TIAM2S Operates Multifaced Talents to Alleviate Radiosensitivity, Restrict Apoptosis, Provoke Cell Propagation, and Escalate Cell Migration for Aggravating Radioresistance-Intensified Cervical Cancer Progression
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Radioresistant Cervical Cancer Cells
2.3. Reverse Transcription (RT) and Quantitative Polymerase Chain Reaction (QPCR)
2.4. siRNA and Plasmid Transfection
2.5. Clonogenic Survival Curve Assay After Irradiation Treatment
2.6. Flow Cytometric Analysis of Annexin V/Propidium Iodide Apoptosis Assay
2.7. BrdU Incorporation Assay
2.8. Time-Lapse Recording of Cell Migration
2.9. Fluorescent Labeling of the Cervical Cancer Cells
2.10. In Vivo Short-Term Locomotion of Tumor Cells to Lungs in Nude Mice Model
2.11. Western Blot
2.12. Statistical Analysis
3. Results
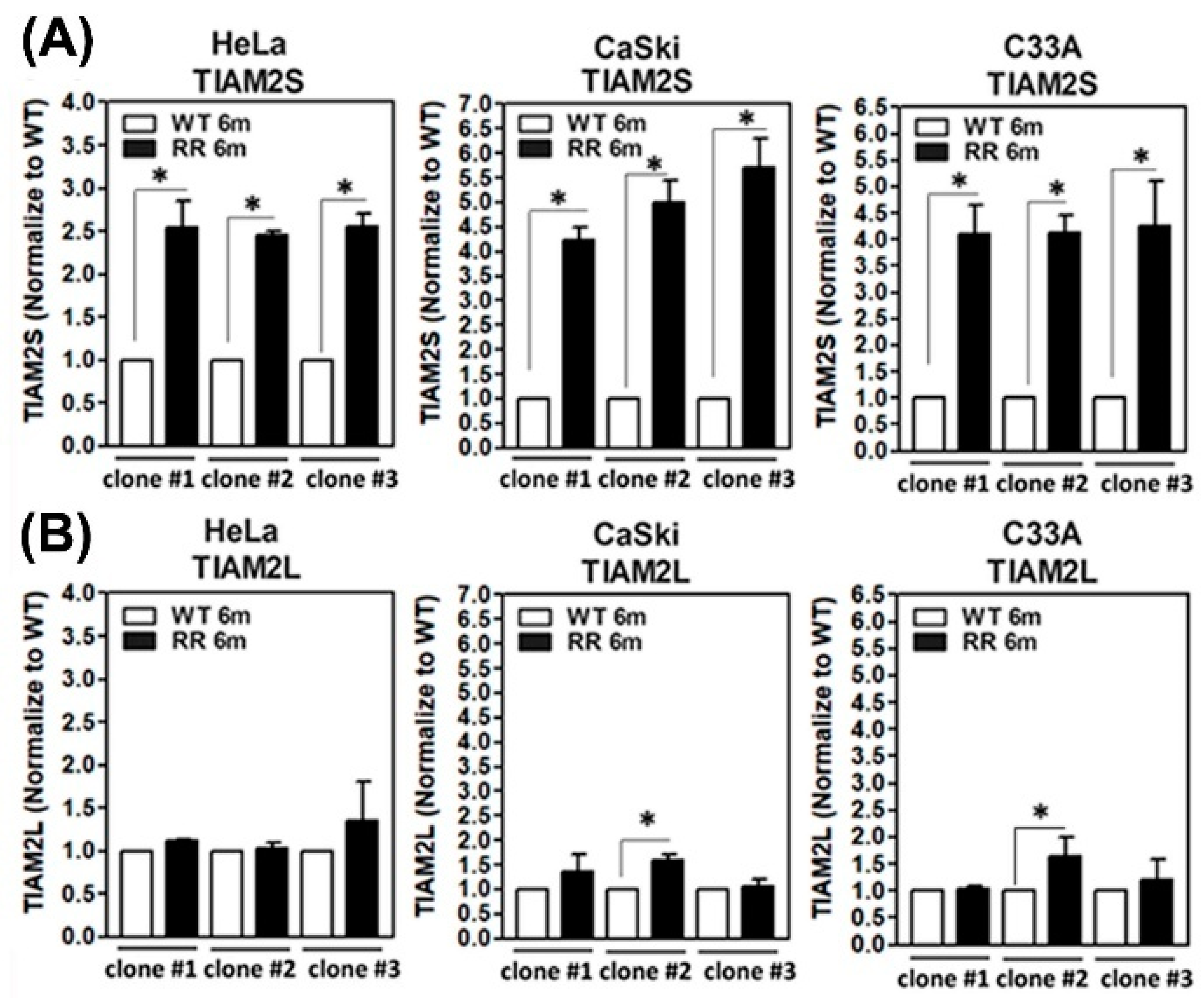
3.1. Amplified TIAM2S Level in Established Radioresistant HeLa, CaSli, and C33A Cell Clones
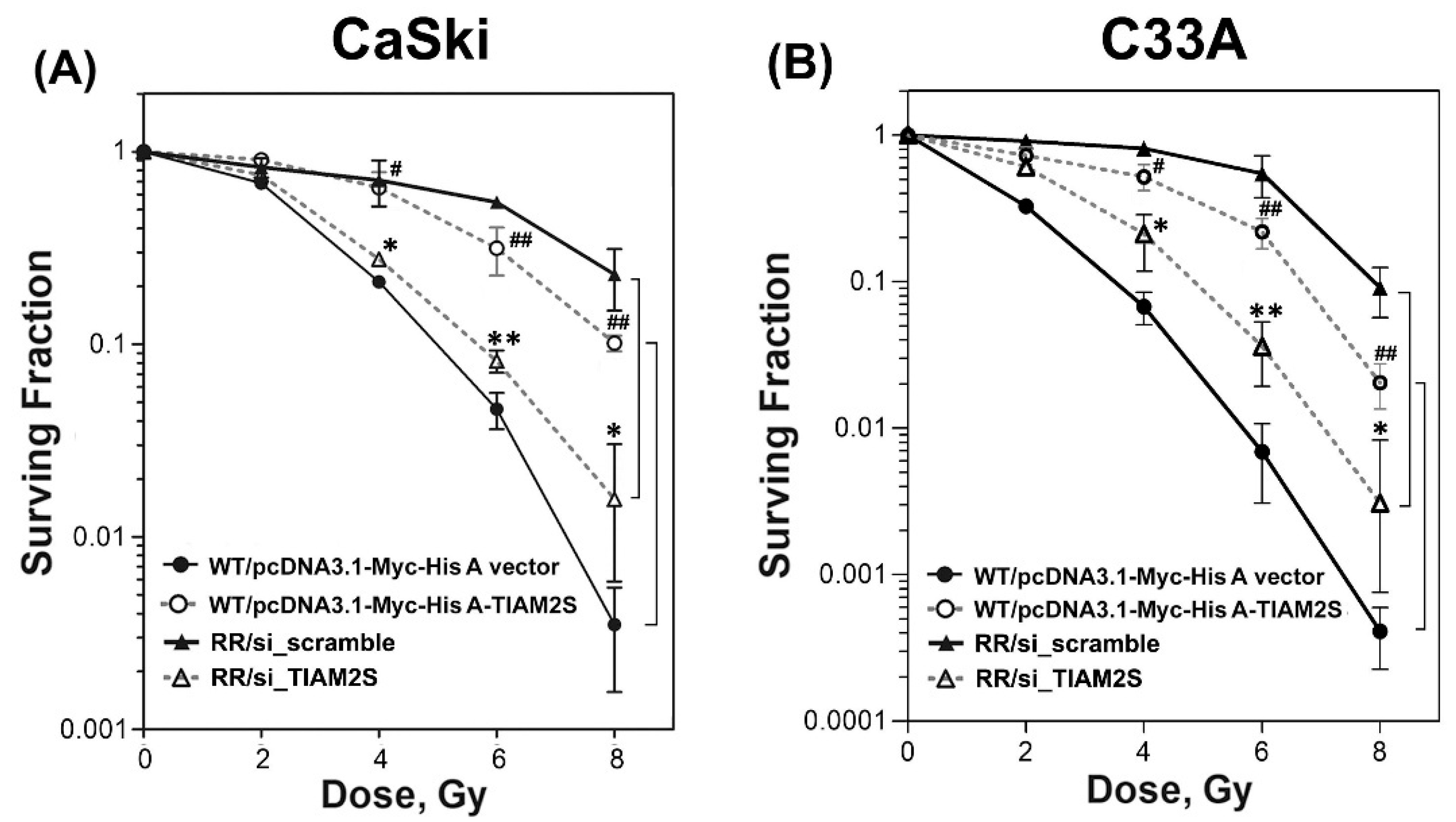
3.2. TIAM2S Manifested as a Radioprotector and Exhibited a Crucial Role in Modulating the Radiosensitivity of Radioresistant CaSki and C33A Cervical Cancer Cells
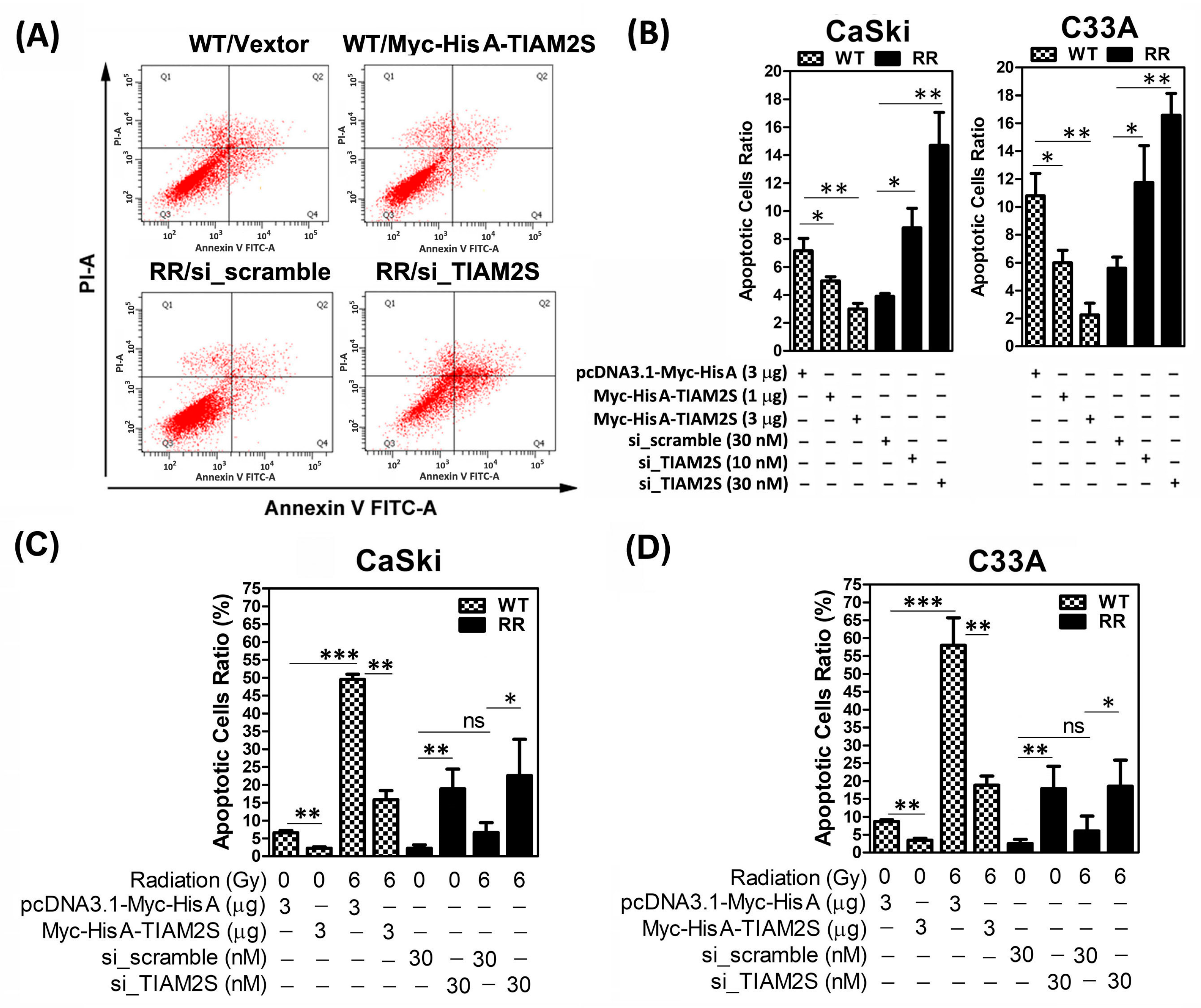
3.3. TIAM2S Played an Anti-Apoptosis Role in Cervical Cancer Cells
3.4. TIAM2S Exhibited Pro-Cell Propagation, While TIAM2S Blockage Effectively Abridged CaSki and C33A Radioresistant Cell Propagation
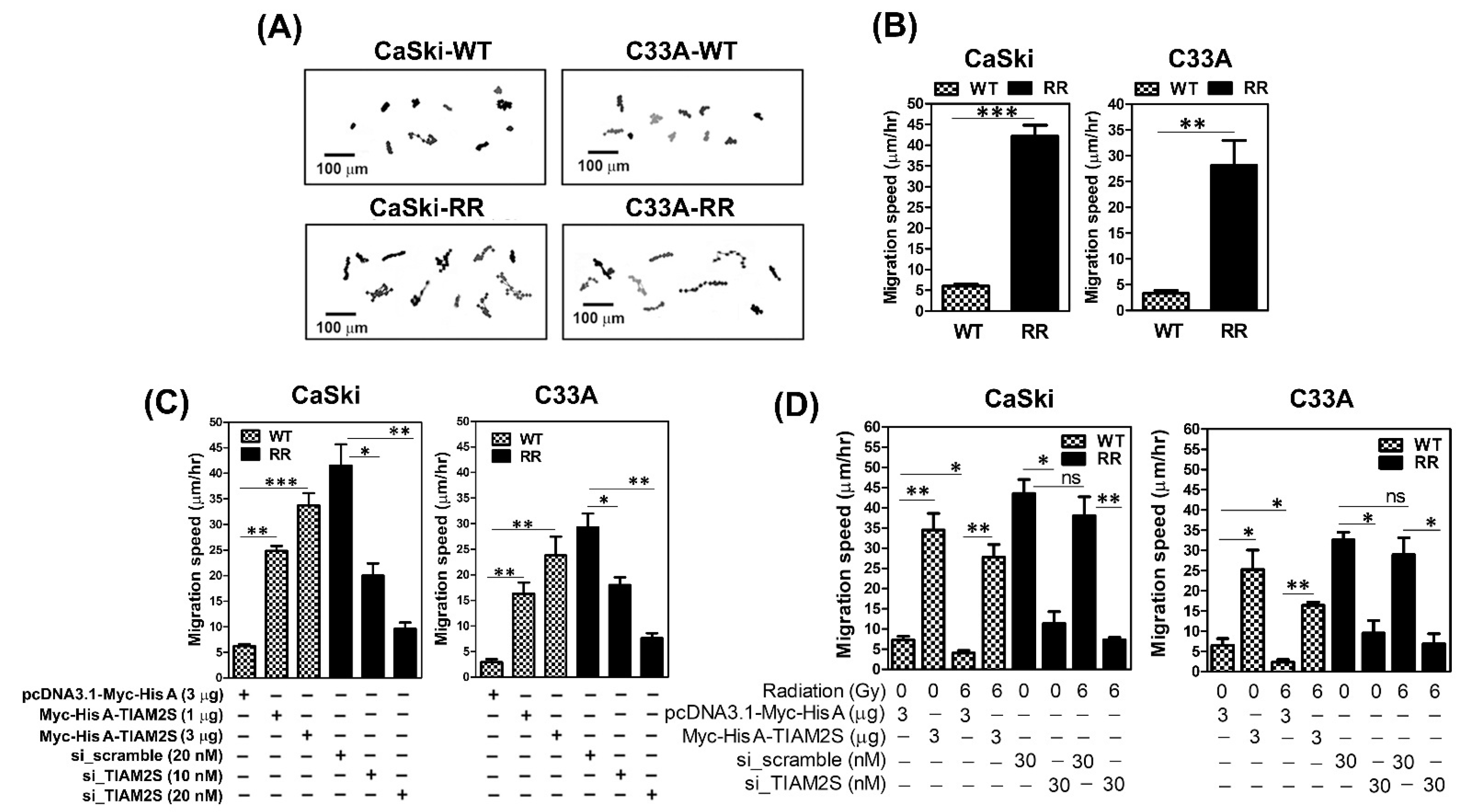
