Decreased Expression of Aquaporins as a Feature of Tubular Damage in Lupus Nephritis
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Selection and Clinical Data Collection
2.2. Immunohistochemistry Staining
2.3. Digital Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical Features
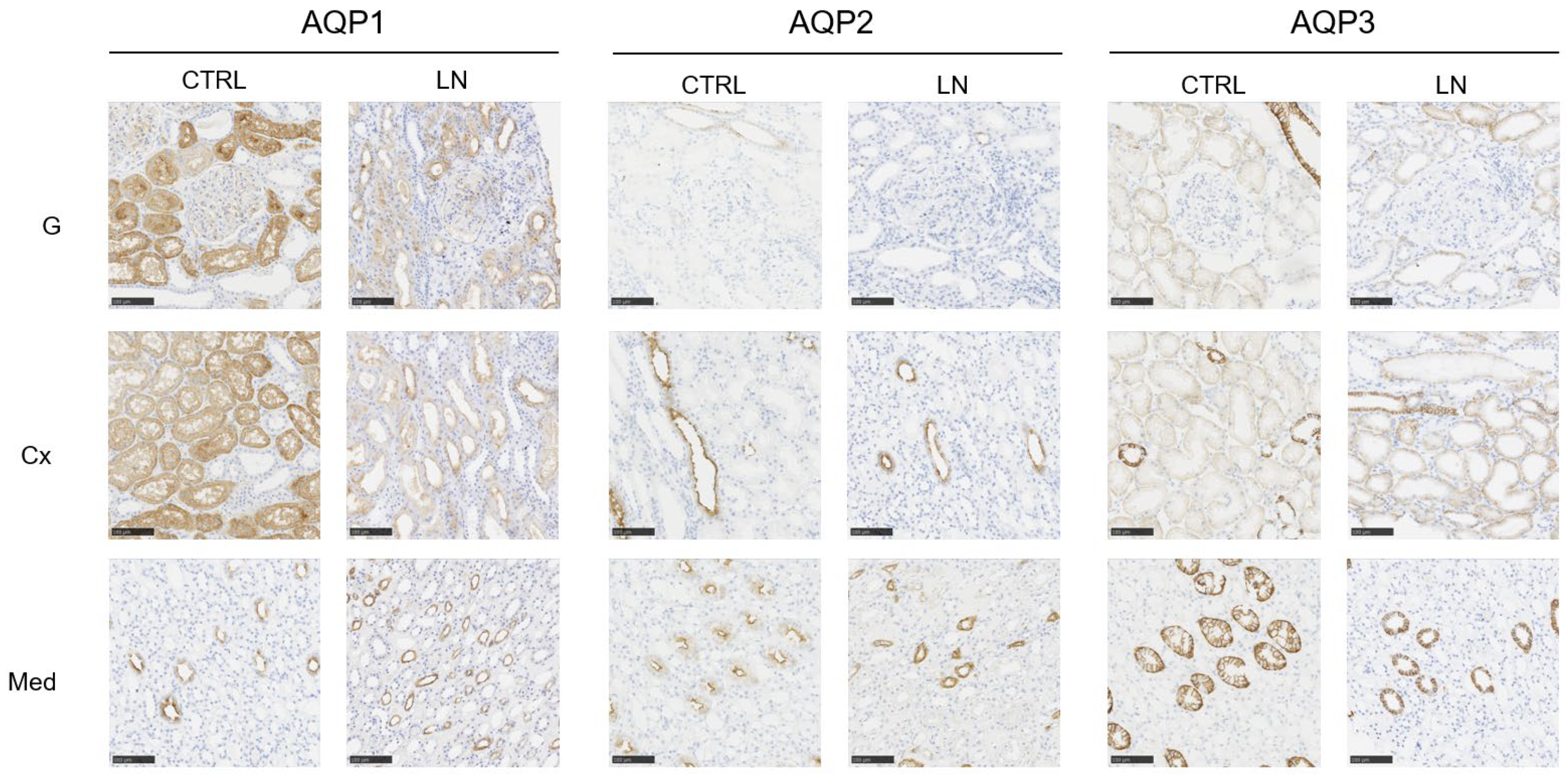
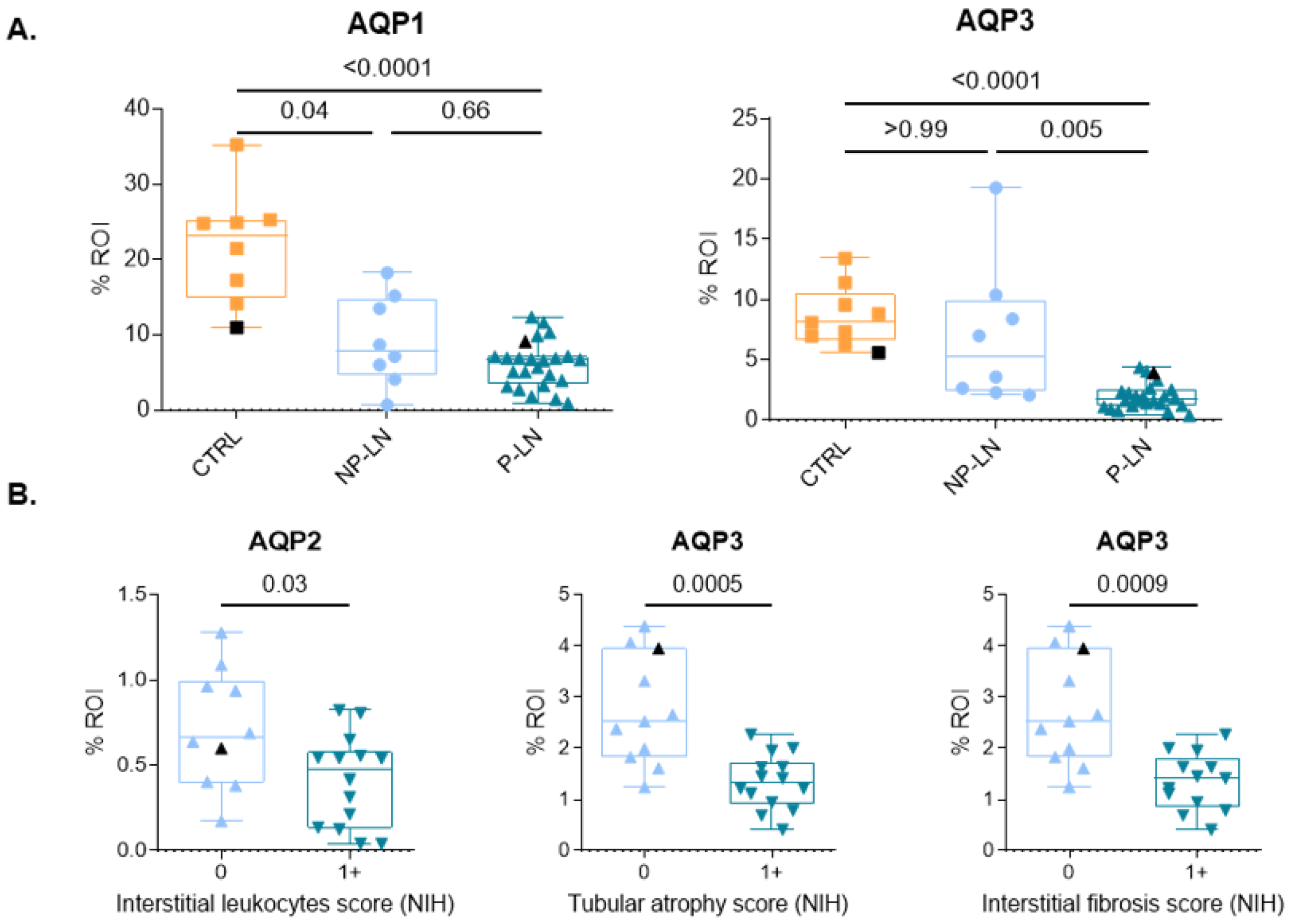
3.2. Analysis of AQP Expression
3.3. Association Between Aquaporin Expression and Tubular Damage
4. Discussion
5. Limitations and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anders, H.J.; Saxena, R.; Zhao, M.H.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus nephritis. Nat. Rev. Dis. Primers. 2020, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Fanouriakis, A.; Kostopoulou, M.; Andersen, J.; Aringer, M.; Arnaud, L.; Bae, S.-C.; Boletis, J.; Bruce, I.N.; Cervera, R.; Doria, A.; et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann. Rheum. Dis. 2023, 83, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Maria, N.I.; Davidson, A. Protecting the kidney in systemic lupus erythematosus: From diagnosis to therapy. Nat. Rev. Rheumatol. 2020, 16, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Hoi, A.; Igel, T.; Mok, C.C.; Arnaud, L. Systemic lupus erythematosus. Lancet 2024, 403, 2326–2338. [Google Scholar] [CrossRef]
- Day, R.E.; Kitchen, P.; Owen, D.S.; Bland, C.; Marshall, L.; Conner, A.C.; Bill, R.M.; Conner, M.T. Human aquaporins: Regulators of transcellular water flow. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1492–1506. [Google Scholar] [CrossRef]
- He, J.; Yang, B. Aquaporins in Renal Diseases. Int. J. Mol. Sci. 2019, 20, 366. [Google Scholar] [CrossRef]
- Su, W.; Cao, R.; Zhang, X.Y.; Guan, Y. Aquaporins in the kidney: Physiology and pathophysiology. Am. J. Physiol. Ren. Physiol. 2020, 318, F193–F203. [Google Scholar] [CrossRef]
- Madeira, A.; Fernández-Veledo, S.; Camps, M.; Zorzano, A.; Moura, T.F.; Ceperuelo-Mallafré, V.; Vendrell, J.; Soveral, G. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity 2014, 22, 2010–2017. [Google Scholar] [CrossRef]
