Isolation and Functional Characterization of Endophytic Bacteria from Muscadine Grape Berries: A Microbial Treasure Trove
Abstract
1. Introduction
2. Material and Methods
2.1. Grape Berry Sample Collection
2.2. Bacterial Isolation and Purification
2.3. Molecular Identification of Isolates
2.4. Growth Curve Analysis
2.5. Biochemical Characterization
2.6. Tolerance Assay to Intestinal Fluids, Bile Salts, Simulated Gastric Juice, and Acidic pH
2.7. Cell Survival Assay
2.8. Microscopy Imaging
2.9. Statistical Analysis
3. Results
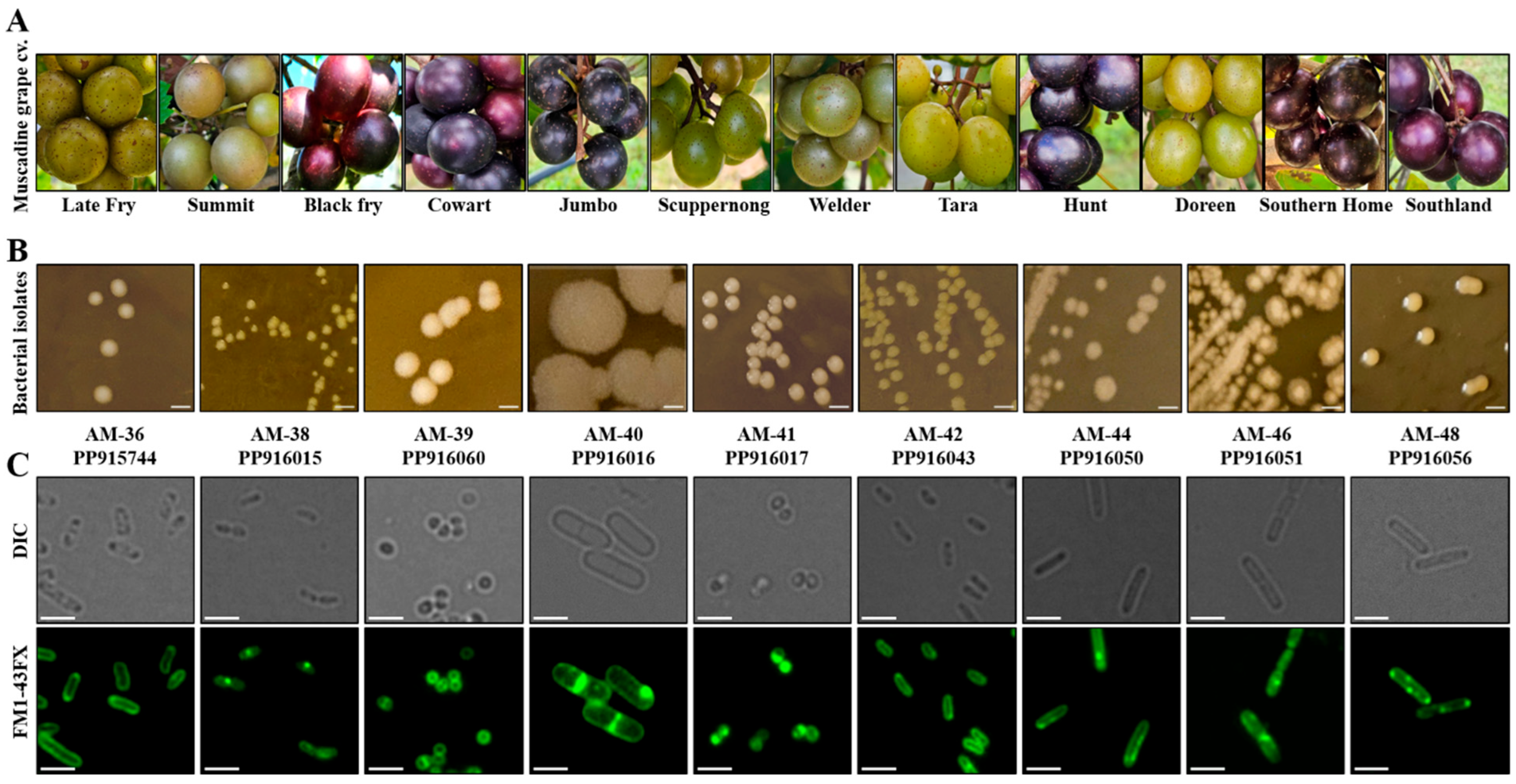
3.1. Isolation and Molecular Identification Revealed Presence of Diverse Bacterial Endophytic Species in Muscadine Berries
3.2. Bacterial Isolates Displayed Variations in Colony Morphology and Cellular Structures
3.3. Bacterial Isolates Could Grow at Varying Temperatures
3.4. Endophytic Isolates Displayed Metabolic Versatility Across Various Biochemical Assays
3.5. Phylum Bacillota Was Predominant in Motility, DNase, and Hemolytic Assays
3.6. Strains AM-40, AM-44, AM-46, and AM-48 Demonstrated Higher Tolerance to Acidic pH
3.7. Isolates Exhibited Distinct Responses for Survival to Varying Stress Conditions
4. Discussion
4.1. Functional Diversity and Biotechnological Potential of Isolates
4.2. Adaptive Survival Strategies of Potential Probiotic Isolates Under Simulated Gastrointestinal Stress Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, J.; Brady, P. The Muscadine Experience: Adding Value to Enhance Profits. Res. Rep. Res. Bull. 2007. [Google Scholar]
- Striegler, R.K.; Morris, J.R.; Carter, P.M.; Clark, J.R.; Threlfall, R.T.; Howard, L.R. Yield, Quality, and Nutraceutical Potential of Selected Muscadine Cultivars Grown in Southwestern Arkansas. HortTechnology 2005, 15, 276–284. [Google Scholar] [CrossRef]
- Chappell, M.C.; Duncan, A.V.; Melo, A.C.; Schaich, C.L.; Pirro, N.T.; Diz, D.I.; Tallant, E.A.; Gallagher, P.E. Targeted UHPLC-MS Analysis Reveals Disparate Polyphenol Composition and Concentration in Muscadine Grape Supplements with Proportional Antioxidant Activity. Antioxidants 2022, 11, 2117. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Johnson, J.V.; Talcott, S.T. Identification of Ellagic Acid Conjugates and Other Polyphenolics in Muscadine Grapes by HPLC-ESI-MS. J. Agric. Food Chem. 2005, 53, 6003–6010. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, P.; Bauer, J.D.; Pollock, S.H.; Gangemi, J.D.; Mayer, E.P.; Ghaffar, A.; Hargrove, J.L.; Hartle, D.K. Antiinflammatory Properties of the Muscadine Grape (Vitis rotundifolia). J. Agric. Food Chem. 2005, 53, 8481–8484. [Google Scholar] [CrossRef]
