Metaboloepigenetics: Role in the Regulation of Flow-Mediated Endothelial (Dys)Function and Atherosclerosis
Abstract
1. Introduction
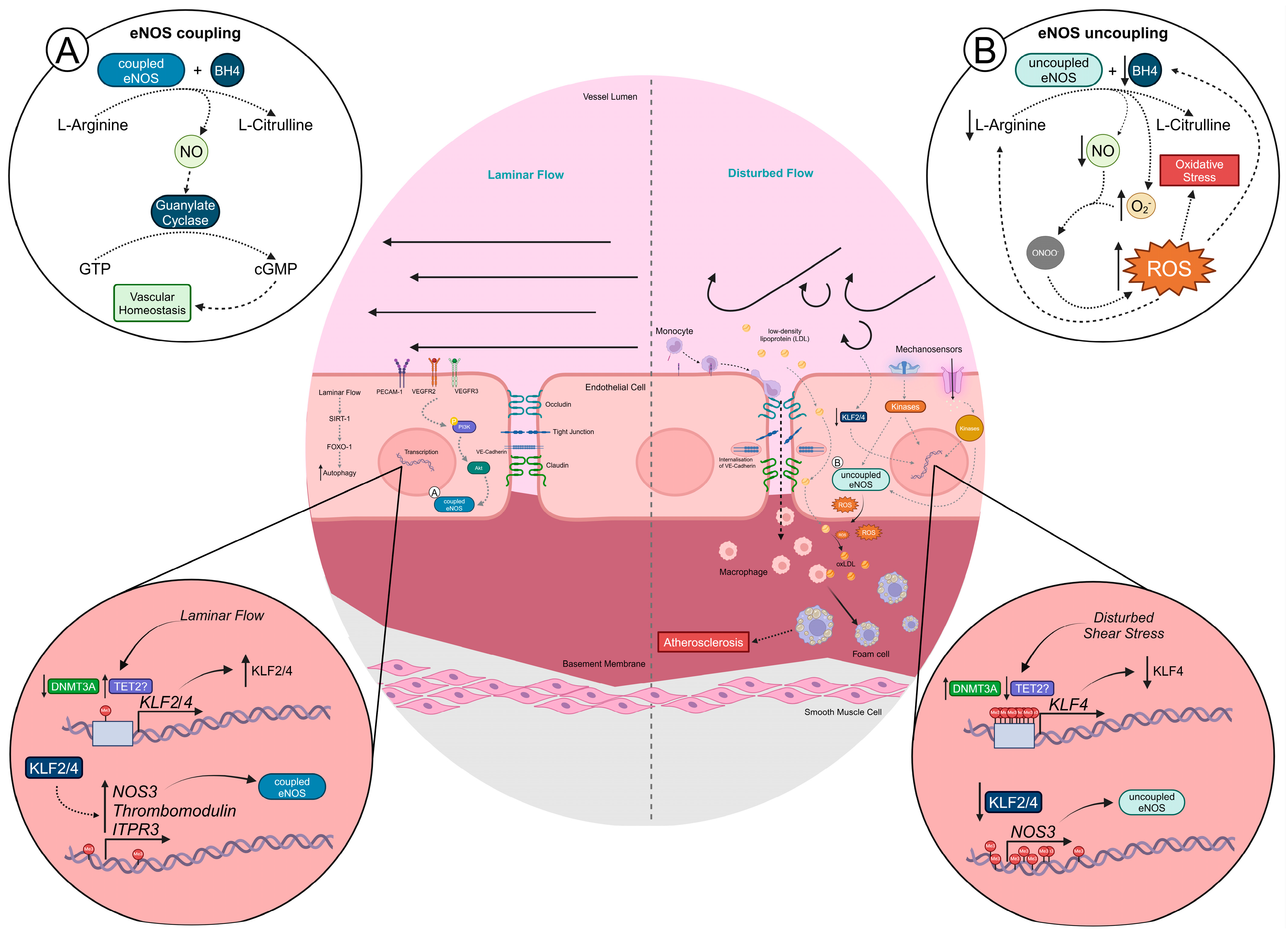
2. Flow-Mediated Endothelial (Dys)Function
3. Epigenetics and Regulation by Metabolism
4. Epigenetic Alterations Mediated by Disturbed Flow
4.1. DNA (De)Methylation
4.2. Histone PTMs
5. A Possible Role for Metaboloepigenetics in Endothelial Dysfunction
5.1. Shear Stress-Mediated EC Metabolism
5.2. Linking the EC Epigenome and Metabolism
5.2.1. Acetyl-CoA and Histone Acetylation
5.2.2. SAM and DNA/Histone Methylation
5.2.3. α-KG and DNA/Histone Demethylation
5.2.4. Lactate and Histone Lactylation
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-OGDD | 2-Oxoglutarate-dependent dioxygenase |
| 5aC | 5-Carboxycytosine |
| 5-Aza | 5-Aza-2′-deoxycytidine |
| 5fC | 5-Formylcytosine |
| 5hmC | 5-Hydroxymethylcytosine |
| 5mC | 5-Methylcysteine |
| α-KG | Alpha-ketoglutarate |
| α-SMA | Alpha smooth muscle actin |
| ACLY | ATP citrate lyase |
| ACSS | Acetyl-CoA synthetase |
| AKT | Also known as PKB (protein kinase B) |
| AP | Activator protein |
| ApoE | Apolipoprotein E |
| ATP | Adenosine triphosphate |
| CoA | Coenzyme A |
| DMR | Differentially methylated region |
| DNA | Deoxyribonucleic acid |
| DNMT | DNA methyltransferase |
| EC | Endothelial cell |
| EndMT | Endothelial–mesenchymal transition |
| eNOS | Endothelial nitric oxide synthase |
| FA | Fatty acid |
| FAK | Focal adhesion kinase |
| FAO | Fatty acid oxidation |
| FASN | Fatty acid synthase |
| HAT | Histone acetyltransferase |
| HDAC | Histone deacetylase |
| HIF | Hypoxia-inducible factor |
| HMT | Histone methyltransferase |
| HUVEC | Human umbilical vein endothelial cell |
| ICAM | Intercellular adhesion molecule |
| IDH | Isocitrate dehydrogenase |
| iPSC | Induced pluripotent stem cell |
| JHDM | Jumonji-domain-containing histone demethylase |
| JNK | c-Jun NH2-terminal kinase |
| KLF | Krüppel-like factor |
| LCA | Left common carotid artery |
| LDH | Lactate dehydrogenase |
| LDL | Low-density lipoprotein |
| Ldlr | Low-density lipoprotein receptor |
| MAPK | Mitogen-activated protein kinases |
| MAT | S-adenosylmethionine synthetase |
| MCP | Monocyte chemoattractant protein |
| MeDIP-seq | Methylated DNA immunoprecipitation sequencing |
| mTOR | Mammalian target of rapamycin |
| NAD+ | Oxidized nicotinamide adenine dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of B cells |
| NO | Nitric oxide |
| NRF | Nuclear factor erythroid |
| OXPHOS | Oxidative phosphorylation |
| PCL | Partial carotid ligation |
| PCR | Polymerase chain reaction |
| PECAM | Platelet endothelial cell adhesion molecule |
| PGC-1α | Peroxisome proliferator gamma coactivator-1α |
| PI3K | Phosphoinositide 3-kinase |
| PINK1 | Phosphatase and tensin homolog (PTEN)-induced kinase 1 |
| PKN1 | Serine/threonine-protein kinase N1 |
| PPAR | Peroxisome proliferator-activated receptor |
| PTM | Post-translational modification |
| RCA | Right carotid artery |
| ROS | Reactive oxygen species |
| SAH | S-adenosylhomocysteine |
| SAM | S-adenosylmethionine |
| SIRT | Sirtuin |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
| TCA | Tricarboxylic acid |
| TET | Ten-eleven translocation |
| TGF | Transforming growth factor |
| UTR | Untranslated region |
| VCAM | Vascular adhesion molecule |
| VE | Vascular endothelial |
| VEGFR | Vascular endothelial growth factor receptor |
| VSMC | Vascular smooth muscle cell |
| YAP | Yes-associated protein |
References
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent Developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Nedkoff, L.; Briffa, T.; Zemedikun, D.; Herrington, S.; Wright, F.L. Global Trends in Atherosclerotic Cardiovascular Disease. Clin. Ther. 2023, 45, 1087–1091. [Google Scholar] [CrossRef]
- Luca, A.C.; David, S.G.; David, A.G.; Țarcă, V.; Pădureț, I.-A.; Mîndru, D.E.; Roșu, S.T.; Roșu, E.V.; Adumitrăchioaiei, H.; Bernic, J.; et al. Atherosclerosis from Newborn to Adult—Epidemiology, Pathological Aspects, and Risk Factors. Life 2023, 13, 2056. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, D.; Birukov, K.G. Mechanosensing and Mechanoregulation of Endothelial Cell Functions. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2019; pp. 873–904. [Google Scholar] [CrossRef]
- Lim, X.R.; Harraz, O.F. Mechanosensing by Vascular Endothelium. Annu. Rev. Physiol. 2024, 86, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, I.A.; Baek, K.I.; Kim, Y.; Park, C.; Jo, H. Flow-Induced Reprogramming of Endothelial Cells in Atherosclerosis. Nat. Rev. Cardiol. 2023, 20, 738–753. [Google Scholar] [CrossRef]
- Fernández-Friera, L.; Peñalvo, J.L.; Fernández-Ortiz, A.; Ibañez, B.; López-Melgar, B.; Laclaustra, M.; Oliva, B.; Mocoroa, A.; Mendiguren, J.; Martínez de Vega, V.; et al. Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort. Circulation 2015, 131, 2104–2113. [Google Scholar] [CrossRef]
