Combinational Inhibition of MEK and AKT Synergistically Induces Melanoma Stem Cell Apoptosis and Blocks NRAS Tumor Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. CRISPR-Cas9 Deletion of CD133
2.3. Generation of Doxycycline (Dox)-Inducible Cells
2.4. Quantitative Reverse Transcription PCR (qRT-PCR)
2.5. Immunoblot Analysis
2.6. Drug Treatment and Cell Viability Assays
2.7. Apoptosis Assays: Annexin V-APC/Sytox Blue Staining and Flow Cytometry
2.8. Immunofluorescence Staining and Imaging
2.9. Clonogenic Assays
2.10. Mouse Xenografting
2.11. Statistical Analysis
3. Results
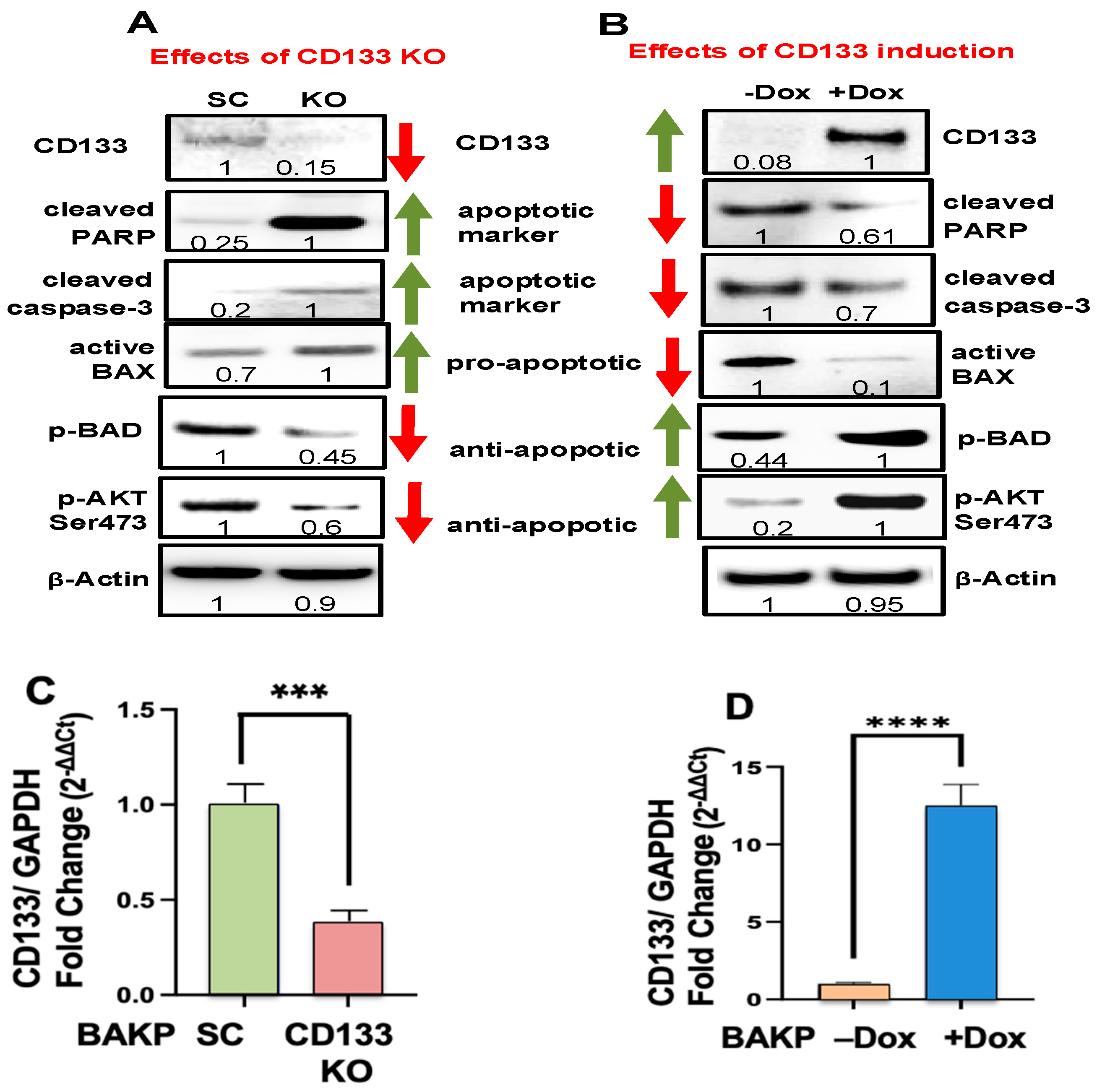
3.1. CRISPR-Cas9 KO of CD133 in BAKP Melanoma Cells Increases Trametinib-Induced Apoptosis via Downregulation of Pro-Survival p-BAD and p-AKT
3.2. Dox-Induced CD133 Activates AKT and Suppresses Trametinib-Induced Apoptosis
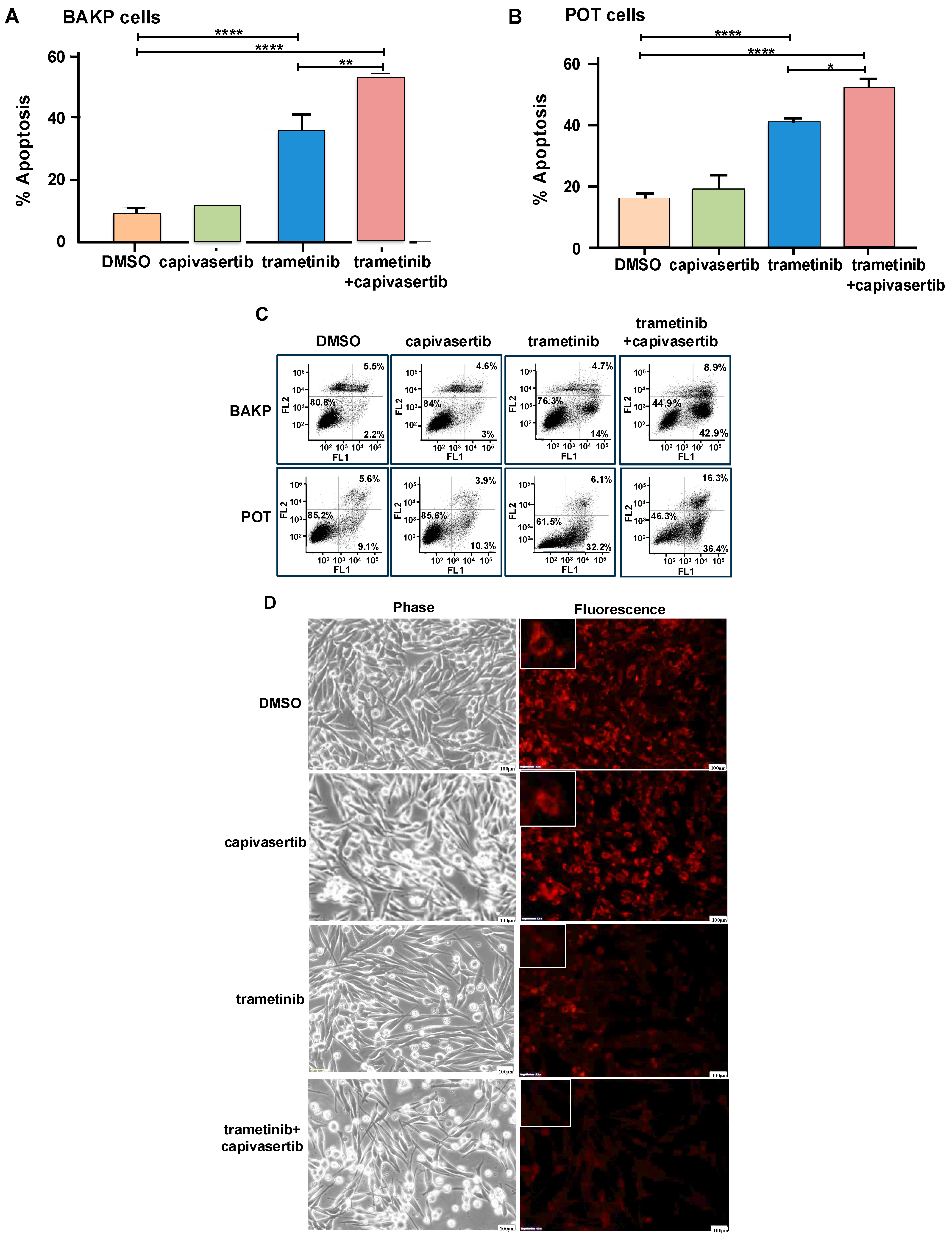
3.3. The Simultaneous Inhibition of MAPK and AKT Pathways by a Combination of Trametinib+ Capivasertib Is Significantly More Cytotoxic in Different NRAS Melanoma Cell Lines
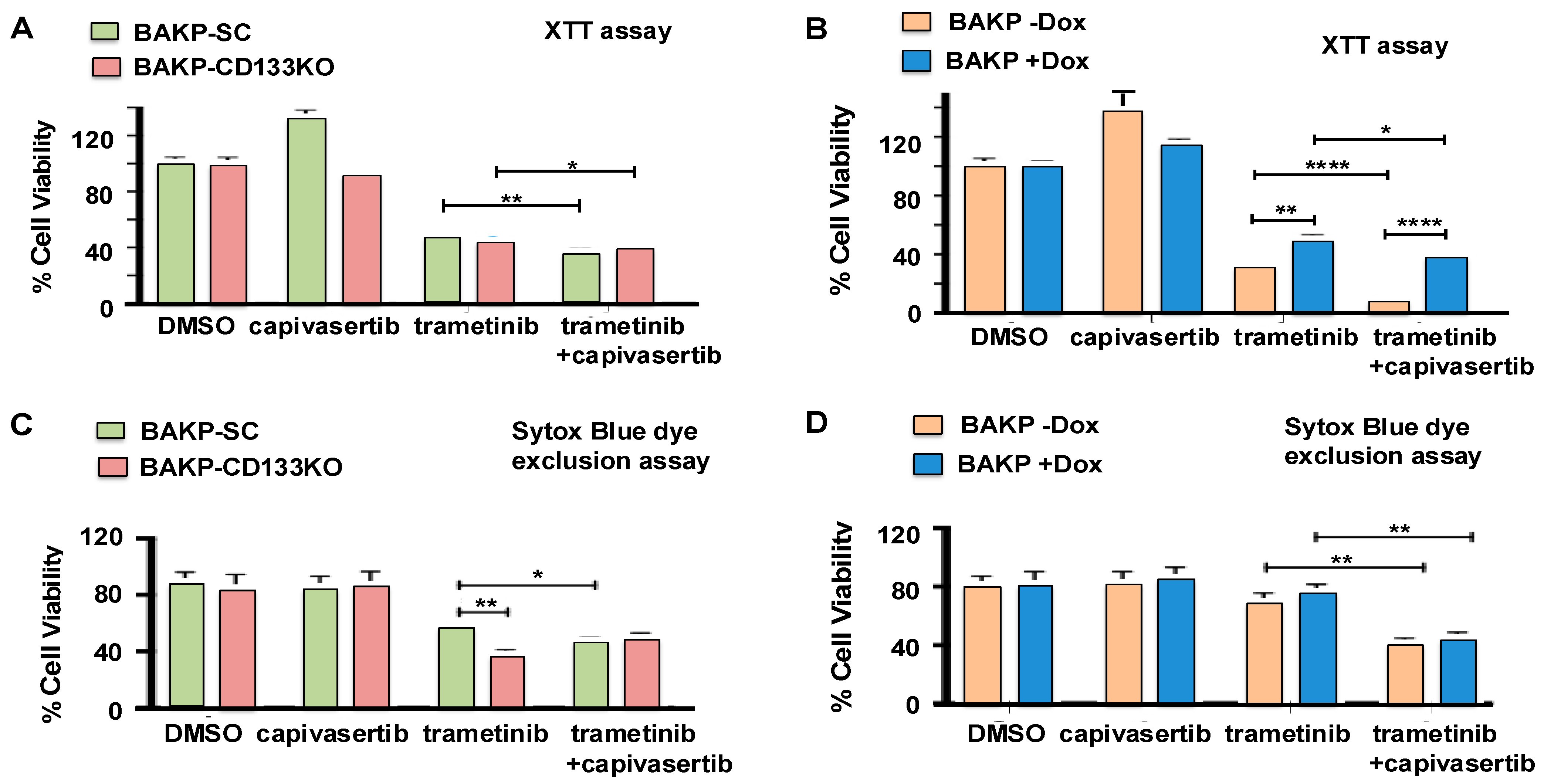
3.4. Effects of CD133 Knockout or Induced CD133 Expression on Cell Viability of BAKP Cells Treated with MEKi Trametinib and AKT Inhibitor Capivasertib, Alone or in Combination
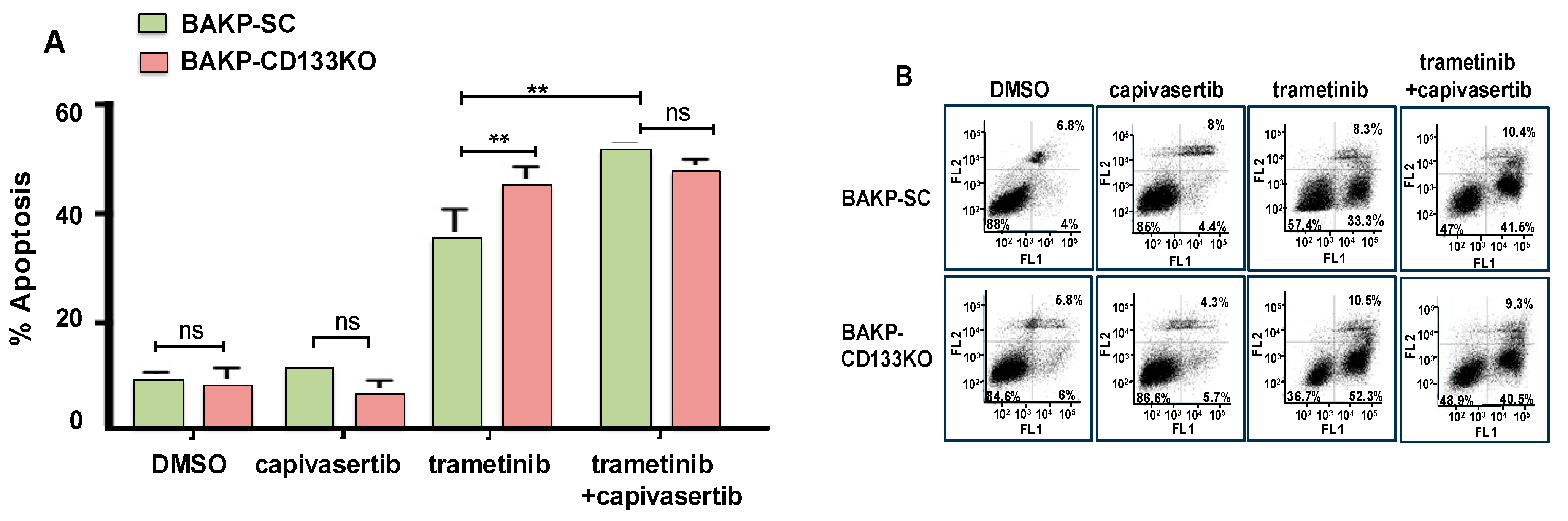
3.5. Effects of CD133 KO on Apoptosis Induction in Response to Trametinib and Capivasertib in Single or Dual Combination as Assessed by Annexin Flow Cytometric Assays
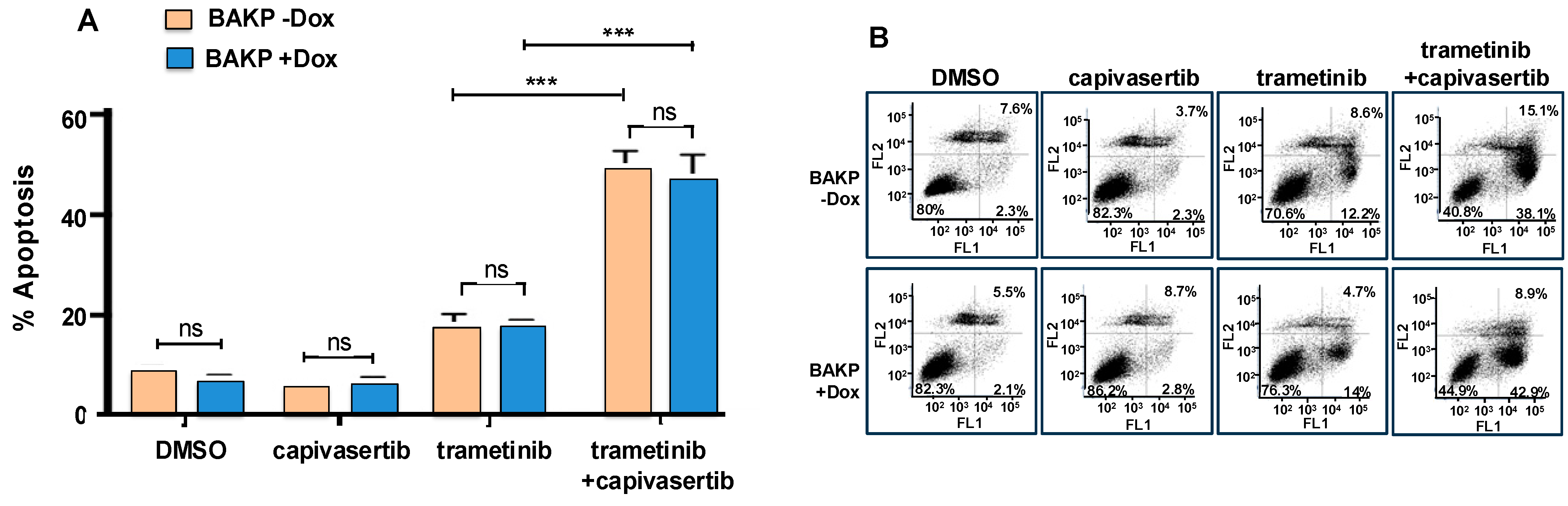
3.6. The Combination of Capivasertib and Trametinib Induces Maximal Cytotoxicity and Apoptosis, Which Cannot Be Reversed by Increased CD133 Expression in Dox-Inducible Cells
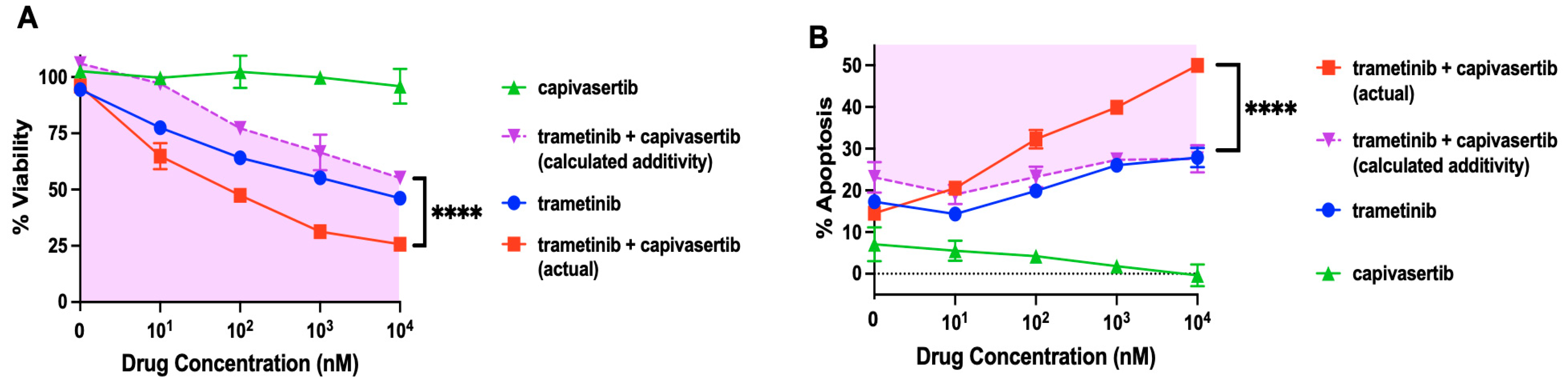
3.7. Capivasertib and Trametinib Function Synergistically in Melanoma Cells
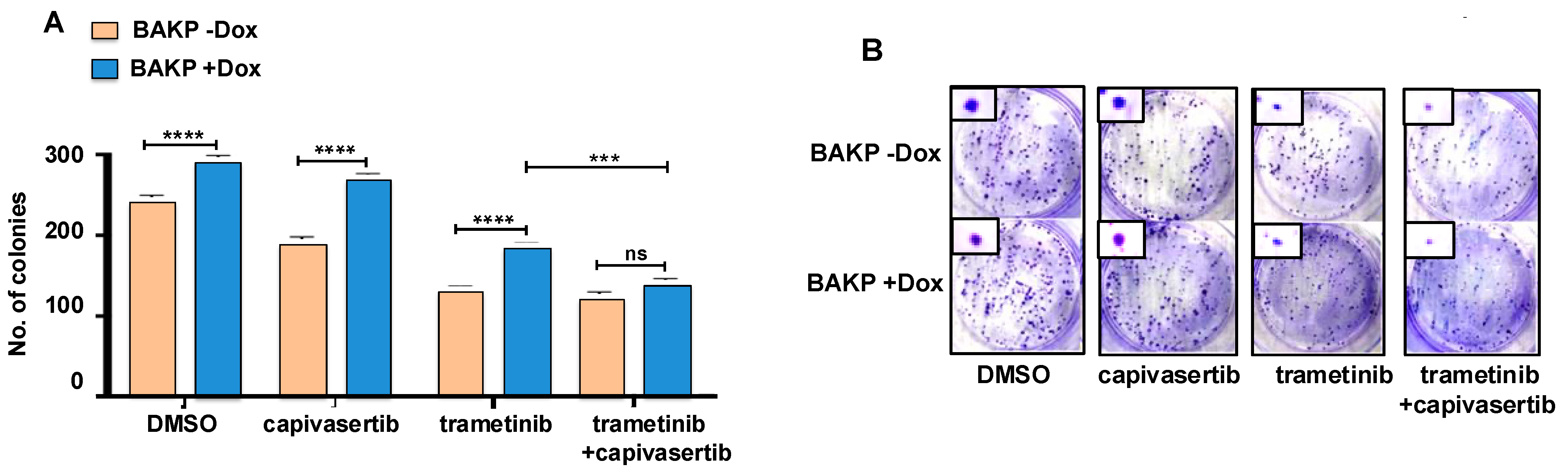