- Mellen, P.B.; Daniel, K.R.; Brosnihan, K.B.; Hansen, K.J.; Herrington, D.M. Effect of Muscadine Grape Seed Supplementation on Vascular Function in Subjects with or at Risk for Cardiovascular Disease: A Randomized Crossover Trial. J. Am. Coll. Nutr. 2010, 29, 469–475. [Google Scholar] [CrossRef]
- Messeha, S.S.; Agarwal, M.; Gendy, S.G.; Mehboob, S.B.; Soliman, K.F.A. The Anti-Obesogenic Effects of Muscadine Grapes through Ciliary Neurotrophic Factor Receptor (Cntfr) and Histamine Receptor H1 (Hrh1) Genes in 3T3-L1 Differentiated Mouse Cells. Nutrients 2024, 16, 1817. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Gu, L. Antioxidant Capacity, Phenolic Content, and Profiling of Phenolic Compounds in the Seeds, Skin, and Pulp of Vitis rotundifolia (Muscadine Grapes) As Determined by HPLC-DAD-ESI-MS(n). J. Agric. Food Chem. 2010, 58, 4681–4692. [Google Scholar] [CrossRef]
- Xu, C.; Yagiz, Y.; Zhao, L.; Simonne, A.; Lu, J.; Marshall, M.R. Fruit Quality, Nutraceutical and Antimicrobial Properties of 58 Muscadine Grape Varieties (Vitis rotundifolia Michx.) Grown in United States. Food Chem. 2017, 215, 149–156. [Google Scholar] [CrossRef]
- Yi, W.; Fischer, J.; Akoh, C.C. Study of Anticancer Activities of Muscadine Grape Phenolics in Vitro. J. Agric. Food Chem. 2005, 53, 8804–8812. [Google Scholar] [CrossRef]
- Blanc, S.; Wiedemann-Merdinoglu, S.; Dumas, V.; Mestre, P.; Merdinoglu, D. A Reference Genetic Map of Muscadinia Rotundifolia and Identification of Ren5, a New Major Locus for Resistance to Grapevine Powdery Mildew. Theor. Appl. Genet. 2012, 125, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Louime, C.; Lu, J.; Onokpise, O.; Vasanthaiah, H.K.N.; Kambiranda, D.; Basha, S.M.; Yun, H.K. Resistance to Elsinoë Ampelina and Expression of Related Resistant Genes in Vitis Rotundifolia Michx. Grapes. Int. J. Mol. Sci. 2011, 12, 3473–3488. [Google Scholar] [CrossRef] [PubMed]
- Olien, W.C. The Muscadine Grape: Botany, Viticulture, History, and Current Industry. HortScience 1990, 25, 732–739. [Google Scholar] [CrossRef]
- Riaz, S.; Tenscher, A.C.; Ramming, D.W.; Walker, M.A. Using a Limited Mapping Strategy to Identify Major QTLs for Resistance to Grapevine Powdery Mildew (Erysiphe necator) and Their Use in Marker-Assisted Breeding. Theor. Appl. Genet. 2011, 122, 1059–1073. [Google Scholar] [CrossRef]
- Ruel, J.J.; Walker, M.A. Resistance to Pierce’s Disease in Muscadinia Rotundifolia and Other Native Grape Species. Am. J. Enol. Vitic. 2006, 57, 158–165. [Google Scholar] [CrossRef]
- Staudt, G.; Kassemeyer, H. Evaluation of Downy Mildew Resistance in Various Accessions of Wild Vitis Species. VITIS J. Grapevine Res. 1995, 34, 225. [Google Scholar] [CrossRef]
- Hickey, C.C.; Smith, E.D.; Cao, S.; Conner, P. Muscadine (Vitis Rotundifolia Michx., Syn. Muscandinia Rotundifolia (Michx.) Small): The Resilient, Native Grape of the Southeastern U.S. Agriculture 2019, 9, 131. [Google Scholar] [CrossRef]