- Laclaustra, M.; Casasnovas, J.A.; Fernández-Ortiz, A.; Fuster, V.; León-Latre, M.; Jiménez-Borreguero, L.J.; Pocovi, M.; Hurtado-Roca, Y.; Ordovas, J.M.; Jarauta, E.; et al. Femoral and Carotid Subclinical Atherosclerosis Association with Risk Factors and Coronary Calcium. J. Am. Coll. Cardiol. 2016, 67, 1263–1274. [Google Scholar] [CrossRef]
- Tang, H.; Zeng, Z.; Shang, C.; Li, Q.; Liu, J. Epigenetic Regulation in Pathology of Atherosclerosis: A Novel Perspective. Front. Genet. 2021, 12, 810689. [Google Scholar] [CrossRef]
- Sum, H.; Brewer, A.C. Epigenetic Modifications as Therapeutic Targets in Atherosclerosis: A Focus on DNA Methylation and Non-Coding RNAs. Front. Cardiovasc. Med. 2023, 10, 1183181. [Google Scholar] [CrossRef]
- Xu, S.; Pelisek, J.; Jin, Z.G. Atherosclerosis Is an Epigenetic Disease. Trends Endocrinol. Metab. 2018, 29, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.W.; Paneni, F.; Jandeleit-Dahm, K.A.M. Cell-Specific Epigenetic Changes in Atherosclerosis. Clin. Sci. 2021, 135, 1165–1187. [Google Scholar] [CrossRef] [PubMed]
- Stroope, C.; Nettersheim, F.S.; Coon, B.; Finney, A.C.; Schwartz, M.A.; Ley, K.; Rom, O.; Yurdagul, A. Dysregulated Cellular Metabolism in Atherosclerosis: Mediators and Therapeutic Opportunities. Nat. Metab. 2024, 6, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.H.; Subramaniam, N.; Marsden, P.A. Epigenetic Determinants of Flow-Mediated Vascular Endothelial Gene Expression. Hypertension 2019, 74, 467–476. [Google Scholar] [CrossRef]
- Pal, S.; Tyler, J.K. Epigenetics and Aging. Sci. Adv. 2016, 2, 1600584. [Google Scholar] [CrossRef]
- Dai, Z.; Ramesh, V.; Locasale, J.W. The Evolving Metabolic Landscape of Chromatin Biology and Epigenetics. Nat. Rev. Genet. 2020, 21, 737–753. [Google Scholar] [CrossRef]
- Keating, S.T.; El-Osta, A. Metaboloepigenetics in Cancer, Immunity, and Cardiovascular Disease. Cardiovasc. Res. 2023, 119, 357–370. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Finley, L.W.S. Metabolic Signatures of Cancer Cells and Stem Cells. Nat. Metab. 2019, 1, 177–188. [Google Scholar] [CrossRef]
- Chisolm, D.A.; Weinmann, A.S. Connections Between Metabolism and Epigenetics in Programming Cellular Differentiation. Annu. Rev. Immunol. 2018, 36, 221–246. [Google Scholar] [CrossRef]
- Theodorou, K.; Boon, R.A. Endothelial Cell Metabolism in Atherosclerosis. Front. Cell Dev. Biol. 2018, 6, 82. [Google Scholar] [CrossRef]
- Michiels, C. Endothelial Cell Functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.D.; Kumar, S.; Jo, H. The Role of Endothelial Mechanosensitive Genes in Atherosclerosis and Omics Approaches. Arch. Biochem. Biophys. 2016, 591, 111–131. [Google Scholar] [CrossRef]
- Dunn, J.; Qiu, H.; Kim, S.; Jjingo, D.; Hoffman, R.; Kim, C.W.; Jang, I.; Son, D.J.; Kim, D.; Pan, C.; et al. Flow-Dependent Epigenetic DNA Methylation Regulates Endothelial Gene Expression and Atherosclerosis. J. Clin. Investig. 2014, 124, 3187–3199. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.; Ni, C.-W.; Rezvan, A.; Suo, J.; Budzyn, K.; Llanos, A.; Harrison, D.; Giddens, D.; Jo, H. Partial Carotid Ligation Is a Model of Acutely Induced Disturbed Flow, Leading to Rapid Endothelial Dysfunction and Atherosclerosis. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H1535–H1543. [Google Scholar] [CrossRef]
- Sala, F.; Aranda, J.; Rotllan, N.; Ramírez, C.; Aryal, B.; Elia, L.; Condorelli, G.; Catapano, A.L.; Fernández-Hernando, C.; Norata, G.D. MiR-143/145 Deficiency Attenuates the Progression of Atherosclerosis in Ldlr-/- Mice. Thromb. Haemost. 2014, 112, 796–802. [Google Scholar] [CrossRef]
- Berk, B.C. Atheroprotective Signaling Mechanisms Activated by Steady Laminar Flow in Endothelial Cells. Circulation 2008, 117, 1082–1089. [Google Scholar] [CrossRef]
- Abe, J.; Berk, B.C. Novel Mechanisms of Endothelial Mechanotransduction. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lu, H.; Liang, W.; Hu, W.; Zhang, J.; Chen, Y.E. Krüppel-like Factors and Vascular Wall Homeostasis. J. Mol. Cell Biol. 2017, 9, 352–363. [Google Scholar] [CrossRef]
- He, M.; Huang, T.-S.; Li, S.; Hong, H.-C.; Chen, Z.; Martin, M.; Zhou, X.; Huang, H.-Y.; Su, S.-H.; Zhang, J.; et al. Atheroprotective Flow Upregulates ITPR3 (Inositol 1,4,5-Trisphosphate Receptor 3) in Vascular Endothelium via KLF4 (Krüppel-Like Factor 4)-Mediated Histone Modifications. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 902–914. [Google Scholar] [CrossRef]
- Zhou, G.; Hamik, A.; Nayak, L.; Tian, H.; Shi, H.; Lu, Y.; Sharma, N.; Liao, X.; Hale, A.; Boerboom, L.; et al. Endothelial Kruppel-like Factor 4 Protects against Atherothrombosis in Mice. J. Clin. Investig. 2012, 122, 4727–4731. [Google Scholar] [CrossRef]
- Orsenigo, F.; Giampietro, C.; Ferrari, A.; Corada, M.; Galaup, A.; Sigismund, S.; Ristagno, G.; Maddaluno, L.; Young Koh, G.; Franco, D.; et al. Phosphorylation of VE-Cadherin Is Modulated by Haemodynamic Forces and Contributes to the Regulation of Vascular Permeability in Vivo. Nat. Commun. 2012, 3, 1208. [Google Scholar] [CrossRef]