3.8. Long-Term Cell Survival Is Decreased by Trametinib or the Combination of Capivasertib + Trametinib, an Effect Which Is Reversed by Dox-Induced CD133 Expression
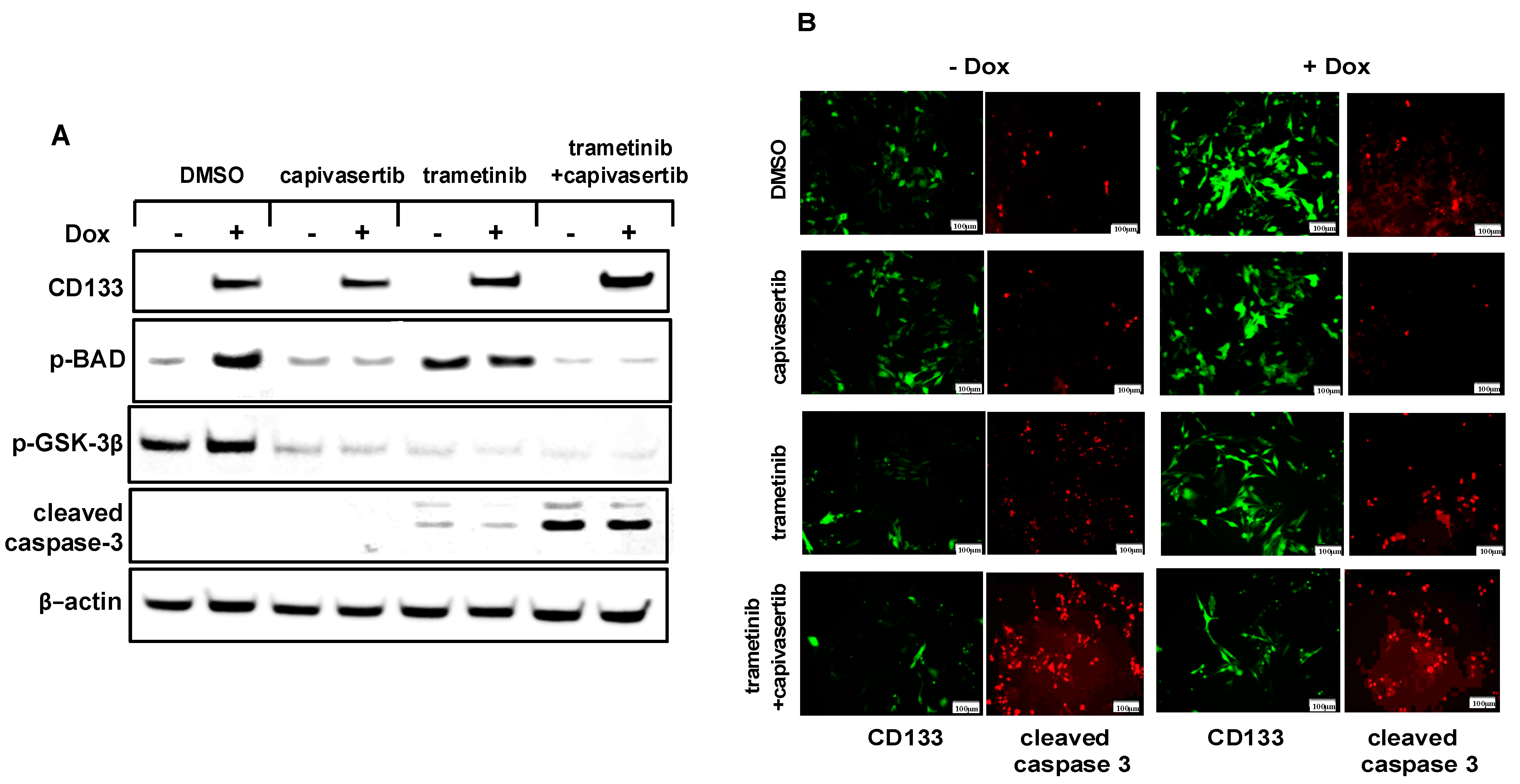
3.9. The Combination of Capivasertib + Trametinib Inhibits the Phosphorylation of BAD and GSK-3β and Increases Caspase-3 Activation in Melanoma Cells
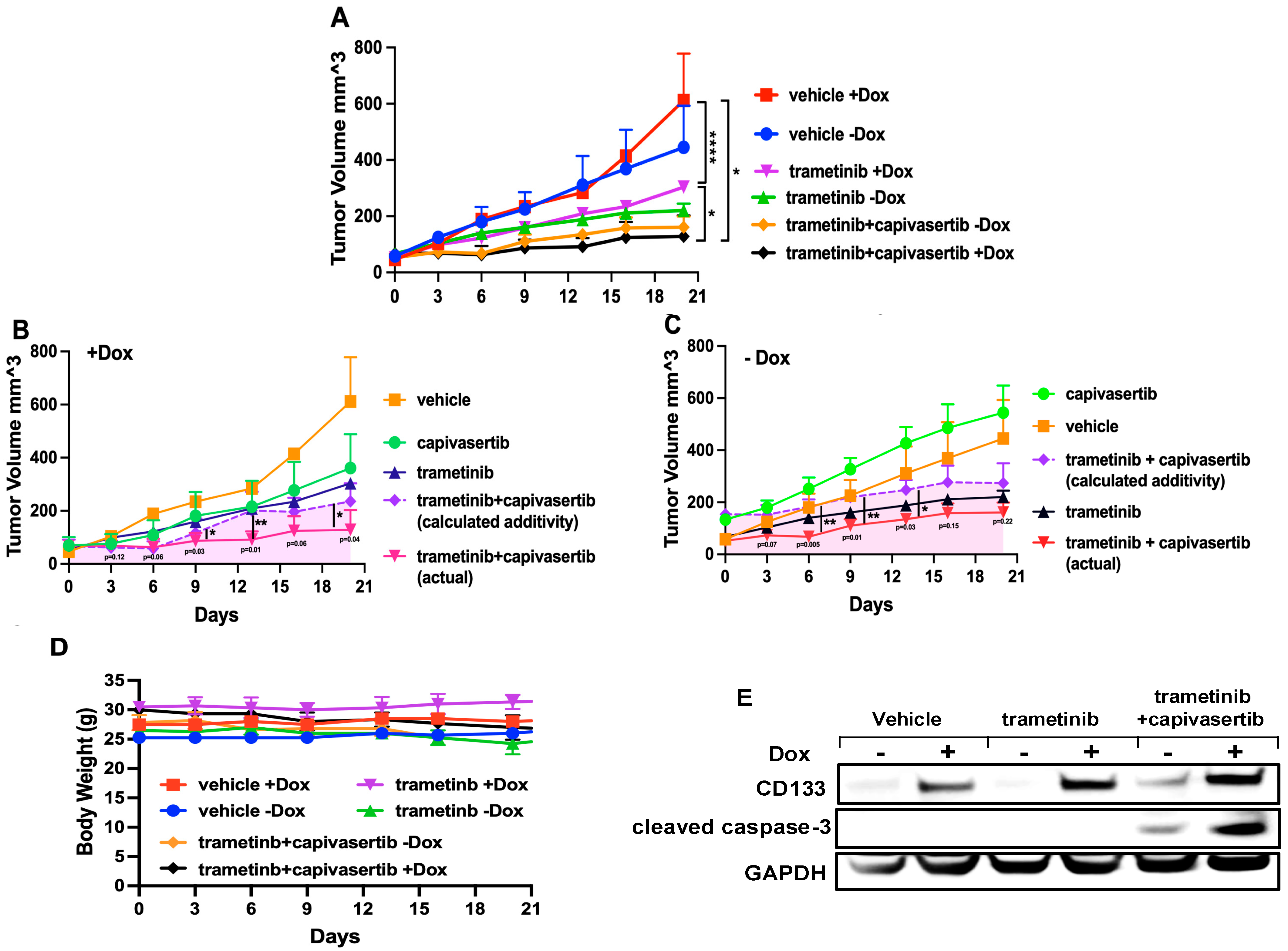
3.10. Capivasertib and Trametinib Function Synergistically in Melanoma Xenografts
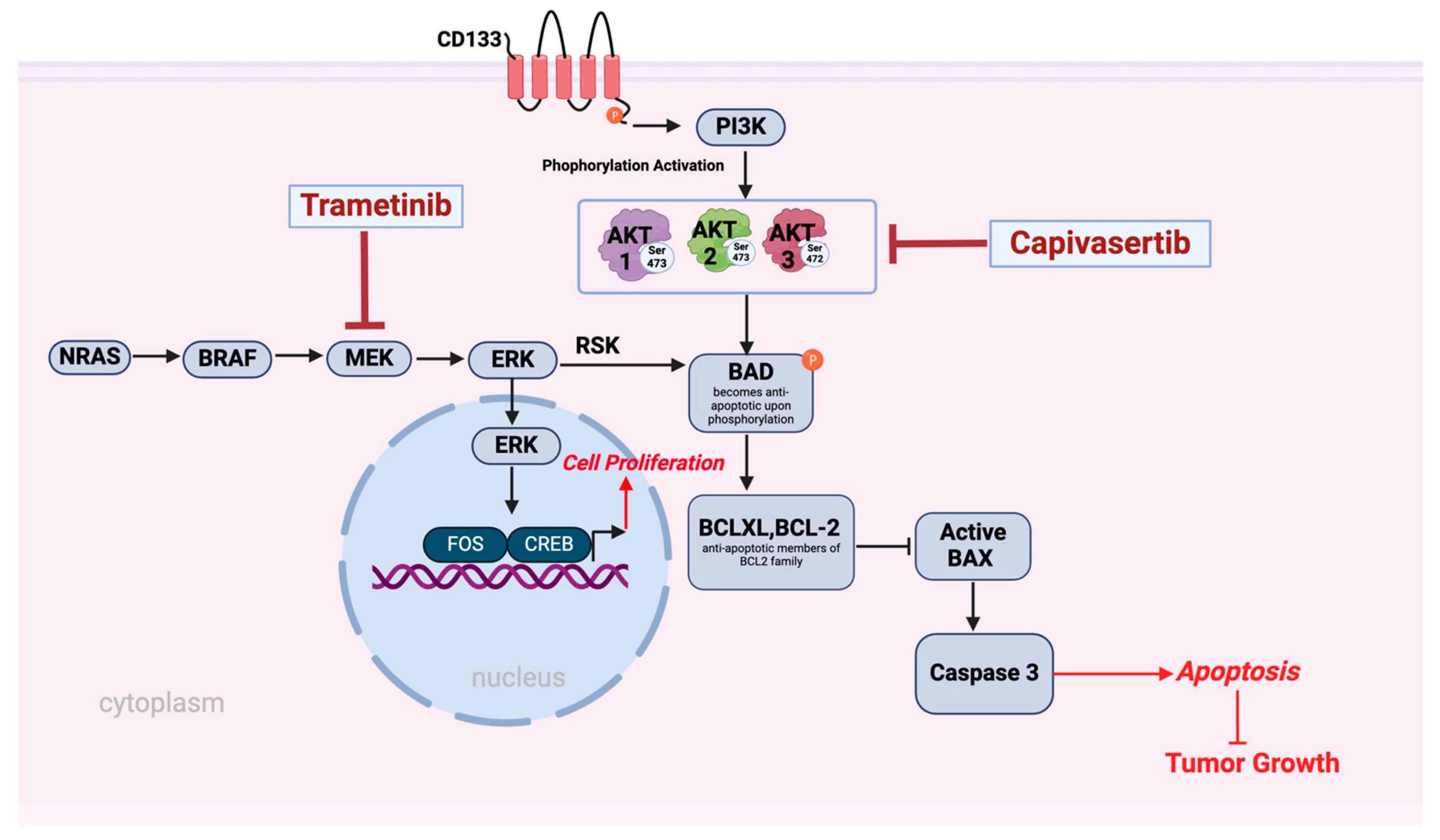
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SEER. Cancer Statistics-Reports on Cancer-Cancer Stat Facts-Melanoma of the Skin; Surveillance, Epidemiology, and End Results (SEER) Program: Bethesda, MD, USA, 2024. Available online: www.seer.cancer.gov (accessed on 1 December 2024).
- Parkin, D.M.; Mesher, D.; Sasieni, P. 13. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br. J. Cancer 2011, 105 (Suppl. S2), S66–S69. [Google Scholar] [CrossRef]
- Conforti, C.; Zalaudek, I. Epidemiology and Risk Factors of Melanoma: A Review. Dermatol. Pract. Concept. 2021, 11, e2021161S. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.Y.; Miller, D.M.; Tsao, H. Somatic driver mutations in melanoma. Cancer 2017, 123, 2104–2117. [Google Scholar] [CrossRef] [PubMed]
- Simbulan-Rosenthal, C.M.; Haribabu, Y.; Vakili, S.; Kuo, L.W.; Clark, H.; Dougherty, R.; Alobaidi, R.; Carney, B.; Sykora, P.; Rosenthal, D.S. Employing CRISPR-Cas9 to Generate CD133 Synthetic Lethal Melanoma Stem Cells. Int. J. Mol. Sci. 2022, 23, 2333. [Google Scholar] [CrossRef]
- Cancer Genome Atlas, N. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef]
- Subbiah, V.; Baik, C.; Kirkwood, J.M. Clinical Development of BRAF plus MEK Inhibitor Combinations. Trends Cancer 2020, 6, 797–810. [Google Scholar] [CrossRef]
