Autologous Paracrine Prostasin–Matriptase Serine Protease Interaction in Lymphoid Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
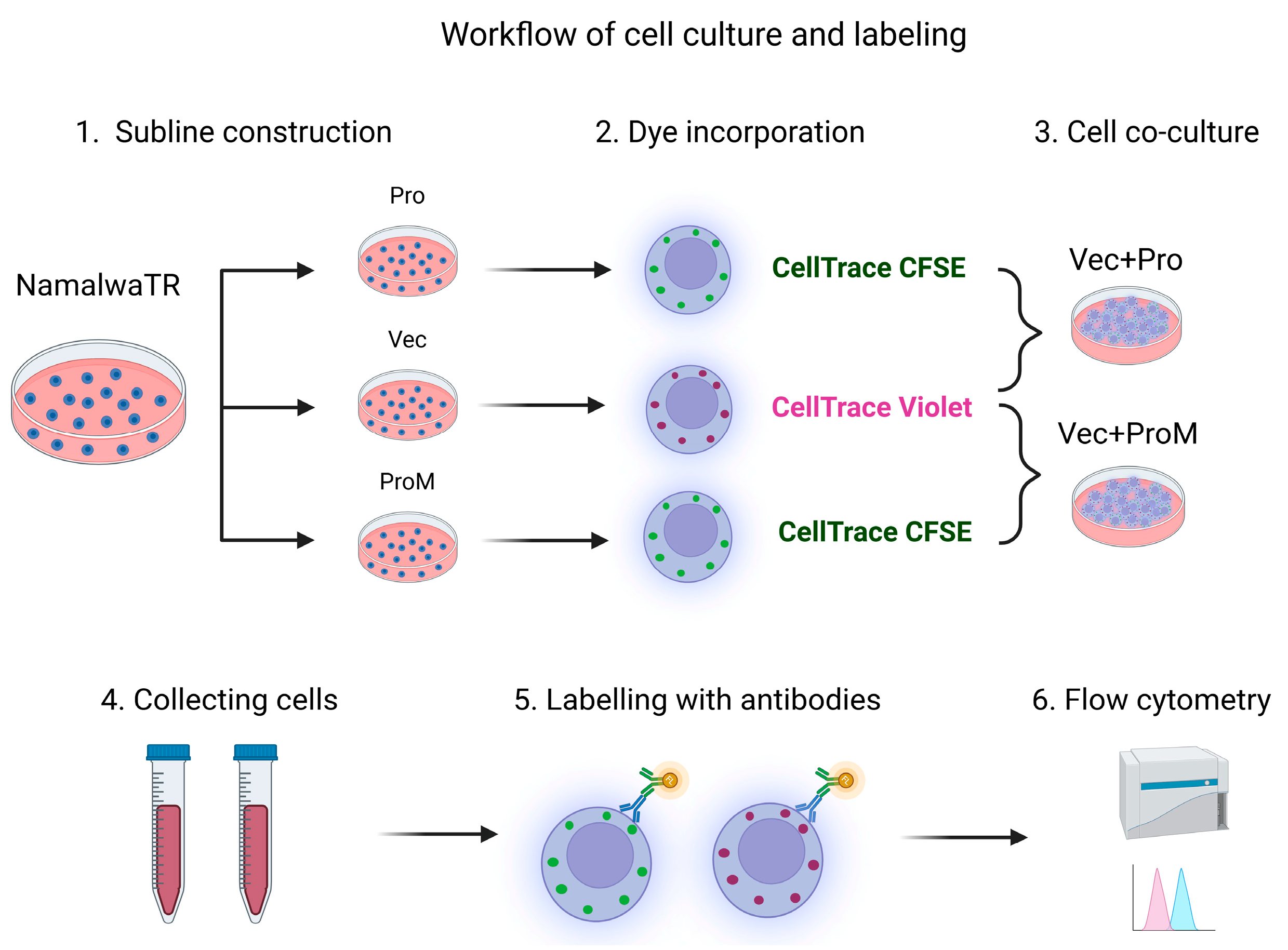
2.2. Establishment of Sublines Over-Expressing Prostasin or Matriptase
2.3. Autologous Cell–Cell Co-Cultures
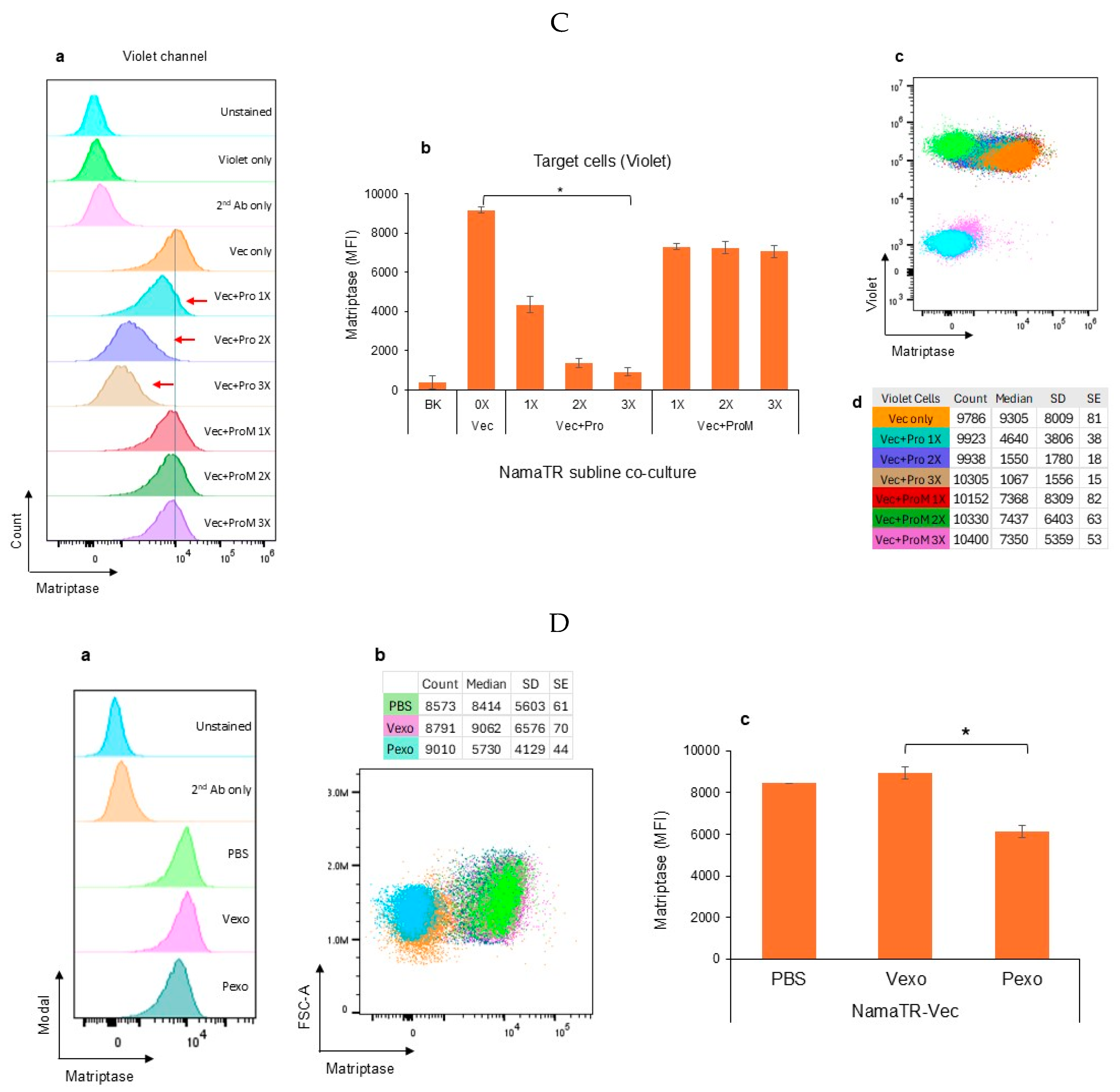
2.4. Exosome Isolation and Co-Cultures
2.5. Cell-Surface Marker Screening
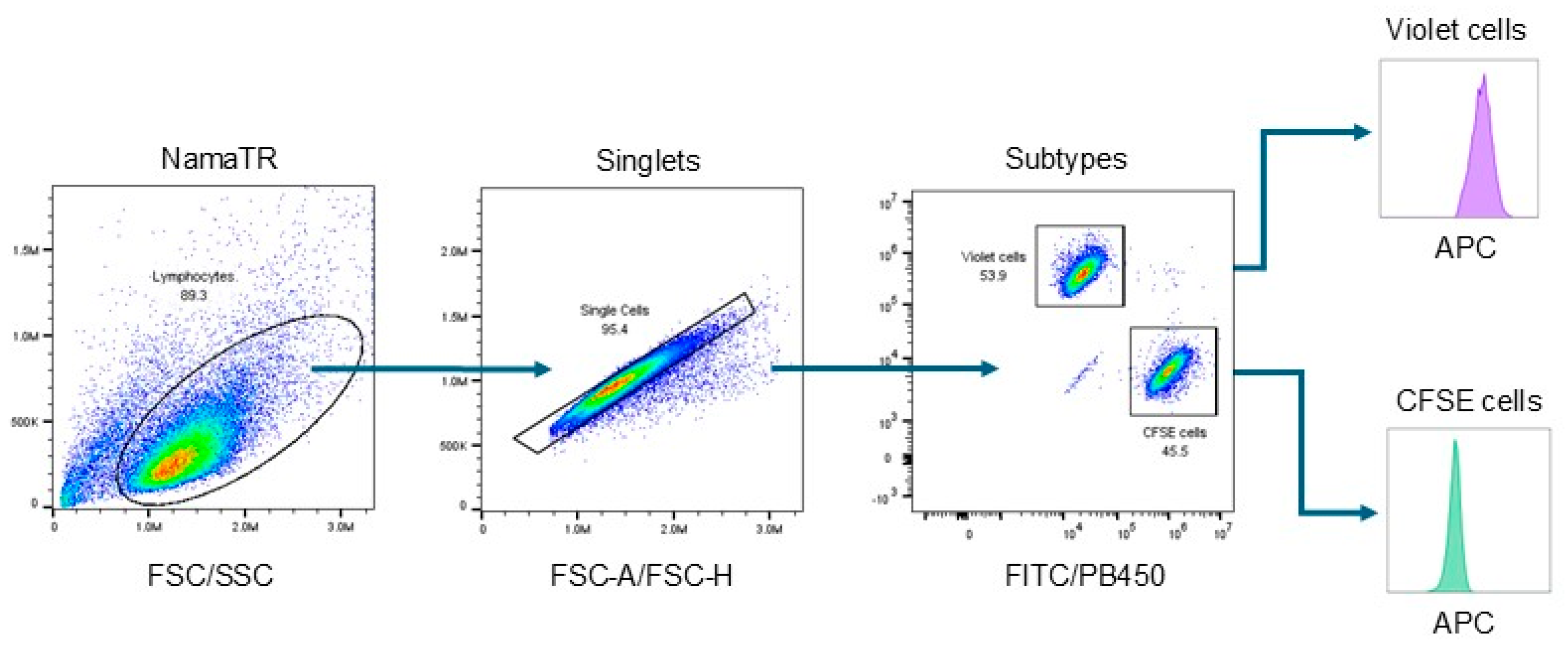
2.6. Flow Cytometry Antibodies and Data Analysis
2.7. Gene Set Expression Analysis
2.8. Statistical Analysis
3. Results
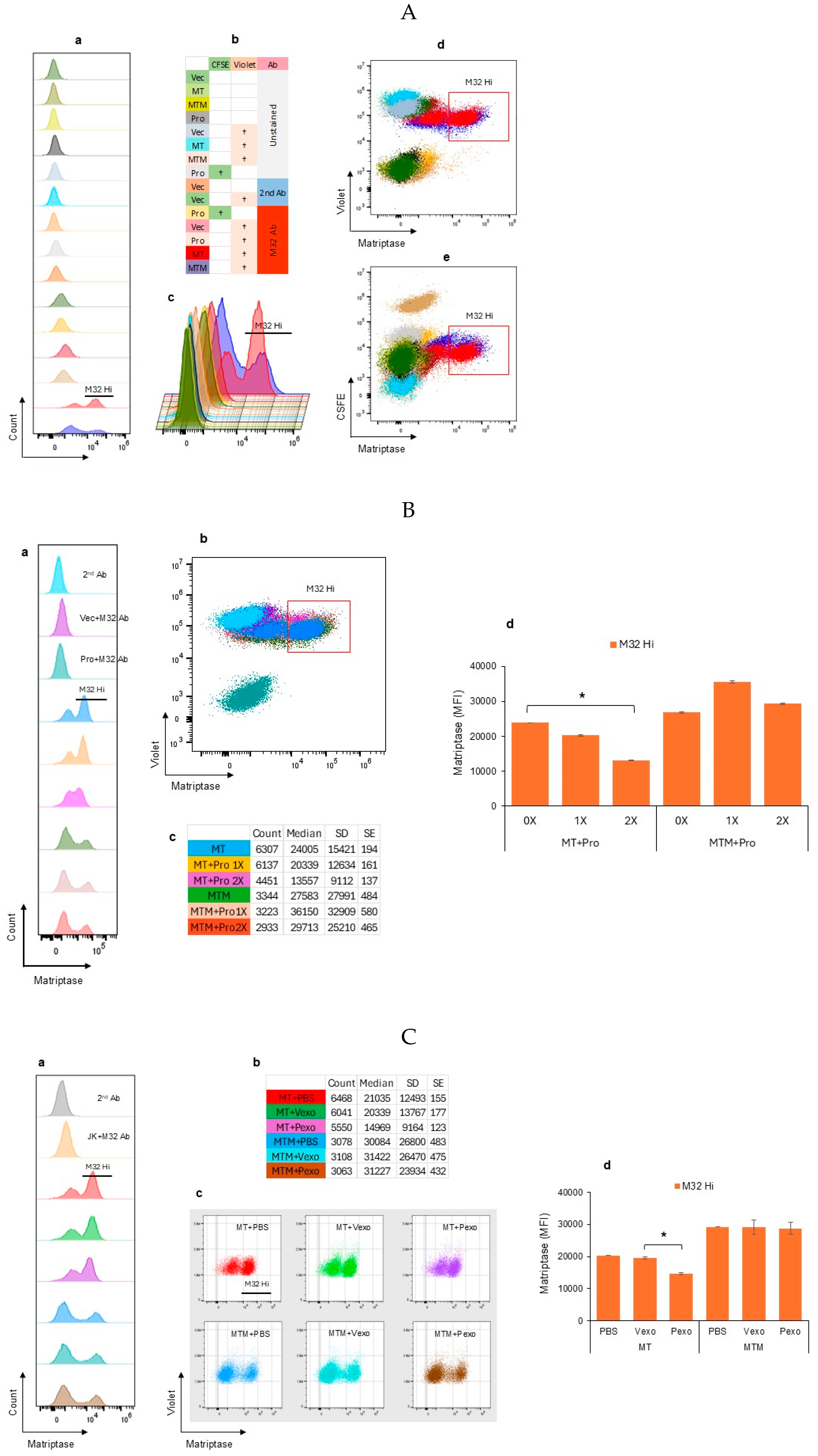
3.1. Autologous Prostasin–Matriptase Serine Protease Interaction Reduced the Endogenous Matriptase Content in the Namalwa Human Burkitt Lymphoma Cells
3.2. Autologous Prostasin–Matriptase Serine Protease Interaction Reduced the Recombinant Matriptase Content in the Jurkat Human Acute Leukemic T Cells
3.3. Prostasin Exosomes Induced Changes in the Cluster Differentiation Molecules (CDs) in Activated Human Peripheral Mononuclear Cells (PBMCs)
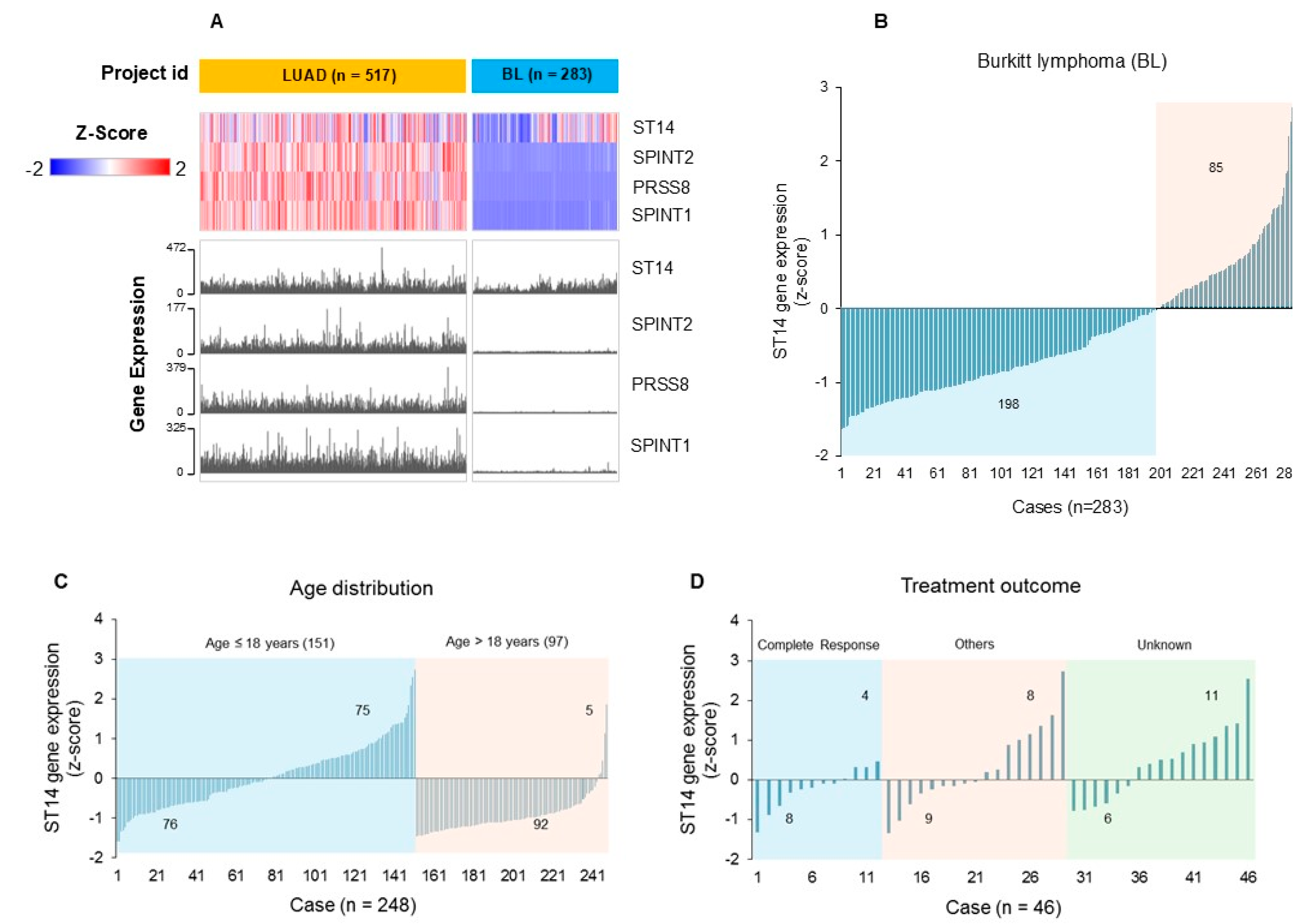
3.4. Gene Expression Profiling (RNA-Seq) of ST14, PRSS8, SPINT1 and SPINT2 (SPSS) Reveals Distinctive Patterns of Expression in Hematological Versus Epithelial Cells
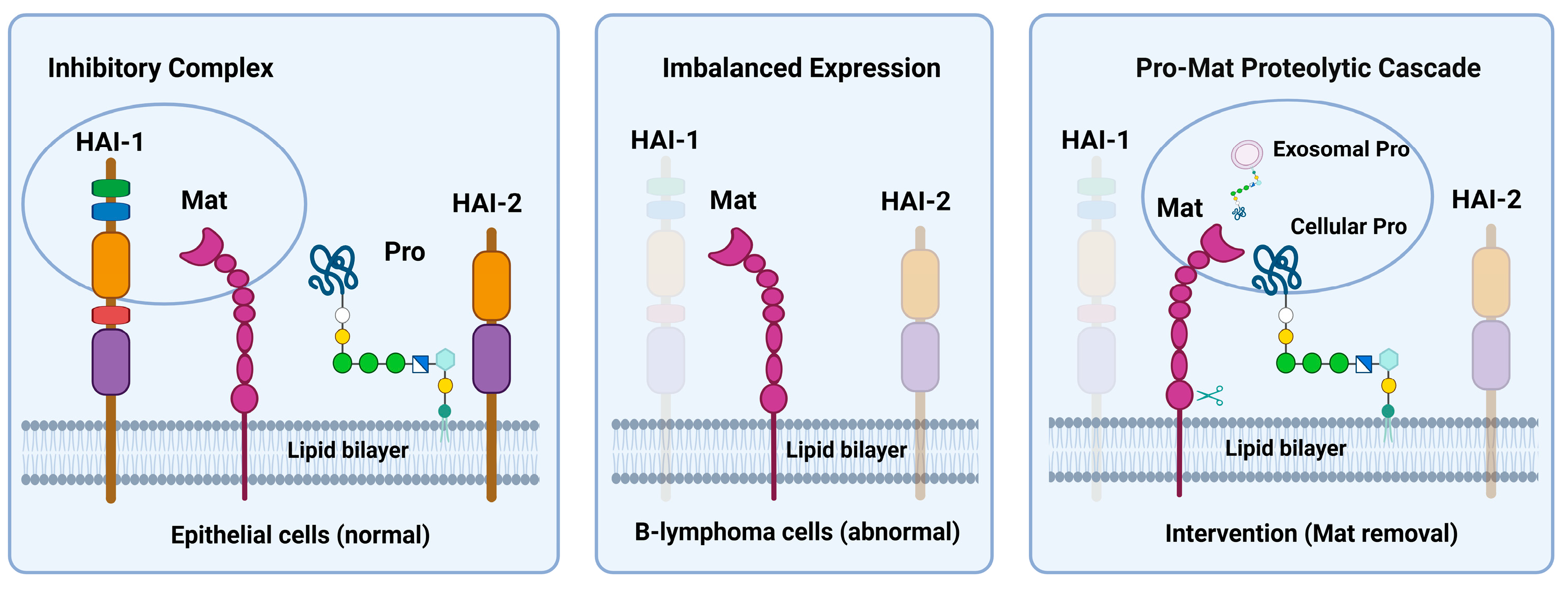
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawlings, N.D.; Salvesen, G. (Eds.) Handbook of Proteolytic Enzymes, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; ISBN 9780123822192. [Google Scholar]
- Chen, L.M. Prostasin in Human Health and Disease; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2023; ISBN 978-981-126-814-4. [Google Scholar]
