Abstract
Small extracellular vesicles (sEVs), including exosomes as a subtype, with a diameter typically less than 200 nm and originating from the endosomal system, are capable of transporting a diverse array of bioactive molecules, including proteins, nucleic acids, and lipids, thereby facilitating intercellular communication and modulating cellular functions. Vascular dementia (VaD) represents a form of cognitive impairment attributed to cerebrovascular disease, characterized by a complex and multifaceted pathophysiological mechanism. Currently, the therapeutic approach to VaD predominantly emphasizes symptom management, as no specific pharmacological treatment exists to cure the condition. Recent investigations have illuminated the significant role of sEVs in the pathogenesis of vascular dementia. This review seeks to provide a comprehensive analysis of the characteristics and functions of sEVs, with a particular focus on their involvement in vascular dementia and its underlying mechanisms. The objective is to advance the understanding of the interplays between sEVs and vascular dementia, thereby offering novel insights for future research and therapeutic strategies.
1. Introduction
Vascular dementia, a condition resulting from cerebrovascular disorders, is marked by progressive cognitive decline, neurodegeneration, and memory impairments [1]. It is prevalent among the elderly population, with its incidence rising linearly with advancing age [2]. Presently, vascular dementia is recognized as the second most prevalent form of dementia, following Alzheimer’s disease, accounting for at least 20% of cases [3]. Epidemiological research has delineated multiple risk factors associated with vascular dementia (VaD), such as hypertension, hyperlipidemia, diabetes mellitus, smoking, and cardiovascular diseases, thereby highlighting the critical need for preventive measures and early intervention strategies [4,5].
Contemporary therapeutic strategies for vascular dementia (VaD) predominantly focus on addressing vascular risk factors and enhancing cerebral blood flow [6,7]. Pharmacological treatments, including acetylcholinesterase inhibitors and memantine, have demonstrated limited effectiveness in the management of VaD [8]. Additionally, non-pharmacological interventions, such as cognitive rehabilitation and lifestyle modifications, are integral components of the treatment framework [3,9,10]. Nevertheless, these therapeutic approaches present significant limitations. Pharmacological agents aimed at alleviating cognitive symptoms in vascular dementia (VaD) frequently provide only modest benefits and are often accompanied by adverse effects. In contrast, non-pharmacological interventions, while potentially beneficial, may necessitate substantial resources and may not be feasible for all patients. Moreover, there are currently no specific disease-modifying treatments available for VaD, nor are there effective strategies to decelerate or reverse the damage associated with this condition [11]. Consequently, comprehending the pathogenesis of vascular dementia and identifying effective strategies for its prevention and treatment are of paramount importance [12].
The pathophysiological mechanisms underlying vascular dementia are intricate, encompassing the multifaceted interplay between vascular and neurodegenerative factors. Chronic cerebral hypoperfusion, small vessel disease, and microvascular injury collectively contribute to neuronal damage and white matter degeneration, thereby impairing cognitive function [13,14]. Furthermore, an increased permeability of the blood–brain barrier and the dysregulation of cerebral blood flow result in the accumulation of neurotoxic waste products, exacerbating cerebral damage [15,16]. Additionally, neuroinflammation and oxidative stress are two intertwined processes in the pathogenesis of vascular dementia [17]. Microglia and astrocyte activation in neuroinflammation triggers the release of inflammatory mediators [18]. These mediators not only directly damage neurons but also disrupt the blood–brain barrier, facilitating the entry of harmful substances and further aggravating the disease [19,20]. Meanwhile, oxidative stress, resulting from an imbalance in free radical production and scavenging, elevates intracellular free radical levels [21]. This leads to cellular damage, apoptosis, and the impairment of neuronal survival, thereby promoting neurodegeneration and worsening cognitive function [22,23]. Notably, oxidative stress can activate the neuroinflammatory response, and neuroinflammation in turn can exacerbate oxidative stress by generating more reactive oxygen species (ROS) [24]. The two processes interact with each other, forming a vicious cycle that accelerates the progression of vascular dementia [25].
Small extracellular vesicles (sEVs) have emerged as important mediators in the pathophysiology of neurodegenerative diseases, including VaD [26,27]. They can transport bioactive molecules between cells, influencing the cellular environment and disease progression [28,29]. Within the context of VaD, sEVs serve a dual role, where they act as carriers for the regulation of pathological signals and hold potential as therapeutic agents [30,31]. It should be noted that exosomes, a subset of sEVs, have often been misidentified in previous studies. When referring to vesicles with an unclear subcellular origin, it is more accurate to use the term “sEVs”. This review adheres to the updated MISEV rules to ensure the proper use of terminology.
2. Overview of Small Extracellular Vesicles
2.1. Release and Composition of sEVs
sEVs are extracellular vesicles with diameters often less than 200 nm [32]. They en-capsulate various genetic materials like mRNA, miRNA, lncRNA, DNA, along with proteins and lipids [33]. Exosomes, a subset of sEVs, are formed through a specific biogenesis process. Early endosome formation marks the beginning of exosome biogenesis [34,35]. Plasma membrane folding leads to the formation of early endosomes, which subsequently undergo a series of intracellular maturation processes to develop into late endosomes. These late endosomes eventually give rise to multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs) [34,36,37,38]. MVBs are capable of fusing with lysosomes, resulting in complete degradation. Conversely, when MVBs fuse with the plasma membrane, they release their intraluminal vesicles (ILVs) via exocytosis thus forming exosomes (Figure 1) [29,39,40]. sEVs are found in a wide range of bodily fluids, including saliva, urine, blood, cerebrospinal fluid, amniotic fluid, and breast milk [41]. The density of sEVs has been reported to vary between 1.13 and 1.19 g/mL [42]. sEVs are characterized by the presence of certain membrane proteins such as CD63, CD9, and CD81 (tetraspanins), along with Alix and TSG101, proteins related to the endosomal sorting complexes required for transport, or ESCRT, involved in the synthesis of multivesicular bodies, and the proteins HSP70 and HSP90, known as heat shock proteins. Examples of these proteins are considered sEV marker proteins due to their prevalent abundance [43,44].
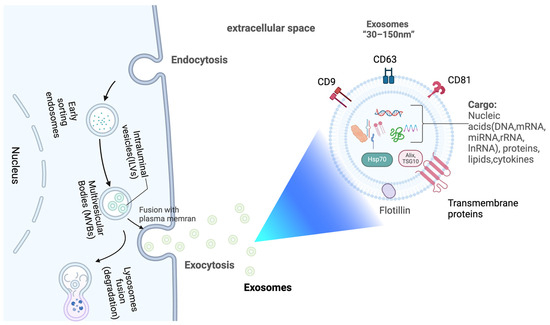
Figure 1.
Release and composition of exosomes. Created with BioRender.com.
2.2. Biological Function of sEVs
As carriers of bioactive molecules, sEVs are important for the communication between cells [34,45]. This communication fosters the exchange of information and signaling molecules, thereby regulating a myriad of physiological and pathological processes [46,47]. As conveyors of immune response, sEVs regulate immune responses by transporting immunomodulatory molecules such as cytokines, chemokines, and microRNAs to immune cells [48,49,50]. They are involved in antigen presentation, immune cell activation, and the establishment of immune tolerance, thereby influencing immune surveillance and inflammatory responses [51,52,53]. sEVs contribute to tissue repair and regeneration through different strategies, such as promoting cell multiplication, migration, and differentiation, overseeing extracellular matrix modification, lessening inflammation, and encouraging angiogenesis [54,55,56,57]. Furthermore, sEVs have gained recognition as a potential source of biomarkers for disease diagnosis, prognosis, and monitoring. These nanovesicles encapsulate a diverse array of biologically active molecules, such as proteins, nucleic acids, lipids, and metabolites, which mirror the physiological and pathological conditions of their originating cells [58,59]. Due to their distinct molecular profiles, sEVs display disease-specific attributes and can be identified and examined in various bodily fluids, offering a non-invasive approach for biomarker discovery [60,61,62].
2.3. Advantages of sEVs Compared to Source Cells
sEVs present several advantages over their parental cells in therapeutic and diagnostic applications [63]. In comparison to the original cells, sEVs exhibit enhanced safety profiles as they do not proliferate, thereby mitigating risks such as immune rejection and tumorigenesis that are often associated with cell therapy [64]. Furthermore, sEVs are devoid of cell surface antigens and maintain a natural cell membrane structure, which contributes to their low immunogenicity and diminishes the probability of eliciting immune responses upon administration [34,65]. sEVs encapsulate bioactive molecules within a lipid bilayer membrane, thereby affording protection against enzymatic degradation and environmental factors, which enhances their stability [66,67]. These vesicles can be derived from a variety of cell types, including mesenchymal stem cells [68], dendritic cells [69], and tumor cells [70], thus providing a diverse array of cellular sources for the loading of therapeutic cargo and the achievement of targeting specificity. Furthermore, sEVs are able to be perpetually produced by cells that can divide without limit, thereby ensuring a sufficient supply [71]. They are also readily storable [72], with the ability to be preserved at −80 °C for prolonged durations [66]. sEVs exhibit significant tissue penetration capabilities and can traverse the blood–brain barrier, facilitating the focused transport of medicinal compounds to specific tissues or organs [73].
