Multiple Intra-Articular Injections of Adipose-Derived Mesenchymal Stem Cells for Canine Osteoarthritis Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Detection of Morphology and Proliferative Capacity of Canine ADSCs
2.2. Isolation of Adipose-Derived Stem Cells
2.3. Multipotent Differentiation Detection
2.4. Preparation and Treatment of Mouse OA Model
2.5. Preparation and Treatment of Canine OA Model
2.6. Histological Analysis
2.7. Scratch Assay
2.8. Immunofluorescence
2.9. Statistical Analysis
3. Results
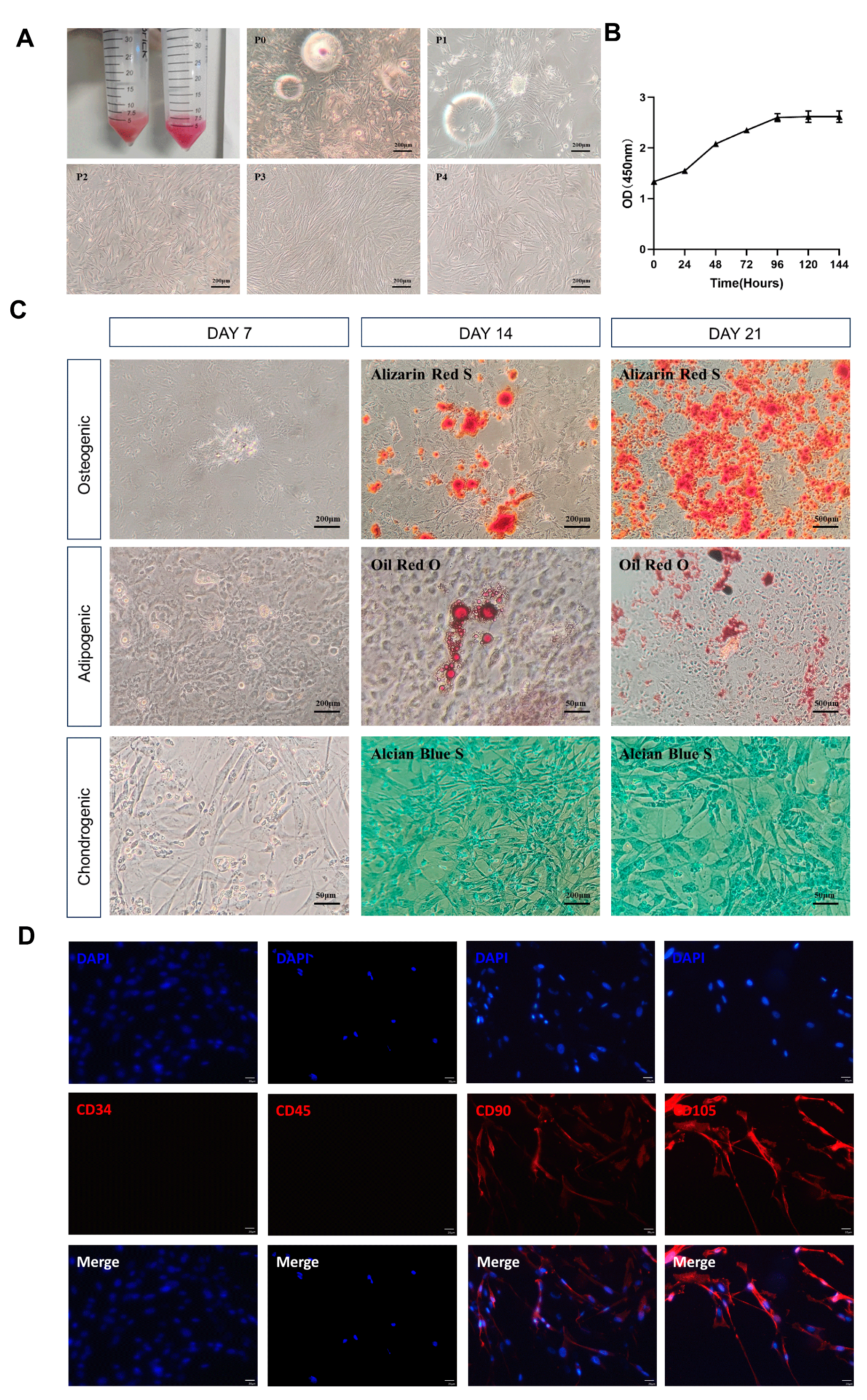
3.1. Characteristic Identification of Canine ADSCs
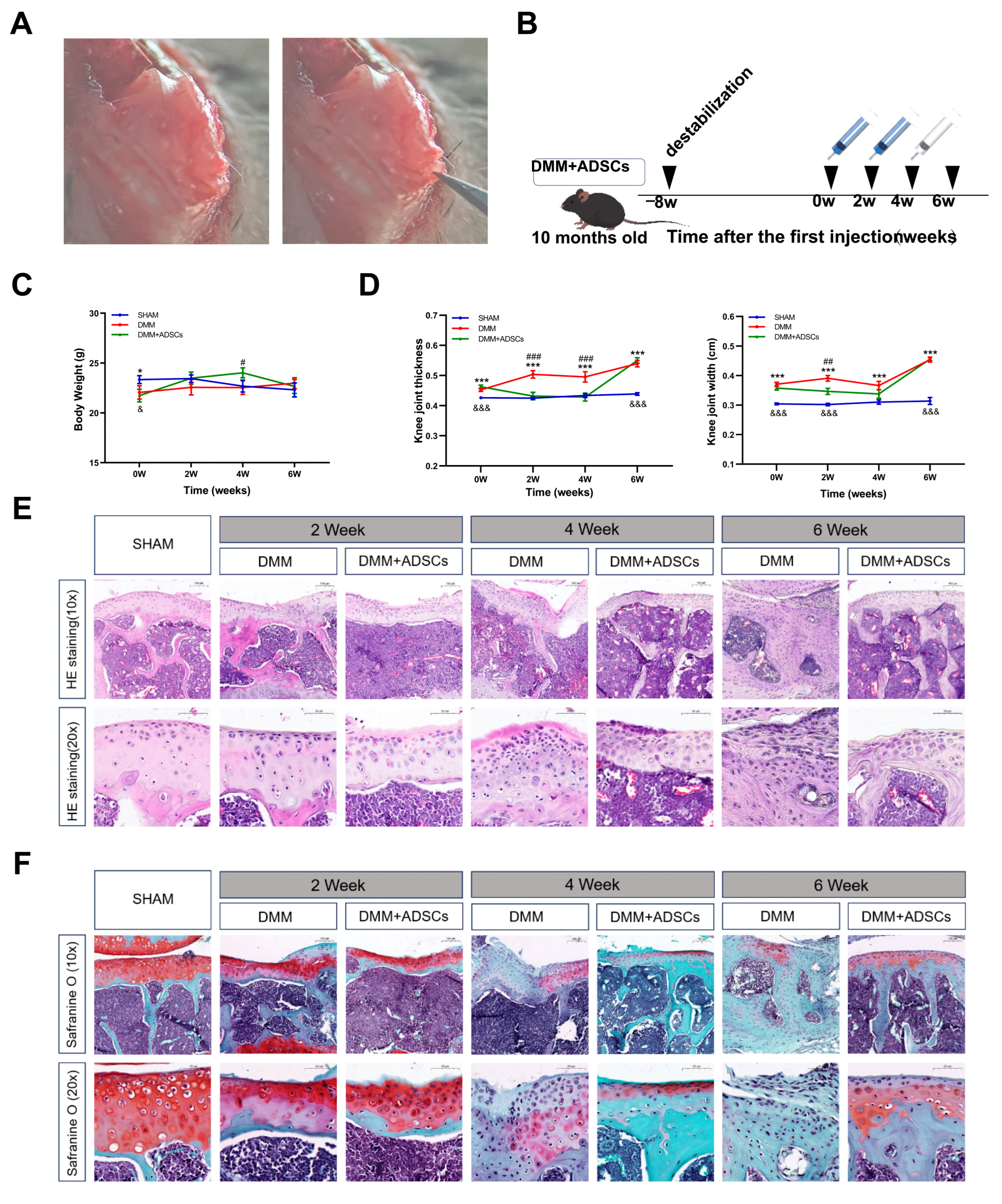
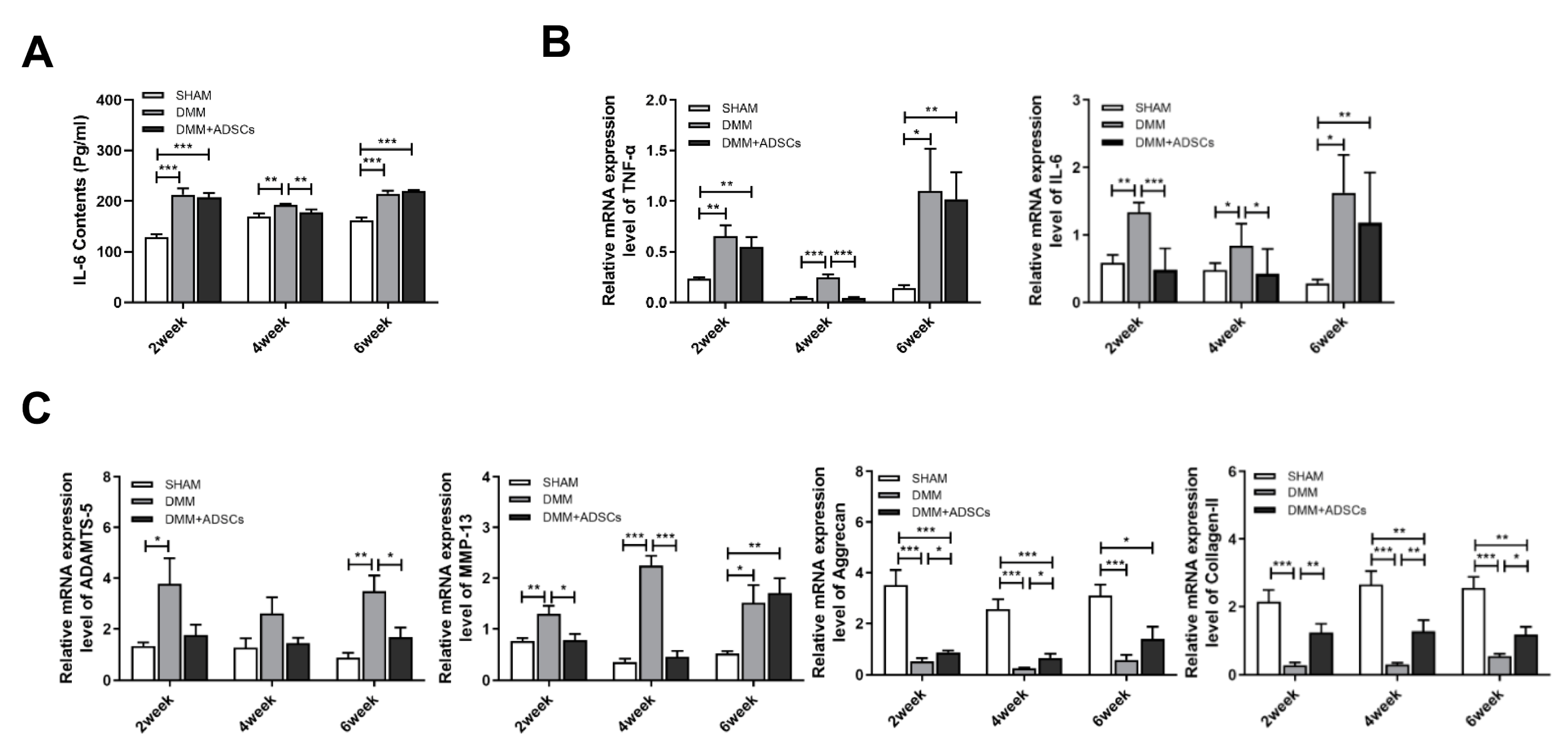
3.2. Comparison of the Therapeutic Effects of ADSCs at Different Time Points on Mouse Osteoarthritis
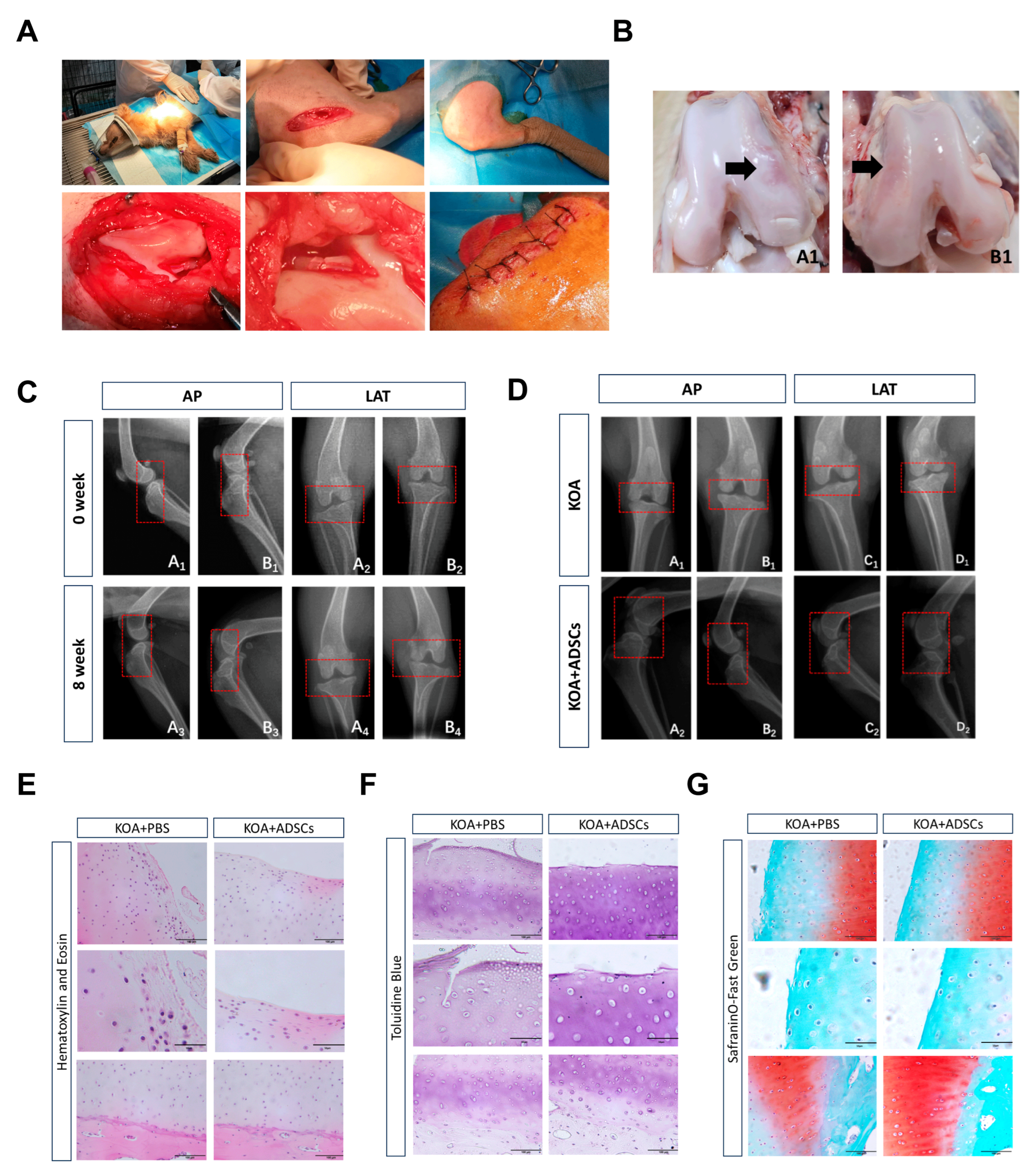
3.3. Clinical Manifestations During Dog Modeling and ADSC Treatment
3.4. Comparison with ADSC Treatment of Canine Osteoarthritis
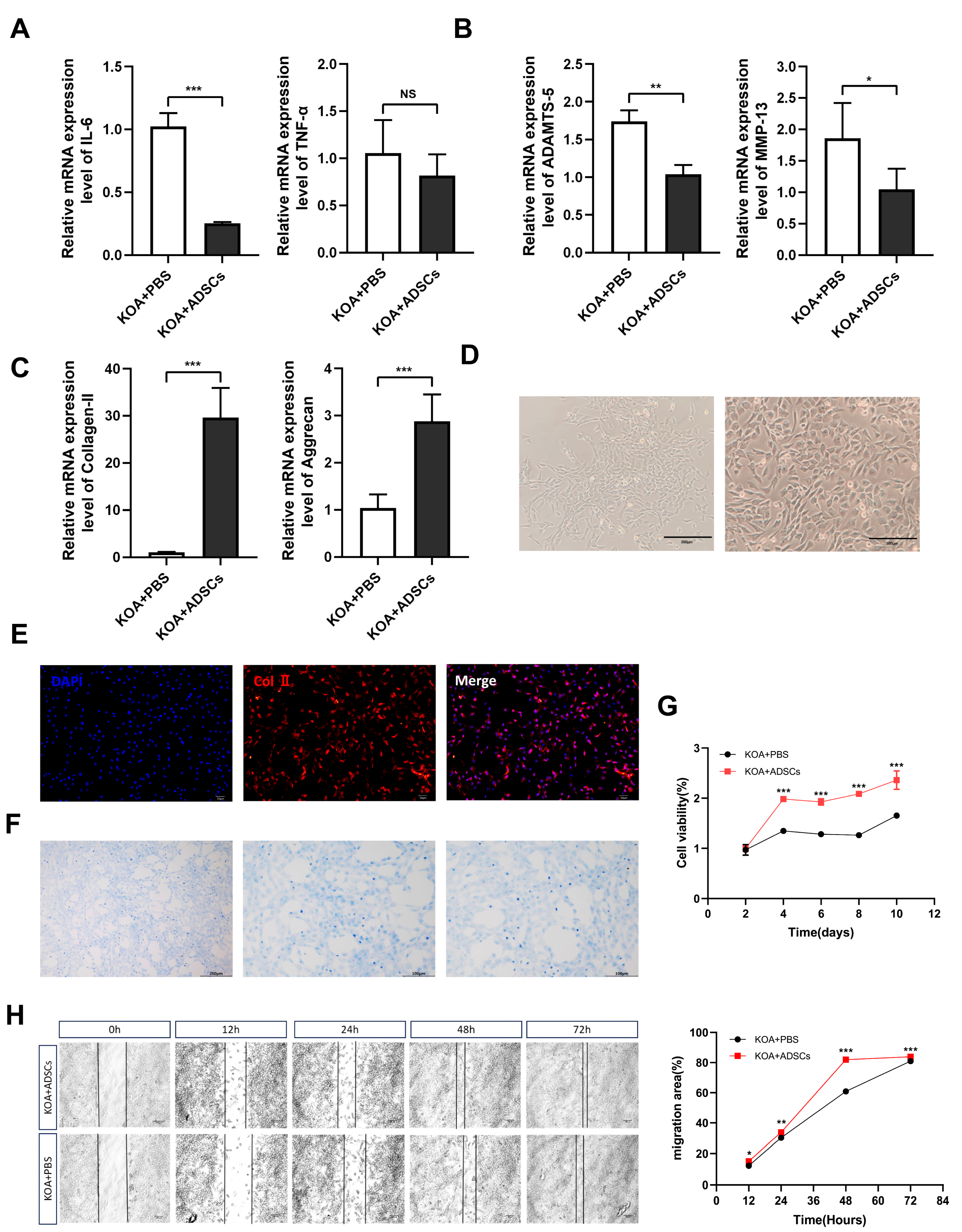
3.5. In Vitro Culture, Proliferation, and Migration Ability Detection of OA Canine Chondrocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, K.L.; O’neill, D.G.; Brodbelt, D.C.; Church, D.B.; Meeson, R.L.; Sargan, D.; Summers, J.F.; Zulch, H.; Collins, L.M. Prevalence, duration and risk factors for appendicular osteoarthritis in a UK dog population under primary veterinary care. Sci. Rep. 2018, 8, 5641. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.-M.; Koh, K.-H.; Jeon, I.-H. Total Elbow Arthroplasty: Clinical Outcomes, Complications, and Revision Surgery. Clin. Orthop. Surg. 2019, 11, 369–379. [Google Scholar] [CrossRef]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hunter, D.J.; Schofield, D.; Callander, E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014, 10, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Poulet, B.; Staines, K.A. New developments in osteoarthritis and cartilage biology. Curr. Opin. Pharmacol. 2016, 28, 8–13. [Google Scholar] [CrossRef]