- Millholland, R.D. Muscadine Grapes: Some Important Diseases and Their Control. Plant Dis. 1991, 75, 113. [Google Scholar] [CrossRef]
- Sun, D.; Qu, J.; Huang, Y.; Lu, J.; Yin, L. Analysis of Microbial Community Diversity of Muscadine Grape Skins. Food Res. Int. 2021, 145, 110417. [Google Scholar] [CrossRef]
- Burragoni, S.G.; Jeon, J. Applications of Endophytic Microbes in Agriculture, Biotechnology, Medicine, and Beyond. Microbiol. Res. 2021, 245, 126691. [Google Scholar] [CrossRef]
- Tiwari, P.; Kang, S.; Bae, H. Plant-Endophyte Associations: Rich yet under-Explored Sources of Novel Bioactive Molecules and Applications. Microbiol. Res. 2023, 266, 127241. [Google Scholar] [CrossRef] [PubMed]
- Aleynova, O.A.; Suprun, A.R.; Nityagovsky, N.N.; Dubrovina, A.S.; Kiselev, K.V. The Influence of the Grapevine Bacterial and Fungal Endophytes on Biomass Accumulation and Stilbene Production by the In Vitro Cultivated Cells of Vitis Amurensis Rupr. Plants 2021, 10, 1276. [Google Scholar] [CrossRef] [PubMed]
- Chhipa, H.; Kaushik, N. Fungal and Bacterial Diversity Isolated from Aquilaria Malaccensis Tree and Soil, Induces Agarospirol Formation within 3 Months after Artificial Infection. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Deepa, N.; Trivedi, P.K.; Singh, B.K.; Srivastava, V.; Singh, A. Plants and Endophytes Interaction: A “Secret Wedlock” for Sustainable Biosynthesis of Pharmaceutically Important Secondary Metabolites. Microb. Cell Factories 2023, 22, 226. [Google Scholar] [CrossRef]
- Shukla, N.; Singh, D.; Tripathi, A.; Kumari, P.; Gupta, R.K.; Singh, S.; Shanker, K.; Singh, A. Synergism of Endophytic Bacillus Subtilis and Klebsiella Aerogenes Modulates Plant Growth and Bacoside Biosynthesis in Bacopa Monnieri. Front. Plant Sci. 2022, 13, 896856. [Google Scholar] [CrossRef]
- Tripathi, P.; Tripathi, A.; Singh, A.; Yadav, V.; Shanker, K.; Khare, P.; Kalra, A. Differential Response of Two Endophytic Bacterial Strains Inoculation on Biochemical and Physiological Parameters of Bacopa Monnieri L. under Arsenic Stress Conditions. J. Hazard. Mater. Adv. 2022, 6, 100055. [Google Scholar] [CrossRef]
- Yang, H.-R.; Yuan, J.; Liu, L.-H.; Zhang, W.; Chen, F.; Dai, C.-C. Endophytic Pseudomonas Fluorescens Induced Sesquiterpenoid Accumulation Mediated by Gibberellic Acid and Jasmonic Acid in Atractylodes Macrocephala Koidz Plantlets. Plant Cell Tiss. Organ. Cult. 2019, 138, 445–457. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Li, X.; Zheng, J.-Y.; Dai, C.-C. Volatiles Released by Endophytic Pseudomonas fluorescens Promoting the Growth and Volatile Oil Accumulation in Atractylodes lancea. Plant Physiol. Biochem. 2016, 101, 132–140. [Google Scholar] [CrossRef]
