Angiotensin-(1-7) Provides Potent Long-Term Neurorepair/Neuroregeneration in a Rodent White Matter Stroke Model: Nonarteritic Ischemic Optic Neuropathy (rNAION)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. rNAION Induction
2.3. Optic Nerve Head Imaging-Optic Nerve Edema Analysis
2.4. Ang-(1-7) and Vehicle Treatments
2.5. OptoMotry-Based Visual Acuity Assessment
2.6. Transcranial Electrode Implantation
2.7. Visual Evoked Potentials
2.8. Tissue Isolation
2.9. Immunohistochemistry
2.10. RGC Stereology
2.11. Statistical Calculations
3. Results
3.1. Optic Nerve Edema Following rNAION and Randomization
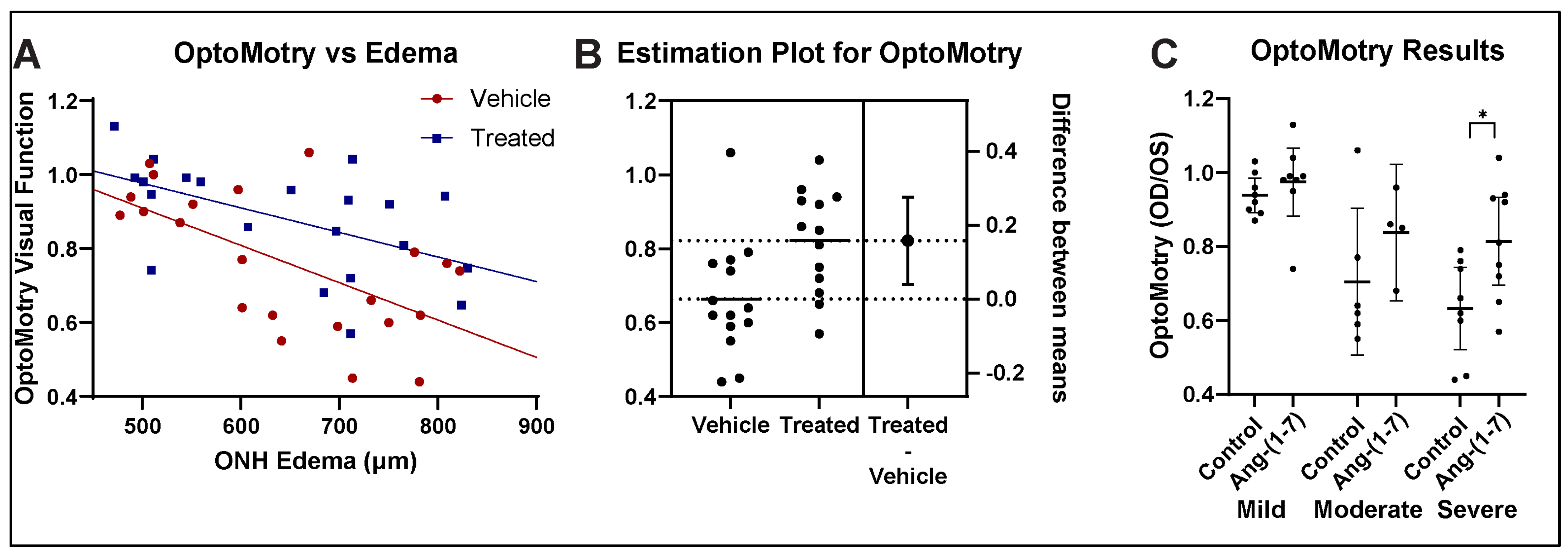
3.2. Ang-(1-7) Improves Visual Acuity Following rNAION: OptoMotry
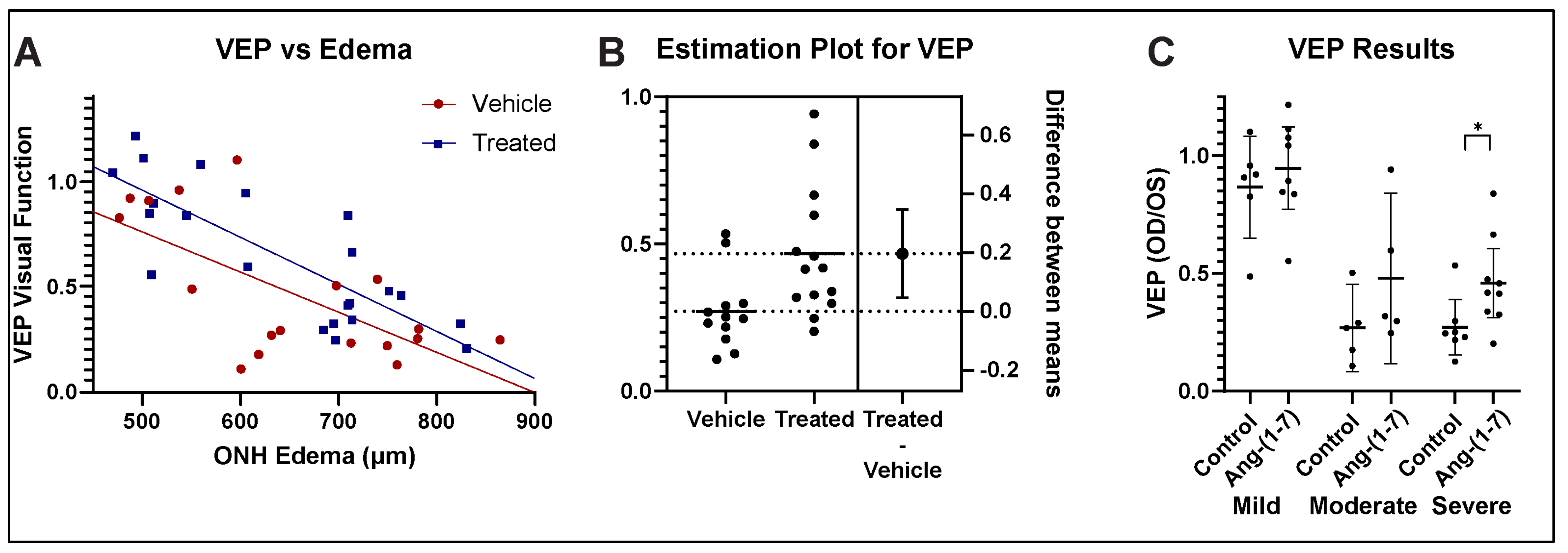
3.3. Ang-(1-7) Improves Visual Function After rNAION: fVEP Amplitude Analysis
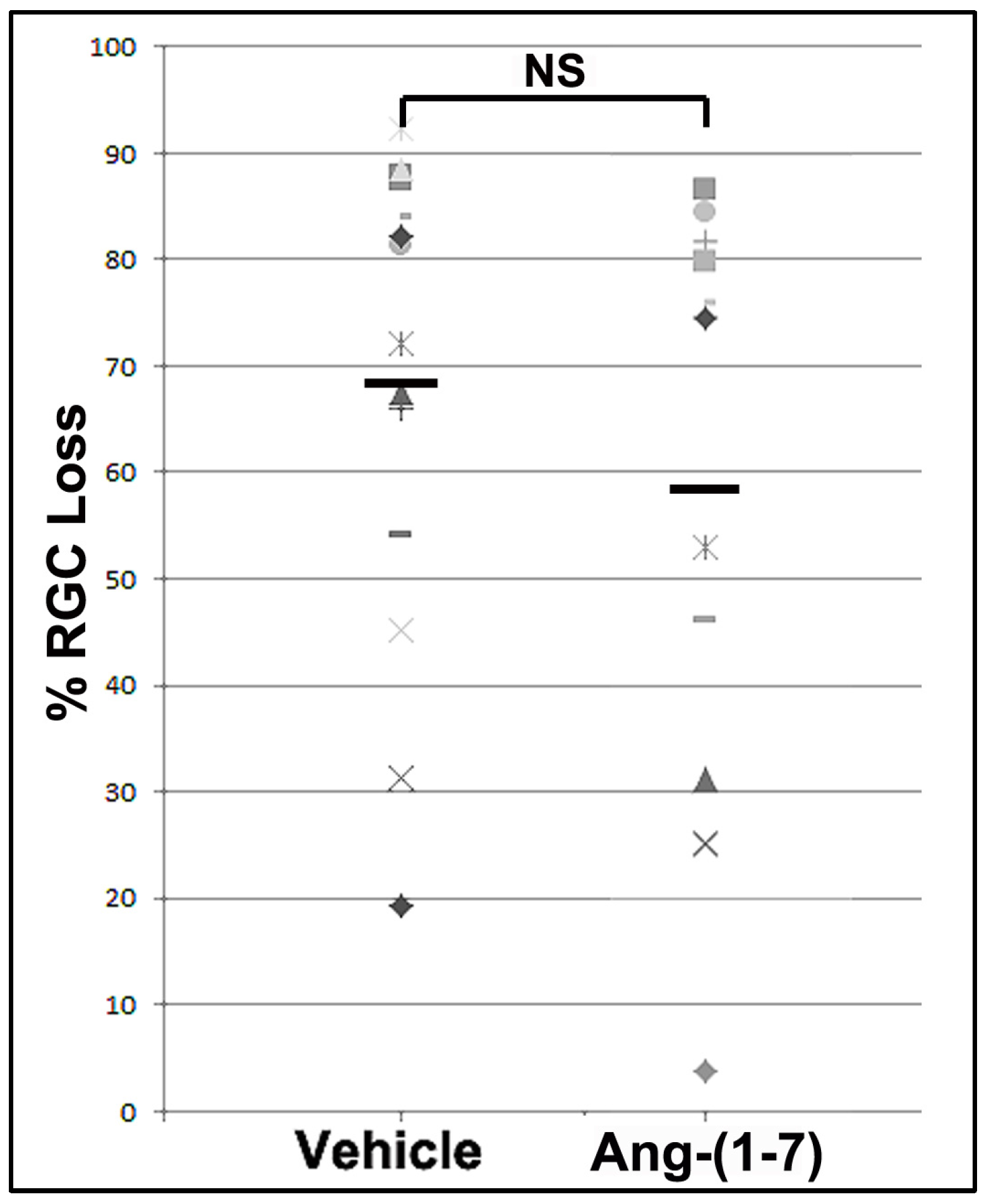
3.4. Ang-(1-7) Effects Are Not Explained by Increasing RGC Survival
3.5. Ang-(1-7) Treatment Effects on Cellular ONH Inflammation After rNAION Induction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salvetat, M.L.; Pellegrini, F.; Spadea, L.; Salati, C.; Zeppieri, M. Non-Arteritic Anterior Ischemic Optic Neuropathy (NA-AION): A Comprehensive Overview. Vision 2023, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.C. Pathogenesis of Nonarteritic Anterior Ischemic Optic Neuropathy. J. Neuro-Ophthalmol. 2003, 23, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.R.; Arnold, A.C. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy. Eye 2014, 29, 65–79. [Google Scholar] [CrossRef]
- Newman, N.J.; Scherer, R.; Langenberg, P.; Kelman, S.; Feldon, S.; Kaufman, D.; Dickersin, K. The fellow eye in naion: Report from the ischemic optic neuropathy decompression trial follow-up study. Arch. Ophthalmol. 2002, 134, 317–328. [Google Scholar] [CrossRef]
- Johnson, M.A.; Mehrabian, Z.; Guo, Y.; Ghosh, J.; Brigell, M.G.; Bernstein, S.L. Anti-NOGO Antibody Neuroprotection in a Rat Model of NAION. Transl. Vis. Sci. Technol. 2021, 10, 12. [Google Scholar] [CrossRef]