- Garcia-Alvarez, A.; Ortiz, C.; Munoz-Couselo, E. Current Perspectives and Novel Strategies of NRAS-Mutant Melanoma. Onco Targets Ther. 2021, 14, 3709–3719. [Google Scholar] [CrossRef] [PubMed]
- Atefi, M.; Titz, B.; Avramis, E.; Ng, C.; Wong, D.J.L.; Lassen, A.; Cerniglia, M.; Escuin-Ordinas, H.; Foulad, D.; Comin-Anduix, B.; et al. Combination of pan-RAF and MEK inhibitors in NRAS mutant melanoma. Mol. Cancer 2015, 14, 27. [Google Scholar] [CrossRef]
- de Braud, F.; Dooms, C.; Heist, R.S.; Lebbe, C.; Wermke, M.; Gazzah, A.; Schadendorf, D.; Rutkowski, P.; Wolf, J.; Ascierto, P.A.; et al. Initial Evidence for the Efficacy of Naporafenib in Combination with Trametinib in NRAS-Mutant Melanoma: Results From the Expansion Arm of a Phase Ib, Open-Label Study. J. Clin. Oncol. 2023, 41, 2651–2660. [Google Scholar] [CrossRef]
- Pleskač, P.; Fargeas, C.A.; Veselska, R.; Corbeil, D.; Skoda, J. Emerging roles of prominin-1 (CD133) in the dynamics of plasma membrane architecture and cell signaling pathways in health and disease. Cell. Mol. Biol. Lett. 2024, 29, 41. [Google Scholar] [CrossRef]
- Glumac, P.M.; LeBeau, A.M. The role of CD133 in cancer: A concise review. Clin. Transl. Med. 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef]
- Galli, R.; Binda, E.; Orfanelli, U.; Cipelletti, B.; Gritti, A.; De Vitis, S.; Fiocco, R.; Foroni, C.; Dimeco, F.; Vescovi, A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004, 64, 7011–7021. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Aljitawi, O.; Van Veldhuizen, P. Prostate Cancer Stem Cells: The Role of CD133. Cancers 2022, 14, 5448. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G.; Roz, L.; Perego, P.; Tortoreto, M.; Fontanella, E.; Gatti, L.; Pratesi, G.; Fabbri, A.; Andriani, F.; Tinelli, S.; et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc. Natl. Acad. Sci. USA 2009, 106, 16281–16286. [Google Scholar] [CrossRef]