3. The Role of sEVs in the Treatment of Vascular Dementia
3.1. Vascular Injury
Vascular injury constitutes a critical pathological mechanism in vascular dementia (VaD), which is characterized by damage to the cerebral blood vessels, thereby diminishing cerebral blood flow and oxygenation, ultimately culminating in cognitive deficits [74,75,76]. sEVs are integral to promoting vascular recovery, thereby contributing positively to the alleviation of vascular dementia (Figure 2) (Table 1). Research indicates that sEVs harbor potent angiogenic paracrine effectors, which facilitate the repair of ischemic tissue-related pathologies by stimulating the production of angiogenic proteins [77]. Notably, mesenchymal stem cell (MSC)-derived sEVs have been demonstrated to enhance angiogenesis through multiple pathways. These sEVs are enriched with growth factors and cytokines, comprising the epidermal growth factor (EGF), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF), which are essential for facilitating endothelial cell proliferation and migration [77]. Furthermore, sEVs contribute to neovascularization by modulating angiogenesis-related signaling pathways. Specifically, human umbilical cord blood-derived sEVs stimulate angiogenesis by triggering the PI3K/Akt and ERK1/2 signaling pathways via miR-21-3p, thereby expediting the healing of skin wounds [78]. MiR-26a, which is enriched in SHED aggregate-derived sEVs, facilitates angiogenesis in SHED and HUVEC by adjusting the TGF-β/SMAD2/3 signaling pathway [79]. MSC-derived sEVs have been shown to facilitate angiogenesis in human brain microvascular endothelial cells (HBMECs) by upregulating ICAM1 expression and activating the SMAD3 and P38MAPK signaling pathways [80]. Furthermore, the study identified that the miRNA components within sEVs may play an important part in managing angiogenesis. Ratajczak et al. demonstrated that the sEVs extracted from CD133+ cells express mRNAs of several pro-angiogenic factors, such as the kit ligand, insulin-like growth factor-1, vascular endothelial growth factor, basic fibroblast growth factor, and interleukin-8, thereby facilitating angiogenesis both in vitro and in vivo [81]. Similarly, Min Gong and colleagues verified that MSC-derived sEVs, containing pro-angiogenic microRNAs such as miR-30b, miR-30c, miR-424, and let-7f, can boost the expression of pro-angiogenic factors [82]. Furthermore, the study identified that endothelial cells require miR-214 for the secretion of sEVs, which play a role in inhibiting cellular aging and promoting angiogenesis, thereby underscoring the critical role of miRNA in the process of angiogenesis [83].
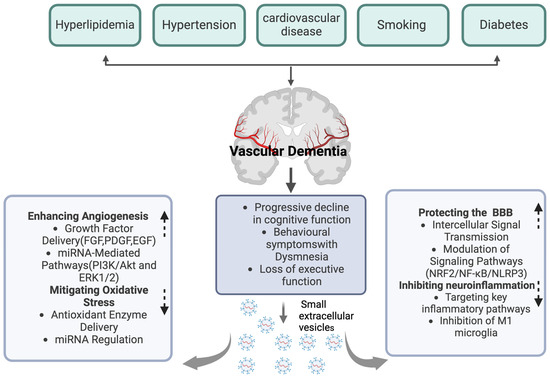
Figure 2.
The role of sEVs in the treatment of vascular dementia. Created with BioRender.com.

Table 1.
Mechanisms and Research Advances of sEVs in Alleviating Vascular Injury for the Treatment of Vascular Dementia.
3.2. Blood–Brain Barrier Dysfunction
In vascular dementia, the disruption of the blood–brain barrier (BBB) function leads to disordered metabolite transport across the BBB, thereby jeopardizing brain health and adversely affecting cognitive function [84,85]. sEVs are integral to the amelioration of BBB dysfunction (Figure 2) (Table 2). They can penetrate the BBB through several mechanisms. One primary method is receptor-mediated endocytosis [86]. sEVs express specific ligands on their surface that can bind to receptors on the endothelial cells of the BBB [87]. For instance, some sEVs carry integrin proteins, which can interact with receptors on the BBB endothelial cells, promoting the internalization of sEVs into the cells [88]. Another key mechanism involves the fusion of sEVs with the cell membrane [89]. The lipid bilayer of sEVs can directly fuse with the plasma membrane of BBB endothelial cells, releasing their contents into the cells and thus influencing BBB function [90]. Empirical evidence indicates that sEVs facilitate intercellular signal transmission by transporting specific microRNAs, thereby influencing the structural integrity and functional capacity of the BBB. For instance, research utilizing a mouse model of middle cerebral artery occlusion (MCAO) demonstrated that endothelial cells can receive considerable quantities of microRNAs, like miR-132-3p, via MSC-derived sEVs [91]. This process promotes Ras and PI3K phosphorylation through the inhibition of RASA1, thereby enhancing endothelial cell proliferation and mitigating damage to the BBB [91]. Pericyte-derived sEVs with miR-210 have been shown to enhance BBB function following spinal cord injury by modulating the JAK1/STAT3 signaling pathway, thereby underscoring the potential role of sEVsin neuroprotection [92]. Similarly, prolonged exercise-induced sEVs with miR-532-5p have been demonstrated to restore BBB function in mouse models of Alzheimer’s disease through the downregulation of EPHA4 [93]. Furthermore, the nasal administration of microglial sEVs overexpressing miRNA-124 have been found to improve BBB integrity and reduce neuronal death by modulating neuroinflammation [94]. Additionally, sEVs have the capacity to activate protective signaling pathways, thereby reinforcing BBB integrity. Research indicates that neural stem cell-derived sEVs from induced pluripotent stem cells (hiPSC–NSC–sEVs) can enhance BBB integrity and decrease leukocyte infiltration by activating the PI3K/AKT/MCP-1 signaling pathway in astrocytes thus improving neurological function following cerebral hemorrhage [95]. Moreover, sEVs possess the ability to influence the expression levels of genes related to the stability of the BBB. In a rodent model of traumatic brain injury (TBI), the administration of sEVs significantly upregulated the expression of the genes associated with BBB stability, featuring occludin, claudin-5, TJP1, RUNX1, and LAMB [91].

Table 2.
Mechanisms and Research Advances of sEVs in Alleviating BBB Dysfunction for the Treatment of Vascular Dementia.
3.3. Neuroinflammatory
Within the vascular dementia model, it is commonly observed that the stimulation of microglia and astrocytes is dysregulated, leading to an upregulation of pro-inflammatory factors and impaired neural tissue repair [96,97,98]. sEVs are essential in modulating neuroinflammatory processes, thereby aiding in the alleviation of vascular dementia (Figure 2) (Table 3). Research indicates that sEVs have the potential to mitigate the neuroinflammation induced by M1 microglia. For instance, bone marrow mesenchymal stem cell-derived sEVs (BMSCs-sEVs) enriched with miR-146a-5p have been shown to inhibit M1 microglial polarization by downregulating the expression of NFAT5 and IRAK1 [99]. Human umbilical cord mesenchymal stem cell-derived sEVs, specifically containing miR-146a-5p, mitigate neuroinflammation mediated by microglia through the suppression of the IRAK1/TRAF6 signaling pathway [100]. Additionally, mesenchymal stem cell-derived sEVs overexpressing microRNA-223-3p alleviate cerebral ischemia/reperfusion injury by inhibiting the CysLT2R-mediated signaling pathway and reducing the pro-inflammatory response associated with microglial M1 polarization [101]. Moreover, sEVs have the capacity to target critical inflammatory signaling pathways to mitigate neuroinflammation. Notably, the NF-κB pathway is a pivotal signaling mechanism involved in the regulation of various pro-inflammatory cytokines [102,103,104]. Studies have demonstrated that, in the context of treating certain neurodegenerative diseases, sEVs can attenuate the suppression of pro-inflammatory factor release by inhibiting the NF-κB pathway, thereby diminishing the neuroinflammatory response [105,106,107,108]. For example, umbilical cord mesenchymal stem cell-derived sEVs have been shown to inhibit the NF-κB/MAPK signaling pathway and attenuate the inflammatory response thus facilitating the recovery process in spinal cord injury [105]. In an Alzheimer’s disease (AD) mouse model, bone marrow-derived mesenchymal stem cell (BM-MSC)-derived sEVs downregulate NF-κB in the astrocytes through miR-146a, thereby reducing inflammation, enhancing synaptogenesis, and ameliorating the cognitive deficits in the AD mice [106]. Bone marrow mesenchymal stromal cell-derived sEVs mitigate neuroinflammation through the inhibition of the TLR2/IRAK1/NF-κB signaling pathway, consequently enhancing the M2/M1 microglial ratio [107]. Similarly, hypoxia-preconditioned mesenchymal stem cell-derived sEVs with miR-216a-5p exert anti-inflammatory effects by downregulating the TLR4/NF-κB/PI3K/AKT signaling pathway, thereby facilitating the polarization of microglia from the M1 to the M2 phenotype [108]. Moreover, adipose stem cell-derived sEVs with miR-188-3p have the potential to confer neuroprotection by inhibiting the autophagy mediated by cyclin-dependent kinase 5 (CDK5) and the inflammation mediated by NLRP3, as demonstrated in recent studies [109].

Table 3.
Mechanisms and Research Advances of sEVs in Alleviating Neuroinflammation for the Treatment of Vascular Dementia.
3.4. Oxidative Stress
Oxidative stress is a critical factor in the pathogenesis of vascular dementia. It arises from an imbalance characterized by the excessive production or diminished scavenging capacity of free radicals [110]. This imbalance is widely recognized as a fundamental pathological mechanism underlying various neurodegenerative diseases, including Alzheimer’s disease (AD) and vascular dementia [111,112]. sEVs are crucial in modulating oxidative stress, thereby contributing positively to the amelioration of vascular dementia (Figure 2) (Table 4). Initially, sEVs mitigate oxidative stress through the regulation of specific genes and signaling pathways. For instance, research indicates that mesenchymal stem cell-derived sEVs can markedly diminish oxidative stress and neuroinflammation by modulating the NRF2/NF-κB/NLRP3 signaling pathway, consequently enhancing neurological function [113]. Human umbilical cord mesenchymal stem cell-derived sEVs (hucMSC-sEVs) have been shown to mitigate oxidative stress and apoptosis through the GPX1-mediated neutralization of hydrogen peroxide [114]. Furthermore, serum-derived sEVs containing miR-137 have the potential to influence neuronal oxidative stress in Parkinson’s disease by modifying OXR1 expression [115]. Moreover, the miRNA constituents within sEVs may play a crucial role in the regulation of oxidation-induced stress. As an illustration, mesenchymal stem cell-derived sEVs can transport specific miRNAs, such as miR-125a-5p, which are capable of inhibiting the endoplasmic reticulum stress induced by high glucose levels [116]. Hippocampal neural stem cell-derived sEVs have been shown to ameliorate the cognitive deficits in rat models of vascular dementia mediated by the miR-34b-5p/CALB1 signaling pathway, which is fundamental to modulate oxidative stress [117]. Similarly, cardiac progenitor cell-derived sEVs with a high concentration of miR-935 have demonstrated a protective effect on cardiomyocytes, safeguarding them against the apoptosis and necrosis induced by oxidative stress, thus mitigating oxidative stress-related damage to some extent [118]. Furthermore, sEVs possess the capability to mitigate oxidative stress by transporting antioxidant enzymes and other antioxidant molecules. As an example, induced pluripotent stem cell-derived sEVs have been proven to convey Necrostatin-1, which alleviates oxidative stress and mitochondrial dysfunction in heart failure by modulating the PARP1/AIFM1 axis [119]. Additionally, certain sEVs are capable of delivering antioxidants such as glutathione, thereby contributing to the reduction in reactive oxygen species levels within cells and diminishing oxidative stress-induced neuronal damage [120]. sEVs also possess the ability to mediate antioxidant effects by transporting mitochondrial NAD-dependent deacetylase sirtuin-3 (SIRT3) [121].