- You, Y.; Klistorner, A.; Thie, J.; Graham, S.L. Improving reproducibility of VEP recording in rats: Electrodes, stimulus source and peak analysis. Doc. Ophthalmol. 2011, 123, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.; Guo, Y.; Mehrabian, Z.; Miller, N.R.; Bernstein, S.L. A method to refine flash visual evoked potential analysis in the rat: Enhanced visual function analysis and sample size reduction. TVST 2024, 13. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Simoes e Silva, A.C.; Maric, C.; Silva, D.M.R.; Machado, R.P.; de Buhr, I.; Heringer-Walther, S.; Pinheiro, S.V.B.; Lopes, M.T.; Bader, M.; et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. USA 2003, 100, 8258–8263. [Google Scholar] [CrossRef] [PubMed]
- Tetzner, A.; Gebolys, K.; Meinert, C.; Klein, S.; Uhlich, A.; Trebicka, J.; Villacañas, Ó.; Walther, T. G-Protein–Coupled Receptor MrgD Is a Receptor for Angiotensin-(1–7) Involving Adenylyl Cyclase, cAMP, and Phosphokinase A. Hypertension 2016, 68, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Karnik, S.S.; Singh, K.D.; Tirupula, K.; Unal, H. Significance of angiotensin 1–7 coupling with MAS1 receptor and other GPCRs to the renin-angiotensin system: IUPHAR Review. Br. J. Pharmacol. 2017, 174, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Lund, B.; Stone, R.; Levy, A.; Lee, S.; Amundson, E.; Kashani, N.; Rodgers, K.; Kelland, E. Reduced disease severity following therapeutic treatment with angiotensin 1–7 in a mouse model of multiple sclerosis. Neurobiol. Dis. 2019, 127, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.-X.; Xue, X.; Gao, L.; Lu, J.-L.; Zhou, J.-S.; Jiang, T.; Zhang, Y.-D. Involvement of angiotensin-(1–7) in the neuroprotection of captopril against focal cerebral ischemia. Neurosci. Lett. 2018, 687, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Annoni, F.; Moro, F.; Caruso, E.; Zoerle, T.; Taccone, F.S.; Zanier, E.R. Angiotensin-(1–7) as a Potential Therapeutic Strategy for Delayed Cerebral Ischemia in Subarachnoid Hemorrhage. Front. Immunol. 2022, 13, 841692. [Google Scholar] [CrossRef]
- Bennion, D.M.; Jones, C.H.; Donnangelo, L.L.; Graham, J.T.; Isenberg, J.D.; Dang, A.N.; Rodriguez, V.; Sinisterra, R.D.M.; Sousa, F.B.; Santos, R.A.S.; et al. Neuroprotection by post-stroke administration of an oral formulation of angiotensin-(1–7) in ischaemic stroke. Exp. Physiol. 2018, 103, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Regenhardt, R.W.; Desland, F.; Mecca, A.P.; Pioquinto, D.J.; Afzal, A.; Mocco, J.; Sumners, C. Anti-inflammatory effects of angiotensin-(1-7) in ischemic stroke. Neuropharmacology 2013, 71, 154–163. [Google Scholar] [CrossRef]