- Anderson, K.L.; Zulch, H.; O’Neill, D.G.; Meeson, R.L.; Collins, L.M. Risk Factors for Canine Osteoarthritis and Its Predisposing Arthropathies: A Systematic Review. Front. Veter. Sci. 2020, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.; Lippuner, K.; Keel, M.; Shintani, N. An educational review of cartilage repair: Precepts & practice—Myths & misconceptions—Progress & prospects. Osteoarthr. Cartil. 2015, 23, 334–350. [Google Scholar] [CrossRef]
- Pye, C.; Bruniges, N.; Peffers, M.; Comerford, E. Advances in the pharmaceutical treatment options for canine osteoarthritis. J. Small Anim. Pract. 2022, 63, 721–738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allaith, S.; Tucker, L.J.; Innes, J.F.; Arthurs, G.; Vezzoni, A.; Morrison, S.; Onyett, J.; Stork, C.K.; Witte, P.; Denny, H.; et al. Outcomes and complications reported from a multiuser canine hip replacement registry over a 10-year period. Veter. Surg. 2023, 52, 196–208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem. Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef]
- Kim, K.-I.; Lee, M.C.; Lee, J.H.; Moon, Y.-W.; Lee, W.-S.; Lee, H.-J.; Hwang, S.-C.; In, Y.; Shon, O.-J.; Bae, K.-C.; et al. Clinical Efficacy and Safety of the Intra-articular Injection of Autologous Adipose-Derived Mesenchymal Stem Cells for Knee Osteoarthritis: A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Sports Med. 2023, 51, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Maioli, M.; Basoli, V.; Santaniello, S.; Cruciani, S.; Delitala, A.P.; Pinna, R.; Milia, E.; Grillari-Voglauer, R.; Fontani, V.; Rinaldi, S.; et al. Osteogenesis from Dental Pulp Derived Stem Cells: A Novel Conditioned Medium Including Melatonin within a Mixture of Hyaluronic, Butyric, and Retinoic Acids. Stem Cells Int. 2016, 2016, 2056416. [Google Scholar] [CrossRef] [PubMed]