- Ait Barka, E.; Nowak, J.; Clément, C. Enhancement of Chilling Resistance of Inoculated Grapevine Plantlets with a Plant Growth-Promoting Rhizobacterium, Burkholderia phytofirmans Strain PsJN. Appl. Environ. Microbiol. 2006, 72, 7246–7252. [Google Scholar] [CrossRef]
- Fernandez, O.; Vandesteene, L.; Feil, R.; Baillieul, F.; Lunn, J.E.; Clément, C. Trehalose Metabolism Is Activated upon Chilling in Grapevine and Might Participate in Burkholderia phytofirmans Induced Chilling Tolerance. Planta 2012, 236, 355–369. [Google Scholar] [CrossRef]
- Hopkins, D.L. Biological Control of Pierce’s Disease in the Vineyard with Strains of Xylella Fastidiosa Benign to Grapevine. Plant Dis. 2005, 89, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Aleynova, O.A.; Nityagovsky, N.N.; Ananev, A.A.; Suprun, A.R.; Ogneva, Z.V.; Dneprovskaya, A.A.; Beresh, A.A.; Tyunin, A.P.; Dubrovina, A.S.; Kiselev, K.V. The Endophytic Microbiome of Wild Grapevines Vitis Amurensis Rupr. and Vitis Coignetiae Pulliat Growing in the Russian Far East. Plants 2023, 12, 2952. [Google Scholar] [CrossRef] [PubMed]
- Stępniewska, Z.; Kuźniar, A. Endophytic Microorganisms—Promising Applications in Bioremediation of Greenhouse Gases. Appl. Microbiol. Biotechnol. 2013, 97, 9589–9596. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xiao, X.; Xi, Q.; Wan, Y.; Chen, L.; Zeng, G.; Liu, C.; Guo, H.; Chen, J. Enhancement of Cadmium Bioremediation by Endophytic Bacterium Bacillus Sp. L14 Using Industrially Used Metabolic Inhibitors (DCC or DNP). J. Hazard. Mater. 2011, 190, 1079–1082. [Google Scholar] [CrossRef]
- Germaine, K.J.; Keogh, E.; Ryan, D.; Dowling, D.N. Bacterial Endophyte-Mediated Naphthalene Phytoprotection and Phytoremediation. FEMS Microbiol. Lett. 2009, 296, 226–234. [Google Scholar] [CrossRef]
- Agarwal, M.; Rathore, R.S.; Black, A.; Xu, X.; Seaman, J.; Chauhan, A. Announcing the Availability of a Culture Collection of Uranium-Resistant Microbial Assemblages (CURMA) Obtained from Metalliferous Soils of the Savannah River Site, USA. Microbiol. Resour. Announc. 2020, 9, e00551-20. [Google Scholar] [CrossRef]
- Gendy, S.; Pathak, A.; Agarwal, M.; Rathore, R.S.; Chauhan, A. Draft Genome Sequence of Mercury-Resistant Serratia Sp. Strain SRS-8-S-2018. Microbiol. Resour. Announc. 2020, 9, 10–1128. [Google Scholar] [CrossRef]
- Agarwal, M.; Rathore, R.S.; Chauhan, A. A Rapid and High Throughput MIC Determination Method to Screen Uranium Resistant Microorganisms. Methods Protoc. 2020, 3, 21. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An Efficient Microbiological Growth Medium for Screening Phosphate Solubilizing Microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Shomi, F.Y.; Uddin, M.B.; Zerin, T. Isolation and Characterization of Nitrogen-Fixing Bacteria from Soil Sample in Dhaka, Bangladesh. Stamford J. Microbiol. 2021, 11, 11–13. [Google Scholar] [CrossRef]