- Caolo, V.; Peacock, H.M.; Kasaai, B.; Swennen, G.; Gordon, E.; Claesson-Welsh, L.; Post, M.J.; Verhamme, P.; Jones, E.A.V. Shear Stress and VE-Cadherin. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2174–2183. [Google Scholar] [CrossRef]
- Colgan, O.C.; Ferguson, G.; Collins, N.T.; Murphy, R.P.; Meade, G.; Cahill, P.A.; Cummins, P.M. Regulation of Bovine Brain Microvascular Endothelial Tight Junction Assembly and Barrier Function by Laminar Shear Stress. Am. J. Physiol.-Heart Circ. Physiol. 2007, 292, H3190–H3197. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, J.-Y.; Li, B.; Tian, X.Y.; Chen, L.-J.; Huang, Y.; Liu, J.; Deng, D.; Lau, C.W.; Wan, S.; et al. Integrin-YAP/TAZ-JNK Cascade Mediates Atheroprotective Effect of Unidirectional Shear Flow. Nature 2016, 540, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Matthys, K.E.; Bult, H. Nitric Oxide Function in Atherosclerosis. Mediat. Inflamm. 1997, 6, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Dzobo, K.E.; Hanford, K.M.L.; Kroon, J. Vascular Metabolism as Driver of Atherosclerosis: Linking Endothelial Metabolism to Inflammation. Immunometabolism 2021, 3, e210020. [Google Scholar] [CrossRef]
- Shaik, S.S.; Soltau, T.D.; Chaturvedi, G.; Totapally, B.; Hagood, J.S.; Andrews, W.W.; Athar, M.; Voitenok, N.N.; Killingsworth, C.R.; Patel, R.P.; et al. Low Intensity Shear Stress Increases Endothelial ELR+ CXC Chemokine Production via a Focal Adhesion Kinase-P38β MAPK-NF-ΚB Pathway. J. Biol. Chem. 2009, 284, 5945–5955. [Google Scholar] [CrossRef]
- Sun, X.; Fu, Y.; Gu, M.; Zhang, L.; Li, D.; Li, H.; Chien, S.; Shyy, J.Y.-J.; Zhu, Y. Activation of Integrin A5 Mediated by Flow Requires Its Translocation to Membrane Lipid Rafts in Vascular Endothelial Cells. Proc. Natl. Acad. Sci. USA 2016, 113, 769–774. [Google Scholar] [CrossRef]
- Chen, L.J.; Li, J.Y.S.; Nguyen, P.; He, M.; Chen, Z.B.; Subramaniam, S.; Chien, S.; Shyy, J.Y.J. Single-Cell RNA Sequencing Unveils Unique Transcriptomic Signatures of Endothelial Cells and Role of ENO1 in Response to Disturbed Flow. Proc. Natl. Acad. Sci. USA 2024, 121, e2318904121. [Google Scholar] [CrossRef]
- Souilhol, C.; Serbanovic-Canic, J.; Fragiadaki, M.; Chico, T.J.; Ridger, V.; Roddie, H.; Evans, P.C. Endothelial Responses to Shear Stress in Atherosclerosis: A Novel Role for Developmental Genes. Nat. Rev. Cardiol. 2019, 17, 52–63. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Gantner, B.N.; Palma, F.R.; Kayzuka, C.; Lacchini, R.; Foltz, D.R.; Backman, V.; Kelleher, N.; Shilatifard, A.; Bonini, M.G. Histone Oxidation as a New Mechanism of Metabolic Control over Gene Expression. Trends Genet. 2024, 40, 739–746. [Google Scholar] [CrossRef]
- Iguchi-Ariga, S.M.; Schaffner, W. CpG Methylation of the CAMP-Responsive Enhancer/Promoter Sequence TGACGTCA Abolishes Specific Factor Binding as Well as Transcriptional Activation. Genes. Dev. 1989, 3, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Shi, Y. Histone Methylation: A Dynamic Mark in Health, Disease and Inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Y. TET-Mediated Active DNA Demethylation: Mechanism, Function and Beyond. Nat. Rev. Genet. 2017, 18, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G.; McKnight, S.L. Influence of Metabolism on Epigenetics and Disease. Cell 2013, 153, 56–69. [Google Scholar] [CrossRef]
- Cornacchia, D.; Zhang, C.; Zimmer, B.; Chung, S.Y.; Fan, Y.; Soliman, M.A.; Tchieu, J.; Chambers, S.M.; Shah, H.; Paull, D.; et al. Lipid Deprivation Induces a Stable, Naive-to-Primed Intermediate State of Pluripotency in Human PSCs. Cell Stem Cell 2019, 25, 120–136.e10. [Google Scholar] [CrossRef]
- Chalkiadaki, A.; Guarente, L. Sirtuins Mediate Mammalian Metabolic Responses to Nutrient Availability. Nat. Rev. Endocrinol. 2012, 8, 287–296. [Google Scholar] [CrossRef]
- Johnston, R.A.; Aracena, K.A.; Barreiro, L.B.; Lea, A.J.; Tung, J. DNA Methylation-Environment Interactions in the Human Genome. Elife 2024, 12, RP89371. [Google Scholar] [CrossRef]
- Yang, Q.; Li, X.; Li, R.; Peng, J.; Wang, Z.; Jiang, Z.; Tang, X.; Peng, Z.; Wang, Y.; Wei, D. Low Shear Stress Inhibited Endothelial Cell Autophagy Through TET2 Downregulation. Ann. Biomed. Eng. 2016, 44, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Z.; Manduchi, E.; Stoeckert, C.J.; Davies, P.F. Arterial Endothelial Methylome: Differential DNA Methylation in Athero-Susceptible Disturbed Flow Regions in Vivo. BMC Genom. 2015, 16, 506. [Google Scholar] [CrossRef]
- Miriyala, S.; Gongora Nieto, M.C.; Mingone, C.; Smith, D.; Dikalov, S.; Harrison, D.G.; Jo, H. Bone Morphogenic Protein-4 Induces Hypertension in Mice. Circulation 2006, 113, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.F.; Heumüller, A.W.; Tombor, L.; Hofmann, P.; Muhly-Reinholz, M.; Fischer, A.; Günther, S.; Kokot, K.E.; Okada, H.; Hassel, D.; et al. The Histone Demethylase JMJD2B Regulates Endothelial-to-Mesenchymal Transition. Proc. Natl. Acad. Sci. USA 2020, 117, 4180–4187. [Google Scholar] [CrossRef] [PubMed]
- Huddleson, J.P.; Srinivasan, S.; Ahmad, N.; Lingrel, J.B. Fluid Shear Stress Induces Endothelial KLF2 Gene Expression through a Defined Promoter Region. Biol. Chem. 2004, 385, 723–729. [Google Scholar] [CrossRef]