- In’t Anker, P.S.; Scherjon, S.A.; der Keur, C.K.; de Groot-Swings, G.M.J.S.; Claas, F.H.J.; Fibbe, W.E.; Kanhai, H.H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Kubosch, E.J.; Lang, G.; Furst, D.; Kubosch, D.; Izadpanah, K.; Rolauffs, B.; Sudkamp, N.P.; Schmal, H. The Potential for Synovium-derived Stem Cells in Cartilage Repair. Curr. Stem Cell Res. Ther. 2018, 13, 174–184. [Google Scholar] [CrossRef] [PubMed]
- da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef]
- Diekman, B.O.; Wu, C.-L.; Louer, C.R.; Furman, B.D.; Huebner, J.L.; Kraus, V.B.; Olson, S.A.; Guilak, F. Intra-articular delivery of purified mesenchymal stem cells from C57BL/6 or MRL/MpJ superhealer mice prevents posttraumatic arthritis. Cell Transplant. 2013, 22, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Toghraie, F.; Razmkhah, M.; Gholipour, M.A.; Faghih, Z.; Chenari, N.; Nezhad, S.T.; Dehghani, S.N.; Ghaderi, A. Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch. Iran Med. 2012, 15, 495–499. [Google Scholar] [PubMed]
- Miao, Z.; Lu, Z.; Luo, S.; Lei, D.; He, Y.; Wu, H.; Zhao, J.; Zheng, L. Murine and Chinese cobra venom-derived nerve growth factor stimulate chondrogenic differentiation of BMSCs in vitro: A comparative study. Mol. Med. Rep. 2018, 18, 3341–3349. [Google Scholar] [CrossRef]
- Dechêne, L.; Colin, M.; Demazy, C.; Fransolet, M.; Niesten, A.; Arnould, T.; Serteyn, D.; Dieu, M.; Renard, P. Characterization of the Proteins Secreted by Equine Muscle-Derived Mesenchymal Stem Cells Exposed to Cartilage Explants in Osteoarthritis Model. Stem Cell Rev. Rep. 2023, 19, 550–567. [Google Scholar] [CrossRef]