3.5. TIAM2S Accelerated the Cell Migrative Properties of CaSki and C33A Radioresistant Cells, as Confirmed by Time-Lapse Recording Cell Movement Assay
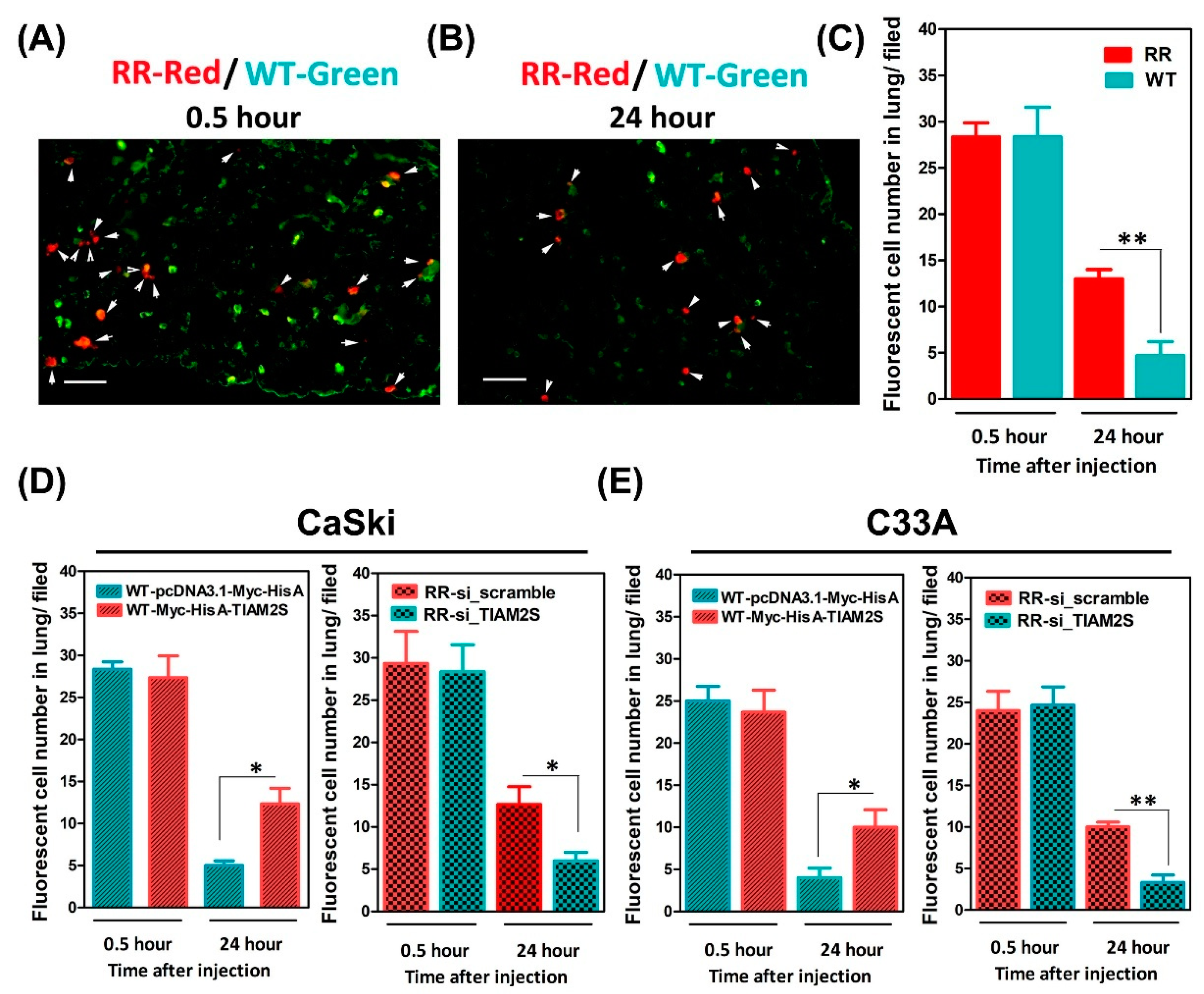
3.6. Escalated Lung Locomotion After Radioresistant Cells’ Injection; TIAM2S Abrogation Noticeably Interrupted Lung Locomotion in BALB/c Nude Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar]
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 64–84. [Google Scholar] [CrossRef]
- The, L. Eliminating cervical cancer. Lancet 2020, 395, 312. [Google Scholar]
- Jiang, W.; Huang, G.; Pan, S.; Chen, X.; Liu, T.; Yang, Z.; Chen, T.; Zhu, X. TRAIL-driven targeting and reversing cervical cancer radioresistance by seleno-nanotherapeutics through regulating cell metabolism. Drug Resist. Updat. 2024, 72, 101033. [Google Scholar] [CrossRef] [PubMed]
- Janicek, M.F.; Averette, H.E. Cervical cancer: Prevention, diagnosis, and therapeutics. CA Cancer J. Clin. 2001, 51, 92–114. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Cheng, X. Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 2016, 27, e43. [Google Scholar] [CrossRef] [PubMed]
- Beadle, B.M.; Jhingran, A.; Yom, S.S.; Ramirez, P.T.; Eifel, P.J. Patterns of Regional Recurrence After Definitive Radiotherapy for Cervical Cancer. Int. J. Radiat. Oncol. 2010, 76, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-H.; Tsai, C.-S.; Lai, C.-H.; Chang, T.-C.; Wang, C.-C.; Chou, H.