- List, K.; Bugge, T.H.; Szabo, R. Matriptase: Potent proteolysis on the cell surface. Mol. Med. 2006, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Tseng, I.C.; Chou, F.P.; Su, S.F.; Chen, Y.W.; Johnson, M.D.; Dickson, R.B. Zymogen activation, inhibition, and ectodomain shedding of matriptase. Front Biosci. 2008, 13, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Yuan, Z.; Tang, Y.; Li, J.; Liu, X.; Sun, X.; Chen, S.; Ye, X.; Zeng, Z.; Zhang, X.K.; et al. Endocytic activation and exosomal secretion of matriptase stimulate the second wave of EGF signaling to promote skin and breast cancer invasion. Cell Rep. 2024, 43, 114002. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Anders, J.; Johnson, M.; Sang, Q.A.; Dickson, R.B. Molecular cloning of cDNA for matriptase, a matrix-degrading serine protease with trypsin-like activity. J. Biol. Chem. 1999, 274, 18231–18236. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Shuman, M.A.; Craik, C.S. Reverse biochemistry: Use of macromolecular protease inhibitors to dissect complex biological processes and identify a membrane-type serine protease in epithelial cancer and normal tissue. Proc. Natl. Acad. Sci. USA 1999, 96, 11054–11061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, J.X.; Chao, L.; Chao, J. Prostasin is a novel human serine proteinase from seminal fluid. Purification, tissue distribution, and localization in prostate gland. J. Biol. Chem. 1994, 269, 18843–18848. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Skinner, M.L.; Kauffman, S.W.; Chao, J.; Chao, L.; Thaler, C.D.; Chai, K.X. Prostasin is a glycosylphosphatidylinositol-anchored active serine protease. J. Biol. Chem. 2001, 276, 21434–21442. [Google Scholar] [CrossRef] [PubMed]