- Shah, K.; Drury, T.; Roic, I.; Hansen, P.; Malin, M.; Boyd, R.; Sumer, H.; Ferguson, R. Outcome of Allogeneic Adult Stem Cell Therapy in Dogs Suffering from Osteoarthritis and Other Joint Defects. Stem Cells Int. 2018, 2018, 7309201. [Google Scholar] [CrossRef] [PubMed]
- Kriston-Pál, É.; Czibula, Á.; Gyuris, Z.; Balka, G.; Seregi, A.; Sükösd, F.; Süth, M.; Kiss-Tóth, E.; Haracska, L.; Uher, F.; et al. Characterization and therapeutic application of canine adipose mesenchymal stem cells to treat elbow osteoarthritis. Can. J. Vet. Res. 2017, 81, 73–78. [Google Scholar] [PubMed]
- Iismaa, S.E.; Kaidonis, X.; Nicks, A.M.; Bogush, N.; Kikuchi, K.; Naqvi, N.; Harvey, R.P.; Husain, A.; Graham, R.M. Comparative regenerative mechanisms across different mammalian tissues. npj Regen. Med. 2018, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Man, G.S.; Mologhianu, G. Osteoarthritis pathogenesis—A complex process that involves the entire joint. J. Med. Life 2014, 7, 37–41. [Google Scholar] [PubMed]
- Toma, C.; Wagner, W.R.; Bowry, S.; Schwartz, A.; Villanueva, F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: In vivo observations of cell kinetics. Circ. Res. 2009, 104, 398–402. [Google Scholar] [CrossRef]
- Kidd, S.; Spaeth, E.; Dembinski, J.L.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, V.L.; Weil, M.; Andreeff, M.; Marini, F.C. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem. Cells 2009, 27, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Harman, R.; Carlson, K.; Gaynor, J.; Gustafson, S.; Dhupa, S.; Clement, K.; Hoelzler, M.; McCarthy, T.; Schwartz, P.; Adams, C. A Prospective, Randomized, Masked, and Placebo-Controlled Efficacy Study of Intraarticular Allogeneic Adipose Stem Cells for the Treatment of Osteoarthritis in Dogs. Front. Veter. Sci. 2016, 3, 81. [Google Scholar] [CrossRef]