- Hamik, A.; Lin, Z.; Kumar, A.; Balcells, M.; Sinha, S.; Katz, J.; Feinberg, M.W.; Gerszten, R.E.; Edelman, E.R.; Jain, M.K. Kruppel-like Factor 4 Regulates Endothelial Inflammation. J. Biol. Chem. 2007, 282, 13769–13779. [Google Scholar] [CrossRef]
- Hosoya, T.; Maruyama, A.; Kang, M.-I.; Kawatani, Y.; Shibata, T.; Uchida, K.; Itoh, K.; Yamamoto, M. Differential Responses of the Nrf2-Keap1 System to Laminar and Oscillatory Shear Stresses in Endothelial Cells. J. Biol. Chem. 2005, 280, 27244–27250. [Google Scholar] [CrossRef]
- Topper, J.N.; Cai, J.; Falb, D.; Gimbrone, M.A. Identification of Vascular Endothelial Genes Differentially Responsive to Fluid Mechanical Stimuli: Cyclooxygenase-2, Manganese Superoxide Dismutase, and Endothelial Cell Nitric Oxide Synthase Are Selectively up-Regulated by Steady Laminar Shear Stress. Proc. Natl. Acad. Sci. USA 1996, 93, 10417–10422. [Google Scholar] [CrossRef]
- Kant, S.; Tran, K.V.; Kvandova, M.; Caliz, A.D.; Yoo, H.J.; Learnard, H.; Dolan, A.C.; Craige, S.M.; Hall, J.D.; Jiménez, J.M.; et al. PGC1α Regulates the Endothelial Response to Fluid Shear Stress via Telomerase Reverse Transcriptase Control of Heme Oxygenase-1. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 19–34. [Google Scholar] [CrossRef]
- Wu, C.; Huang, R.-T.; Kuo, C.-H.; Kumar, S.; Kim, C.W.; Lin, Y.-C.; Chen, Y.-J.; Birukova, A.; Birukov, K.G.; Dulin, N.O.; et al. Mechanosensitive PPAP2B Regulates Endothelial Responses to Atherorelevant Hemodynamic Forces. Circ. Res. 2015, 117, E41–E53. [Google Scholar] [CrossRef]
- Topper, J.N.; Gimbrone, M.A., Jr. Blood Flow and Vascular Gene Expression: Fluid Shear Stress as a Modulator of Endothelial Phenotype. Mol. Med. Today 1999, 5, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Demos, C.; Johnson, J.; Andueza, A.; Park, C.; Kim, Y.; Villa-Roel, N.; Kang, D.-W.; Kumar, S.; Jo, H. Sox13 Is a Novel Flow-Sensitive Transcription Factor That Prevents Inflammation by Repressing Chemokine Expression in Endothelial Cells. Front. Cardiovasc. Med. 2022, 9, 979745. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Jung, Y.Y.; Seo, Y.S.; Park, H.; Lee, D.H.; Kim, S.; Roh, Y.-S.; Han, S.B.; Yoon, D.Y.; Hong, J.T. Interleukin-32α Inhibits Endothelial Inflammation, Vascular Smooth Muscle Cell Activation, and Atherosclerosis by Upregulating Timp3 and Reck through Suppressing MicroRNA-205 Biogenesis. Theranostics 2017, 7, 2186–2203. [Google Scholar] [CrossRef]
- Lee, S.; Schleer, H.; Park, H.; Jang, E.; Boyer, M.; Tao, B.; Gamez-Mendez, A.; Singh, A.; Folta-Stogniew, E.; Zhang, X.; et al. Genetic or Therapeutic Neutralization of ALK1 Reduces LDL Transcytosis and Atherosclerosis in Mice. Nat. Cardiovasc. Res. 2023, 2, 438–448. [Google Scholar] [CrossRef]
- Chang, K.; Weiss, D.; Suo, J.; Vega, J.D.; Giddens, D.; Taylor, W.R.; Jo, H. Bone Morphogenic Protein Antagonists Are Coexpressed with Bone Morphogenic Protein 4 in Endothelial Cells Exposed to Unstable Flow In Vitro in Mouse Aortas and in Human Coronary Arteries. Circulation 2007, 116, 1258–1266. [Google Scholar] [CrossRef]
- Shyy, Y.J.; Hsieh, H.J.; Usami, S.; Chien, S. Fluid Shear Stress Induces a Biphasic Response of Human Monocyte Chemotactic Protein 1 Gene Expression in Vascular Endothelium. Proc. Natl. Acad. Sci. USA 1994, 91, 4678–4682. [Google Scholar] [CrossRef]
- Kostopoulos, C.G.; Spiroglou, S.G.; Varakis, J.N.; Apostolakis, E.; Papadaki, H.H. Chemerin and CMKLR1 Expression in Human Arteries and Periadventitial Fat: A Possible Role for Local Chemerin in Atherosclerosis? BMC Cardiovasc. Disord. 2014, 14, 56. [Google Scholar] [CrossRef]
- Itoh, S.; Lemay, S.; Osawa, M.; Che, W.; Duan, Y.; Tompkins, A.; Brookes, P.S.; Sheu, S.-S.; Abe, J. Mitochondrial Dok-4 Recruits Src Kinase and Regulates NF-ΚB Activation in Endothelial Cells. J. Biol. Chem. 2005, 280, 26383–26396. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Hsu, P.-P.; Chen, B.P.; Yuan, S.; Usami, S.; Shyy, J.Y.-J.; Li, Y.-S.; Chien, S. Molecular Mechanism of Endothelial Growth Arrest by Laminar Shear Stress. Proc. Natl. Acad. Sci. USA 2000, 97, 9385–9389. [Google Scholar] [CrossRef]
- Björck, H.M.; Renner, J.; Maleki, S.; Nilsson, S.F.E.; Kihlberg, J.; Folkersen, L.; Karlsson, M.; Ebbers, T.; Eriksson, P.; Länne, T. Characterization of Shear-Sensitive Genes in the Normal Rat Aorta Identifies Hand2 as a Major Flow-Responsive Transcription Factor. PLoS ONE 2012, 7, e52227. [Google Scholar] [CrossRef]
- Feng, S.; Bowden, N.; Fragiadaki, M.; Souilhol, C.; Hsiao, S.; Mahmoud, M.; Allen, S.; Pirri, D.; Ayllon, B.T.; Akhtar, S.; et al. Mechanical Activation of Hypoxia-Inducible Factor 1a Drives Endothelial Dysfunction at Atheroprone Sites. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2087–2101. [Google Scholar] [CrossRef] [PubMed]
- Arderiu, G.; Cuevas, I.; Chen, A.; Carrio, M.; East, L.; Boudreau, N.J. HoxA5 Stabilizes Adherens Junctions Via Increased Akt1. Cell Adhes. Migr. 2007, 1, 185–195. [Google Scholar] [CrossRef][Green Version]
