martinez, A.; Luca, C.C.; Keane, R.W.; de Rivero Vaccari, J.P. Proof-of-Principle Study of Inflammasome Signaling Proteins as Diagnostic Biomarkers of the Inflammatory Response in Parkinson’s Disease. Pharmaceuticals 2023, 16, 883. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Ng, W.L.; Goh, S.Y.; Gulam, M.Y.; Wang, L.F.; Tan, E.K.; Ahn, M.; Chao, Y.X. Targeting the inflammasome in Parkinson’s disease. Front. Aging Neurosci. 2022, 14, 957705. [Google Scholar] [CrossRef] [PubMed]
- McManus, R.M.; Latz, E. NLRP3 inflammasome signalling in Alzheimer’s disease. Neuropharmacology 2024, 252, 109941. [Google Scholar] [CrossRef]
- Vontell, R.T.; Gober, R.; Dallmeier, J.; Brzostowicki, D.; Barreda, A.; Blennow, K.; Zetterberg, H.; Kvartsberg, H.; Gultekin, S.H.; de Rivero Vaccari, J.P.; et al. Association of region-specific hippocampal reduction of neurogranin with inflammasome proteins in post mortem brains of Alzheimer’s disease. Alzheimers Dement. 2024, 10, e12444. [Google Scholar] [CrossRef]
- Cyr, B.; Hadad, R.; Keane, R.W.; de Rivero Vaccari, J.P. The Role of Non-canonical and Canonical Inflammasomes in Inflammaging. Front. Mol. Neurosci. 2022, 15, 774014. [Google Scholar] [CrossRef]
- Mejias, N.H.; Martinez, C.C.; Stephens, M.E.; de Rivero Vaccari, J.P. Contribution of the inflammasome to inflammaging. J. Inflamm. 2018, 15, 23. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Kanneganti, T.D. The inflammasome: A remote control for metabolic syndrome. Cell Res. 2012, 22, 1095–1098. [Google Scholar] [CrossRef]
- Pahwa, R.; Singh, A.; Adams-Huet, B.; Devaraj, S.; Jialal, I. Increased inflammasome activity in subcutaneous adipose tissue of patients with metabolic syndrome. Diabetes Metab. Res. Rev. 2021, 37, e3383. [Google Scholar] [CrossRef]
- Miller, K.B.; Mi, K.L.; Nelson, G.A.; Norman, R.B.; Patel, Z.S.; Huff, J.L. Ionizing radiation, cerebrovascular disease, and consequent dementia: A review and proposed framework relevant to space radiation exposure. Front. Physiol. 2022, 13, 1008640. [Google Scholar] [CrossRef]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef] [PubMed]
- Tobin, B.W.; Uchakin, P.N.; Leeper-Woodford, S.K. Insulin secretion and sensitivity in space flight: Diabetogenic effects. Nutrition 2002, 18, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Childress, S.D.; Williams, T.C.; Francisco, D.R. NASA Space Flight Human-System Standard: Enabling human spaceflight missions by supporting astronaut health, safety, and performance. NPJ Microgravity 2023, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; McCoy, T.; Gazda, D.; Morgan, J.L.; Heer, M.; Zwart, S.R. Space flight calcium: Implications for astronaut health, spacecraft operations, and Earth. Nutrients 2012, 4, 2047–2068. [Google Scholar] [CrossRef]
- Yasuda, H.; Komiyama, T.; Fujitaka, K. Probability of hippocampus cell hits by high-LET space radiation in a low-Earth-orbit mission (STS-91). Phys. Med. 2001, 17 (Suppl. S1), 166–169. [Google Scholar]
- Robinson, N.T.M.; Descamps, L.A.L.; Russell, L.E.; Buchholz, M.O.; Bicknell, B.A.; Antonov, G.K.; Lau, J.Y.N.; Nutbrown, R.; Schmidt-Hieber, C.; Hausser, M. Targeted Activation of Hippocampal Place Cells Drives Memory-Guided Spatial Behavior. Cell 2020, 183, 1586–1599.e1510. [Google Scholar] [CrossRef]
- Tidmore, A.; Dutta, S.M.; Fesshaye, A.S.; Russell, W.K.; Duncan, V.D.; Britten, R.A. Space Radiation-Induced Alterations in the Hippocampal Ubiquitin-Proteome System. Int. J. Mol. Sci. 2021, 22, 7713. [Google Scholar] [CrossRef]