- Pan, H.; Huang, W.; Wang, Z.; Ren, F.; Luo, L.; Zhou, J.; Tian, M.; Tang, L. The ACE2-Ang-(1-7)-Mas Axis Modulates M1/M2 Macrophage Polarization to Relieve CLP-Induced Inflammation via TLR4-Mediated NF-ĸb and MAPK Pathways. J. Inflamm. Res. 2021, 14, 2045–2060. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhu, D.; Ji, L.; Tian, M.; Xu, C.; Shi, J. Angiotensin-(1-7) improves cognitive function in rats with chronic cerebral hypoperfusion. Brain Res. 2014, 1573, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Dang, R.; Yang, M.; Cui, C.; Wang, C.; Zhang, W.; Geng, C.; Han, W.; Jiang, P. Activation of angiotensin-converting enzyme 2/angiotensin (1–7)/mas receptor axis triggers autophagy and suppresses microglia proinflammatory polarization via forkhead box class O1 signaling. Aging Cell 2021, 20, e13480. [Google Scholar] [CrossRef]
- Wheeler-Schilling, T.H.; Kohler, K.; Sautter, M.; Guenther, E. Angiotensin II receptor subtype gene expression and cellular localization in the retina and non-neuronal ocular tissues of the rat. Eur. J. Neurosci. 1999, 11, 3387–3394. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.; Verma, A.; Li, Q. Expression and cellular localization of the Mas receptor in the adult and developing mouse retina. Mol. Vis. 2014, 20, 1443–1455. [Google Scholar] [PubMed]
- Prusky, G.T.; Harker, K.; Douglas, R.M.; Whishaw, I.Q. Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behav. Brain Res. 2002, 136, 339–348. [Google Scholar] [CrossRef]
- Bernstein, S.L.; Guo, Y.; Kelman, S.E.; Flower, R.W.; Johnson, M.A. Functional and Cellular Responses in a Novel Rodent Model of Anterior Ischemic Optic Neuropathy. Investig. Opthalmology Vis. Sci. 2003, 44, 4153–4162. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Mehrabian, Z.; Johnson, M.A.; Miller, N.R.; Henderson, A.D.; Hamlyn, J.; Bernstein, S.L. Biomarkers of lesion severity in a rodent model of nonarteritic anterior ischemic optic neuropathy (rNAION). PLoS ONE 2021, 16, e0243186. [Google Scholar] [CrossRef] [PubMed]
- Douglas, R.; ALAM, N.; Silver, B.; Mcgill, T.; Tschetter, W.; Prusky, G. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis. Neurosci. 2005, 22, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain, a Stereotaxic Atlas, 4th ed.; Academic Press: New York, NY, USA, 2001. [Google Scholar]