- Nagel, T.; Resnick, N.; Atkinson, W.J.; Dewey, C.F.; Gimbrone, M.A. Shear Stress Selectively Upregulates Intercellular Adhesion Molecule-1 Expression in Cultured Human Vascular Endothelial Cells. J. Clin. Investig. 1994, 94, 885–891. [Google Scholar] [CrossRef]
- Knights, A.J.; Yang, L.; Shah, M.; Norton, L.J.; Green, G.S.; Stout, E.S.; Vohralik, E.J.; Crossley, M.; Quinlan, K.G.R. Krüppel-like Factor 3 (KLF3) Suppresses NF-ΚB–Driven Inflammation in Mice. J. Biol. Chem. 2020, 295, 6080–6091. [Google Scholar] [CrossRef] [PubMed]
- Kadohama, T.; Nishimura, K.; Hoshino, Y.; Sasajima, T.; Sumpio, B.E. Effects of Different Types of Fluid Shear Stress on Endothelial Cell Proliferation and Survival. J. Cell Physiol. 2007, 212, 244–251. [Google Scholar] [CrossRef]
- Pamukcu, B.; Lip, G.Y.H.; Shantsila, E. The Nuclear Factor—Kappa B Pathway in Atherosclerosis: A Potential Therapeutic Target for Atherothrombotic Vascular Disease. Thromb. Res. 2011, 128, 117–123. [Google Scholar] [CrossRef]
- van der Heiden, K.; Cuhlmann, S.; Luong, L.A.; Zakkar, M.; Evans, P.C. Role of Nuclear Factor ΚB in Cardiovascular Health and Disease. Clin. Sci. 2010, 118, 593–605. [Google Scholar] [CrossRef]
- Jeon, H.; Boo, Y.C. Laminar Shear Stress Enhances Endothelial Cell Survival through a NADPH Oxidase 2-Dependent Mechanism. Biochem. Biophys. Res. Commun. 2013, 430, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Song, H.; Mowbray, A. Role of NADPH Oxidases in Disturbed Flow- and BMP4- Induced Inflammation and Atherosclerosis. Antioxid. Redox Signal 2006, 8, 1609–1619. [Google Scholar] [CrossRef]
- Gray, S.P.; Di Marco, E.; Okabe, J.; Szyndralewiez, C.; Heitz, F.; Montezano, A.C.; de Haan, J.B.; Koulis, C.; El-Osta, A.; Andrews, K.L.; et al. NADPH Oxidase 1 Plays a Key Role in Diabetes Mellitus–Accelerated Atherosclerosis. Circulation 2013, 127, 1888–1902. [Google Scholar] [CrossRef]
- Craige, S.M.; Kant, S.; Reif, M.; Chen, K.; Pei, Y.; Angoff, R.; Sugamura, K.; Fitzgibbons, T.; Keaney, J.F. Endothelial NADPH Oxidase 4 Protects ApoE-/- Mice from Atherosclerotic Lesions. Free Radic. Biol. Med. 2015, 89, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Langbein, H.; Brunssen, C.; Hofmann, A.; Cimalla, P.; Brux, M.; Bornstein, S.R.; Deussen, A.; Koch, E.; Morawietz, H. NADPH Oxidase 4 Protects against Development of Endothelial Dysfunction and Atherosclerosis in LDL Receptor Deficient Mice. Eur. Heart J. 2016, 37, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seo, M.; Kim, S.K.; Bae, Y.S. Flagellin-Induced NADPH Oxidase 4 Activation Is Involved in Atherosclerosis. Sci. Rep. 2016, 6, 25437. [Google Scholar] [CrossRef]
- Müller, L.; Keil, R.; Glaß, M.; Hatzfeld, M. Plakophilin 4 Controls the Spatio-Temporal Activity of RhoA at Adherens Junctions to Promote Cortical Actin Ring Formation and Tissue Tension. Cell. Mol. Life Sci. 2024, 81, 291. [Google Scholar] [CrossRef]
- Hu, S.; Liu, Y.; You, T.; Heath, J.; Xu, L.; Zheng, X.; Wang, A.; Wang, Y.; Li, F.; Yang, F.; et al. Vascular Semaphorin 7A Upregulation by Disturbed Flow Promotes Atherosclerosis Through Endothelial Β1 Integrin. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 335–343. [Google Scholar] [CrossRef]
- Peier, M.; Walpen, T.; Christofori, G.; Battegay, E.; Humar, R. Sprouty2 Expression Controls Endothelial Monolayer Integrity and Quiescence. Angiogenesis 2013, 16, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-F.; Cheng, S.-H.; Lin, Y.-S.; Shentu, T.-P.; Huang, R.-T.; Zhu, J.; Chen, Y.-T.; Kumar, S.; Lin, M.-S.; Kao, H.-L.; et al. Targeting Mechanosensitive Endothelial TXNDC5 to Stabilize ENOS and Reduce Atherosclerosis in Vivo. Sci. Adv. 2022, 8, abl8096. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Huang, Y.-T.; Huang, T.-S.; Pang, W.; Zhu, J.-J.; Liu, Y.-F.; Tang, R.-Z.; Zhao, C.-R.; Yao, W.-J.; Li, Y.-S.; et al. The Mammalian Target of Rapamycin and DNA Methyltransferase 1 Axis Mediates Vascular Endothelial Dysfunction in Response to Disturbed Flow. Sci. Rep. 2017, 7, 14996. [Google Scholar] [CrossRef]
- Jiang, Y.-Z.; Jiménez, J.M.; Ou, K.; McCormick, M.E.; Zhang, L.-D.; Davies, P.F. Hemodynamic Disturbed Flow Induces Differential DNA Methylation of Endothelial Kruppel-Like Factor 4 Promoter In Vitro and In Vivo. Circ. Res. 2014, 115, 32–43. [Google Scholar] [CrossRef]
- Li, A.F.; Tan, L.L.; Zhang, S.L.; Tao, J.; Wang, Z.; Wei, D. Low Shear Stress-Induced Endothelial Mesenchymal Transformation via the down-Regulation of TET2. Biochem. Biophys. Res. Commun. 2021, 545, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yang, Q.; Li, A.-F.; Li, R.-Q.; Wang, Z.; Liu, L.-S.; Ren, Z.; Zheng, X.-L.; Tang, X.-Q.; Li, G.-H.; et al. Tet Methylcytosine Dioxygenase 2 Inhibits Atherosclerosis via Upregulation of Autophagy in ApoE−/− Mice. Oncotarget 2016, 7, 76423–76436. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.S.; Turgeon, P.J.; Man, H.-S.J.; Dubinsky, M.K.; Ho, J.J.D.; El-Rass, S.; Wang, Y.-D.; Wen, X.-Y.; Marsden, P.A. Histone Acetyltransferase 7 (KAT7)-Dependent Intragenic Histone Acetylation Regulates Endothelial Cell Gene Regulation. J. Biol. Chem. 2018, 293, 4381–4402. [Google Scholar] [CrossRef]