- Cabon, Q.; Febre, M.; Gomez, N.; Cachon, T.; Pillard, P.; Carozzo, C.; Saulnier, N.; Robert, C.; Livet, V.; Rakic, R.; et al. Long-Term Safety and Efficacy of Single or Repeated Intra-Articular Injection of Allogeneic Neonatal Mesenchymal Stromal Cells for Managing Pain and Lameness in Moderate to Severe Canine Osteoarthritis Without Anti-inflammatory Pharmacological Support: Pilot Clinical Study. Front. Veter. Sci. 2019, 6, 10. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Centeno, C.J.; Al-Sayegh, H.; Freeman, M.D.; Smith, J.; Murrell, W.D.; Bubnov, R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int. Orthop. 2016, 40, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ezzelarab, M.B.; Cooper, D.K. Do mesenchymal stem cells function across species barriers? Relevance for xenotransplantation. Xenotransplantation 2012, 19, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, N.; Viguier, E.; Perrier-Groult, E.; Chenu, C.; Pillet, E.; Roger, T.; Maddens, S.; Boulocher, C. Intra-articular administration of xenogeneic neonatal Mesenchymal Stromal Cells early after meniscal injury down-regulates metalloproteinase gene expression in synovium and prevents cartilage degradation in a rabbit model of osteoarthritis. Osteoarthr. Cartil. 2015, 23, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noël, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017, 7, 16214. [Google Scholar] [CrossRef]
- Shang, Z.; Wanyan, P.; Zhang, B.; Wang, M.; Wang, X. A systematic review, umbrella review, and quality assessment on clinical translation of stem cell therapy for knee osteoarthritis: Are we there yet? Stem. Cell Res. Ther. 2023, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Alhambra, D.; Judge, A.; Javaid, M.K.; Cooper, C.; Diez-Perez, A.; Arden, N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2014, 73, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, A.; Rahman, H.S.; Markov, A.; Endjun, J.J.; Zekiy, A.O.; Chartrand, M.S.; Beheshtkhoo, N.; Kouhbanani, M.A.J.; Marofi, F.; Nikoo, M.; et al. Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res. Ther. 2021, 12, 297. [Google Scholar] [CrossRef]