- Song, S.; Pei, G.; Du, Y.; Wu, J.; Ni, X.; Wang, S.; Jiang, B.; Luo, M.; Yu, J. Interaction between CD133 and PI3K-p85 promotes chemoresistance in gastric cancer cells. Am. J. Transl. Res. 2018, 10, 304–314. [Google Scholar] [PubMed]
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007, 445, 106–110. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, A.; Nagaki, M.; Aoki, H.; Motohashi, T.; Kunisada, T.; Moriwaki, H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem. Biophys. Res. Commun. 2006, 351, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.N.; Zeng, T.T.; He, F.; Chen, S.P.; Ma, S.; Bi, J.; Zhu, X.F.; Guan, X.Y. CD133+ liver cancer stem cells resist interferon-gamma-induced autophagy. BMC Cancer 2016, 16, 15. [Google Scholar] [CrossRef]
- Barzegar Behrooz, A.; Syahir, A.; Ahmad, S. CD133: Beyond a cancer stem cell biomarker. J. Drug Target. 2019, 27, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Kemper, K.; Sprick, M.R.; de Bree, M.; Scopelliti, A.; Vermeulen, L.; Hoek, M.; Zeilstra, J.; Pals, S.T.; Mehmet, H.; Stassi, G.; et al. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res. 2010, 70, 719–729. [Google Scholar] [CrossRef]
- Giebel, B.; Corbeil, D.; Beckmann, J.; Höhn, J.; Freund, D.; Giesen, K.; Fischer, J.; Kögler, G.; Wernet, P. Segregation of lipid raft markers including CD133 in polarized human hematopoietic stem and progenitor cells. Blood 2004, 104, 2332–2338. [Google Scholar] [CrossRef]
- Corbeil, D.; Marzesco, A.-M.; Wilsch-Bräuninger, M.; Huttner, W.B. The intriguing links between prominin-1 (CD133), cholesterol-based membrane microdomains, remodeling of apical plasma membrane protrusions, extracellular membrane particles, and (neuro)epithelial cell differentiation. FEBS Lett. 2010, 584, 1659–1664. [Google Scholar] [CrossRef]