- Qiu, Z.; Jiang, T.; Li, Y.; Wang, W.; Yang, B. Aquaporins in Urinary System. In Advances in Experimental Medicine and Biology; Yang, B., Ed.; Springer Nature: Singapore, 2023; pp. 155–177. [Google Scholar] [CrossRef]
- Missner, A.; Kügler, P.; Saparov, S.M.; Sommer, K.; Mathai, J.C.; Zeidel, M.L.; Pohl, P. Carbon Dioxide Transport through Membranes. J. Biol. Chem. 2008, 283, 25340–25347. [Google Scholar] [CrossRef]
- Pallone, T.L.; Kishore, B.K.; Nielsen, S.; Agre, P.; Knepper, M.A. Evidence that aquaporin-1 mediates NaCl-induced water flux across descending vasa recta. Am. J. Physiol. Ren. Physiol. 1997, 272, F587–F596. [Google Scholar] [CrossRef]
- Ma, T.; Yang, B.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Severely Impaired Urinary Concentrating Ability in Transgenic Mice Lacking Aquaporin-1 Water Channels. J. Biol. Chem. 1998, 273, 4296–4299. [Google Scholar] [CrossRef] [PubMed]
- Meli, R.; Pirozzi, C.; Pelagalli, A. New Perspectives on the Potential Role of Aquaporins (AQPs) in the Physiology of Inflammation. Front Physiol. 2018, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, C.; Summer, S.N.; Falk, S.; Wang, W.; Ljubanovic, D.; Schrier, R.W. Role of AQP1 in endotoxemia-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2008, 294, F1473–F1480. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, B.; Tang, K.; Dong, X.; Xue, L.; Su, G.; Jin, Y. Aquaporin 1 alleviates acute kidney injury via PI3K-mediated macrophage M2 polarization. Inflamm. Res. 2020, 69, 509–521. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Yu, G.; Liu, Q.; Jin, Y. Cytoprotective effect of aquaporin 1 against lipopolysaccharide-induced apoptosis and inflammation of renal epithelial HK-2 cells. Exp. Ther. Med. 2018, 15, 4243–4252. [Google Scholar] [CrossRef]