- Nadal-Nicola’s, F.M.; Jime’nez-Lo’pez, M.; Sobrado-Calvo, P.; Nieto-Lo’pez, L.; Ca’novas-Marti’nez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a Marker of Retinal Ganglion Cells: Qualitative and Quantitative Time Course Studies in Naïve and Optic Nerve–Injured Retinas. Investig. Opthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef]
- Levene, H. On the Power Function of Tests of Randomness Based on Runs up and Down. Ann. Math. Stat. 1952, 23, 34–56. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice-Hall: Hoboken, NY, USA, 1999. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Creel, D.J. Visual and Auditory Anomalies Associated with Albinism. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Jones, B., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Heiduschka, P.; Schraermeyer, U. Comparison of visual function in pigmented and albino rats by electroretinography and visual evoked potentials. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 1559–1573. [Google Scholar] [CrossRef]
- Askenase, M.H.; Goods, B.A.; Beatty, H.E.; Steinschneider, A.F.; Velazquez, S.E.; Osherov, A.; Landreneau, M.J.; Carroll, S.L.; Tran, T.B.; Avram, V.S.; et al. Longitudinal transcriptomics define the stages of myeloid activation in the living human brain after intracerebral hemorrhage. Sci. Immunol. 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Fel, A.; Simonutti, M.; Froger, N.; Ivkovic, I.; Lehoang, P.; Bernstein, S.; Bodaghi, B.; Sahel, J.; Paques, M.; Picaud, S.; et al. Minocycline as a new neuroprotective agent in a rodent model of NAION. Acta Ophthalmol. 2013, 91. [Google Scholar] [CrossRef]
- Miller, N.R.; Johnson, M.A.; Nolan, T.; Guo, Y.; Bernstein, A.M.; Bernstein, S.L. Sustained Neuroprotection from a Single In-travitreal Injection of PGJ2 in a Non-Human Primate Model of Nonarteritic Anterior Ischemic Optic Neuropathy. Invest. Ophthalmol. Vis. Sci. 2014, 55, 7047–7056. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.-T.; Huang, T.-L.; Huang, S.-P.; Chang, C.-H.; Tsai, R.-K. Early applications of granulocyte colony-stimulating factor (G-CSF) can stabilize the blood-optic nerve barrier and further ameliorate optic nerve inflammation in a rat model of anterior ischemic optic neuropathy (rAION). Dis. Model. Mech. 2016, 9, 1193–1202. [Google Scholar] [CrossRef]
- Levin, L.A. Mechanisms of optic neuropathy. Curr. Opin. Ophthalmol. 1997, 8, 9–15. [Google Scholar] [CrossRef]