- Blaber, E.; Marcal, H.; Burns, B.P. Bioastronautics: The influence of microgravity on astronaut health. Astrobiology 2010, 10, 463–473. [Google Scholar] [CrossRef]
- Bondar, R.L. Astronaut aids health research. Interview by Marilyn Rubin and Sarah Gueldner. Reflections 1995, 21, 12. [Google Scholar]
- Chancellor, J.C.; Scott, G.B.; Sutton, J.P. Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life 2014, 4, 491–510. [Google Scholar] [CrossRef]
- Green, D.A. SpaceX must share astronaut health data to boost space biomedicine. Nature 2024, 634, 782. [Google Scholar] [CrossRef] [PubMed]
- Hamm, P.B.; Nicogossian, A.E.; Pool, S.L.; Wear, M.L.; Billica, R.D. Design and current status of the longitudinal study of astronaut health. Aviat. Space Environ. Med. 2000, 71, 564–570. [Google Scholar] [PubMed]
- Oluwafemi, F.A.; Abdelbaki, R.; Lai, J.C.; Mora-Almanza, J.G.; Afolayan, E.M. A review of astronaut mental health in manned missions: Potential interventions for cognitive and mental health challenges. Life Sci. Space Res. 2021, 28, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.E.; Pepper, L.J.; Hamm, P.B.; Gilbert, S.L. Longitudinal study of astronaut health: Mortality in the years 1959–1991. Radiat. Res. 1993, 133, 257–264. [Google Scholar] [CrossRef]
- Smith, K.; Mercuri, J. Microgravity and Radiation Effects on Astronaut Intervertebral Disc Health. Aerosp. Med. Hum. Perform. 2021, 92, 342–352. [Google Scholar] [CrossRef]
- Vafapour, H.; Rafiepour, P.; Moradgholi, J.; Mortazavi, S. Evaluating the biological impact of shelters on astronaut health during different solar particle events: A Geant4-DNA simulation study. Radiat. Environ. Biophys. 2025, 1–14. [Google Scholar] [CrossRef]
- Waisberg, E.; Ong, J.; Masalkhi, M.; Cools, B.; Lee, R.; Vinken, M.; Lee, A.G. Hepatotoxicity in spaceflight: Intrinsic and extrinsic risks for astronaut metabolic health. Ir. J. Med. Sci. 2025, 1–4. [Google Scholar] [CrossRef]
- Kiffer, F.; Carr, H.; Groves, T.; Anderson, J.E.; Alexander, T.; Wang, J.; Seawright, J.W.; Sridharan, V.; Carter, G.; Boerma, M.; et al. Effects of (1)H + (16)O Charged Particle Irradiation on Short-Term Memory and Hippocampal Physiology in a Murine Model. Radiat. Res. 2018, 189, 53–63. [Google Scholar] [CrossRef]
- Chatterjee, S.; Pietrofesa, R.A.; Park, K.; Tao, J.Q.; Carabe-Fernandez, A.; Berman, A.T.; Koumenis, C.; Sielecki, T.; Christofidou-Solomidou, M. LGM2605 Reduces Space Radiation-Induced NLRP3 Inflammasome Activation and Damage in In Vitro Lung Vascular Networks. Int. J. Mol. Sci. 2019, 20, 176. [Google Scholar] [CrossRef]
- Choi, S.Y.; Saravia-Butler, A.; Shirazi-Fard, Y.; Leveson-Gower, D.; Stodieck, L.S.; Cadena, S.M.; Beegle, J.; Solis, S.; Ronca, A.; Globus, R.K. Validation of a New Rodent Experimental System to Investigate Consequences of Long Duration Space Habitation. Sci. Rep. 2020, 10, 2336. [Google Scholar] [CrossRef]
- Holley, J.M.; Stanbouly, S.; Pecaut, M.J.; Willey, J.S.; Delp, M.; Mao, X.W. Characterization of gene expression profiles in the mouse brain after 35 days of spaceflight mission. NPJ Microgravity 2022, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Aboghazleh, R.; Boyajian, S.D.; Atiyat, A.; Udwan, M.; Al-Helalat, M.; Al-Rashaideh, R. Rodent brain extraction and dissection: A comprehensive approach. MethodsX 2024, 12, 102516. [Google Scholar] [CrossRef] [PubMed]
- Meyerhoff, J.; Muhie, S.; Chakraborty, N.; Naidu, L.; Sowe, B.; Hammamieh, R.; Jett, M.; Gautam, A. Microdissection of Mouse Brain into Functionally and Anatomically Different Regions. J. Vis. Exp. 2021, 168, e61941. [Google Scholar] [CrossRef]