- Zanotti, L.; Sarukhan, A.; Dander, E.; Castor, M.; Cibella, J.; Soldani, C.; Trovato, A.E.; Ploia, C.; Luca, G.; Calvitti, M.; et al. Encapsulated mesenchymal stem cells for in vivo immunomodulation. Leukemia 2013, 27, 500–503. [Google Scholar] [CrossRef]
- An, X.; Wang, J.; Xu, K.; Zhao, R.C.; Su, J. Perspectives on Osteoarthritis Treatment with Mesenchymal Stem Cells and Radix Achyranthis Bidentatae. Aging Dis. 2024, 15, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Maccario, R.; Moretta, A.; Cometa, A.; Montagna, D.; Comoli, P.; Locatelli, F.; Podestà, M.; Frassoni, F. Human mesenchymal stem cells and cyclosporin a exert a synergistic suppressive effect on in vitro activation of alloantigen-specific cytotoxic lymphocytes. Biol. Blood Marrow Transplant. 2005, 11, 1031–1032. [Google Scholar] [CrossRef] [PubMed]
- de la Garza-Rodea, A.S.; Verweij, M.C.; Boersma, H.; van der Velde-van Dijke, I.; de Vries, A.A.F.; Hoeben, R.C.; van Bekkum, D.W.; Wiertz, E.J.H.J.; Knaän-Shanzer, S. Exploitation of herpesvirus immune evasion strategies to modify the immunogenicity of human mesenchymal stem cell transplants. PLoS ONE 2011, 6, e14493. [Google Scholar] [CrossRef]
- Matas, J.; Orrego, M.; Amenabar, D.; Infante, C.; Tapia-Limonchi, R.; Cadiz, M.I.; Alcayaga-Miranda, F.; González, P.L.; Muse, E.; Khoury, M.; et al. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl. Med. 2019, 8, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Conaghan, P.G.; Cook, A.D.; Hamilton, J.A.; Tak, P.P. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat. Rev. Rheumatol. 