Role of Hemocytes in the Aging of Drosophila Male Germline
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Fly Stocks and Maintenance
2.2. Immunohistochemistry
2.3. Fluorescence Microscopy
2.4. Electron Microscopy
2.5. Western Blot
2.6. PCR Experiments
2.7. TUNEL
2.8. Evaluation, Statistics
3. Results
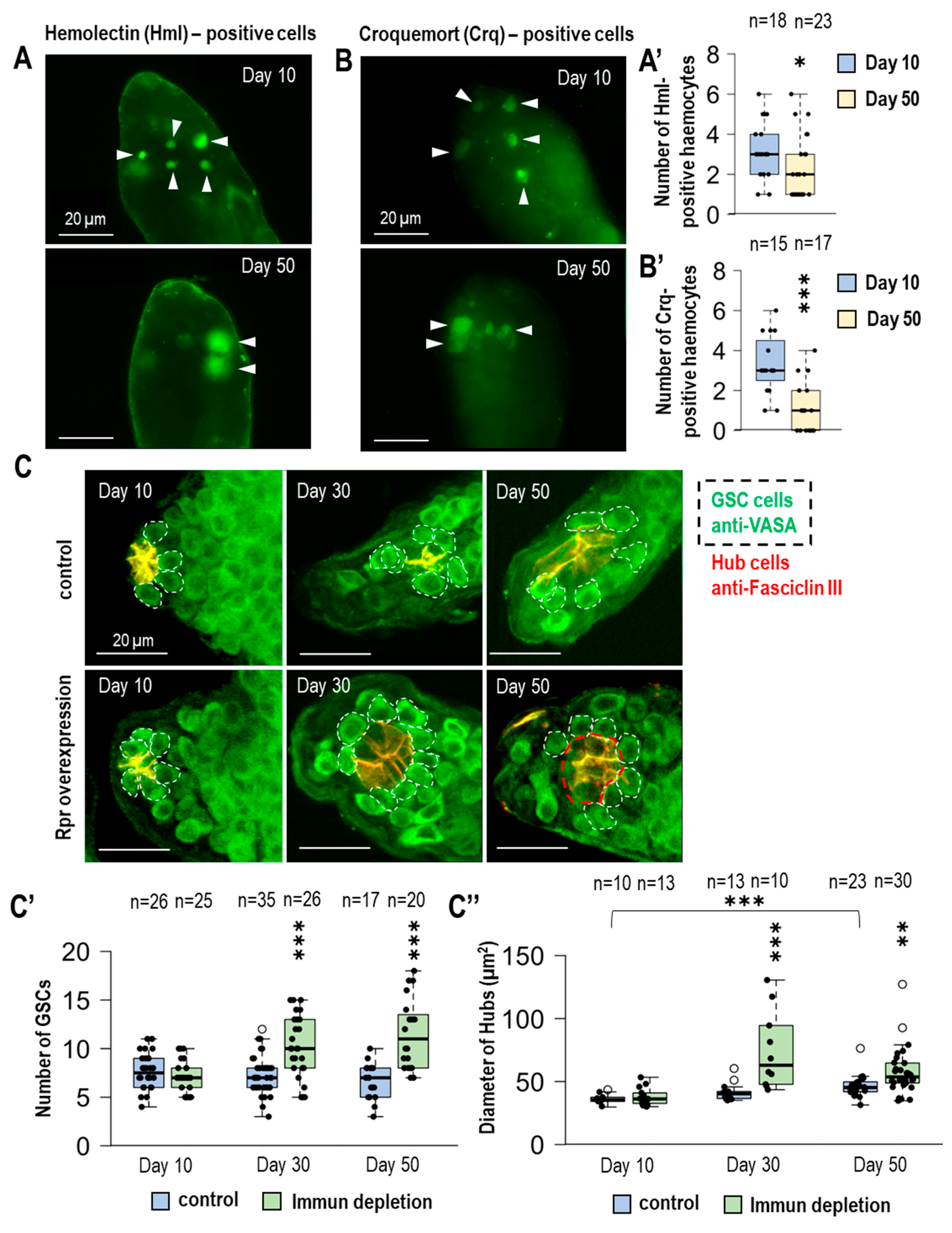
3.1. The Number of Immune Cells Located in the Testes Varies During Aging
3.2. Immunodeficiency Results in an Increasing Number of GSCs in Aging Fruit Flies
3.3. The Size of the Hub Is Changing During Aging
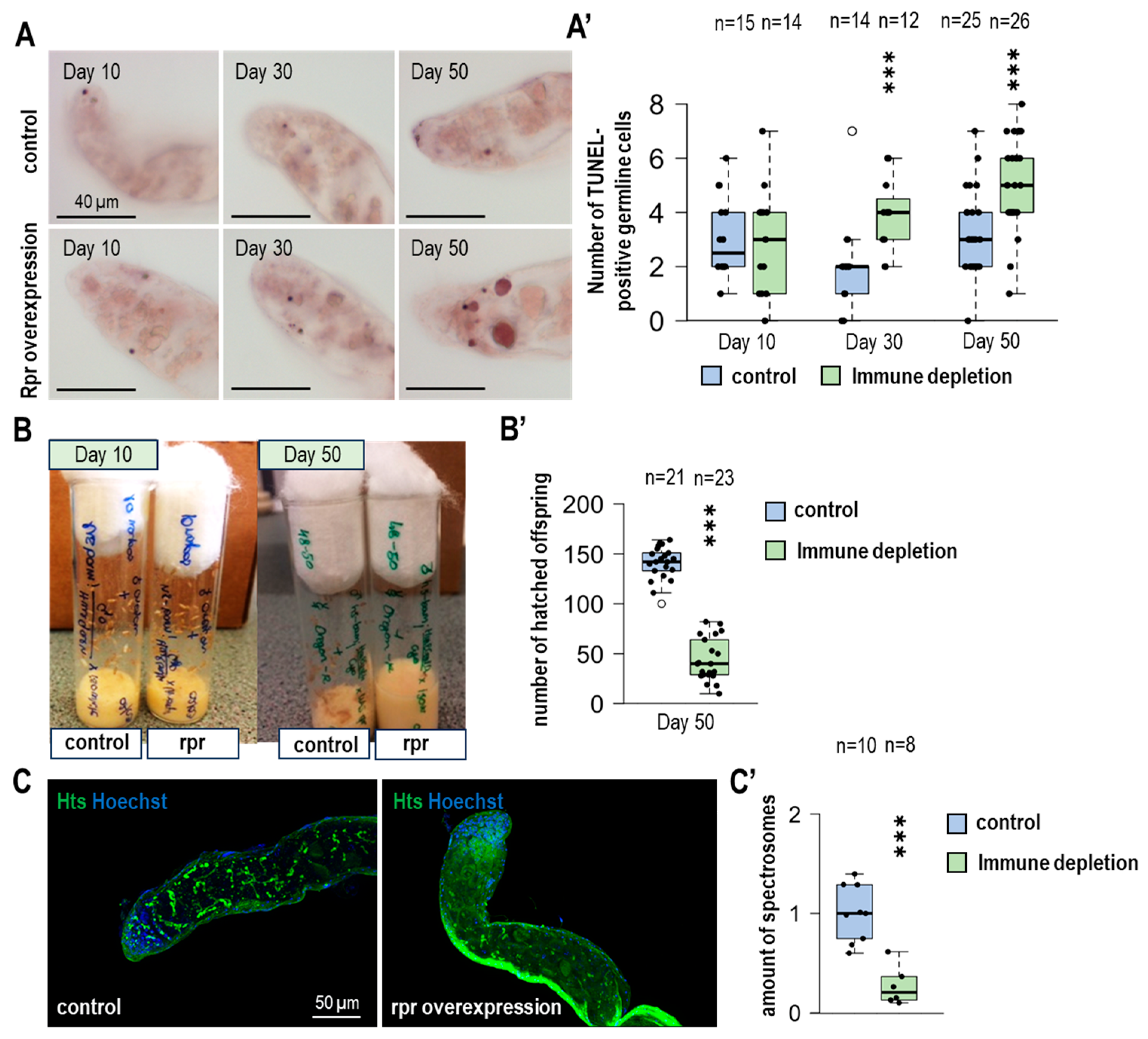
3.4. The Number of Apoptotic GSCs Is Increasing Due to the Lack of Hemocytes
3.5. In the Lack of Immune Cells, Fertility Is Decreasing During Aging
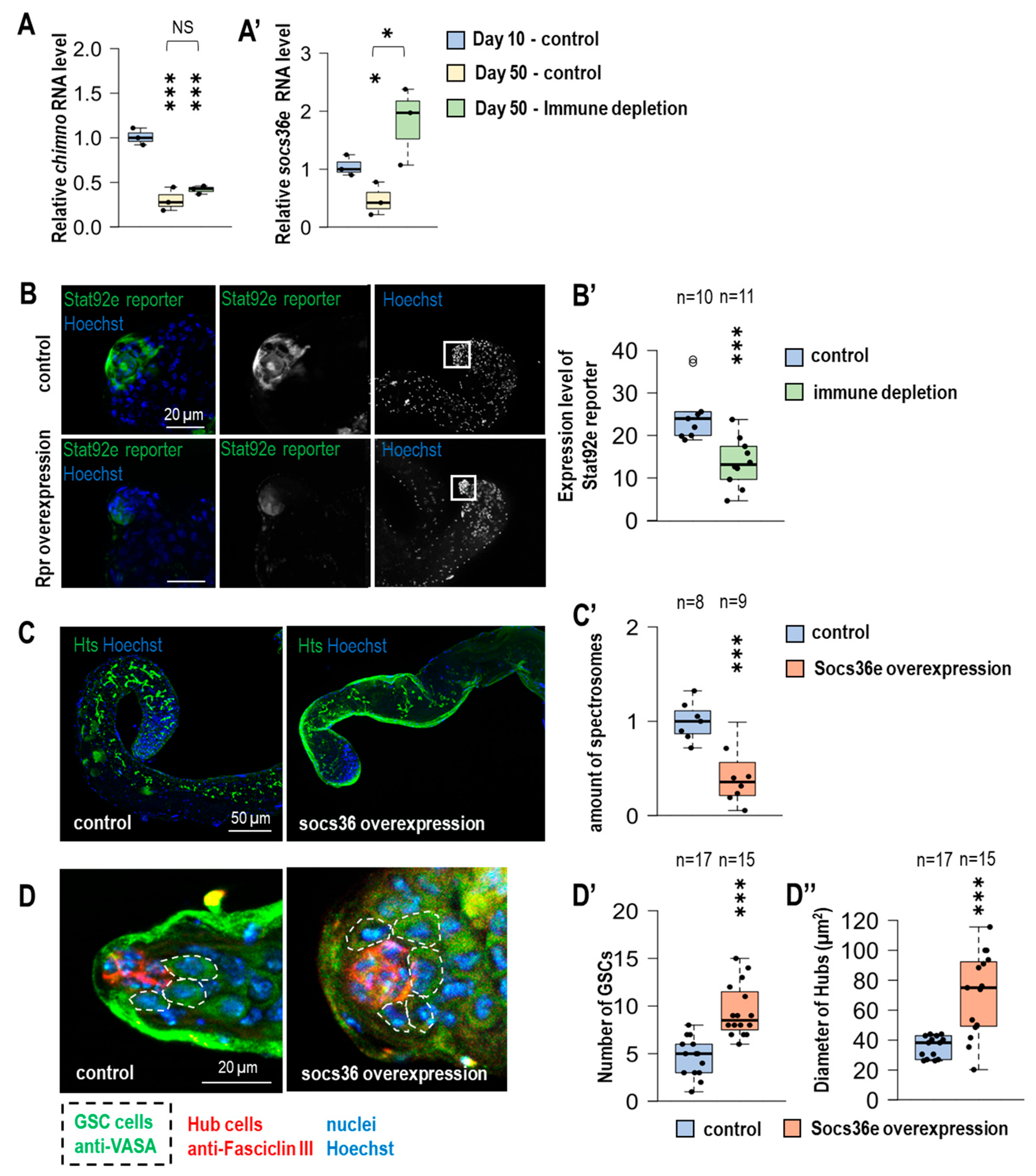
3.6. Expression of JAK-STAT Target Genes Decreases Under Immune-Depleted Circumstances in the Testes of Advanced-Aged Fruit Flies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lenhart, K.F.; Capozzoli, B.; Warrick, G.S.D.; DiNardo, S. Diminished Jak/STAT Signaling Causes Early-Onset Aging Defects in Stem Cell Cytokinesis. Curr. Biol. 2019, 29, 256–267.e3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wallenfang, M.R.; Nayak, R.; DiNardo, S. Dynamics of the male germline stem cell population during aging of Drosophila melanogaster. Aging Cell 2006, 5, 297–304. [Google Scholar] [CrossRef] [PubMed]
- de Cuevas, M.; Matunis, E.L. The stem cell niche: Lessons from the Drosophila testis. Development 2011, 138, 2861–2869. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demarco, R.S.; Eikenes, Å.H.; Haglund, K.; Jones, D.L. Investigating spermatogenesis in Drosophila melanogaster. Methods 2014, 68, 218–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boyle, M.; Wong, C.; Rocha, M.; Jones, D.L. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell 2007, 1, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Losick, V.P.; Morris, L.X.; Fox, D.T.; Spradling, A. Drosophila stem cell niches: A decade of discovery suggests a unified view of stem cell regulation. Dev. Cell 2011, 21, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Dansereau, D.A.; Lasko, P. The development of germline stem cells in Drosophila. Methods Mol. Biol. 2008, 450, 3–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeidler, M.P.; Bausek, N. The Drosophila JAK-STAT pathway. JAK-STAT 2013, 2, e25353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stec, W.; Vidal, O.; Zeidler, M.P. Drosophila SOCS36E negatively regulates JAK/STAT pathway signaling via two separable mechanisms. Mol. Biol. Cell 2013, 24, 3000–3009. [Google Scholar] [CrossRef]
- Letourneau, M.; Lapraz, F.; Sharma, A.; Vanzo, N.; Waltzer, L.; Crozatier, M. Drosophila hematopoiesis under normal conditions and in response to immune stress. FEBS Lett. 2016, 590, 4034–4051. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Kobayashi, S.; Nakato, H. Drosophila glypicans regulate the germline stem cell niche. J. Cell Biol. 2009, 187, 473–480. [Google Scholar] [CrossRef]
- Manzéger, A.; Tagscherer, K.; Lőrincz, P.; Szaker, H.; Lukácsovich, T.; Pilz, P.; Kméczik, R.; Csikós, G.; Erdélyi, M.; Sass, M.; et al. Condition-dependent functional shift of two Drosophila Mtmr lipid phosphatases in autophagy control. Autophagy 2021, 17, 4010–4028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bach, E.A.; Ekas, L.A.; Ayala-Camargo, A.; Flaherty, M.S.; Lee, H.; Perrimon, N.; Baeg, G.H. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr. Patterns 2007, 7, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Varga, V.B.; Schuller, D.; Szikszai, F.; Szinyákovics, J.; Puska, G.; Vellai, T.; Kovács, T. Autophagy is required for spermatogonial differentiation in the Drosophila testis. Biol. Futur. 2022, 73, 187–204. [Google Scholar] [CrossRef] [PubMed]