-H.; Lee, S.P.; Hsueh, S. Recurrent squamous cell carcinoma of cervix after definitive radiotherapy. Int. J. Radiat. Oncol. 2004, 60, 249–257. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Leng, S.; Martin, K.A.; Kim, E.; Gorman, S.; Duhl, D.M. Cloning and Characterization of T-Cell Lymphoma Invasion and Metastasis 2 (TIAM2), a Novel Guanine Nucleotide Exchange Factor Related to TIAM1. Genomics 1999, 61, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Leeuwen, F.N.; Kain, H.E.; Kammen, R.A.; Michiels, F.; Kranenburg, O.W.; Collard, J.G. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J. Cell Biol. 1997, 139, 797–807. [Google Scholar] [CrossRef]
- Stankiewicz, T.R.; Linseman, D.A. Rho family GTPases: Key players in neuronal development, neuronal survival, and neurodegeneration. Front. Cell. Neurosci. 2014, 8, 314. [Google Scholar] [CrossRef]
- Chu, C.; Chen, J.; Chuang, P.; Su, C.; Chan, Y.; Yang, Y.; Chiang, Y.; Su, Y.; Gean, P.; Sun, H.S. TIAM2S as a novel regulator for serotonin level enhances brain plasticity and locomotion behavior. FASEB J. 2020, 34, 3267–3288. [Google Scholar] [CrossRef]
- Chu, C.; Chen, J.; Chan, Y.; Lu, W.; Huang, Y.; Mao, P.; Sze, C.; Sun, H.S. TIAM2S-positive microglia enhance inflammation and neurotoxicity through soluble ICAM-1-mediated immune priming. FASEB J. 2023, 37, e23242. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.H.; Su, C.H.; Hsiao, Y.H.; Yu, C.C.; Liao, Y.C.; Mao, P.C.; Chen, J.S.; Sun, H.S. Overexpression of TIAM2S, a Critical Regulator for the Hippocampal-Medial Prefrontal Cortex Network, Progresses Age-Related Spatial Memory Impairment. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2024, 79, glae191. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-H.; Chuang, P.-C.; Huang, E.-Y.; Yang, K.D. Radiation-Induced Increase in Cell Migration and Metastatic Potential of Cervical Cancer Cells Operates Via the K-Ras Pathway. Am. J. Pathol. 2012, 180, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.; Wu, M.; Shoji, Y.; Tsai, S. Downregulation of CD36 results in reduced phagocytic ability of peritoneal macrophages of women with endometriosis. J. Pathol. 2009, 219, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.C.; Sun, H.S.; Chen, T.M.; Tsai, S.J. Prostaglandin E2 induces fibroblast growth factor 9 via EP3-dependent protein kinase Cdelta and Elk-1 signaling. Mol. Cell. Biol. 2006, 26, 8281–8292. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Su, I.; Leu, Y.; Young, K.; Sun, H.S. Expression of T-cell lymphoma invasion and metastasis 2 (TIAM2) promotes proliferation and invasion of liver cancer. Int. J. Cancer 2011, 130, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Iwasaki, S.; Yoshino, H. Effects and Related Mechanisms of the Senolytic Agent ABT-263 on the Survival of Ir-radiated A549 and Ca9-22 Cancer Cells. Int. J. Mol. Sci. 2021, 22, 13233. [Google Scholar] [CrossRef]
- Hu, J.; Verkman, A.S. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006, 20, 1892–1894. [Google Scholar] [CrossRef]