2019, 15, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Eymard, F.; Pigenet, A.; Citadelle, D.; Flouzat-Lachaniette, C.; Poignard, A.; Benelli, C.; Berenbaum, F.; Chevalier, X.; Houard, X. Induction of an inflammatory and prodegradative phenotype in autologous fibroblast-like synoviocytes by the infrapatellar fat pad from patients with knee osteoarthritis. Arthritis Rheumatol. 2014, 66, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B. Osteoarthritis and cartilage: The role of cytokines. Curr. Rheumatol. Rep. 2000, 2, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; McKelvey, K.; Shen, K.; Minhas, N.; March, L.; Park, S.-Y.; Jackson, C.J. Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatology 2014, 53, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Tetlow, L.C.; Adlam, D.J.; Woolley, D.E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: Associations with degenerative changes. Arthritis. Rheum. 2001, 44, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Lakin, B.A.; Snyder, B.D.; Grinstaff, M.W. Assessing Cartilage Biomechanical Properties: Techniques for Evaluating the Functional Performance of Cartilage in Health and Disease. Annu. Rev. Biomed. Eng. 2017, 19, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-K.; Li, G.-W.; Zeng, C.; Lin, C.-X.; Huang, L.-S.; Huang, G.-X.; Zhao, C.; Feng, S.-Y.; Fang, H. Mechanical stress contributes to osteoarthritis development through the activation of transforming growth factor beta 1 (TGF-β1). Bone Jt. Res. 2018, 7, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Kyurkchiev, D.; Bochev, I.; Ivanova-Todorova, E.; Mourdjeva, M.; Oreshkova, T.; Belemezova, K.; Kyurkchiev, S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem. Cells 2014, 6, 552–570. [Google Scholar] [CrossRef] [PubMed]