- Asvapromtada, S.; Sonoda, H.; Kinouchi, M.; Oshikawa, S.; Takahashi, S.; Hoshino, Y.; Sinlapadeelerdkul, T.; Yokota-Ikeda, N.; Matsuzaki, T.; Ikeda, M. Characterization of urinary exosomal release of aquaporin-1 and -2 after renal ischemia-reperfusion in rats. Am. J. Physiol. Ren. Physiol. 2018, 314, F584–F601. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Yaguchi, T.; Shimizu, K.; Kita, A.; Ishibashi, K.; Takata, K. The distribution and function of aquaporins in the kidney: Resolved and unresolved questions. Anat. Sci. Int. 2017, 92, 187–199. [Google Scholar] [CrossRef]
- Höcherl, K.; Schmidt, C.; Kurt, B.; Bucher, M. Inhibition of NF-κB ameliorates sepsis-induced downregulation of aquaporin-2/V 2 receptor expression and acute renal failure in vivo. Am. J. Physiol. Ren. Physiol. 2010, 298, F196–F204. [Google Scholar] [CrossRef]
- Chen, B.; Zang, C.; Zhang, J.; Wang, W.; Wang, J.-G.; Zhou, H.; Fu, Y. The Changes of Aquaporin 2 in the Graft of Acute Rejection Rat Renal Transplantation Model. Transplant. Proc. 2010, 42, 1884–1887. [Google Scholar] [CrossRef]
- Kanno, K.; Sasaki, S.; Hirata, Y.; Ishikawa, S.-E.; Fushimi, K.; Nakanishi, S.; Bichet, D.G.; Marumo, F. Urinary Excretion of Aquaporin-2 in Patients with Diabetes Insipidus. N. Engl. J. Med. 1995, 332, 1540–1545. [Google Scholar] [CrossRef]
- Lei, L.; Wang, W.; Jia, Y.; Su, L.; Zhou, H.; Verkman, A.S.; Yang, B. Aquaporin-3 deletion in mice results in renal collecting duct abnormalities and worsens ischemia-reperfusion injury. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.J.; Leader, J.P.; Walker, R.J. Aquaporin Expression in Normal Human Kidney and in Renal Disease. J. Am. Soc. Nephrol. 2003, 14, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Bajema, I.M.; Wilhelmus, S.; Alpers, C.E.; Bruijn, J.A.; Colvin, R.B.; Cook, H.T.; D’agati, V.D.; Ferrario, F.; Haas, M.; Jennette, J.C.; et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: Clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018, 93, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; Soyfoo, M. Aquaporins: Unexpected actors in autoimmune diseases. Autoimmun. Rev. 2022, 21, 103131. [Google Scholar] [CrossRef]
- da Silva, I.V.; Soveral, G. Aquaporins in Immune Cells and Inflammation: New Targets for Drug Development. Int. J. Mol. Sci. 2021, 22, 1845. [Google Scholar] [CrossRef]