- Franklin, K.B. The Mouse Brain: In Stereotaxic Coordinates, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Cyr, B.; de Rivero Vaccari, J.P. Methods to Study Inflammasome Activation in the Central Nervous System: Immunoblotting and Immunohistochemistry. Methods Mol. Biol. 2023, 2696, 223–238. [Google Scholar] [CrossRef]
- Scott, X.O.; Chen, S.H.; Hadad, R.; Yavagal, D.; Peterson, E.C.; Starke, R.M.; Dietrich, W.D.; Keane, R.W.; de Rivero Vaccari, J.P. Cohort study on the differential expression of inflammatory and angiogenic factors in thrombi, cerebral and peripheral plasma following acute large vessel occlusion stroke. J. Cereb. Blood Flow Metab. 2022, 42, 1827–1839. [Google Scholar] [CrossRef]
- Cyr, B.; de Rivero Vaccari, J.P. Sex Differences in the Inflammatory Profile in the Brain of Young and Aged Mice. Cells 2023, 12, 1372. [Google Scholar] [CrossRef]
- Crucian, B.; Stowe, R.P.; Mehta, S.; Quiriarte, H.; Pierson, D.; Sams, C. Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity 2015, 1, 15013. [Google Scholar] [CrossRef]
- Baqai, F.P.; Gridley, D.S.; Slater, J.M.; Luo-Owen, X.; Stodieck, L.S.; Ferguson, V.; Chapes, S.K.; Pecaut, M.J. Effects of spaceflight on innate immune function and antioxidant gene expression. J. Appl. Physiol. 2009, 106, 1935–1942. [Google Scholar] [CrossRef]
- Carregosa, D.; Loncarevic-Vasiljkovic, N.; Feliciano, R.; Moura-Louro, D.; Mendes, C.S.; Dos Santos, C.N. Locomotor and gait changes in the LPS model of neuroinflammation are correlated with inflammatory cytokines in blood and brain. J. Inflamm. 2024, 21, 39. [Google Scholar] [CrossRef]
- Yang, J.; Ran, M.; Li, H.; Lin, Y.; Ma, K.; Yang, Y.; Fu, X.; Yang, S. New insight into neurological degeneration: Inflammatory cytokines and blood-brain barrier. Front. Mol. Neurosci. 2022, 15, 1013933. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Q.; Liu, S.; Wang, L.; Yu, M.; Lu, X.; Yang, L.; Lei, W.; Chen, G. Association of inflammatory cytokines with magnetic resonance imaging features of the brain in patients with depression. Brain Res. Bull. 2024, 219, 111131. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.P.; Iwaniec, U.T.; Turner, R.T. Evidence for increased thermogenesis in female C57BL/6J mice housed aboard the international space station. NPJ Microgravity 2021, 7, 23. [Google Scholar] [CrossRef]
- Beheshti, A.; Chakravarty, K.; Fogle, H.; Fazelinia, H.; Silveira, W.A.D.; Boyko, V.; Polo, S.L.; Saravia-Butler, A.M.; Hardiman, G.; Taylor, D.; et al. Multi-omics analysis of multiple missions to space reveal a theme of lipid dysregulation in mouse liver. Sci. Rep. 2019, 9, 19195. [Google Scholar] [CrossRef]
- Li, K.; Desai, R.; Scott, R.T.; Steele, J.R.; Machado, M.; Demharter, S.; Hoarfrost, A.; Braun, J.L.; Fajardo, V.A.; Sanders, L.M.; et al. Explainable machine learning identifies multi-omics signatures of muscle response to spaceflight in mice. NPJ Microgravity 2023, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Vitry, G.; Finch, R.; McStay, G.; Behesti, A.; Dejean, S.; Larose, T.; Wotring, V.; da Silveira, W.A. Muscle atrophy phenotype gene expression during spaceflight is linked to a metabolic crosstalk in both the liver and the muscle in mice. iScience 2022, 25, 105213. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Bhopale, V.M.; Hu, J.; Yang, M. Increased carbon dioxide levels stimulate neutrophils to produce microparticles and activate the nucleotide-binding domain-like receptor 3 inflammasome. Free Radic. Biol. Med. 2017, 106, 406–416. [Google Scholar] [CrossRef]