- Ogale, S.; Yadav, K.S.; Navale, S. Screening of Endophytic Bacteria from the Pharmacologically Important Medicinal Plant Gloriosa Superba for Their Multiple Plant Growth Promoting Properties. Pharma Innov. J. 2018, 7, 208–214. [Google Scholar]
- Tittsler, R.P.; Sandholzer, L.A. The Use of Semi-Solid Agar for the Detection of Bacterial Motility. J. Bacteriol. 1936, 31, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Rathore, R.S.; Jagoe, C.; Chauhan, A. Multiple Lines of Evidences Reveal Mechanisms Underpinning Mercury Resistance and Volatilization by Stenotrophomonas Sp. MA5 Isolated from the Savannah River Site (SRS), USA. Cells 2019, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Mitter, B.; Colli-Mull, J.G.; Gangl, H.; Sessitsch, A. Endophytes of Grapevine Flowers, Berries, and Seeds: Identification of Cultivable Bacteria, Comparison with Other Plant Parts, and Visualization of Niches of Colonization. Microb. Ecol. 2011, 62, 188–197. [Google Scholar] [CrossRef]
- Nisiotou, A.A.; Rantsiou, K.; Iliopoulos, V.; Cocolin, L.; Nychas, G.-J.E. Bacterial Species Associated with Sound and Botrytis-Infected Grapes from a Greek Vineyard. Int. J. Food Microbiol. 2011, 145, 432–436. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Zhong, Q.; Zhuang, X.; Bai, Z. Microbial Community Analyses Associated with Nine Varieties of Wine Grape Carposphere Based on High-Throughput Sequencing. Microorganisms 2019, 7, 668. [Google Scholar] [CrossRef]
- Bruisson, S.; Zufferey, M.; L’Haridon, F.; Trutmann, E.; Anand, A.; Dutartre, A.; De Vrieze, M.; Weisskopf, L. Endophytes and Epiphytes From the Grapevine Leaf Microbiome as Potential Biocontrol Agents Against Phytopathogens. Front. Microbiol. 2019, 10, 2726. [Google Scholar] [CrossRef]
- Ek-Ramos, M.J.; Gomez-Flores, R.; Orozco-Flores, A.A.; Rodríguez-Padilla, C.; González-Ochoa, G.; Tamez-Guerra, P. Bioactive Products From Plant-Endophytic Gram-Positive Bacteria. Front. Microbiol. 2019, 10, 463. [Google Scholar] [CrossRef]
- Kordowska-Wiater, M.; Pytka, M.; Stój, A.; Kubik-Komar, A.; Wyrostek, J.; Waśko, A. A Metagenetic Insight into Microbial Diversity of Spontaneously Fermented Polish Red Wines and an Analysis of Selected Physicochemical Properties. Appl. Sci. 2022, 12, 4373. [Google Scholar] [CrossRef]
- Liang, L.; Ma, Y.; Jiang, Z.; Sam, F.E.; Peng, S.; Li, M.; Wang, J. Dynamic Analysis of Microbial Communities and Flavor Properties in Merlot Wines Produced from Inoculation and Spontaneous Fermentation. Food Res. Int. 2023, 164, 112379. [Google Scholar] [CrossRef]