- Kim, S.; Takahashi, H.; Lin, W.-W.; Descargues, P.; Grivennikov, S.; Kim, Y.; Luo, J.-L.; Karin, M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009, 457, 102–106. [Google Scholar] [CrossRef]
- Liang, M.; Sheng, L.; Ke, Y.; Wu, Z. The research progress on radiation resistance of cervical cancer. Front. Oncol. 2024, 14, 1380448. [Google Scholar] [CrossRef]
- Huang, E.-Y.; Wang, C.-J.; Chen, H.-C.; Fang, F.-M.; Huang, Y.-J.; Wang, C.-Y.; Hsu, H.-C. Multivariate Analysis of Para-Aortic Lymph Node Recurrence After Definitive Radiotherapy for Stage IB-IVA Squamous Cell Carcinoma of Uterine Cervix. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, H.; Perez, C.A.; Grigsby, P.W.; Lockett, M.A. Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sang, N. Hypoxia-Inducible Factor-1: A Critical Player in the Survival Strategy of Stressed Cells. J. Cell. Biochem. 2016, 117, 267–278. [Google Scholar] [CrossRef]
- Kim, T.W. Nodakenin Induces ROS-Dependent Apoptotic Cell Death and ER Stress in Radioresistant Breast Cancer. Antioxidants 2023, 12, 492. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Ko, S.G. A Novel PPARgamma Modulator Falcarindiol Mediates ER Stress-Mediated Apoptosis by Regulating NOX4 and Overcomes Radioresistance in Breast Cancer. Antioxidants 2024, 13, 1533. [Google Scholar] [CrossRef] [PubMed]
- Halazonetis, T.D.; Gorgoulis, V.G.; Bartek, J. An Oncogene-Induced DNA Damage Model for Cancer Development. Science 2008, 319, 1352–1355. [Google Scholar] [CrossRef]