- Moyer, E.L.; Dumars, P.M.; Sun, G.S.; Martin, K.J.; Heathcote, D.G.; Boyle, R.D.; Skidmore, M.G. Evaluation of rodent spaceflight in the NASA animal enclosure module for an extended operational period (up to 35 days). NPJ Microgravity 2016, 2, 16002. [Google Scholar] [CrossRef]
- Stoecklein, V.M.; Osuka, A.; Ishikawa, S.; Lederer, M.R.; Wanke-Jellinek, L.; Lederer, J.A. Radiation exposure induces inflammasome pathway activation in immune cells. J. Immunol. 2015, 194, 1178–1189. [Google Scholar] [CrossRef]
- Feldmeyer, L.; Keller, M.; Niklaus, G.; Hohl, D.; Werner, S.; Beer, H.D. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr. Biol. 2007, 17, 1140–1145. [Google Scholar] [CrossRef]
- Tungjai, M.; Whorton, E.B.; Rithidech, K.N. Persistence of apoptosis and inflammatory responses in the heart and bone marrow of mice following whole-body exposure to (2)(8)Silicon ((2)(8)Si) ions. Radiat. Environ. Biophys. 2013, 52, 339–350. [Google Scholar] [CrossRef]
- Yan, X.; Sasi, S.P.; Gee, H.; Lee, J.; Yang, Y.; Mehrzad, R.; Onufrak, J.; Song, J.; Enderling, H.; Agarwal, A.; et al. Cardiovascular risks associated with low dose ionizing particle radiation. PLoS ONE 2014, 9, e110269. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, C.; Hassler, D.M.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S.; Weigle, G.; Bottcher, S.; Bohm, E.; et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, H.; Liu, Z.; Lin, L.; Wang, L.; Xie, M.; Li, D.; Zhang, J.; Zhang, R. Endoplasmic reticulum stress-dependent activation of iNOS/NO-NF-kappaB signaling and NLRP3 inflammasome contributes to endothelial inflammation and apoptosis associated with microgravity. FASEB J. 2020, 34, 10835–10849. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Niu, M.; Sun, T.; Li, M.; Jiang, X.; Duan, H.; Zhang, T.; Zhang, J.; Xie, F.; Song, R.; et al. X-ray irradiation reduces ATP-dependent activation of NLRP3 inflammasome by inhibiting TWIK2 activity in macrophages. Immunol. Lett. 2024, 272, 106967. [Google Scholar] [CrossRef]
- Chen, Z.; Stanbouly, S.; Nishiyama, N.C.; Chen, X.; Delp, M.D.; Qiu, H.; Mao, X.W.; Wang, C. Spaceflight decelerates the epigenetic clock orchestrated with a global alteration in DNA methylome and transcriptome in the mouse retina. Precis. Clin. Med. 2021, 4, 93–108. [Google Scholar] [CrossRef]
- Emam, A.; Wu, X.; Xu, S.; Wang, L.; Liu, S.; Wang, B. Stalled replication fork protection limits cGAS-STING and P-body-dependent innate immune signalling. Nat. Cell Biol. 2022, 24, 1154–1164. [Google Scholar] [CrossRef]
- Bellone, J.A.; Gifford, P.S.; Nishiyama, N.C.; Hartman, R.E.; Mao, X.W. Long-term effects of simulated microgravity and/or chronic exposure to low-dose gamma radiation on behavior and blood-brain barrier integrity. NPJ Microgravity 2016, 2, 16019. [Google Scholar] [CrossRef]
- Pani, G.; Verslegers, M.; Quintens, R.; Samari, N.; de Saint-Georges, L.; van Oostveldt, P.; Baatout, S.; Benotmane, M.A. Combined Exposure to Simulated Microgravity and Acute or Chronic Radiation Reduces Neuronal Network Integrity and Survival. PLoS ONE 2016, 11, e0155260. [Google Scholar] [CrossRef]
- Radstake, W.E.; Gautam, K.; Miranda, S.; Vermeesen, R.; Tabury, K.; Rehnberg, E.; Buset, J.; Janssen, A.; Leysen, L.; Neefs, M.; et al. The Effects of Combined Exposure to Simulated Microgravity, Ionizing Radiation, and Cortisol on the In Vitro Wound Healing Process. Cells 2023, 12, 246. [Google Scholar] [CrossRef]