Table 4.
Mechanisms and Research Advances of sEVs in Reducing Oxidative Stress for the Treatment of Vascular Dementia.
4. The Potential of sEVs in the Diagnosis and Treatment of Vascular Dementia
4.1. sEVs as Diagnostic Markers
The exploration of sEVs as non-invasive biomarkers for vascular dementia represents a field of ongoing research. sEVs isolated from blood or cerebrospinal fluid (CSF) have the potential to mirror the pathological conditions of the brain and vasculature [122,123]. Alterations in the concentrations of specific sEVs components, including particular proteins or microRNAs, have been correlated with the advancement of vascular dementia. For instance, Yang et al. identified an elevation in serum sEV-associated miR-135a levels and a reduction in miR-193b levels in patients diagnosed with vascular dementia [124]. Zhao et al. observed an elevation in plasma sEV-miRNA-223-3p levels among patients diagnosed with cerebral small vessel disease, noting a significant upregulation of miRNA-223-3p expression concomitant with the onset of cognitive impairment [125]. Additionally, another study reported a marked reduction in the serum sEV-associated miR-23a, miR-29a, and miR-130b levels among the subjects with vascular dementia compared to the healthy controls [126]. In patients with vascular dementia, the circulating sEV-associated miRNA-154-5p was found to be upregulated, a finding that was corroborated in a rat model of vascular dementia caused by vascular occlusion. This upregulation of miR-154-5p was observed to significantly impair endothelial progenitor cell (EPC) function and inhibit angiogenesis in the vascular dementia rat model [12]. Furthermore, Elahi and his colleagues discovered that, in comparison to individuals without white matter hyperintensity, the concentrations of certain proteins, including LAT1 and GLUT1, were significantly elevated in the plasma sEVs of patients suffering from cSVD or endothelial disease [127]. Subsequent research has demonstrated that sEVs are detectable in various body fluids, including blood, urine, and cerebrospinal fluid, thereby offering the potential for their use as non-invasive diagnostic tools [128]. In the context of Alzheimer’s disease research, sEVs have been employed to identify early molecular alterations, a technique that could similarly be applied to the early diagnosis of vascular dementia [129]. Overall, sEVs exhibit significant promise as diagnostic markers for vascular dementia. Future investigations should aim to identify specific sEV markers across different dementia types to facilitate more precise diagnoses and personalized therapeutic approaches.
4.2. sEVs as Vehicles for Drug Delivery
sEVs represent a promising medication delivery method for the administration of therapeutic agents and pharmaceuticals [130]. Their intrinsic nano-scale dimensions and membrane-encapsulated architecture confer protection against drug degradation and metabolism, thereby enhancing the stability and targeting efficiency of drugs in vivo [131,132,133]. Furthermore, the sEV surface can be modified and functionalized to facilitate the targeted delivery and controlled release of drugs, thereby augmenting therapeutic efficacy [134,135,136]. sEVs present numerous advantages as drug delivery vehicles. Firstly, they possess the capability to efficiently encapsulate and safeguard pharmaceutical agents from degradation or metabolic processes within the body, thereby enhancing the stability and bioavailability of these drugs [137,138]. Secondly, the nano-scale dimensions and membrane-encapsulated architecture of sEVs support the delivery of drugs to designated cells or tissues via mechanisms such as cell membrane fusion or receptor-mediated endocytosis [139,140]. This targeted approach has the potential to elevate the local concentration of the drug while minimizing the adverse effects on non-target tissues [139,141,142]. Furthermore, sEVs have the capability to modulate the physiological functions and signaling pathways of cells through interactions with the target cells, thereby enhancing the therapeutic efficacy [143,144]. Applying sEVs in the management of neurological diseases has demonstrated significant potential. Research indicates that sEVs are capable of traversing the blood–brain barrier to deliver therapeutic agents to the central nervous system, thereby ameliorating the symptoms associated with neurodegenerative conditions such as Alzheimer’s disease [145,146]. Furthermore, sEVs serve as effective carriers for anti-inflammatory drugs in the management of neuroinflammation-related disorders [147]. In the context of vascular dementia, sEVs can mitigate the neuronal damage induced by cerebrovascular disease through the delivery of antioxidants or anti-inflammatory molecules. Studies have revealed that sEVs confer neuroprotective effects by modulating neuronal survival and function [148,149]. Additionally, sEVs can influence the pathological processes of vascular dementia by transporting specific microRNAs or proteins [12]. In summary, sEVs hold considerable promise as drug delivery vehicles in the treatment of vascular dementia. Future investigations should aim to elucidate the precise mechanisms and application strategies of sEVs across various nervous system disorders to facilitate the advancement of more efficacious remedial interventions.
5. Challenges and Limitations of sEV-Based Therapies
5.1. Standardization and Normalization Issues
As a novel therapeutic modality, sEVs confront challenges in the standardization and regularization of their clinical applications. In the intricate processes of sEV isolation, purification, and quality control, the lack of a standardized framework constitutes a remarkable impediment [150]. sEVs are commonly isolated from diverse biological samples, such as cell culture supernatants, blood, and other bodily fluids [151]. Currently, a wide variety of isolation techniques are available, including ultracentrifugation, size-exclusion chromatography, and immunoaffinity-based methods [152]. For example, ultracentrifugation, a widely used technique, is both time-consuming and may cause damage to sEVs due to the high-speed centrifugal forces [153,154]. Size-exclusion chromatography can yield relatively pure sEVs but demands sophisticated equipment and careful calibration [155,156]. Apart from isolation, the purification of sEVs also lacks standardization. After isolation, subsequent purification steps are essential for eliminating residual contaminants. Some laboratories may adopt additional filtration steps, like using a 0.22 μm filter to remove larger particles [157], while others may rely on precipitation methods, such as using polyethylene glycol to precipitate sEVs [158]. These different treatment approaches result in variances in the purity levels of sEV preparations, thereby influencing the interpretation of experimental results. The quality control measures for sEVs remain in a state of disorder. Well-established, standardized criteria for assessing the quality of sEV preparations have not been established yet. Parameters such as sEV concentration (which can be measured by nanoparticle tracking analysis), size distribution, and the presence or absence of contaminants should be precisely defined and measured [159,160,161]. Without standardized quality control, it is extremely arduous to compare the results of different studies. In summary, the establishment of unified standards for sEV isolation, purification, and quality control is of crucial importance for promoting the clinical translation of sEV-based therapies. Although the International Society for Extracellular Vesicles (ISEV) has made efforts in formulating some general guidelines, much more work is required to fully standardize these aspects of sEV research [162].
5.2. Production and Storage Challenges
The development of large-scale and highly efficient production and storage technologies for small extracellular vesicles represents a significant challenge in their therapeutic applications. Currently, the preparation methods of small extracellular vesicles (sEVs) are faced with the limitations of high cost and low yield [163]. Ultracentrifugation is a widely used technique. Although it can obtain sEVs, this method is time-consuming, requires expensive equipment, and has a relatively low yield [164]. To address the bottlenecks in large-scale production, researchers are exploring a variety of innovative strategies. One approach is the use of microfluidic devices. These devices have the potential to achieve the continuous and high-throughput production of sEVs and can better control the separation process [165]. By precisely controlling the liquid flow in the microchannels, sEVs can be effectively separated from complex biological mixtures [166]. Moreover, compared with traditional methods, microfluidic systems require less starting material, making them more cost-effective in large-scale production [35]. Another approach is to engineer the extracellular environment to enhance the secretion and function of small extracellular vesicles [167]. For instance, research has demonstrated that magnetic iron oxide nanoparticles can enhance the production of sEVs by upregulating the genes associated with the transportation and secretion of sEVs [168]. Meanwhile, the application of 3D cell culture systems is also being explored. These systems can better mimic the in vivo microenvironment, promoting cell growth and sEV production [169]. They have the potential to improve the yield of sEVs while maintaining their quality and function [170]. Furthermore, the issues regarding the storage and stability of sEVs also need to be addressed. Currently, the storage method at −80 °C is the most commonly adopted approach [171]. Although this extremely low temperature helps to slow down biochemical reactions and maintain the integrity of sEVs to a certain extent, it requires the use of specialized and energy-consuming equipment, such as ultra-low-temperature freezers [172]. Additionally, even under the storage condition of −80 °C, long-term storage may still affect the integrity and function of sEVs [173]. Over time, some studies have shown that the bioactive molecules associated with sEVs may gradually lose their activity thus leading to the loss of potential therapeutic effects [172]. To solve these storage-related problems, some researchers are exploring the use of cryoprotectants. These substances can be added to the sEV suspension to protect sEVs from damage during the freezing and thawing processes [174]. Another emerging method is lyophilization, which involves removing water through freeze-drying and converting sEVs into a dry and stable form [175]. This technology has the potential to extend the shelf-life of sEVs and simplify their storage and transportation. However, the use of cryoprotectants and lyophilization technology are still in the experimental stage, and a large amount of research is still needed to optimize these methods.