- Fu, H.-C.; Chuang, I.-C.; Yang, Y.-C.; Chuang, P.-C.; Lin, H.; Ou, Y.-C.; Chang Chien, C.-C.; Huang, H.-S.; Kang, H.-Y. Low P16INK4A Expression Associated with High Expression of Cancer Stem Cell Markers Predicts Poor Prognosis in Cervical Cancer after Radiotherapy. Int. J. Mol. Sci. 2018, 19, 2541. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhang, C.; Chen, H.; Ren, M.; Liu, X. Cathepsins Trigger Cell Death and Regulate Radioresistance in Glioblastoma. Cells 2022, 11, 4108. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Zhao, Y.; Moran, M.S.; Yang, Q.; Liu, Q.; Yuan, C.; Hong, S. Metadherin regulates radioresistance in cervical cancer cells. Oncol. Rep. 2012, 27, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, S.; Tian, G.; Zhao, L.; Wang, H.; Li, Y.; Shen, Y.; Han, L. KLK5 is associated with the radioresistance, aggression, and progression of cervical cancer. Gynecol. Oncol. 2022, 166, 138–147. [Google Scholar] [CrossRef]
- Sun, Q.; Guo, Y.; Liu, X.; Czauderna, F.; Carr, M.I.; Zenke, F.T.; Blaukat, A.; Vassilev, L.T. Therapeutic Implications of p53 Status on Cancer Cell Fate Following Exposure to Ionizing Radiation and the DNA-PK Inhibitor M3814. Mol. Cancer Res. 2019, 17, 2457–2468. [Google Scholar] [CrossRef] [PubMed]