- Bulgari, D.; Minio, A.; Casati, P.; Quaglino, F.; Delledonne, M.; Bianco, P.A. Curtobacterium sp. Genome Sequencing Underlines Plant Growth Promotion-Related Traits. Genome Announc. 2014, 2, e00592-14. [Google Scholar] [CrossRef] [PubMed]
- Ferrigo, D.; Causin, R.; Raiola, A. Effect of Potential Biocontrol Agents Selected among Grapevine Endophytes and Commercial Products on Crown Gall Disease. BioControl 2017, 62, 821–833. [Google Scholar] [CrossRef]
- Campisano, A.; Antonielli, L.; Pancher, M.; Yousaf, S.; Pindo, M.; Pertot, I. Bacterial Endophytic Communities in the Grapevine Depend on Pest Management. PLoS ONE 2014, 9, e112763. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Lee, J.-H.; Jeong, D.-W. Food-Derived Coagulase-Negative Staphylococcus as Starter Cultures for Fermented Foods. Food Sci. Biotechnol. 2020, 29, 1023–1035. [Google Scholar] [CrossRef]
- de Freire Bastos, M.d.C.; Miceli de Farias, F.; Carlin Fagundes, P.; Varella Coelho, M.L. Staphylococcins: An Update on Antimicrobial Peptides Produced by Staphylococci and Their Diverse Potential Applications. Appl. Microbiol. Biotechnol. 2020, 104, 10339–10368. [Google Scholar] [CrossRef]
- Aoki, T.; Aoki, Y.; Ishiai, S.; Otoguro, M.; Suzuki, S. Impact of Bacillus Cereus NRKT on Grape Ripe Rot Disease through Resveratrol Synthesis in Berry Skin. Pest. Manag. Sci. 2017, 73, 174–180. [Google Scholar] [CrossRef]
- Feng, B.; Ding, C.; Li, P.; Fu, L. Combined Application of the Endophyte Bacillus K1 and Sodium Dehydroacetate Alleviates Postharvest Gray Mold in Grapes. Food Microbiol. 2025, 125, 104637. [Google Scholar] [CrossRef]
- Hamaoka, K.; Aoki, Y.; Suzuki, S. Isolation and Characterization of Endophyte Bacillus Velezensis KOF112 from Grapevine Shoot Xylem as Biological Control Agent for Fungal Diseases. Plants 2021, 10, 1815. [Google Scholar] [CrossRef]
- Nigris, S.; Baldan, E.; Tondello, A.; Zanella, F.; Vitulo, N.; Favaro, G.; Guidolin, V.; Bordin, N.; Telatin, A.; Barizza, E.; et al. Biocontrol Traits of Bacillus Licheniformis GL174, a Culturable Endophyte of Vitis Vinifera Cv. Glera. BMC Microbiol. 2018, 18, 133. [Google Scholar] [CrossRef]
- Zeng, Q.; Xie, J.; Li, Y.; Gao, T.; Zhang, X.; Wang, Q. Comprehensive Genomic Analysis of the Endophytic Bacillus Altitudinis Strain GLB197, a Potential Biocontrol Agent of Grape Downy Mildew. Front. Genet. 2021, 12, 729603. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Li, Y.; Fu, X.; Wang, Q. Screening and Characterization of Endophytic Bacillus for Biocontrol of Grapevine Downy Mildew. Crop Prot. 2017, 96, 173–179. [Google Scholar] [CrossRef]