- Preisser, L.; Teillet, L.; Aliotti, S.; Gobin, R.; Berthonaud, V.; Chevalier, J.; Corman, B.; Verbavatz, J.-M. Downregulation of aquaporin-2 and -3 in aging kidney is independent of V(2) vasopressin receptor. Am. J. Physiol. Ren. Physiol. 2000, 279, F144–F152. [Google Scholar] [CrossRef]

| Clinical Data at Diagnosis | ||
|---|---|---|
| Variable (n) | Median [Q1–Q3] | Normal Range (Unit) |
| Age (37) | 35 [28–41] | (years) |
| Female/Male ratio (37) | 36/1 | |
| Previous LN (>2 y) | 10.8% (4/37) | |
| Diabetes | 2.7% (1/37) | |
| Hypertension | 13.5% (5/37) | |
| Tobacco | 5.4% (2/37) | |
| Flare/New diagnosis (37) | 78.4% (29/37)/21.6% (8/37) | |
| SLEDAI-2k (37) | 16 [14–22] | |
| Urea (37) | 35.3 [23–62] | 17–48 (mg/dL) |
| Creatinine (37) | 0.8 [0.6–1.2] | 0.5–0.9 (mg/dL) |
| eGFR (37) | 100 [50.5–112] | >90 (mL/min/1.73 m2) |
| eGFR distribution | ||
| >90 | 51.4% (18/35) | |
| 60–90 | 17.1% (6/35) | |
| 15–60 | 25.7% (9/35) | |
| <15 | 5.7% (2/35) | |
| ESR (31) | 53 [35–77] | <20 (mm/h) |
| CRP (36) | 0.86 [0.43–6.90] | <5 (mg/L) |
| C3 (34) | 58.5 [37–76.75] | 80–164 (mg/dL) |
| C4 (35) | 8 [5.5–14.5] | 10–40 (mg/dL) |
| ANA titer (37) | 1/1280 [1/640–1/2560] | <1/160 |
| Anti-dsDNA (34) | 175 [42.25–466.5] | <30 (U/mL) |
| Anti-Sm positivity (35) | 34.3% (12/35) | |
| Antiphospholipids positivity (30) | 26.7% (8/30) | |
| Leucocyturia (30) | 64 [11.5–172] | <30 (WBC/µL) |
| Hematuria (31) | 63 [24–175.5] | <30 (RBC/µL) |
| Urine protein-to-creatinine | 2.00 [1.10–3.40] | <0.5 (g/g) |
| ratio (PCR) (37) | ||
| Nephrotic range (NR) | 21.6% (8/37) | |
| LN classification distribution (ISN/RPS 2018) | ||
| Class | ||
| I | 2.7% (1/37) | |
| II | 2.7% (1/37) | |
| III | 21.6% (8/37) | |
| IV | 40.5% (15/37) | |
| V | 21.6% (8/37) | |
| III + V | 5.4% (2/37) | |
| IV + V | 5.4% (2/37) | |
| Class III/IV NIH indices (ISN/RPS 2018) | ||
| NIH activity | 9 [6–10] | |
| NIH chronicity | 2 [1–4] | |
| Clinical data during follow-up | ||
| Variable (n) | Median [Q1–Q3] | Normal range (units) |
| PCR at 3 months (27) | 1 [0.65–1.70] | <0.5 (g/g) |