- List, K.; Haudenschild, C.C.; Szabo, R.; Chen, W.; Wahl, S.M.; Swaim, W.; Engelholm, L.H.; Behrendt, N.; Bugge, T.H. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene 2002, 21, 3765–3779. [Google Scholar] [CrossRef] [PubMed]
- Leyvraz, C.; Charles, R.P.; Rubera, I.; Guitard, M.; Rotman, S.; Breiden, B.; Sandhoff, K.; Hummler, E. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J. Cell Biol. 2005, 170, 487–496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hummler, E.; Dousse, A.; Rieder, A.; Stehle, J.C.; Rubera, I.; Osterheld, M.C.; Beermann, F.; Frateschi, S.; Charles, R.P. The channel-activating protease CAP1/Prss8 is required for placental labyrinth maturation. PLoS ONE 2013, 8, e55796. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oberst, M.D.; Williams, C.A.; Dickson, R.B.; Johnson, M.D.; Lin, C.Y. The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. J. Biol. Chem. 2003, 278, 26773–26779. [Google Scholar] [CrossRef] [PubMed]
- Friis, S.; Sales, K.U.; Schafer, J.M.; Vogel, L.K.; Kataoka, H.; Bugge, T.H. The protease inhibitor HAI-2, but not HAI-1, regulates matriptase activation and shedding through prostasin. J. Biol. Chem. 2014, 289, 22319–22332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tseng, C.C.; Jia, B.; Barndt, R.; Gu, Y.; Chen, C.Y.; Tseng, I.C.; Su, S.F.; Wang, J.K.; Johnson, M.D.; Lin, C.Y. Matriptase shedding is closely