5.3. Regulatory and Ethical Concerns
The application of sEVs, especially those derived from stem cells or engineered for gene delivery, has given rise to a multitude of regulatory and ethical considerations that demand careful attention. On the one hand, when using sEVs derived from stem cells, there may be risks of tumorigenesis and immune rejection. Although sEVs themselves have relatively low immunogenicity, the bioactive molecules they carry may trigger an immune response [176]. In allogeneic transplantation, the recipient’s immune system may recognize the sEVs from donor stem cells as foreign substances, leading to a rejection reaction [177,178]. Additionally, if the immunomodulatory effect of sEVs is excessive, it may result in an over-suppression of the immune system, increasing the risks of infection and tumorigenesis [179]. On the other hand, engineered sEVs for gene delivery must strictly adhere to regulatory requirements to ensure their safety and efficacy. These sEVs are designed to carry genetic materials such as DNA or RNA and deliver them to specific cells to modify gene expression [180]. However, this process must undergo rigorous pre-clinical and clinical trials to verify its safety and effectiveness [181]. Meanwhile, ethical issues such as the source of cells for sEV production and potential off-target effects also need to be resolved. When using human cells as the source, strict ethical standards should be followed to ensure the informed consent of donors and the protection of their privacy [182]. The natural characteristics of sEVs may cause them to accumulate in non-target tissues or organs [183]. Researchers need to ensure that sEV-based therapies can precisely target the intended cells and minimize adverse reactions [184]. Currently, regulatory authorities are still in the process of formulating comprehensive guidelines for sEV-based therapies. More research and discussions are required to establish clear ethical and regulatory frameworks.
5.4. Delivery-Related Challenges
sEVs can be delivered through various routes, each with its own advantages and limitations. Intravenous (IV) delivery is a common and convenient method. Given that sEVs can cross the blood–brain barrier (BBB), IV delivery has the potential to reach the brain and treat vascular dementia [185]. However, it is not entirely clear if IV delivery is sufficient. After an intravenous injection, sEVs may be rapidly cleared from the circulation by the reticuloendothelial system (RES), reducing their availability to reach the target site in the brain [186,187]. To enhance the effectiveness of IV-delivered sEVs, strategies such as surface modification can be employed. For example, coating sEVs with polyethylene glycol (PEG) can increase their circulation time by preventing RES uptake [188]. Additionally, conjugating targeting ligands to the sEV surface can improve their specificity for brain-related cells, enhancing their ability to cross the BBB and reach the affected areas in the brain [189]. Another potential route is intranasal delivery. This route allows sEVs to bypass the BBB to some extent and directly reach the central nervous system through the olfactory nerve pathway [190]. Intranasal delivery has shown promise in pre-clinical studies, as it can deliver sEVs to the brain with relatively high efficiency and may reduce the systemic side effects associated with IV delivery [191,192,193]. However, challenges remain in optimizing the formulation for intranasal delivery to ensure proper absorption and distribution within the nasal cavity and subsequent transport to the brain. Local delivery methods, such as direct injection into the brain parenchyma or into the cerebrospinal fluid (CSF), can also be considered [194,195,196]. These methods can achieve high local concentrations of sEVs at the target site, but they are invasive and carry risks such as infection, tissue damage, and potential immune responses at the injection site. Therefore, the careful consideration of the benefits and risks is required when choosing a delivery route for sEV-based therapies in vascular dementia treatment.
6. Conclusions and Future Directions
sEVs present substantial potential as diagnostic biomarkers and therapeutic agents for vascular dementia. Their capacity to mirror disease status and deliver targeted therapy positions them as valuable instruments in the management of vascular dementia. However, numerous challenges, including issues related to standardization, production, storage, regulation, and delivery, need to be overcome. Future research should focus on addressing these challenges. This includes developing standardized methods for sEV isolation, purification, and quality control; exploring new production technologies to increase yields and reduce costs; studying optimal storage conditions to maintain sEV stability; establishing clear regulatory and ethical guidelines for sEV-based therapies; and further investigating effective delivery routes. Further investigation of the role and mechanisms of sEVs in vascular dementia will provide a theoretical and experimental basis for the development of novel diagnostic methods and therapeutic strategies. Ongoing research in sEV biology, alongside advancements in scientific technology, may yield more effective solutions to this complex issue. sEV therapy represents an innovative therapeutic approach aimed at preventing or decelerating the progression of vascular dementia. This strategy holds the potential to provide more efficacious treatment options for individuals afflicted with vascular dementia, thereby enhancing their quality of life.
Author Contributions
H.C., C.D. and Y.Y. conceived and designed the manuscript. Y.Y., C.D., F.A., Y.H., H.L., Y.L. (Yizhou Liu), D.H., X.C., Q.Z., J.X. and Y.L. (Yajie Li) searched the literature. Y.Y. prepared the manuscript draft. The other authors helped with the manuscript preparation. H.C. and C.D. supervised, reviewed, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Key Research and Development Project of Hubei Province, China (2022BCA028).
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
We would like to thank our colleagues for their suggestions for the preparation of the manuscript.
Conflicts of Interest
The authors declare no competing interests.
References
- Ghassab-Abdollahi, N.; Mobasseri, K.; Dehghani Ahmadabad, A.; Nadrian, H.; Mirghafourvand, M. The effects of Huperzine A on dementia and mild cognitive impairment: An overview of systematic reviews. Phytother. Res. 2021, 35, 4971–4987. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, A.; Aschwanden, D.; Passamonti, L.; Toschi, N.; Stephan, Y.; Luchetti, M.; Lee, J.H.; Sesker, A.; O’Suilleabhain, P.S.; Sutin, A.R. Is neuroticism differentially associated with risk of Alzheimer’s disease, vascular dementia, and frontotemporal dementia? J. Psychiatr. Res. 2021, 138, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef]
- Chang Wong, E.; Chang Chui, H. Vascular Cognitive Impairment and Dementia. Continuum 2022, 28, 750–780. [Google Scholar] [CrossRef]
- Rundek, T.; Tolea, M.; Ariko, T.; Fagerli, E.A.; Camargo, C.J. Vascular Cognitive Impairment (VCI). Neurotherapeutics 2022, 19, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Librizzi, D.; Cabanel, N.; Zavorotnyy, M.; Riehl, E.; Kircher, T.; Luster, M.; Hooshyar Yousefi, B. Clinical Relevance of [(18)F]Florbetaben and [(18)F]FDG PET/CT Imaging on the Management of Patients with Dementia. Molecules 2021, 26, 1282. [Google Scholar] [CrossRef]
- van der Flier, W.M.; Skoog, I.; Schneider, J.A.; Pantoni, L.; Mok, V.; Chen, C.L.H.; Scheltens, P. Vascular cognitive impairment. Nat. Rev. Dis. Primers 2018, 4, 18003. [Google Scholar] [CrossRef]
- Sinha, K.; Sun, C.; Kamari, R.; Bettermann, K. Current status and future prospects of pathophysiology-based neuroprotective drugs for the treatment of vascular dementia. Drug Discov. Today 2020, 25, 793–799. [Google Scholar] [CrossRef]
- Bahar-Fuchs, A.; Clare, L.; Woods, B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database Syst. Rev. 2013, 2013, Cd003260. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, L.; Tu, Y.; Wei, J.; Zhang, J.; Jiang, G.; Shi, Q.; Ying, H. Circulating exo-miR-154-5p regulates vascular dementia through endothelial progenitor cell-mediated angiogenesis. Front. Cell Neurosci. 2022, 16, 881175. [Google Scholar] [CrossRef]
- Smith, E.E.; Beaudin, A.E. New insights into cerebral small vessel disease and vascular cognitive impairment from MRI. Curr. Opin. Neurol. 2018, 31, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Thrippleton, M.J.; Blair, G.W.; Dickie, D.A.; Marshall, I.; Hamilton, I.; Doubal, F.N.; Chappell, F.; Wardlaw, J.M. Small vessel disease is associated with altered cerebrovascular pulsatility but not resting cerebral blood flow. J. Cereb. Blood Flow. Metab. Off. J. Int. Soc. Cereb. Blood Flow. Metab. 2020, 40, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Barry Erhardt, E.; Pesko, J.C.; Prestopnik, J.; Thompson, J.; Caprihan, A.; Rosenberg, G.A. Biomarkers identify the Binswanger type of vascular cognitive impairment. J. Cereb. Blood Flow. Metab. Off. J. Int. Soc. Cereb. Blood Flow. Metab. 2019, 39, 1602–1612. [Google Scholar] [CrossRef]