- Zacchigna, S.; Oh, H.; Wilsch-Brauninger, M.; Missol-Kolka, E.; Jaszai, J.; Jansen, S.; Tanimoto, N.; Tonagel, F.; Seeliger, M.; Huttner, W.B.; et al. Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J. Neurosci. 2009, 29, 2297–2308. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.R.; Greenwood, H.; Dudley, P.; Crafter, C.; Yu, D.-H.; Zhang, J.; Li, J.; Gao, B.; Ji, Q.; Maynard, J.; et al. Preclinical Pharmacology of AZD5363, an Inhibitor of AKT: Pharmacodynamics, Antitumor Activity, and Correlation of Monotherapy Activity with Genetic Background. Mol. Cancer Ther. 2012, 11, 873–887. [Google Scholar] [CrossRef]
- Simbulan-Rosenthal, C.M.; Dougherty, R.; Vakili, S.; Ferraro, A.M.; Kuo, L.W.; Alobaidi, R.; Aljehane, L.; Gaur, A.; Sykora, P.; Glasgow, E.; et al. CRISPR-Cas9 Knockdown and Induced Expression of CD133 Reveal Essential Roles in Melanoma Invasion and Metastasis. Cancers 2019, 11, 1490. [Google Scholar] [CrossRef]
- Simbulan-Rosenthal, C.M.; Dakshanamurthy, S.; Gaur, A.; Chen, Y.S.; Fang, H.B.; Abdussamad, M.; Zhou, H.; Zapas, J.; Calvert, V.; Petricoin, E.F.; et al. The repurposed anthelmintic mebendazole in combination with trametinib suppresses refractory NRASQ61K melanoma. Oncotarget 2017, 8, 12576–12595. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yang, J.; Gong, H.; Lin, Y.; Liu, J. Trametinib Inhibits the Growth and Aerobic Glycolysis of Glioma Cells by Targeting the PKM2/c-Myc Axis. Front. Pharmacol. 2021, 12, 760055. [Google Scholar] [CrossRef] [PubMed]
- Eberlein, C.; Williamson, S.C.; Hopcroft, L.; Ros, S.; Moss, J.I.; Kerr, J.; van Weerden, W.M.; de Bruin, E.C.; Dunn, S.; Willis, B.; et al. Capivasertib combines with docetaxel to enhance anti-tumour activity through inhibition of AKT-mediated survival mechanisms in prostate cancer. Br. J. Cancer 2024, 130, 1377–1387. [Google Scholar] [CrossRef]