- Ali, M.A.; Ahmed, T.; Ibrahim, E.; Rizwan, M.; Chong, K.P.; Yong, J.W.H. A Review on Mechanisms and Prospects of Endophytic Bacteria in Biocontrol of Plant Pathogenic Fungi and Their Plant Growth-Promoting Activities. Heliyon 2024, 10, e31573. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Zou, C.; Xu, X.; Ke, Z.; Hou, J.; Jiang, G.; Fan, C.; Gong, J.; Wei, J. Complete Genome Sequence of Biocontrol Strain Paenibacillus Peoriae HJ-2 and Further Analysis of Its Biocontrol Mechanism. BMC Genom. 2022, 23, 161. [Google Scholar] [CrossRef] [PubMed]
- Rybakova, D.; Cernava, T.; Köberl, M.; Liebminger, S.; Etemadi, M.; Berg, G. Endophytes-Assisted Biocontrol: Novel Insights in Ecology and the Mode of Action of Paenibacillus. Plant Soil. 2016, 405, 125–140. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, X.; Li, J.; Shi, Y.; Chai, A.; Fan, T.; Li, B.; Li, L. Comparative Genomics Insights into a Novel Biocontrol Agent Paenibacillus Peoriae Strain ZF390 against Bacterial Soft Rot. Biology 2022, 11, 1172. [Google Scholar] [CrossRef]
- Verginer, M.; Leitner, E.; Berg, G. Production of Volatile Metabolites by Grape-Associated Microorganisms. J. Agric. Food Chem. 2010, 58, 8344–8350. [Google Scholar] [CrossRef]
- von Cosmos, N.H.; Watson, B.A.; Fellman, J.K.; Mattinson, D.S.; Edwards, C.G. Characterization of Bacillus megaterium, Bacillus pumilus, and Paenibacillus polymyxa Isolated from a Pinot Noir Wine from Western Washington State. Food Microbiol. 2017, 67, 11–16. [Google Scholar] [CrossRef]
- Ramey, B.E.; Koutsoudis, M.; von Bodman, S.B.; Fuqua, C. Biofilm Formation in Plant–Microbe Associations. Curr. Opin. Microbiol. 2004, 7, 602–609. [Google Scholar] [CrossRef]
- Moon, S.-G.; Kothari, D.; Lee, W.-D.; Kim, J.-I.; Kim, K.-I.; Kim, Y.-G.; Ga, G.-W.; Kim, E.-J.; Kim, S.-K. Potential Probiotic Acceptability of a Novel Strain of Paenibacillus Konkukensis SK 3146 and Its Dietary Effects on Growth Performance, Intestinal Microbiota, and Meat Quality in Broilers. Animals 2022, 12, 1471. [Google Scholar] [CrossRef]
- Karasz, D.C.; Weaver, A.I.; Buckley, D.H.; Wilhelm, R.C. Conditional Filamentation as an Adaptive Trait of Bacteria and Its Ecological Significance in Soils. Environ. Microbiol. 2022, 24, 1–17. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, C.; Wang, E.; Raza, A.; Yin, C. Bacillus amyloliquefaciens as an Excellent Agent for Biofertilizer and Biocontrol in Agriculture: An Overview for Its Mechanisms. Microbiol. Res. 2022, 259, 127016. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.; Imre, K.; Arslan-Acaroz, D.; Istanbullugil, F.R.; Fang, Y.; Ros, G.; Zhu, K.; Acaroz, U. Mechanisms of Probiotic Bacillus against Enteric Bacterial Infections. One Health Adv. 2023, 1, 21. [Google Scholar] [CrossRef]