coupled with matriptase zymogen activation and requires de novo proteolytic cleavage likely involving its own activity. PLoS ONE 2017, 12, e0183507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimomura, T.; Denda, K.; Kitamura, A.; Kawaguchi, T.; Kito, M.; Kondo, J.; Kagaya, S.; Qin, L.; Takata, H.; Miyazawa, K.; et al. Hepatocyte growth factor activator inhibitor, a novel Kunitz-type serine protease inhibitor. J. Biol. Chem. 1997, 272, 6370–6376. [Google Scholar] [CrossRef] [PubMed]
- Marlor, C.W.; Delaria, K.A.; Davis, G.; Muller, D.K.; Greve, J.M.; Tamburini, P.P. Identification and cloning of human placental bikunin, a novel serine protease inhibitor containing two Kunitz domains. J. Biol. Chem. 1997, 272, 12202–12208. [Google Scholar] [CrossRef] [PubMed]
- Oberst, M.D.; Chen, L.Y.; Kiyomiya, K.; Williams, C.A.; Lee, M.S.; Johnson, M.D.; Dickson, R.B.; Lin, C.Y. HAI-1 regulates activation and expression of matriptase, a membrane-bound serine protease. Am. J. Physiol. Cell Physiol. 2005, 289, C462–C470. [Google Scholar] [CrossRef] [PubMed]
- Benaud, C.M.; Oberst, M.; Dickson, R.B.; Lin, C.Y. Deregulated activation of matriptase in breast cancer cells. Clin. Exp. Metastasis 2002, 19, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Szabo, R.; Uzzun Sales, K.; Kosa, P.; Shylo, N.A.; Godiksen, S.; Hansen, K.K.; Friis, S.; Gutkind, J.S.; Vogel, L.K.; Hummler, E.; et al. Reduced prostasin (CAP1/PRSS8) activity eliminates HAI-1 and HAI-2 deficiency-associated developmental defects by preventing matriptase activation. PLoS Genet. 2012, 8, e1002937. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nimishakavi, S.; Besprozvannaya, M.; Raymond, W.W.; Craik, C.S.; Gruenert, D.C.; Caughey, G.H. Activity and inhibition of prostasin and matriptase on apical and basolateral surfaces of human airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L97–L106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, H.H.; Xu, Y.; Lai, H.; Yang, X.; Tseng, C.C.; Lai, Y.J.; Pan, Y.; Zhou, E.; Johnson, M.D.; Wang, J.K.; et al. Differential subcellular localization renders HAI-2 a matriptase inhibitor in breast cancer cells but not in mammary epithelial cells. PLoS ONE 2015, 10, e0120489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larsen, B.R.; Steffensen, S.D.; Nielsen, N.V.; Friis, S.; Godiksen, S.; Bornholdt, J.; Soendergaard, C.; Nonboe, A.W.; Andersen, M.N.; Poulsen, S.S.; et al. Hepatocyte growth factor activator inhibitor-2 prevents shedding of matriptase. Exp. Cell Res. 2013, 319, 918–929. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.P.; Kao, C.Y.; Chang, S.C.; Chiu, Y.L.; Chen, Y.J.; Chen, M.G.; Chang, C.C.; Lin, Y.W.; Chiang, C.P.; Wang, J.K.; et al. Tissue distribution and subcellular localizations determine in vivo functional relationship among prostasin, matriptase, HAI-1, and HAI-2 in human skin. PLoS ONE 2018, 13, e0192632. [Google Scholar]
- Martin, C.E.; List, K. Cell surface-anchored serine proteases in cancer progression and metastasis. Cancer Metastasis Rev. 2019, 38, 357–387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J Clin. 2024, 74, 12–49. [Google Scholar]