- Simbulan-Rosenthal, C.M.; Daher, A.; Trabosh, V.; Chen, W.C.; Gerstel, D.; Soeda, E.; Rosenthal, D.S. Id3 induces a caspase-3- and -9-dependent apoptosis and mediates UVB sensitization of HPV16 E6/7 immortalized human keratinocytes. Oncogene 2006, 25, 3649–3660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bliss, C.I. THE TOXICITY OF POISONS APPLIED JOINTLY1. Ann. Appl. Biol. Int. J. AAB 1939, 26, 585–615. [Google Scholar] [CrossRef]
- Duarte, D.; Vale, N. Evaluation of synergism in drug combinations and reference models for future orientations in oncology. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100110. [Google Scholar] [CrossRef]
- Simbulan-Rosenthal, C.M.; Gaur, A.; Zhou, H.; AbdusSamad, M.; Qin, Q.; Dougherty, R.; Aljehane, L.; Kuo, L.W.; Vakili, S.; Karna, K.; et al. CD133 Is Associated with Increased Melanoma Cell Survival after Multikinase Inhibition. J. Oncol. 2019, 2019, 6486173. [Google Scholar] [CrossRef]
- He, B.; Liang, J.; Qin, Q.; Zhang, Y.; Shi, S.; Cao, J.; Zhang, Z.; Bie, Q.; Zhao, R.; Wei, L.; et al. IL-13/IL-13RA2 signaling promotes colorectal cancer stem cell tumorigenesis by inducing ubiquitinated degradation of p53. Genes. Dis. 2024, 11, 495–508. [Google Scholar] [CrossRef]
- Wei, Y.; Jiang, Y.; Zou, F.; Liu, Y.; Wang, S.; Xu, N.; Xu, W.; Cui, C.; Xing, Y.; Liu, Y.; et al. Activation of PI3K/Akt pathway by CD133-p85 interaction promotes tumorigenic capacity of glioma stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 6829–6834. [Google Scholar] [CrossRef]
- Shimozato, O.; Waraya, M.; Nakashima, K.; Souda, H.; Takiguchi, N.; Yamamoto, H.; Takenobu, H.; Uehara, H.; Ikeda, E.; Matsushita, S.; et al. Receptor-type protein tyrosine phosphatase kappa directly dephosphorylates CD133 and regulates downstream AKT activation. Oncogene 2015, 34, 1949–1960. [Google Scholar] [CrossRef]