- Brown, R.; Benveniste, H.; Black, S.E.; Charpak, S.; Dichgans, M.; Joutel, A.; Nedergaard, M.; Smith, K.J.; Zlokovic, B.V.; Wardlaw, J.M. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc. Res. 2018, 114, 1462–1473. [Google Scholar] [CrossRef]
- Yang, C.; He, Y.; Ren, S.; Ding, Y.; Liu, X.; Li, X.; Sun, H.; Jiao, D.; Zhang, H.; Wang, Y.; et al. Hydrogen Attenuates Cognitive Impairment in Rat Models of Vascular Dementia by Inhibiting Oxidative Stress and NLRP3 Inflammasome Activation. Adv. Healthc. Mater. 2024, 13, e2400400. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Lee, E.C.; Hong, D.Y.; Lee, D.H.; Park, S.W.; Lee, J.Y.; Jeong, J.H.; Kim, E.Y.; Chung, H.M.; Hong, K.S.; Park, S.P.; et al. Inflammation and Rho-Associated Protein Kinase-Induced Brain Changes in Vascular Dementia. Biomedicines 2022, 10, 446. [Google Scholar] [CrossRef]
- Ritson, M.; Wheeler-Jones, C.P.D.; Stolp, H.B. Endothelial dysfunction in neurodegenerative disease: Is endothelial inflammation an overlooked druggable target? J. Neuroimmunol. 2024, 391, 578363. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. CB 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Zhou, Z.F.; Zhu, Y.G.; Wan, Z.K.; Yang, M.W.; Hong, F.F.; Yang, S.L. Pharmacological Treatment of Vascular Dementia: A Molecular Mechanism Perspective. Aging Dis. 2021, 12, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Dmytriv, T.R.; Duve, K.V.; Storey, K.B.; Lushchak, V.I. Vicious cycle of oxidative stress and neuroinflammation in pathophysiology of chronic vascular encephalopathy. Front. Physiol. 2024, 15, 1443604. [Google Scholar] [CrossRef]
- Huber, C.C.; Wang, H. Pathogenic and therapeutic role of exosomes in neurodegenerative disorders. Neural Regen. Res. 2024, 19, 75–79. [Google Scholar] [CrossRef]
- Rastogi, S.; Sharma, V.; Bharti, P.S.; Rani, K.; Modi, G.P.; Nikolajeff, F.; Kumar, S. The Evolving Landscape of Exosomes in Neurodegenerative Diseases: Exosomes Characteristics and a Promising Role in Early Diagnosis. Int. J. Mol. Sci. 2021, 22, 440. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Nouri, Z.; Barfar, A.; Perseh, S.; Motasadizadeh, H.; Maghsoudian, S.; Fatahi, Y.; Nouri, K.; Yektakasmaei, M.P.; Dinarvand, R.; Atyabi, F. Exosomes as therapeutic and drug delivery vehicle for neurodegenerative diseases. J. Nanobiotechnol. 2024, 22, 463. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, L.; Zhang, L.; Shen, C.; Wei, S.; Wang, L.; Qiu, Y.; Li, C.; Xiong, Y.; Zhang, X. Emerging role of mesenchymal stem cells-derived extracellular vesicles in vascular dementia. Front. Aging Neurosci. 2024, 16, 1329357. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Balbi, C.; Vassalli, G. Exosomes: Beyond stem cells for cardiac protection and repair. Stem Cells 2020, 38, 1387–1399. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Lin, S.; Yu, Z.; Chen, D.; Wang, Z.; Miao, J.; Li, Q.; Zhang, D.; Song, J.; Cui, D. Progress in Microfluidics-Based Exosome Separation and Detection Technologies for Diagnostic Applications. Small 2020, 16, e1903916. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. CMLS 2018, 75, 193–208. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, E.; Boing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Simons, M.; Raposo, G. Exosomes--vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Breakefield, X.O.; Frederickson, R.M.; Simpson, R.J. Gesicles: Microvesicle “cookies” for transient information transfer between cells. Mol. Ther. 2011, 19, 1574–1576. [Google Scholar] [CrossRef]
- Février, B.; Raposo, G. Exosomes: Endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 2004, 16, 415–421. [Google Scholar] [CrossRef]
- Boriachek, K.; Islam, M.N.; Möller, A.; Salomon, C.; Nguyen, N.T.; Hossain, M.S.A.; Yamauchi, Y.; Shiddiky, M.J.A. Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small 2018, 14, 1702153. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef]
- Zhang, M.; Johnson-Stephenson, T.K.; Wang, W.; Wang, Y.; Li, J.; Li, L.; Zen, K.; Chen, X.; Zhu, D. Mesenchymal stem cell-derived exosome-educated macrophages alleviate systemic lupus erythematosus by promoting efferocytosis and recruitment of IL-17(+) regulatory T cell. Stem Cell Res. Ther. 2022, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.L.; Feng, Y.; Wen, Y.; Wu, W.J.; Ni, H.F.; Li, Z.L.; Zhou, L.T.; Wang, B.; Zhang, J.D.; Crowley, S.D.; et al. Exosomal CCL2 from Tubular Epithelial Cells Is Critical for Albumin-Induced Tubulointerstitial Inflammation. J. Am. Soc. Nephrol. JASN 2018, 29, 919–935. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, T.; Zhang, C.; Ji, H.; Tong, X.; Xia, R.; Wang, W.; Ma, Z.; Shi, X. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit. Care 2021, 25, 356. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Poggio, M.; Hu, T.; Pai, C.C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell 2019, 177, 414–427.e413. [Google Scholar] [CrossRef]
- Bai, K.; Lee, C.L.; Liu, X.; Li, J.; Cao, D.; Zhang, L.; Hu, D.; Li, H.; Hou, Y.; Xu, Y.; et al. Human placental exosomes induce maternal systemic immune tolerance by reprogramming circulating monocytes. J. Nanobiotechnol. 2022, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cheng, J.; Shi, W.; Ren, B.; Zhao, F.; Shi, Y.; Yang, P.; Duan, X.; Zhang, J.; Fu, X.; et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020, 106, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Li, T.; Shen, K.; Wang, K.J.; Tian, C.; Hu, D. Adipose Mesenchymal Stem Cell Derived Exosomes Promote Keratinocytes and Fibroblasts Embedded in Collagen/Platelet-Rich Plasma Scaffold and Accelerate Wound Healing. Adv. Mater. 2023, 35, e2303642. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Hao, Z.; Zhou, P.; Wang, P.; Fang, S.; Li, L.; Xu, S.; Xia, Y. Umbilical Mesenchymal Stem Cell-Derived Exosome-Encapsulated Hydrogels Accelerate Bone Repair by Enhancing Angiogenesis. ACS Appl. Mater. Interfaces 2021, 13, 18472–18487. [Google Scholar] [CrossRef]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Xu, Y.X.; Pu, S.D.; Li, X.; Yu, Z.W.; Zhang, Y.T.; Tong, X.W.; Shan, Y.Y.; Gao, X.Y. Exosomal ncRNAs: Novel therapeutic target and biomarker for diabetic complications. Pharmacol. Res. 2022, 178, 106135. [Google Scholar] [CrossRef]
- Li, K.; Lin, Y.; Luo, Y.; Xiong, X.; Wang, L.; Durante, K.; Li, J.; Zhou, F.; Guo, Y.; Chen, S.; et al. A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: A multicenter prospective study. Mol. Cancer 2022, 21, 21. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, D.; Ren, Q.; Hu, Y.; Wang, J.; Hao, J.; Wang, H.; Zhao, X.; Wang, X.; Song, C.; et al. Identification of a Non-Invasive Urinary Exosomal Biomarker for Diabetic Nephropathy Using Data-Independent Acquisition Proteomics. Int. J. Mol. Sci. 2023, 24, 13560. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Hong, Q.; Zhou, Y.; Chen, X.; Li, L.; Wang, M.; Chen, W.; Xie, X.; Zhuang, D.; Lai, M.; et al. Circulating plasma and exosome levels of the miR-320 family as a non-invasive biomarker for methamphetamine use disorder. Front. Psychiatry 2023, 14, 1160341. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, Y.; Lai, R.C.; Tan, S.S.; Choo, A.B.; Lim, S.K. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014, 23, 1233–1244. [Google Scholar] [CrossRef]
- Lu, M.; Peng, L.; Ming, X.; Wang, X.; Cui, A.; Li, Y.; Wang, X.; Meng, D.; Sun, N.; Xiang, M.; et al. Enhanced wound healing promotion by immune response-free monkey autologous iPSCs and exosomes vs. their allogeneic counterparts. EBioMedicine 2019, 42, 443–457. [Google Scholar] [CrossRef]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Zhuang, W.Z.; Lin, Y.H.; Su, L.J.; Wu, M.S.; Jeng, H.Y.; Chang, H.C.; Huang, Y.H.; Ling, T.Y. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021, 28, 28. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tsuchiya, A.; Terai, S. The development of mesenchymal stem cell therapy in the present, and the perspective of cell-free therapy in the future. Clin. Mol. Hepatol. 2021, 27, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jiang, X.; Li, H.; Zhang, C.; Zhang, Z.; Wu, C.; Zhang, J.; Hu, J.; Zhang, J. The role of mesenchymal stem cell-derived EVs in diabetic wound healing. Front. Immunol. 2023, 14, 1136098. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Peng, Y.; Du, Y.; Yang, Z.; Qi, X. Dendritic cell derived exosomes loaded neoantigens for personalized cancer immunotherapies. J. Control Release 2023, 353, 423–433. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Faghihkhorasani, F.; Fakhr, S.S.; Moghaddam, P.R.; Yazdani, E.; Kheradmand, Z.; Rezaei-Tazangi, F.; Adelian, S.; Mobarak, H.; Hamblin, M.R.; et al. Tumor-derived exosomal non-coding RNAs as diagnostic biomarkers in cancer. Cell. Mol. Life Sci. CMLS 2022, 79, 572. [Google Scholar] [CrossRef]
- Xunian, Z.; Kalluri, R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020, 111, 3100–3110. [Google Scholar] [CrossRef]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.T.; Skupien-Rabian, B.; Chen, X.; et al. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, X.; Li, J.; Zhu, A.; Du, Y.; Zeng, W.; Guo, Y.; Di, L.; Wang, R. Immune Exosomes Loading Self-Assembled Nanomicelles Traverse the Blood-Brain Barrier for Chemo-immunotherapy against Glioblastoma. ACS Nano 2023, 17, 1464–1484. [Google Scholar] [CrossRef] [PubMed]