- Huang, T.-L.; Wen, Y.-T.; Chang, C.-H.; Chang, S.-W.; Lin, K.-H.; Tsai, R.-K. Early Methylprednisolone Treatment Can Stabilize the Blood-Optic Nerve Barrier in a Rat Model of Anterior Ischemic Optic Neuropathy (rAION). Investig. Opthalmol. Vis. Sci. 2017, 58, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, H.; Golalipour, M.; Doozandeh, A.; Pakravan, M.; Yaseri, M.; Esfandiari, H. The effect of systemic erythropoietin and oral prednisolone on recent-onset non-arteritic anterior ischemic optic neuropathy: A randomized clinical trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Gao, L.; Lu, J.; Zhang, Y.-D. ACE2-Ang-(1-7)-Mas Axis in Brain: A Potential Target for Prevention and Treatment of Ischemic Stroke. Curr. Neuropharmacol. 2013, 11, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Arroja, M.M.C.; Reid, E.; Roy, L.A.; Vallatos, A.V.; Holmes, W.M.; Nicklin, S.A.; Work, L.M.; McCabe, C. Assessing the effects of Ang-(1-7) therapy following transient middle cerebral artery occlusion. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nassir, C.M.N.C.M.; Zolkefley, M.K.I.; Ramli, M.D.; Norman, H.H.; Hamid, H.A.; Mustapha, M. Neuroinflammation and COVID-19 Ischemic Stroke Recovery—Evolving Evidence for the Mediating Roles of the ACE2/Angiotensin-(1–7)/Mas Receptor Axis and NLRP3 Inflammasome. Int. J. Mol. Sci. 2022, 23, 3085. [Google Scholar] [CrossRef] [PubMed]
- Tetzner, A.; Naughton, M.; Gebolys, K.; Eichhorst, J.; Sala, E.; Villacañas, Ó.; Walther, T. Decarboxylation of Ang-(1–7) to Ala1-Ang-(1–7) leads to significant changes in pharmacodynamics. Eur. J. Pharmacol. 2018, 833, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, C.; Roks, A.J.; Westermann, D.; Schumacher, S.-M.; Escher, F.; Schoemaker, R.G.; Reudelhuber, T.L.; van Gilst, W.H.; Schultheiss, H.-P.; et al. Circulating Rather Than Cardiac Angiotensin-(1-7) Stimulates Cardioprotection After Myocardial Infarction. Circ. Hear. Fail. 2010, 3, 286–293. [Google Scholar] [CrossRef]
- Alenina, N.; Bader, M.; Walther, T. Imprinting of the Murine Mas Protooncogene Is Restricted to Its Antisense RNA. Biochem. Biophys. Res. Commun. 2002, 290, 1072–1078. [Google Scholar] [CrossRef]