- Sanzari, J.K.; Romero-Weaver, A.L.; James, G.; Krigsfeld, G.; Lin, L.; Diffenderfer, E.S.; Kennedy, A.R. Leukocyte activity is altered in a ground based murine model of microgravity and proton radiation exposure. PLoS ONE 2013, 8, e71757. [Google Scholar] [CrossRef]
- Shanmugarajan, S.; Zhang, Y.; Moreno-Villanueva, M.; Clanton, R.; Rohde, L.H.; Ramesh, G.T.; Sibonga, J.D.; Wu, H. Combined Effects of Simulated Microgravity and Radiation Exposure on Osteoclast Cell Fusion. Int. J. Mol. Sci. 2017, 18, 2443. [Google Scholar] [CrossRef] [PubMed]
- Yamanouchi, S.; Rhone, J.; Mao, J.H.; Fujiwara, K.; Saganti, P.B.; Takahashi, A.; Hada, M. Simultaneous Exposure of Cultured Human Lymphoblastic Cells to Simulated Microgravity and Radiation Increases Chromosome Aberrations. Life 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.; Olson, M.; Mitchell, C.; Juran, C.M.; Paul, A.M. The Impact of Microgravity on Immunological States. Immunohorizons 2023, 7, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Spatz, J.M.; Fulford, M.H.; Tsai, A.; Gaudilliere, D.; Hedou, J.; Ganio, E.; Angst, M.; Aghaeepour, N.; Gaudilliere, B. Human immune system adaptations to simulated microgravity revealed by single-cell mass cytometry. Sci. Rep. 2021, 11, 11872. [Google Scholar] [CrossRef]
- Krukowski, K.; Grue, K.; Frias, E.S.; Pietrykowski, J.; Jones, T.; Nelson, G.; Rosi, S. Female mice are protected from space radiation-induced maladaptive responses. Brain Behav. Immun. 2018, 74, 106–120. [Google Scholar] [CrossRef]
- Liu, B.; Hinshaw, R.G.; Le, K.X.; Park, M.A.; Wang, S.; Belanger, A.P.; Dubey, S.; Frost, J.L.; Shi, Q.; Holton, P.; et al. Space-like (56)Fe irradiation manifests mild, early sex-specific behavioral and neuropathological changes in wildtype and Alzheimer’s-like transgenic mice. Sci. Rep. 2019, 9, 12118. [Google Scholar] [CrossRef]
- Raber, J.; Yamazaki, J.; Torres, E.R.S.; Kirchoff, N.; Stagaman, K.; Sharpton, T.; Turker, M.S.; Kronenberg, A. Combined Effects of Three High-Energy Charged Particle Beams Important for Space Flight on Brain, Behavioral and Cognitive Endpoints in B6D2F1 Female and Male Mice. Front. Physiol. 2019, 10, 179. [Google Scholar] [CrossRef]
- Rola, R.; Fishman, K.; Baure, J.; Rosi, S.; Lamborn, K.R.; Obenaus, A.; Nelson, G.A.; Fike, J.R. Hippocampal neurogenesis and neuroinflammation after cranial irradiation with (56)Fe particles. Radiat. Res. 2008, 169, 626–632. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, U.; Hadad, R.; Rodriguez, A.A.; Saju, A.; Roy, D.; Gil, M.; Keane, R.W.; Scott, R.T.; Mao, X.W.; de Rivero Vaccari, J.P. Effects of Space Flight on Inflammasome Activation in the Brain of Mice. Cells 2025, 14, 417. https://doi.org/10.3390/cells14060417
Roy U, Hadad R, Rodriguez AA, Saju A, Roy D, Gil M, Keane RW, Scott RT, Mao XW, de Rivero Vaccari JP. Effects of Space Flight on Inflammasome Activation in the Brain of Mice. Cells. 2025; 14(6):417. https://doi.org/10.3390/cells14060417
Chicago/Turabian StyleRoy, Upal, Roey Hadad, Angel A. Rodriguez, Alen Saju, Deepa Roy, Mario Gil, Robert W. Keane, Ryan T. Scott, Xiao W. Mao, and Juan Pablo de Rivero Vaccari. 2025. "Effects of Space Flight on Inflammasome Activation in the Brain of Mice" Cells 14, no. 6: 417. https://doi.org/10.3390/cells14060417
APA StyleRoy, U., Hadad, R., Rodriguez, A. A., Saju, A., Roy, D., Gil, M., Keane, R. W., Scott, R. T., Mao, X. W., & de Rivero Vaccari, J. P. (2025). Effects of Space Flight on Inflammasome Activation in the Brain of Mice. Cells, 14(6), 417. https://doi.org/10.3390/cells14060417