| Muscadine Grape Cultivar | Berry Color | Berry pH | Berry Brix | Bacterial Isolate Identified | Gram Stain |
|---|---|---|---|---|---|
| Late fry | Bronze | 3.22 ± 0.09 | 16.5 ± 0.4 | Tatumella ptyseos AM-36 | Negative |
| Summit | Bronze | 3.47 ± 0.16 | 17.13 ± 0.32 | Tatumella ptyseos AM-37 | Negative |
| Black fry | Black | 3.34 ± 0.12 | 16.33 ± 0.32 | Curtobacterium oryzae AM-38 | Positive |
| Cowart | Black | 3.61 ± 0.18 | 15.56 ± 0.21 | Staphylococcus aureus AM-39 | Positive |
| Bacillus tropicus AM-40 | Positive | ||||
| Jumbo | Black | 3.42 ± 0.09 | 17.6 ± 0.36 | Staphylococcus warneri AM-41 | Positive |
| Curtobacterium citreum AM-42 | Positive | ||||
| Scuppernong | Bronze | 3.52 ± 0.12 | 15.26 ± 0.15 | Curtobacterium oryzae AM-43 | Positive |
| Welder | Bronze | 3.31 ± 0.07 | 17.53 ± 0.40 | Paenibacillus cineris AM-44 | Positive |
| Tara | Bronze | 3.53 ± 0.05 | 15.36 ± 0.41 | Calidifontibacillus erzurumensis AM-45 | Positive |
| Hunt | Black | 3.35 ± 0.04 | 15.5 ± 0.36 | Calidifontibacillus erzurumensis AM-46 | Positive |
| Doreen | Bronze | 3.29 ± 0.04 | 13.46 ± 0.25 | Staphylococcus warneri AM-47 | Positive |
| Bacillus aerius AM-48 | Positive | ||||
| Southern Home | Black | 3.67 ± 0.07 | 16.5 ± 0.4 | Calidifontibacillus erzurumensis AM-49 | Positive |
| Southland | Black | 3.68 ± 0.09 | 15.56 ± 0.21 | Staphylococcus warneri AM-50 | Positive |
| Bacterial Isolate | Accession Number |
|---|---|
| Tatumella ptyseos AM-36 | PP915744 |
| Curtobacterium oryzae AM-38 | PP916015 |
| Staphylococcus aureus AM-39 | PP916060 |
| Bacillus tropicus AM-40 | PP916016 |
| Staphylococcus warneri AM-41 | PP916017 |
| Curtobacterium citreum AM-42 | PP916043 |
| Paenibacillus cineris AM-44 | PP916050 |
| Calidifontibacillus erzurumensis AM-46 | PP916051 |
| Bacillus aerius AM-48 | PP916056 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, M.; Sheikh, M.B. Isolation and Functional Characterization of Endophytic Bacteria from Muscadine Grape Berries: A Microbial Treasure Trove. Cells 2025, 14, 369. https://doi.org/10.3390/cells14050369
Agarwal M, Sheikh MB. Isolation and Functional Characterization of Endophytic Bacteria from Muscadine Grape Berries: A Microbial Treasure Trove. Cells. 2025; 14(5):369. https://doi.org/10.3390/cells14050369
Chicago/Turabian StyleAgarwal, Meenakshi, and Mehboob B. Sheikh. 2025. "Isolation and Functional Characterization of Endophytic Bacteria from Muscadine Grape Berries: A Microbial Treasure Trove" Cells 14, no. 5: 369. https://doi.org/10.3390/cells14050369
APA StyleAgarwal, M., & Sheikh, M. B. (2025). Isolation and Functional Characterization of Endophytic Bacteria from Muscadine Grape Berries: A Microbial Treasure Trove. Cells, 14(5), 369. https://doi.org/10.3390/cells14050369