- Mossanen Parsi, M.; Duval, C.; Ariëns, R.A.S. Vascular Dementia and Crosstalk Between the Complement and Coagulation Systems. Front. Cardiovasc. Med. 2021, 8, 803169. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Duering, M.; Hachinski, V.; Joutel, A.; Pendlebury, S.T.; Schneider, J.A.; Dichgans, M. Vascular Cognitive Impairment and Dementia: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 3326–3344. [Google Scholar] [CrossRef]
- Smith, E.E. Clinical presentations and epidemiology of vascular dementia. Clin. Sci. 2017, 131, 1059–1068. [Google Scholar] [CrossRef]
- Anderson, J.D.; Johansson, H.J.; Graham, C.S.; Vesterlund, M.; Pham, M.T.; Bramlett, C.S.; Montgomery, E.N.; Mellema, M.S.; Bardini, R.L.; Contreras, Z.; et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells 2016, 34, 601–613. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef]
- Wu, M.; Liu, X.; Li, Z.; Huang, X.; Guo, H.; Guo, X.; Yang, X.; Li, B.; Xuan, K.; Jin, Y. SHED aggregate exosomes shuttled miR-26a promote angiogenesis in pulp regeneration via TGF-β/SMAD2/3 signalling. Cell Prolif. 2021, 54, e13074. [Google Scholar] [CrossRef]
- Xue, C.; Li, X.; Ba, L.; Zhang, M.; Yang, Y.; Gao, Y.; Sun, Z.; Han, Q.; Zhao, R.C. MSC-Derived Exosomes can Enhance the Angiogenesis of Human Brain MECs and Show Therapeutic Potential in a Mouse Model of Parkinson’s Disease. Aging Dis. 2021, 12, 1211–1222. [Google Scholar] [CrossRef]
- Ratajczak, J.; Kucia, M.; Mierzejewska, K.; Marlicz, W.; Pietrzkowski, Z.; Wojakowski, W.; Greco, N.J.; Tendera, M.; Ratajczak, M.Z. Paracrine proangiopoietic effects of human umbilical cord blood-derived purified CD133+ cells--implications for stem cell therapies in regenerative medicine. Stem Cells Dev. 2013, 22, 422–430. [Google Scholar] [CrossRef]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.S.; Ashraf, M.; Xu, M. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 2017, 8, 45200–45212. [Google Scholar] [CrossRef] [PubMed]
- van Balkom, B.W.; de Jong, O.G.; Smits, M.; Brummelman, J.; den Ouden, K.; de Bree, P.M.; van Eijndhoven, M.A.; Pegtel, D.M.; Stoorvogel, W.; Würdinger, T.; et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 2013, 121, 3997–4006, s3991-3915. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Andjelkovic, A.V.; Situ, M.; Citalan-Madrid, A.F.; Stamatovic, S.M.; Xiang, J.; Keep, R.F. Blood-Brain Barrier Dysfunction in Normal Aging and Neurodegeneration: Mechanisms, Impact, and Treatments. Stroke 2023, 54, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, K.; Kim, D.H.; Oh, B.K.; Yim, H.; Jo, S.; Choi, C. Strategies for Targeted Delivery of Exosomes to the Brain: Advantages and Challenges. Pharmaceutics 2022, 14, 672. [Google Scholar] [CrossRef]
- Kodali, M.C.; Salim, C.; Ismael, S.; Lebovitz, S.G.; Lin, G.; Liao, F.F. Characterization of exosome-mediated propagation of systemic inflammatory responses into the central nervous system. Mol. Brain 2024, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Spinelli, S.; Tripodi, D.; Corti, N.; Zocchi, E.; Bruschi, M.; Leoni, V.; Dominici, R. Roles, Functions, and Pathological Implications of Exosomes in the Central Nervous System. Int. J. Mol. Sci. 2025, 26, 1345. [Google Scholar] [CrossRef]
- Osaid, Z.; Haider, M.; Hamoudi, R.; Harati, R. Exosomes Interactions with the Blood-Brain Barrier: Implications for Cerebral Disorders and Therapeutics. Int. J. Mol. Sci. 2023, 24, 15635. [Google Scholar] [CrossRef]
- Pan, Q.; Kuang, X.; Cai, S.; Wang, X.; Du, D.; Wang, J.; Wang, Y.; Chen, Y.; Bihl, J.; Chen, Y.; et al. miR-132-3p priming enhances the effects of mesenchymal stromal cell-derived exosomes on ameliorating brain ischemic injury. Stem Cell Res. Ther. 2020, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Yi, J.; Chen, W.; Gu, J.; Miao, S.; Wang, X.; Huang, Y.; Jiang, T.; Li, Q.; Zhou, W.; et al. Pericyte-derived exosomal miR-210 improves mitochondrial function and inhibits lipid peroxidation in vascular endothelial cells after traumatic spinal cord injury by activating JAK1/STAT3 signaling pathway. J. Nanobiotechnol. 2023, 21, 452. [Google Scholar] [CrossRef]
- Liang, X.; Fa, W.; Wang, N.; Peng, Y.; Liu, C.; Zhu, M.; Tian, N.; Wang, Y.; Han, X.; Qiu, C.; et al. Exosomal miR-532-5p induced by long-term exercise rescues blood-brain barrier function in 5XFAD mice via downregulation of EPHA4. Aging Cell 2023, 22, e13748. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Ge, X.; Wang, C.; Yin, Z.; Jia, Z.; Hu, T.; Li, M.; Wang, D.; Han, Z.; Wang, L.; et al. Intranasal Delivery of Gene-Edited Microglial Exosomes Improves Neurological Outcomes after Intracerebral Hemorrhage by Regulating Neuroinflammation. Brain Sci. 2023, 13, 639. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, F.; Han, Z.; Yan, B.; Liao, P.; Yin, Z.; Ge, X.; Li, D.; Zhong, R.; Liu, Q.; et al. Human-induced pluripotent stem cell-derived neural stem cell exosomes improve blood-brain barrier function after intracerebral hemorrhage by activating astrocytes via PI3K/AKT/MCP-1 axis. Neural Regen. Res. 2025, 20, 518–532. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Zhu, Z. Vascular dementia: A microglia’s perspective. Ageing Res. Rev. 2022, 81, 101734. [Google Scholar] [CrossRef] [PubMed]
- Price, B.R.; Norris, C.M.; Sompol, P.; Wilcock, D.M. An emerging role of astrocytes in vascular contributions to cognitive impairment and dementia. J. Neurochem. 2018, 144, 644–650. [Google Scholar] [CrossRef]
- Alavez-Rubio, J.S.; Juarez-Cedillo, T. Microglia as a Possible Alternative Therapeutic for Dementia. J. Alzheimer’s Dis. Rep. 2024, 8, 43–56. [Google Scholar] [CrossRef]
- Duan, S.; Wang, F.; Cao, J.; Wang, C. Exosomes Derived from MicroRNA-146a-5p-Enriched Bone Marrow Mesenchymal Stem Cells Alleviate Intracerebral Hemorrhage by Inhibiting Neuronal Apoptosis and Microglial M1 Polarization. Drug Des. Dev. Ther. 2020, 14, 3143–3158. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, X.; Zhang, R.; Xie, Y.; Feng, Z.; Li, F.; Han, J.; Sun, H.; Ouyang, Q.; Hua, S.; et al. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging 2021, 13, 3060–3079. [Google Scholar] [CrossRef]
- Zhao, Y.; Gan, Y.; Xu, G.; Hua, K.; Liu, D. Exosomes from MSCs overexpressing microRNA-223-3p attenuate cerebral ischemia through inhibiting microglial M1 polarization mediated inflammation. Life Sci. 2020, 260, 118403. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Hanada, T.; Yoshimura, A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor. Rev. 2002, 13, 413–421. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.; Liu, J.; Li, M.; Wang, Y.; Wang, Y. Exosomes derived from umbilical cord-mesenchymal stem cells inhibit the NF-κB/MAPK signaling pathway and reduce the inflammatory response to promote recovery from spinal cord injury. J. Orthop. Surg. Res. 2024, 19, 184. [Google Scholar] [CrossRef]
- Nakano, M.; Kubota, K.; Kobayashi, E.; Chikenji, T.S.; Saito, Y.; Konari, N.; Fujimiya, M. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Sci. Rep. 2020, 10, 10772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Buller, B.A.; Zhang, Z.G.; Zhang, Y.; Lu, M.; Rosene, D.L.; Medalla, M.; Moore, T.L.; Chopp, M. Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Exp. Neurol. 2022, 347, 113895. [Google Scholar] [CrossRef]
- Liu, W.; Rong, Y.; Wang, J.; Zhou, Z.; Ge, X.; Ji, C.; Jiang, D.; Gong, F.; Li, L.; Chen, J.; et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J. Neuroinflamm. 2020, 17, 47. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xing, H.; Wang, Y.; Guo, Y. Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson’s disease. Mol. Ther. Nucleic Acids 2021, 23, 1334–1344. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Trujillo-Rangel, W.; Acuña-Vaca, S.; Padilla-Ponce, D.J.; García-Mercado, F.G.; Torres-Mendoza, B.M.; Pacheco-Moises, F.P.; Escoto-Delgadillo, M.; García-Benavides, L.; Delgado-Lara, D.L.C. Modulation of the Circadian Rhythm and Oxidative Stress as Molecular Targets to Improve Vascular Dementia: A Pharmacological Perspective. Int. J. Mol. Sci. 2024, 25, 4401. [Google Scholar] [CrossRef]
- Lyu, Y.; Meng, Z.; Hu, Y.; Jiang, B.; Yang, J.; Chen, Y.; Zhou, J.; Li, M.; Wang, H. Mechanisms of mitophagy and oxidative stress in cerebral ischemia-reperfusion, vascular dementia, and Alzheimer’s disease. Front. Mol. Neurosci. 2024, 17, 1394932. [Google Scholar]