- Fish, J.E.; Matouk, C.C.; Rachlis, A.; Lin, S.; Tai, S.C.; D’Abreo, C.; Marsden, P.A. The Expression of Endothelial Nitric-Oxide Synthase Is Controlled by a Cell-Specific Histone Code. J. Biol. Chem. 2005, 280, 24824–24838. [Google Scholar] [CrossRef]
- Fish, J.E.; Yan, M.S.; Matouk, C.C.; Bernard, R.S.; Ho, J.J.D.; Gavryushova, A.; Srivastava, D.; Marsden, P.A. Hypoxic Repression of Endothelial Nitric-Oxide Synthase Transcription Is Coupled with Eviction of Promoter Histones. J. Biol. Chem. 2010, 285, 810–826. [Google Scholar] [CrossRef] [PubMed]
- Illi, B.; Nanni, S.; Scopece, A.; Farsetti, A.; Biglioli, P.; Capogrossi, M.C.; Gaetano, C. Shear Stress–Mediated Chromatin Remodeling Provides Molecular Basis for Flow-Dependent Regulation of Gene Expression. Circ. Res. 2003, 93, 155–161. [Google Scholar] [CrossRef]
- Chen, W.; Bacanamwo, M.; Harrison, D.G. Activation of P300 Histone Acetyltransferase Activity Is an Early Endothelial Response to Laminar Shear Stress and Is Essential for Stimulation of Endothelial Nitric-Oxide Synthase MRNA Transcription. J. Biol. Chem. 2008, 283, 16293–16298. [Google Scholar] [CrossRef]
- Danielsson, B.E.; Tieu, K.V.; Spagnol, S.T.; Vu, K.K.; Cabe, J.I.; Raisch, T.B.; Dahl, K.N.; Conway, D.E. Chromatin Condensation Regulates Endothelial Cell Adaptation to Shear Stress. Mol. Biol. Cell 2022, 33, ar101. [Google Scholar] [CrossRef]
- Zampetaki, A.; Zeng, L.; Margariti, A.; Xiao, Q.; Li, H.; Zhang, Z.; Pepe, A.E.; Wang, G.; Habi, O.; Defalco, E.; et al. Histone Deacetylase 3 Is Critical in Endothelial Survival and Atherosclerosis Development in Response to Disturbed Flow. Circulation 2010, 121, 132–142. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Lee, C.-I.; Lin, T.-E.; Lim, S.H.; Zhou, J.; Tseng, Y.-C.; Chien, S.; Chiu, J.-J. Role of Histone Deacetylases in Transcription Factor Regulation and Cell Cycle Modulation in Endothelial Cells in Response to Disturbed Flow. Proc. Natl. Acad. Sci. USA 2012, 109, 1967–1972. [Google Scholar] [CrossRef]
- Hyndman, K.A.; Ho, D.H.; Sega, M.F.; Pollock, J.S. Histone Deacetylase 1 Reduces NO Production in Endothelial Cells via Lysine Deacetylation of NO Synthase 3. Am. J. Physiol.-Heart Circ. Physiol. 2014, 307, H803–H809. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Peng, I.C.; Cui, X.; Li, Y.S.; Chien, S.; Shyy, J.Y.J. Shear Stress, SIRT1, and Vascular Homeostasis. Proc. Natl. Acad. Sci. USA 2010, 107, 10268–10273. [Google Scholar] [CrossRef]
- Katakia, Y.T.; Thakkar, N.P.; Thakar, S.; Sakhuja, A.; Goyal, R.; Sharma, H.; Dave, R.; Mandloi, A.; Basu, S.; Nigam, I.; et al. Dynamic Alterations of H3K4me3 and H3K27me3 at ADAM17 and Jagged-1 Gene Promoters Cause an Inflammatory Switch of Endothelial Cells. J. Cell Physiol. 2022, 237, 992–1012. [Google Scholar] [CrossRef]
- Xu, S.; Xu, Y.; Yin, M.; Zhang, S.; Liu, P.; Koroleva, M.; Si, S.; Little, P.J.; Pelisek, J.; Jin, Z.G. Flow-Dependent Epigenetic Regulation of IGFBP5 Expression by H3K27me3 Contributes to Endothelial Anti-Inflammatory Effects. Theranostics 2018, 8, 3007–3021. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-J.; Liang, G.; Li, R.; Wang, S.; Alnouri, M.W.; Bentsen, M.; Kuenne, C.; Günther, S.; Yan, Y.; Li, Y.; et al. Phosphorylation of Endothelial Histone H3.3 Serine 31 by PKN1 Links Flow-Induced Signaling to Proatherogenic Gene Expression. Nat. Cardiovasc. Res. 2025, 4, 180–196. [Google Scholar] [CrossRef]
- Armache, A.; Yang, S.; Martínez de Paz, A.; Robbins, L.E.; Durmaz, C.; Cheong, J.Q.; Ravishankar, A.; Daman, A.W.; Ahimovic, D.J.; Klevorn, T.; et al. Histone H3.3 Phosphorylation Amplifies Stimulation-Induced Transcription. Nature 2020, 583, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Martire, S.; Gogate, A.A.; Whitmill, A.; Tafessu, A.; Nguyen, J.; Teng, Y.-C.; Tastemel, M.; Banaszynski, L.A. Phosphorylation of Histone H3.3 at Serine 31 Promotes P300 Activity and Enhancer Acetylation. Nat. Genet. 2019, 51, 941–946. [Google Scholar] [CrossRef]
- Doddaballapur, A.; Michalik, K.M.; Manavski, Y.; Lucas, T.; Houtkooper, R.H.; You, X.; Chen, W.; Zeiher, A.M.; Potente, M.; Dimmeler, S.; et al. Laminar Shear Stress Inhibits Endothelial Cell Metabolism via KLF2-Mediated Repression of PFKFB3. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 137–145. [Google Scholar] [CrossRef]
- Rohlenova, K.; Veys, K.; Miranda-Santos, I.; De Bock, K.; Carmeliet, P. Endothelial Cell Metabolism in Health and Disease. Trends Cell Biol. 2018, 28, 224–236. [Google Scholar] [CrossRef]
- Coon, B.G.; Timalsina, S.; Astone, M.; Zhuang, Z.W.; Fang, J.; Han, J.; Themen, J.; Chung, M.; Yang-Klingler, Y.J.; Jain, M.; et al. A Mitochondrial Contribution to Anti-Inflammatory Shear Stress Signaling in Vascular Endothelial Cells. J. Cell Biol. 2022, 221, e202109144. [Google Scholar] [CrossRef]
- Gong, G.; Song, M.; Csordas, G.; Kelly, D.P.; Matkovich, S.J.; Dorn, G.W. Parkin-Mediated Mitophagy Directs Perinatal Cardiac Metabolic Maturation in Mice. Science 2015, 350, aad2459. [Google Scholar] [CrossRef]