- Johnson, M.A.; Miller, N.R.; Nolan, T.; Bernstein, S.L. Peripapillary Retinal Nerve Fiber Layer Swelling Predicts Peripapillary Atrophy in a Primate Model of Nonarteritic Anterior Ischemic Optic NeuropathyPeripapillary RNFL Swelling and Atrophy in pNAION. Invest. Ophthalmol. Vis. Sci. 2016, 57, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, S.-Y.; Wang, Q.-G.; Xu, Z.-H.; Peng, Q.; Chen, S.-Y.; Zhu, L.; Zhang, Y.-D.; Duan, R. AVE 0991 Suppresses Astrocyte-Mediated Neuroinflammation of Alzheimer’s Disease by Enhancing Autophagy. J. Inflamm. Res. 2023, 16, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shi, P.; Sumners, C. Direct anti-inflammatory effects of angiotensin-(1–7) on microglia. J. Neurochem. 2015, 136, 163–171. [Google Scholar] [CrossRef]
- Wosik, K.; Cayrol, R.; Dodelet-Devillers, A.; Berthelet, F.; Bernard, M.; Moumdjian, R.; Bouthillier, A.; Reudelhuber, T.L.; Prat, A. Angiotensin II Controls Occludin Function and Is Required for Blood–Brain Barrier Maintenance: Relevance to Multiple Sclerosis. J. Neurosci. 2007, 27, 9032–9042. [Google Scholar] [CrossRef] [PubMed]
- Slater, B.J.; Mehrabian, Z.; Guo, Y.; Hunter, A.; Bernstein, S.L. Rodent Anterior Ischemic Optic Neuropathy (rAION) Induces Regional Retinal Ganglion Cell Apoptosis with a Unique Temporal Pattern. Investig. Opthalmol. Vis. Sci. 2008, 49, 3671–3676. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, K.M.; Guo, Y.; Mehrabian, Z.; Walther, T.; Miller, N.R.; Bernstein, S.L. Angiotensin-(1-7) Provides Potent Long-Term Neurorepair/Neuroregeneration in a Rodent White Matter Stroke Model: Nonarteritic Ischemic Optic Neuropathy (rNAION). Cells 2025, 14, 289. https://doi.org/10.3390/cells14040289
Woo KM, Guo Y, Mehrabian Z, Walther T, Miller NR, Bernstein SL. Angiotensin-(1-7) Provides Potent Long-Term Neurorepair/Neuroregeneration in a Rodent White Matter Stroke Model: Nonarteritic Ischemic Optic Neuropathy (rNAION). Cells. 2025; 14(4):289. https://doi.org/10.3390/cells14040289
Chicago/Turabian StyleWoo, Kwang Min, Yan Guo, Zara Mehrabian, Thomas Walther, Neil R. Miller, and Steven L. Bernstein. 2025. "Angiotensin-(1-7) Provides Potent Long-Term Neurorepair/Neuroregeneration in a Rodent White Matter Stroke Model: Nonarteritic Ischemic Optic Neuropathy (rNAION)" Cells 14, no. 4: 289. https://doi.org/10.3390/cells14040289
APA StyleWoo, K. M., Guo, Y., Mehrabian, Z., Walther, T., Miller, N. R., & Bernstein, S. L. (2025). Angiotensin-(1-7) Provides Potent Long-Term Neurorepair/Neuroregeneration in a Rodent White Matter Stroke Model: Nonarteritic Ischemic Optic Neuropathy (rNAION). Cells, 14(4), 289. https://doi.org/10.3390/cells14040289