- Che, J.; Wang, H.; Dong, J.; Wu, Y.; Zhang, H.; Fu, L.; Zhang, J. Human umbilical cord mesenchymal stem cell-derived exosomes attenuate neuroinflammation and oxidative stress through the NRF2/NF-κB/NLRP3 pathway. CNS Neurosci. Ther. 2024, 30, e14454. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Tan, Y.; Zou, S.; Zhang, H.; Mao, F.; Gong, A.; Qian, H.; Xu, W. hucMSC Exosome-Derived GPX1 Is Required for the Recovery of Hepatic Oxidant Injury. Mol. Ther. 2017, 25, 465–479. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.; Chen, L.; Jin, Y.; Zhang, G.; Lin, Z.; Du, S.; Fu, Z.; Chen, T.; Qin, Y.; et al. Serum secreted miR-137-containing exosomes affects oxidative stress of neurons by regulating OXR1 in Parkinson’s disease. Brain Res. 2019, 1722, 146331. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, S.; Lin, C.; Yang, S.; Zhang, W. Mesenchymal stem cell-derived exosomes carry miR-125a-5p to improve diabetic keratopathy by regulating endoplasmic reticulum stress. Tissue Cell 2024, 93, 102669. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Hou, X.; Jin, C.; Chen, X.; Pan, C.; Fu, H.; Song, L.; Xue, J. HNSC exosome-derived MIAT improves cognitive disorders in rats with vascular dementia via the miR-34b-5p/CALB1 axis. Am. J. Transl. Res. 2021, 13, 10075–10093. [Google Scholar]
- Aguilar, S.; García-Olloqui, P.; Amigo-Morán, L.; Torán, J.L.; López, J.A.; Albericio, G.; Abizanda, G.; Herrero, D.; Vales, Á.; Rodríguez-Diaz, S.; et al. Cardiac Progenitor Cell Exosomal miR-935 Protects against Oxidative Stress. Cells 2023, 12, 2300. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Liu, B.; Su, X.; Tian, X.; Wang, H. Unlocking cardioprotection: iPSC exosomes deliver Nec-1 to target PARP1/AIFM1 axis, alleviating HF oxidative stress and mitochondrial dysfunction. J. Transl. Med. 2024, 22, 681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, T.; Xue, Y.; Zhan, B.; Lai, Z.; Huang, W.; Peng, X.; Zhou, Y. Research progress of extracellular vesicles and exosomes derived from mesenchymal stem cells in the treatment of oxidative stress-related diseases. Front. Immunol. 2023, 14, 1238789. [Google Scholar] [CrossRef]
- Chierchia, A.; Chirico, N.; Boeri, L.; Raimondi, I.; Riva, G.A.; Raimondi, M.T.; Tunesi, M.; Giordano, C.; Forloni, G.; Albani, D. Secretome released from hydrogel-embedded adipose mesenchymal stem cells protects against the Parkinson’s disease related toxin 6-hydroxydopamine. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik e.V 2017, 121, 113–120. [Google Scholar]
- Hirschberg, Y.; Valle-Tamayo, N.; Dols-Icardo, O.; Engelborghs, S.; Buelens, B.; Vandenbroucke, R.E.; Vermeiren, Y.; Boonen, K.; Mertens, I. Proteomic comparison between non-purified cerebrospinal fluid and cerebrospinal fluid-derived extracellular vesicles from patients with Alzheimer’s, Parkinson’s and Lewy body dementia. J. Extracell. Vesicles 2023, 12, e12383. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Qiu, Q.; Zhang, H.; Chu, L.; Du, Y.; Zhang, J.; Zhou, C.; Liang, F.; Shi, S.; Wang, S.; et al. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2019, 15, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Liu, C.G.; Gao, S.C.; Zhang, Y.; Wang, P.C. The Serum Exosome Derived MicroRNA-135a, -193b, and -384 Were Potential Alzheimer’s Disease Biomarkers. Biomed. Environ. Sci. 2018, 31, 87–96. [Google Scholar]
- Zhao, W.; Sun, W.; Li, S.; Jiao, Y.; Wang, Z.; Wu, T.; Liu, P.; Tan, L.; Yin, C. Exosomal miRNA-223-3p as potential biomarkers in patients with cerebral small vessel disease cognitive impairment. Ann. Transl. Med. 2021, 9, 1781. [Google Scholar] [CrossRef]
- Barbagallo, C.; Mostile, G.; Baglieri, G.; Giunta, F.; Luca, A.; Raciti, L.; Zappia, M.; Purrello, M.; Ragusa, M.; Nicoletti, A. Specific Signatures of Serum miRNAs as Potential Biomarkers to Discriminate Clinically Similar Neurodegenerative and Vascular-Related Diseases. Cell Mol. Neurobiol. 2020, 40, 531–546. [Google Scholar] [CrossRef]
- Elahi, F.M.; Casaletto, K.B.; Altendahl, M.; Staffaroni, A.M.; Fletcher, E.; Filshtein, T.J.; Glymour, M.M.; Miller, B.L.; Hinman, J.D.; DeCarli, C.; et al. “Liquid Biopsy” of White Matter Hyperintensity in Functionally Normal Elders. Front. Aging Neurosci. 2018, 10, 343. [Google Scholar] [CrossRef]
- Street, J.M.; Barran, P.E.; Mackay, C.L.; Weidt, S.; Balmforth, C.; Walsh, T.S.; Chalmers, R.T.; Webb, D.J.; Dear, J.W. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J. Transl. Med. 2012, 10, 5. [Google Scholar] [CrossRef]
- Cano, A.; Esteban-de-Antonio, E.; Bernuz, M.; Puerta, R.; García-González, P.; de Rojas, I.; Olivé, C.; Pérez-Cordón, A.; Montrreal, L.; Núñez-Llaves, R.; et al. Plasma extracellular vesicles reveal early molecular differences in amyloid positive patients with early-onset mild cognitive impairment. J. Nanobiotechnol. 2023, 21, 54. [Google Scholar] [CrossRef]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- Ma, M.; Li, B.; Zhang, M.; Zhou, L.; Yang, F.; Ma, F.; Shao, H.; Li, Q.; Li, X.; Zhang, X. Therapeutic effects of mesenchymal stem cell-derived exosomes on retinal detachment. Exp. Eye Res. 2020, 191, 107899. [Google Scholar] [CrossRef] [PubMed]
- Yari, H.; Mikhailova, M.V.; Mardasi, M.; Jafarzadehgharehziaaddin, M.; Shahrokh, S.; Thangavelu, L.; Ahmadi, H.; Shomali, N.; Yaghoubi, Y.; Zamani, M.; et al. Emerging role of mesenchymal stromal cells (MSCs)-derived exosome in neurodegeneration-associated conditions: A groundbreaking cell-free approach. Stem Cell Res. Ther. 2022, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Liaw, W.S.; Chen, C.A.; Zhou, Q.A. Exosomes—Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. ACS Nano 2022, 16, 17802–17846. [Google Scholar] [CrossRef]
- Xia, C.; Dai, Z.; Jin, Y.; Chen, P. Emerging Antioxidant Paradigm of Mesenchymal Stem Cell-Derived Exosome Therapy. Front. Endocrinol. 2021, 12, 727272. [Google Scholar] [CrossRef]
- Iyaswamy, A.; Thakur, A.; Guan, X.J.; Krishnamoorthi, S.; Fung, T.Y.; Lu, K.; Gaurav, I.; Yang, Z.; Su, C.F.; Lau, K.F.; et al. Fe65-engineered neuronal exosomes encapsulating corynoxine-B ameliorate cognition and pathology of Alzheimer’s disease. Signal Transduct. Target. Ther. 2023, 8, 404. [Google Scholar]
- Mosquera-Heredia, M.I.; Morales, L.C.; Vidal, O.M.; Barceló, E.; Silvera-Redondo, C.; Vélez, J.I.; Garavito-Galofre, P. Exosomes: Potential Disease Biomarkers and New Therapeutic Targets. Biomedicines 2021, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Kimiz-Gebologlu, I.; Oncel, S.S. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control Release 2022, 347, 533–543. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Shao, J.; Zaro, J.; Shen, Y. Advances in Exosome-Based Drug Delivery and Tumor Targeting: From Tissue Distribution to Intracellular Fate. Int. J. Nanomed. 2020, 15, 9355–9371. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. Int. J. Mol. Sci. 2020, 21, 5840. [Google Scholar] [CrossRef] [PubMed]
- Pascua-Maestro, R.; González, E.; Lillo, C.; Ganfornina, M.D.; Falcón-Pérez, J.M.; Sanchez, D. Extracellular Vesicles Secreted by Astroglial Cells Transport Apolipoprotein D to Neurons and Mediate Neuronal Survival Upon Oxidative Stress. Front. Cell Neurosci. 2018, 12, 526. [Google Scholar] [CrossRef]
- Sayeed, N.; Sugaya, K. Exosome mediated Tom40 delivery protects against hydrogen peroxide-induced oxidative stress by regulating mitochondrial function. PLoS ONE 2022, 17, e0272511. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.D.; Ordain, N.S.; Kutscher, H.; Karki, S.; Reynolds, J.L. HIV Neuroinflammation: The Role of Exosomes in Cell Signaling, Prognostic and Diagnostic Biomarkers and Drug Delivery. Front. Cell Dev. Biol. 2021, 9, 637192. [Google Scholar] [CrossRef]
- Guo, L.; Huang, Z.; Huang, L.; Liang, J.; Wang, P.; Zhao, L.; Shi, Y. Surface-modified engineered exosomes attenuated cerebral ischemia/reperfusion injury by targeting the delivery of quercetin towards impaired neurons. J. Nanobiotechnol. 2021, 19, 141. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Li, L.; Zhang, Z.; Zhang, K.; Chu, M.; Liu, Y.; Mao, X.; Wu, D.; Xu, D.; et al. Anti-ferroptosis exosomes engineered for targeting M2 microglia to improve neurological function in ischemic stroke. J. Nanobiotechnol. 2024, 22, 291. [Google Scholar] [CrossRef]
- Vo, N.; Tran, C.; Tran, N.H.B.; Nguyen, N.T.; Nguyen, T.; Ho, D.T.K.; Nguyen, D.D.N.; Pham, T.; Nguyen, T.A.; Phan, H.T.N.; et al. A novel multi-stage enrichment workflow and comprehensive characterization for HEK293F-derived extracellular vesicles. J. Extracell. Vesicles 2024, 13, e12454. [Google Scholar] [CrossRef]
- Bano, R.; Ahmad, F.; Mohsin, M. A perspective on the isolation and characterization of extracellular vesicles from different biofluids. RSC Adv. 2021, 11, 19598–19615. [Google Scholar] [CrossRef]
- Shirejini, S.Z.; Inci, F. The Yin and Yang of exosome isolation methods: Conventional practice, microfluidics, and commercial kits. Biotechnol. Adv. 2022, 54, 107814. [Google Scholar] [CrossRef] [PubMed]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, E.; Bozbeyoglu, N.; Gursel, I.; Korkusuz, F.; Bakan Misirlioglu, F.; Korkusuz, P. Comparative analysis of magnetically activated cell sorting and ultracentrifugation methods for exosome isolation. PLoS ONE 2023, 18, e0282238. [Google Scholar] [CrossRef]
- Nishimura, H.; Hashii, N.; Yamamoto, T.; Sun, Y.; Miura, T.; Sato, Y.; Ishii-Watabe, A. Usefulness of Size-Exclusion Chromatography-Multi-Angle Light Scattering to Assess Particle Composition and Protein Impurities for Quality Control of Therapeutic Exosome Preparations. Pharmaceutics 2024, 16, 1526. [Google Scholar] [CrossRef]