- Ichiseki, T.; Shimasaki, M.; Ueda, Y.; Ueda, S.; Tsuchiya, M.; Souma, D.; Kaneuji, A.; Kawahara, N. Intraarticularly-Injected Mesenchymal Stem Cells Stimulate Anti-Inflammatory Molecules and Inhibit Pain Related Protein and Chondrolytic Enzymes in a Monoiodoacetate-Induced Rat Arthritis Model. Int. J. Mol. Sci. 2018, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Szabó, E.; Fajka-Boja, R.; Kriston-Pál, É.; Hornung, Á.; Makra, I.; Kudlik, G.; Uher, F.; Katona, R.L.; Monostori, É.; Czibula, Á. Licensing by Inflammatory Cytokines Abolishes Heterogeneity of Immunosuppressive Function of Mesenchymal Stem Cell Population. Stem. Cells Dev. 2015, 24, 2171–2180. [Google Scholar] [CrossRef]
- Gabriel, S.E.; Crowson, C.S.; O’Fallon, W.M. Comorbidity in arthritis. J. Rheumatol. 1999, 26, 2475–2479. [Google Scholar]
- Vadhan, A.; Gupta, T.; Hsu, W.-L. Mesenchymal Stem Cell-Derived Exosomes as a Treatment Option for Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 9149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Jian, X.; Yan, Z.; Liu, H.; Zhang, L. Multiple Intra-Articular Injections of Adipose-Derived Mesenchymal Stem Cells for Canine Osteoarthritis Treatment. Cells 2025, 14, 323. https://doi.org/10.3390/cells14050323
Li X, Jian X, Yan Z, Liu H, Zhang L. Multiple Intra-Articular Injections of Adipose-Derived Mesenchymal Stem Cells for Canine Osteoarthritis Treatment. Cells. 2025; 14(5):323. https://doi.org/10.3390/cells14050323
Chicago/Turabian StyleLi, Xianqiang, Xuwei Jian, Ziyin Yan, Huazhen Liu, and Lisheng Zhang. 2025. "Multiple Intra-Articular Injections of Adipose-Derived Mesenchymal Stem Cells for Canine Osteoarthritis Treatment" Cells 14, no. 5: 323. https://doi.org/10.3390/cells14050323
APA StyleLi, X., Jian, X., Yan, Z., Liu, H., & Zhang, L. (2025). Multiple Intra-Articular Injections of Adipose-Derived Mesenchymal Stem Cells for Canine Osteoarthritis Treatment. Cells, 14(5), 323. https://doi.org/10.3390/cells14050323