| 25% decrease at 3 m | 70.4% (19/27) | |
| PCR at 6 months | 0.49 [0.15–1.07] | <0.5 (g/g) |
| 50% decrease at 6 m | 42.3% (11/26) | |
| PCR at 12 months | 0.34 [0.10–1.04] | <0.5 (g/g) |
| <0.5 g/g | 57.7% (15/26) | |
| eGFR at 12 months | 100 [75–100] | >90 (mL/min/1.73 m2) |
| eGFR at 12 months distribution | ||
| >90 | 60% (18/30) | |
| 60–90 | 16.7% (5/30) | |
| 15–60 | 6.7% (2/30) | |
| <15 | 13.3% (4/30) | |
| Clinical Data of Controls | |
|---|---|
| Variable (n) | Median [Q1–Q3] |
| Age (8) | 55 [43–57.5] |
| Female/Male ratio (8) | 7/1 |
| Comorbidities (nephrectomy samples only) | |
| Tobacco (4) | 0% (0/4) |
| Diabetes (4) | 0% (0/4) |
| Hypertension (4) | 100% (4/4) |
| Reason for biopsy | |
| Prior to transplantation | 55.5% (5/9) |
| Nephrectomy | 44.4% (4/9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melchior, M.; Van Eycken, M.; Nicaise, C.; Duquesne, T.; Longueville, L.; Collin, A.; Decaestecker, C.; Salmon, I.; Delporte, C.; Soyfoo, M. Decreased Expression of Aquaporins as a Feature of Tubular Damage in Lupus Nephritis. Cells 2025, 14, 380. https://doi.org/10.3390/cells14050380
Melchior M, Van Eycken M, Nicaise C, Duquesne T, Longueville L, Collin A, Decaestecker C, Salmon I, Delporte C, Soyfoo M. Decreased Expression of Aquaporins as a Feature of Tubular Damage in Lupus Nephritis. Cells. 2025; 14(5):380. https://doi.org/10.3390/cells14050380
Chicago/Turabian StyleMelchior, Maxime, Marie Van Eycken, Charles Nicaise, Thomas Duquesne, Léa Longueville, Amandine Collin, Christine Decaestecker, Isabelle Salmon, Christine Delporte, and Muhammad Soyfoo. 2025. "Decreased Expression of Aquaporins as a Feature of Tubular Damage in Lupus Nephritis" Cells 14, no. 5: 380. https://doi.org/10.3390/cells14050380
APA StyleMelchior, M., Van Eycken, M., Nicaise, C., Duquesne, T., Longueville, L., Collin, A., Decaestecker, C., Salmon, I., Delporte, C., & Soyfoo, M. (2025). Decreased Expression of Aquaporins as a Feature of Tubular Damage in Lupus Nephritis. Cells, 14(5), 380. https://doi.org/10.3390/cells14050380