- Krantz, S.; Kim, Y.M.; Srivastava, S.; Leasure, J.W.; Toth, P.T.; Marsboom, G.; Rehman, J. Mitophagy Mediates Metabolic Reprogramming of Induced Pluripotent Stem Cells Undergoing Endothelial Differentiation. J. Biol. Chem. 2021, 297, 101410. [Google Scholar] [CrossRef]
- Xiong, J.; Kawagishi, H.; Yan, Y.; Liu, J.; Wells, Q.S.; Edmunds, L.R.; Fergusson, M.M.; Yu, Z.-X.; Rovira, I.I.; Brittain, E.L.; et al. A Metabolic Basis for Endothelial-to-Mesenchymal Transition. Mol. Cell 2018, 69, 689–698.e7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Guang, L.; Li, L.; Shyh-Chang, N. Putting Stem Cells on a Low-Fat Diet Switches Their Pluripotent State. Cell Stem Cell 2019, 25, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, K.D.; Rohlenova, K.; Luo, Y.; Carmeliet, P. The Metabolic Engine of Endothelial Cells. Nat. Metab. 2019, 1, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, J.; Ma, Q.; Liu, Z.; Sudhahar, V.; Cao, Y.; Wang, L.; Zeng, X.; Zhou, Y.; Zhang, M.; et al. PRKAA1/AMPKα1-Driven Glycolysis in Endothelial Cells Exposed to Disturbed Flow Protects against Atherosclerosis. Nat. Commun. 2018, 9, 4667. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.H.; Kim, J.; Park, D.Y.; Bae, H.; Lee, D.-H.; Kim, K.H.; Hong, S.P.; Jang, S.P.; Kubota, Y.; et al. YAP/TAZ Regulates Sprouting Angiogenesis and Vascular Barrier Maturation. J. Clin. Investig. 2017, 127, 3441–3461. [Google Scholar] [CrossRef]
- Wu, D.; Huang, R.-T.; Hamanaka, R.B.; Krause, M.; Oh, M.-J.; Kuo, C.-H.; Nigdelioglu, R.; Meliton, A.Y.; Witt, L.; Dai, G.; et al. HIF-1α Is Required for Disturbed Flow-Induced Metabolic Reprogramming in Human and Porcine Vascular Endothelium. Elife 2017, 6, e25217. [Google Scholar] [CrossRef]
- Pirri, D.; Tian, S.; Tardajos-Ayllon, B.; Irving, S.; Donati, F.; Allen, S.P.; Mammoto, T.; Vilahur, G.; Kabir, L.; Bennett, J.; et al. EPAS1 Attenuates Atherosclerosis Initiation at Disturbed Flow Sites Through Endothelial Fatty Acid Uptake. Circ. Res. 2024, 135, 822–837. [Google Scholar] [CrossRef]
- Wai, T. Is Mitochondrial Morphology Important for Cellular Physiology? Trends Endocrinol. Metab. 2024, 35, 854–871. [Google Scholar] [CrossRef]
- Wai, T.; Langer, T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. 2016, 27, 105–117. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, E.; Crown, S.B.; Fox, D.B.; Kitir, B.; Ilkayeva, O.R.; Olsen, C.A.; Grimsrud, P.A.; Hirschey, M.D. Lipids Reprogram Metabolism to Become a Major Carbon Source for Histone Acetylation. Cell Rep. 2016, 17, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Yucel, N.; Wang, Y.X.; Mai, T.; Porpiglia, E.; Lund, P.J.; Markov, G.; Garcia, B.A.; Bendall, S.C.; Angelo, M.; Blau, H.M. Glucose Metabolism Drives Histone Acetylation Landscape Transitions That Dictate Muscle Stem Cell Function. Cell Rep. 2019, 27, 3939–3955.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Y.; Soaita, I.; Lee, H.-W.; Bae, H.; Boutagy, N.; Bostwick, A.; Zhang, R.-M.; Bowman, C.; Xu, Y.; et al. Acetate Controls Endothelial-to-Mesenchymal Transition. Cell Metab. 2023, 35, 1163–1178.e10. [Google Scholar] [CrossRef]
- Toborek, M.; Hennig, B. Dietary Methionine Imbalance, Endothelial Cell Dysfunction and Atherosclerosis. Nutr. Res. 1996, 16, 1251–1266. [Google Scholar] [CrossRef]
- Dann, S.G.; Ryskin, M.; Barsotti, A.M.; Golas, J.; Shi, C.; Miranda, M.; Hosselet, C.; Lemon, L.; Lucas, J.; Karnoub, M.; et al. Reciprocal Regulation of Amino Acid Import and Epigenetic State through Lat1 and EZH2. EMBO J. 2015, 34, 1773–1785. [Google Scholar] [CrossRef]
- Dai, Z.; Mentch, S.J.; Gao, X.; Nichenametla, S.N.; Locasale, J.W. Methionine Metabolism Influences Genomic Architecture and Gene Expression through H3K4me3 Peak Width. Nat. Commun. 2018, 9, 1955. [Google Scholar] [CrossRef]
- Lang, M.B.; Leung, K.-Y.; Greene, N.D.E.; Malone, K.M.; Saginc, G.; Randi, A.M.; Kiprianos, A.; Maughan, R.T.; Pericleous, C.; Mason, J.C. The Actions of Methotrexate on Endothelial Cells Are Dependent on the Shear Stress-Induced Regulation of One Carbon Metabolism. Front. Immunol. 2023, 14, 1209490. [Google Scholar] [CrossRef]
- Go, Y.-M.; Kim, C.W.; Walker, D.I.; Kang, D.W.; Kumar, S.; Orr, M.; Uppal, K.; Quyyumi, A.A.; Jo, H.; Jones, D.P. Disturbed Flow Induces Systemic Changes in Metabolites in Mouse Plasma: A Metabolomics Study Using ApoE−/− Mice with Partial Carotid Ligation. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 308, R62–R72. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.W.; Finley, L.W.S.; Cross, J.R.; Allis, C.D.; Thompson, C.B. Intracellular α-Ketoglutarate Maintains the Pluripotency of Embryonic Stem Cells. Nature 2015, 518, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, F.; Günther, S.; Looso, M.; Kuenne, C.; Zhang, T.; Wiesnet, M.; Klatt, S.; Zukunft, S.; Fleming, I.; et al. Inhibition of Fatty Acid Oxidation Enables Heart Regeneration in Adult Mice. Nature 2023, 622, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Persad, K.L.; Lopaschuk, G.D. Energy Metabolism on Mitochondrial Maturation and Its Effects on Cardiomyocyte Cell Fate. Front. Cell Dev. Biol. 2022, 10, 886393. [Google Scholar] [CrossRef] [PubMed]