- Sample, K.M. DNA repair gene expression is associated with differential prognosis between HPV16 and HPV18 positive cervical cancer patients following radiation therapy. Sci. Rep. 2020, 10, 2774. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, F.; Liu, Y.; Zhao, Q.; Tang, H. DNMT1 recruited by EZH2-mediated silencing of miR-484 contributes to the malignancy of cervical cancer cells through MMP14 and HNF1A. Clin. Epigenetics 2019, 11, 186. [Google Scholar] [CrossRef]
- Xu, T.; Zeng, Y.; Shi, L.; Yang, Q.; Chen, Y.; Wu, G.; Li, G.; Xu, S. Targeting NEK2 impairs oncogenesis and radioresistance via inhibiting the Wnt1/beta-catenin signaling pathway in cervical cancer. J. Exp. Clin. Cancer Res. 2020, 39, 183. [Google Scholar] [CrossRef]
- Samarzija, I.; Beard, P. Hedgehog pathway regulators influence cervical cancer cell proliferation, survival and migration. Biochem. Biophys. Res. Commun. 2012, 425, 64–69. [Google Scholar] [CrossRef]
- Yen, W.; Ke, W.; Hung, J.; Chen, T.; Chen, J.; Sun, H.S. Sp1-mediated ectopic expression of T-cell lymphoma invasion and metastasis 2 in hepatocellular carcinoma. Cancer Med. 2016, 5, 465–477. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Han, C.-G.; Liu, J.-T.; Wang, C.-L.; Wang, Y.; Cheng, L.-Y. TIAM2 Enhances Non-small Cell Lung Cancer Cell Invasion and Motility. Asian Pac. J. Cancer Prev. 2013, 14, 6305–6309. [Google Scholar] [CrossRef]