- DeVorkin, L.; Gorski, S.M. LysoTracker staining to aid in monitoring autophagy in Drosophila. Cold Spring Harb. Protoc. 2014, 2014, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Kovács, T.; Szinyákovics, J.; Billes, V.; Murányi, G.; Varga, V.B.; Bjelik, A.; Légrádi, Á.; Szabó, M.; Sándor, S.; Kubinyi, E.; et al. A conserved MTMR lipid phosphatase increasingly suppresses autophagy in brain neurons during aging. Sci. Rep. 2022, 12, 21817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Billes, V.; Kovács, T.; Manzéger, A.; Lőrincz, P.; Szincsák, S.; Regős, Á.; Kulcsár, P.I.; Korcsmáros, T.; Lukácsovich, T.; Hoffmann, G.; et al. Developmentally regulated autophagy is required for eye formation in Drosophila. Autophagy 2018, 14, 1499–1519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guillou, A.; Troha, K.; Wang, H.; Franc, N.C.; Buchon, N. The Drosophila CD36 Homologue croquemort Is Required to Maintain Immune and Gut Homeostasis during Development and Aging. PLoS Pathog. 2016, 12, e1005961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evans, C.J.; Liu, T.; Banerjee, U. Drosophila hematopoiesis: Markers and methods for molecular genetic analysis. Methods 2014, 68, 242–251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goto, A.; Kumagai, T.; Kumagai, C.; Hirose, J.; Narita, H.; Mori, H.; Kadowaki, T.; Beck, K.; Kitagawa, Y. A Drosophila haemocyte-specific protein, hemolectin, similar to human von Willebrand factor. Biochem. J. 2001, 359 Pt 1, 99–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franc, N.C.; Dimarcq, J.L.; Lagueux, M.; Hoffmann, J.; Ezekowitz, R.A. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity 1996, 4, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Van De Bor, V.; Zimniak, G.; Papone, L.; Cerezo, D.; Malbouyres, M.; Juan, T.; Ruggiero, F.; Noselli, S. Companion Blood Cells Control Ovarian Stem Cell Niche Microenvironment and Homeostasis. Cell Rep. 2015, 13, 546–560. [Google Scholar] [CrossRef]
- Herrera, S.C.; Sainz de la Maza, D.; Grmai, L.; Margolis, S.; Plessel, R.; Burel, M.; O’Connor, M.; Amoyel, M.; Bach, E.A. Proliferative stem cells maintain quiescence of their niche by secreting the Activin inhibitor Follistatin. Dev. Cell 2021, 56, 2284–2294.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fairchild, M.J.; Yang, L.; Goodwin, K.; Tanentzapf, G. Occluding Junctions Maintain Stem Cell Niche Homeostasis in the Fly Testes. Curr. Biol. 2016, 26, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Kyrylkova, K.; Kyryachenko, S.; Leid, M.; Kioussi, C. Detection of apoptosis by TUNEL assay. Methods Mol. Biol. 2012, 887, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, M.S.; Salis, P.; Evans, C.J.; Ekas, L.A.; Marouf, A.; Zavadil, J.; Banerjee, U.; Bach, E.A. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev. Cell 2010, 18, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Amoyel, M.; Anderson, J.; Suisse, A.; Glasner, J.; Bach, E.A. Socs36E Controls Niche Competition by Repressing MAPK Signaling in the Drosophila Testis. PLoS Genet. 2016, 12, e1005815. [Google Scholar] [CrossRef]
- Cheng, J.; Türkel, N.; Hemati, N.; Fuller, M.T.; Hunt, A.J.; Yamashita, Y.M. Centrosome misorientation reduces stem cell division during ageing. Nature 2008, 456, 599–604. [Google Scholar] [CrossRef]
- DeFalco, T.; Potter, S.J.; Williams, A.V.; Waller, B.; Kan, M.J.; Capel, B. Macrophages Contribute to the Spermatogonial Niche in the Adult Testis. Cell Rep. 2015, 12, 1107–1119. [Google Scholar] [CrossRef]