- Simbulan-Rosenthal, C.M.; Islam, N.; Haribabu, Y.; Alobaidi, R.; Shalamzari, A.; Graham, G.; Kuo, L.W.; Sykora, P.; Rosenthal, D.S. CD133 Stimulates Cell Proliferation via the Upregulation of Amphiregulin in Melanoma. Cells 2024, 13, 777. [Google Scholar] [CrossRef] [PubMed]
- Nierengarten, M.B. FDA approves capivasertib with fulvestrant for breast cancer. Cancer 2024, 130, 835–836. [Google Scholar] [CrossRef]
- Parkman, G.L.; Turapov, T.; Kircher, D.A.; Burnett, W.J.; Stehn, C.M.; O’Toole, K.; Culver, K.M.; Chadwick, A.T.; Elmer, R.C.; Flaherty, R.; et al. Genetic Silencing of AKT Induces Melanoma Cell Death via mTOR Suppression. Mol. Cancer Ther. 2024, 23, 301–315. [Google Scholar] [CrossRef]
- Algazi, A.P.; Esteve-Puig, R.; Nosrati, A.; Hinds, B.; Hobbs-Muthukumar, A.; Nandoskar, P.; Ortiz-Urda, S.; Chapman, P.B.; Daud, A. Dual MEK/AKT inhibition with trametinib and GSK2141795 does not yield clinical benefit in metastatic NRAS-mutant and wild-type melanoma. Pigment. Cell Melanoma Res. 2018, 31, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Aasen, S.N.; Parajuli, H.; Hoang, T.; Feng, Z.; Stokke, K.; Wang, J.; Roy, K.; Bjerkvig, R.; Knappskog, S.; Thorsen, F. Effective Treatment of Metastatic Melanoma by Combining MAPK and PI3K Signaling Pathway Inhibitors. Int. J. Mol. Sci. 2019, 20, 4235. [Google Scholar] [CrossRef]
- Maira, S.M.; Pecchi, S.; Huang, A.; Burger, M.; Knapp, M.; Sterker, D.; Schnell, C.; Guthy, D.; Nagel, T.; Wiesmann, M.; et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol. Cancer Ther. 2012, 11, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.; Xu, X.; Davies, M.A.; Gimotty, P.A.; Morrissette, J.J.D.; Tetzlaff, M.T.; Wani, K.M.; Liu, S.; Deng, W.; Buckley, M.; et al. A Multicenter Phase I Study Evaluating Dual PI3K and BRAF Inhibition with PX-866 and Vemurafenib in Patients with Advanced BRAF V600-Mutant Solid Tumors. Clin. Cancer Res. 2018, 24, 22–32. [Google Scholar] [CrossRef]