- Chan, Y.-L.; Lai, W.-C.; Chen, J.-S.; Tseng, J.T.-C.; Chuang, P.-C.; Jou, J.; Lee, C.-T.; Sun, H.S. TIAM2S Mediates Serotonin Homeostasis and Provokes a Pro-Inflammatory Immune Microenvironment Permissive for Colorectal Tumorigenesis. Cancers 2020, 12, 1844. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, P.-C.; Su, W.-H.; Hsieh, C.-H.; Huang, E.-Y. TIAM2S Operates Multifaced Talents to Alleviate Radiosensitivity, Restrict Apoptosis, Provoke Cell Propagation, and Escalate Cell Migration for Aggravating Radioresistance-Intensified Cervical Cancer Progression. Cells 2025, 14, 339. https://doi.org/10.3390/cells14050339
Chuang P-C, Su W-H, Hsieh C-H, Huang E-Y. TIAM2S Operates Multifaced Talents to Alleviate Radiosensitivity, Restrict Apoptosis, Provoke Cell Propagation, and Escalate Cell Migration for Aggravating Radioresistance-Intensified Cervical Cancer Progression. Cells. 2025; 14(5):339. https://doi.org/10.3390/cells14050339
Chicago/Turabian StyleChuang, Pei-Chin, Wen-Hong Su, Ching-Hua Hsieh, and Eng-Yen Huang. 2025. "TIAM2S Operates Multifaced Talents to Alleviate Radiosensitivity, Restrict Apoptosis, Provoke Cell Propagation, and Escalate Cell Migration for Aggravating Radioresistance-Intensified Cervical Cancer Progression" Cells 14, no. 5: 339. https://doi.org/10.3390/cells14050339
APA StyleChuang, P.-C., Su, W.-H., Hsieh, C.-H., & Huang, E.-Y. (2025). TIAM2S Operates Multifaced Talents to Alleviate Radiosensitivity, Restrict Apoptosis, Provoke Cell Propagation, and Escalate Cell Migration for Aggravating Radioresistance-Intensified Cervical Cancer Progression. Cells, 14(5), 339. https://doi.org/10.3390/cells14050339