- Li, C.Y.; Guo, Z.; Wang, Z. TGFbeta receptor saxophone non-autonomously regulates germline proliferation in a Smox/dSmad2-dependent manner in Drosophila testis. Dev. Biol. 2007, 309, 70–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ayyaz, A.; Li, H.; Jasper, H. Haemocytes control stem cell activity in the Drosophila intestine. Nat. Cell Biol. 2015, 17, 736–748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, S.R.; Zheng, Z.; Wang, H.; Oh, S.W.; Chen, X.; Hou, S.X. Competitiveness for the niche and mutual dependence of the germline and somatic stem cells in the Drosophila testis are regulated by the JAK/STAT signaling. J. Cell. Physiol. 2010, 223, 500–510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monahan, A.J.; Starz-Gaiano, M. Socs36E limits STAT signaling via Cullin2 and a SOCS-box independent mechanism in the Drosophila egg chamber. Mech. Dev. 2015, 138 Pt 3, 313–327. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, V.; Szinyákovics, J.; Bebes, A.; Szikszai, F.; Kovács, T. Role of Hemocytes in the Aging of Drosophila Male Germline. Cells 2025, 14, 315. https://doi.org/10.3390/cells14040315
Varga V, Szinyákovics J, Bebes A, Szikszai F, Kovács T. Role of Hemocytes in the Aging of Drosophila Male Germline. Cells. 2025; 14(4):315. https://doi.org/10.3390/cells14040315
Chicago/Turabian StyleVarga, Virginia, Janka Szinyákovics, Anikó Bebes, Fanni Szikszai, and Tibor Kovács. 2025. "Role of Hemocytes in the Aging of Drosophila Male Germline" Cells 14, no. 4: 315. https://doi.org/10.3390/cells14040315
APA StyleVarga, V., Szinyákovics, J., Bebes, A., Szikszai, F., & Kovács, T. (2025). Role of Hemocytes in the Aging of Drosophila Male Germline. Cells, 14(4), 315. https://doi.org/10.3390/cells14040315