- Schmitz, R.; Ceribelli, M.; Pittaluga, S.; Wright, G.; Staudt, L.M. Oncogenic mechanisms in Burkitt lymphoma. Cold Spring Harb. Perspect. Med. 2014, 4, a014282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bishop, P.C.; Rao, V.K.; Wilson, W.H. Burkitt’s lymphoma: Molecular pathogenesis and treatment. Cancer Investig. 2000, 18, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Chou, F.P.; Chen, Y.W.; Zhao, X.F.; Xu-Monette, Z.Y.; Young, K.H.; Gartenhaus, R.B.; Wang, J.K.; Kataoka, H.; Zuo, A.H.; Barndt, R.J.; et al. Imbalanced matriptase pericellular proteolysis contributes to the pathogenesis of malignant B-cell lymphomas. Am. J. Pathol. 2013, 183, 1306–1317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiu, Y.L.; Wu, Y.Y.; Barndt, R.B.; Yeo, Y.H.; Lin, Y.W.; Sytwo, H.P.; Liu, H.C.; Xu, Y.; Jia, B.; Wang, J.K.; et al. Aberrant regulation favours matriptase proteolysis in neoplastic B-cells that co-express HAI-2. J. Enzym. Inhib. Med. Chem. 2019, 34, 692–702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, L.; Liu, M.; Dong, N.; Jiang, Y.; Lin, C.Y.; Huang, M.; Wu, D.; Wu, Q. Matriptase is highly upregulated in chronic lymphocytic leukemia and promotes cancer cell invasion. Leukemia 2013, 27, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- List, K.; Szabo, R.; Molinolo, A.; Sriuranpong, V.; Redeye, V.; Murdock, T.; Burke, B.; Nielsen, B.S.; Gutkind, J.S.; Bugge, T.H. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes. Dev. 2005, 19, 1934–1950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friis, S. Matriptase (ST14, Suppressor of Tumorigenicity 14 Protein). In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Zoratti, G.L.; Tanabe, L.M.; Varela, F.A.; Murray, A.S.; Bergum, C.; Colombo, É.; Lang, J.E.; Molinolo, A.A.; Leduc, R.; Marsault, E.; et al. Targeting matriptase in breast cancer abrogates tumour progression via impairment of stromal-epithelial growth factor signalling. Nat. Commun. 2015, 6, 6776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chatterjee, S.; Smith, E.R.; Hanada, K.; Stevens, V.L.; Mayor, S. GPI anchoring leads to sphingolipid-dependent retention of endocytosed proteins in the recycling endosomal compartment. EMBO J. 2001, 20, 1583–1592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, L.M.; Chai, J.C.; Liu, B.; Strutt, T.M.; McKinstry, K.K.; Chai, K.X. Prostasin regulates PD-L1 expression in human lung cancer cells. Biosci. Rep. 2021, 41, BSR20211370. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Chai, K.X. Exosome-Mediated Activation of the Prostasin-Matriptase Serine Protease Cascade in B Lymphoma Cells. Cancers 2023, 15, 3848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van der Lubbe, N.; Jansen, P.M.; Salih, M.; Fenton, R.A.; van den Meiracker, A.H.; Danser, A.H.; Zietse, R.; Hoorn, E.J. The phosphorylated sodium chloride cotransporter in urinary exosomes is superior to prostasin as a marker for aldosteronism. Hypertension 2012, 60, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, O.; Chiecchi, L.; Pizzolo, F.; Castagna, A.; Raffaelli, R.; Gunasekaran, M.; Guarini, P.; Consoli, L.; Salvagno, G.; Kitamura, K. Urinary prostasin in normotensive individuals: Correlation with the aldosterone to renin ratio