- Evertts, A.G.; Zee, B.M.; DiMaggio, P.A.; Gonzales-Cope, M.; Coller, H.A.; Garcia, B.A. Quantitative Dynamics of the Link between Cellular Metabolism and Histone Acetylation. J. Biol. Chem. 2013, 288, 12142–12151. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhang, K.; Wang, Y.; Gu, Y. Comprehensive Review of Histone Lactylation: Structure, Function, and Therapeutic Targets. Biochem. Pharmacol. 2024, 225, 116331. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic Regulation of Gene Expression by Histone Lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Merkuri, F.; Rothstein, M.; Simoes-Costa, M. Histone Lactylation Couples Cellular Metabolism with Developmental Gene Regulatory Networks. Nat. Commun. 2024, 15, 90. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, Y.; Chen, M.; Tan, Y.; Min, J.; He, X.; Liu, F.; Gu, J.; Jiang, H.; Zheng, L.; et al. ASF1A-Dependent P300-Mediated Histone H3 Lysine 18 Lactylation Promotes Atherosclerosis by Regulating EndMT. Acta Pharm. Sin. B 2024, 14, 3027–3048. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.-H.; Li, N. Altered Metabolism in Cancer: Insights into Energy Pathways and Therapeutic Targets. Mol. Cancer 2024, 23, 203. [Google Scholar] [CrossRef]
- Schoors, S.; De Bock, K.; Cantelmo, A.R.; Georgiadou, M.; Ghesquière, B.; Cauwenberghs, S.; Kuchnio, A.; Wong, B.W.; Quaegebeur, A.; Goveia, J.; et al. Partial and Transient Reduction of Glycolysis by PFKFB3 Blockade Reduces Pathological Angiogenesis. Cell Metab. 2014, 19, 37–48. [Google Scholar] [CrossRef]
- Villa-Roel, N.; Ryu, K.; Gu, L.; Fernandez Esmerats, J.; Kang, D.-W.; Kumar, S.; Jo, H. Hypoxia Inducible Factor 1α Inhibitor PX-478 Reduces Atherosclerosis in Mice. Atherosclerosis 2022, 344, 20–30. [Google Scholar] [CrossRef]

| Shear Stress | Gene | Expression | Effect in ECs | References |
|---|---|---|---|---|
| Laminar | BMPR2 | ↑ | Inhibits NF-κB activation and oxidative stress | [54] |
| KDM4B | ↓ | Induces EndMT | [55] | |
| KLF2 | ↑ | Maintains EC identity | [27,56] | |
| KLF4 | ↑ | [30,57] | ||
| NFE2L2 | ↑ | Reduces oxidative stress | [58] | |
| NOS3 | ↑ | Catalyzes production of NO | [59] | |
| PPARGC1A | ↑ | Promotes mitochondrial biogenesis | [60] | |
| PPAP2B | ↑ | Reduces inflammation | [61] | |
| SOD2 | ↑ | Antioxidative responses | [62] | |
| SOD3 | ↑ | |||
| SOX13 | ↑ | Reduces inflammation | [63] | |
| TIMP3 | ↑ | Reduces extracellular matrix degradation | [64] | |
| Oscillatory | ACVRL1 | ↓ | Regulates angiogenesis, LDL entry and transcytosis | [24,65] |
| ADAMTSL5 | ↓ | Not established | [24] | |
| BMP4 | ↑ | Enhances oxidative stress and inflammation | [66] | |
| CCL2 | ↑ | Enhances immune cell adhesion to ECs | [67] | |
| CMKLR1 | ↓ | Regulates ligand-mediated migration and angiogenesis | [24,68] | |
| DOK4 | ↓ | Regulates NF-κB activation | [24,69] | |
| F2RL1 | ↓ | Regulates angiogenic responses | [24] | |
| GADD45 | ↑ | Activates cell growth and proliferation | [70] | |
| HAND2 | ↑ | Increases extracellular matrix degradation | [71] | |
| HIF1A | ↑ | Enhances aerobic glycolysis | [72] | |
| HOX5 | ↓ | Regulates angiogenesis | [24,73] | |
| ICAM1 | ↑ | Enhances immune cell adhesion to ECs | [74] | |
| KLF3 | ↓ | Regulation of NF-κB-driven inflammation | [24,75] | |
| MAPK1 | ↑ | Activate cell growth and proliferation | [76] | |
| MAPK3 | ↑ | |||
| NFKB | ↑ | Enhances inflammation | [77,78] | |
| NOX1 | ↑ | Promotes production of superoxide | [79,80,81] | |
| NOX2 | ↑ | |||
| NOX4 | ↑/↓ | Promotes production of H2O2 | [82,83,84] | |
| PKP4 | ↓ | Regulates intercellular adhesion | [24,85] | |
| SEMA7A | ↑ | Enhances immune cell adhesion to ECs | [86] | |
| SPRY2 | ↓ | Regulates integrity of endothelial cell monolayer | [24,87] | |
| TMEM184B | ↓ | Not established | [24] | |
| TXNDC5 | ↑ | Impairs eNOS | [88] | |
| VCAM1 | ↑ | Enhances immune cell adhesion to ECs | [74] | |
| YAP1, TAZ | ↑ | Enhances inflammation | [89] | |
| ZFP46 | ↓ | Not established | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, F.; Sum, H.; Yan, D.C.L.; Brewer, A.C. Metaboloepigenetics: Role in the Regulation of Flow-Mediated Endothelial (Dys)Function and Atherosclerosis. Cells 2025, 14, 378. https://doi.org/10.3390/cells14050378
Santos F, Sum H, Yan DCL, Brewer AC. Metaboloepigenetics: Role in the Regulation of Flow-Mediated Endothelial (Dys)Function and Atherosclerosis. Cells. 2025; 14(5):378. https://doi.org/10.3390/cells14050378
Chicago/Turabian StyleSantos, Francisco, Hashum Sum, Denise Cheuk Lee Yan, and Alison C. Brewer. 2025. "Metaboloepigenetics: Role in the Regulation of Flow-Mediated Endothelial (Dys)Function and Atherosclerosis" Cells 14, no. 5: 378. https://doi.org/10.3390/cells14050378
APA StyleSantos, F., Sum, H., Yan, D. C. L., & Brewer, A. C. (2025). Metaboloepigenetics: Role in the Regulation of Flow-Mediated Endothelial (Dys)Function and Atherosclerosis. Cells, 14(5), 378. https://doi.org/10.3390/cells14050378