- Kantor, A.; Daud, A.; Munster, P.N.; Ea, R.; Algazi, A.P. A phase I/II trial of BKM120 combined with vemurafenib (PLX4032) in BRAFV600E/k mutant advanced melanoma. J. Clin. Oncol. 2012, 30, TPS8602. [Google Scholar] [CrossRef]
- Grover, R.; Drall, S.; Poonia, N.; Kumar Jain, G.; Aggarwal, G.; Lather, V.; Kesharwani, P.; Pandita, D.; Goyal, R.K. CD44 and CD133 aptamer directed nanocarriers for cancer stem cells targeting. Eur. Polym. J. 2023, 183, 111770. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alobaidi, R.; Islam, N.; Olkey, T.; Haribabu, Y.; Shamo, M.; Sykora, P.; Simbulan-Rosenthal, C.M.; Rosenthal, D.S. Combinational Inhibition of MEK and AKT Synergistically Induces Melanoma Stem Cell Apoptosis and Blocks NRAS Tumor Growth. Cells 2025, 14, 248. https://doi.org/10.3390/cells14040248
Alobaidi R, Islam N, Olkey T, Haribabu Y, Shamo M, Sykora P, Simbulan-Rosenthal CM, Rosenthal DS. Combinational Inhibition of MEK and AKT Synergistically Induces Melanoma Stem Cell Apoptosis and Blocks NRAS Tumor Growth. Cells. 2025; 14(4):248. https://doi.org/10.3390/cells14040248
Chicago/Turabian StyleAlobaidi, Ryyan, Nusrat Islam, Toni Olkey, Yogameenakshi Haribabu, Mathew Shamo, Peter Sykora, Cynthia M. Simbulan-Rosenthal, and Dean S. Rosenthal. 2025. "Combinational Inhibition of MEK and AKT Synergistically Induces Melanoma Stem Cell Apoptosis and Blocks NRAS Tumor Growth" Cells 14, no. 4: 248. https://doi.org/10.3390/cells14040248
APA StyleAlobaidi, R., Islam, N., Olkey, T., Haribabu, Y., Shamo, M., Sykora, P., Simbulan-Rosenthal, C. M., & Rosenthal, D. S. (2025). Combinational Inhibition of MEK and AKT Synergistically Induces Melanoma Stem Cell Apoptosis and Blocks NRAS Tumor Growth. Cells, 14(4), 248. https://doi.org/10.3390/cells14040248