- Díaz Ludovico, I.; Powell, S.M.; Many, G.; Bramer, L.; Sarkar, S.; Stratton, K.; Liu, T.; Shi, T.; Qian, W.J.; Burnum-Johnson, K.E.; et al. A fast and sensitive size-exclusion chromatography method for plasma extracellular vesicle proteomic analysis. Proteomics 2024, 24, e2400025. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhu, D.; Wang, J.; Wu, X. A highly efficient method for isolating urinary exosomes. Int. J. Mol. Med. 2019, 43, 83–90. [Google Scholar] [CrossRef]
- Ludwig, A.K.; De Miroschedji, K.; Doeppner, T.R.; Börger, V.; Ruesing, J.; Rebmann, V.; Durst, S.; Jansen, S.; Bremer, M.; Behrmann, E.; et al. Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales. J. Extracell. Vesicles 2018, 7, 1528109. [Google Scholar] [CrossRef]
- Tong, M.; Brown, O.S.; Stone, P.R.; Cree, L.M.; Chamley, L.W. Flow speed alters the apparent size and concentration of particles measured using NanoSight nanoparticle tracking analysis. Placenta 2016, 38, 29–32. [Google Scholar] [CrossRef]
- Varga, Z.; Yuana, Y.; Grootemaat, A.E.; van der Pol, E.; Gollwitzer, C.; Krumrey, M.; Nieuwland, R. Towards traceable size determination of extracellular vesicles. J. Extracell. Vesicles 2014, 3, 23298. [Google Scholar] [CrossRef]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Confounding factors in vesicle uptake studies using fluorescent lipophilic membrane dyes. J. Extracell. Vesicles 2017, 6, 1388731. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, D.; Shetty, A.K. MISEV2023 provides an updated and key reference for researchers studying the basic biology and applications of extracellular vesicles. Stem Cells Transl. Med. 2024, 13, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ai, H.; Qian, K.; Li, G.; Zhang, S.; Zou, Y.; Lei, C.; Fu, W.; Hu, S. Small extracellular vesicles purification and scale-up. Front. Immunol. 2024, 15, 1344681. [Google Scholar] [CrossRef] [PubMed]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: Yield, purity and functional potential. J. Extracell. Vesicles 2019, 8, 1560809. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, M.; Xiao, Q.; Wang, H.; Chi, C.; Lin, T.; Wang, Y.; Yi, X.; Zhu, L. Recent Advances in Microfluidic-Based Extracellular Vesicle Analysis. Micromachines 2024, 15, 630. [Google Scholar] [CrossRef]
- Saar, K.L.; Müller, T.; Charmet, J.; Challa, P.K.; Knowles, T.P.J. Enhancing the Resolution of Micro Free Flow Electrophoresis through Spatially Controlled Sample Injection. Anal. Chem. 2018, 90, 8998–9005. [Google Scholar] [CrossRef]
- Phan, J.; Kumar, P.; Hao, D.; Gao, K.; Farmer, D.; Wang, A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J. Extracell. Vesicles 2018, 7, 1522236. [Google Scholar] [CrossRef]
- Yang, X.; Yi, Z.; Liang, Y.; Tong, S. Magnetic Iron Oxide Nanoparticles Enhance Exosome Production by Upregulating Exosome Transport and Secretion Pathways. ACS Appl. Mater. Interfaces 2024, 16, 67235–67245. [Google Scholar] [CrossRef]
- Chen, W.; Wu, P.; Jin, C.; Chen, Y.; Li, C.; Qian, H. Advances in the application of extracellular vesicles derived from three-dimensional culture of stem cells. J. Nanobiotechnol. 2024, 22, 215. [Google Scholar] [CrossRef]
- Johnston, J.; Stone, T., Jr.; Wang, Y. Biomaterial-enabled 3D cell culture technologies for extracellular vesicle manufacturing. Biomater. Sci. 2023, 11, 4055–4072. [Google Scholar] [CrossRef]
- Yuan, F.; Li, Y.M.; Wang, Z. Preserving extracellular vesicles for biomedical applications: Consideration of storage stability before and after isolation. Drug Deliv. 2021, 28, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The impact of storage on extracellular vesicles: A systematic study. J. Extracell. Vesicles 2022, 11, e12162. [Google Scholar] [CrossRef]
- Cheng, Y.; Zeng, Q.; Han, Q.; Xia, W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell 2019, 10, 295–299. [Google Scholar] [CrossRef]
- Villa, F.; Quarto, R.; Tasso, R. Extracellular Vesicles as Natural, Safe and Efficient Drug Delivery Systems. Pharmaceutics 2019, 11, 557. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Richter, M.; Heinrich, E.; Koch, M.; Fuhrmann, G.; Friess, W. Enhancing the Stabilization Potential of Lyophilization for Extracellular Vesicles. Adv. Healthc. Mater. 2022, 11, e2100538. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, J.; Liu, G.; Wolfram, J. Immunogenicity of Extracellular Vesicles. Adv. Mater. 2024, 36, e2403199. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Arif, T.; Mahmood, R.; Harris, D.T. Stem Cell-Based Acellular Therapy: Insight into Biogenesis, Bioengineering and Therapeutic Applications of Exosomes. Biomolecules 2024, 14, 792. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, S.; Guo, W.Z.; Li, X.K. The Unique Immunomodulatory Properties of MSC-Derived Exosomes in Organ Transplantation. Front. Immunol. 2021, 12, 659621. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Kandimalla, R.; Wallen, M.; Tyagi, N.; Wilcher, S.; Yan, J.; Schultz, D.J.; Spencer, W.; et al. Exosome-mediated delivery of RNA and DNA for gene therapy. Cancer Lett. 2021, 505, 58–72. [Google Scholar] [CrossRef]
- Abou-El-Enein, M.; Cathomen, T.; Ivics, Z.; June, C.H.; Renner, M.; Schneider, C.K.; Bauer, G. Human Genome Editing in the Clinic: New Challenges in Regulatory Benefit-Risk Assessment. Cell Stem Cell 2017, 21, 427–430. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laventhal, N.T.; Rivkees, S.A.; Opipari, V.P. Hope vs. caution: Ethical and regulatory considerations for neonatal stem cell therapies. Pediatr. Res. 2018, 83, 557–558. [Google Scholar] [CrossRef]
- Fu, P.; Yin, S.; Cheng, H.; Xu, W.; Jiang, J. Engineered Exosomes for Drug Delivery in Cancer Therapy: A Promising Approach and Application. Curr. Drug Deliv. 2024, 21, 817–827. [Google Scholar] [CrossRef]
- Pang, J.L.; Shao, H.; Xu, X.G.; Lin, Z.W.; Chen, X.Y.; Chen, J.Y.; Mou, X.Z.; Hu, P.Y. Targeted drug delivery of engineered mesenchymal stem/stromal-cell-derived exosomes in cardiovascular disease: Recent trends and future perspectives. Front. Bioeng. Biotechnol. 2024, 12, 1363742. [Google Scholar] [CrossRef]
- Hu, G.; Xia, Y.; Zhang, J.; Chen, Y.; Yuan, J.; Niu, X.; Zhao, B.; Li, Q.; Wang, Y.; Deng, Z. ESC-sEVs Rejuvenate Senescent Hippocampal NSCs by Activating Lysosomes to Improve Cognitive Dysfunction in Vascular Dementia. Adv. Sci. 2020, 7, 1903330. [Google Scholar] [CrossRef]
- Matsumoto, A.; Takahashi, Y.; Chang, H.Y.; Wu, Y.W.; Yamamoto, A.; Ishihama, Y.; Takakura, Y. Blood concentrations of small extracellular vesicles are determined by a balance between abundant secretion and rapid clearance. J. Extracell. Vesicles 2020, 9, 1696517. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, Y.; Zeng, H.; Wang, C.; Xu, C.; Wang, Q.; Wang, H.; Li, S.; Chen, J.; Xiao, C.; et al. Mechano-boosting nanomedicine antitumour efficacy by blocking the reticuloendothelial system with stiff nanogels. Nat. Commun. 2023, 14, 1437. [Google Scholar] [CrossRef]
- Patras, L.; Ionescu, A.E.; Munteanu, C.; Hajdu, R.; Kosa, A.; Porfire, A.; Licarete, E.; Rauca, V.F.; Sesarman, A.; Luput, L.; et al. Trojan horse treatment based on PEG-coated extracellular vesicles to deliver doxorubicin to melanoma in vitro and in vivo. Cancer Biol. Ther. 2022, 23, 1–16. [Google Scholar]
- Haroon, K.; Ruan, H.; Zheng, H.; Wu, S.; Liu, Z.; Shi, X.; Tang, Y.; Yang, G.Y.; Zhang, Z. Bio-clickable, small extracellular vesicles-COCKTAIL therapy for ischemic stroke. J. Control Release 2023, 363, 585–596. [Google Scholar] [CrossRef]
- Gotoh, S.; Kawabori, M.; Fujimura, M. Intranasal administration of stem cell-derived exosomes for central nervous system diseases. Neural Regen. Res. 2024, 19, 1249–1255. [Google Scholar] [CrossRef]
- Ikeda, T.; Kawabori, M.; Zheng, Y.; Yamaguchi, S.; Gotoh, S.; Nakahara, Y.; Yoshie, E.; Fujimura, M. Intranasal Administration of Mesenchymal Stem Cell-Derived Exosome Alleviates Hypoxic-Ischemic Brain Injury. Pharmaceutics 2024, 16, 446. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Huang, M.; Zheng, M.; Dai, C.; Song, Q.; Zhang, Q.; Li, Q.; Gu, X.; Chen, H.; Jiang, G.; et al. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer’s disease. J. Control Release 2020, 327, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Deng, X.; Liu, M.; He, M.; Long, W.; Xu, Z.; Zhang, K.; Liu, T.; So, K.F.; Fu, Q.L.; et al. Intranasal delivery of BDNF-loaded small extracellular vesicles for cerebral ischemia therapy. J. Control Release 2023, 357, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ding, N.; Luo, R.; Zhang, Q.; Li, Z.; Zhao, F.; Zhang, S.; Zhang, X.; Zhou, T.; Wang, H.; et al. Exosomes from young healthy human plasma promote functional recovery from intracerebral hemorrhage via counteracting ferroptotic injury. Bioact. Mater. 2023, 27, 1–14. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhou, Y.J.; Gu, P.; Zhao, W.; Chen, H.X.; Wu, R.Y.; Zhou, L.Y.; Cui, Q.Z.; Sun, S.K.; Zhang, L.Q.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate Parkinson’s disease and neuronal damage through inhibition of microglia. Neural Regen. Res. 2023, 18, 2291–2300. [Google Scholar]
- Wang, J.; Li, L.; Zhang, Z.; Zhang, X.; Zhu, Y.; Zhang, C.; Bi, Y. Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance. Cell Metab. 2022, 34, 1264–1279.e8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).