and urinary sodium. Hypertens. Res. 2013, 36, 528–533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qi, Y.; Wang, X.; Rose, K.L.; MacDonald, W.H.; Zhang, B.; Schey, K.L.; Luther, J.M. Activation of the Endogenous Renin-Angiotensin-Aldosterone System or Aldosterone Administration Increases Urinary Exosomal Sodium Channel Excretion. J. Am. Soc. Nephrol. 2016, 27, 646–656. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zachar, R.; Jensen, B.L.; Svenningsen, P. Dietary Na+ intake in healthy humans changes the urine extracellular vesicle prostasin abundance while the vesicle excretion rate, NCC, and ENaC are not altered. Am. J. Physiol. Renal Physiol. 2019, 317, F1612–F1622. [Google Scholar] [CrossRef] [PubMed]
- Krishnamachary, B.; Cook, C.; Kumar, A.; Spikes, L.; Chalise, P.; Dhillon, N.K. Extracellular vesicle-mediated endothelial apoptosis and EV-associated proteins correlate with COVID-19 disease severity. J. Extracell. Vesicles 2021, 10, e12117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fontana, S.; Mauceri, R.; Novara, M.E.; Alessandro, R.; Campisi, G. Protein Cargo of Salivary Small Extracellular Vesicles as Potential Functional Signature of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2021, 22, 11160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rossi, M.; Breman, E. Engineering strategies to safely drive CAR T-cells into the future. Front. Immunol. 2024, 15, 1411393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Posey, A.D., Jr.; Young, R.M.; June, C.H. Future perspectives on engineered T cells for cancer. Trends Cancer 2024, 10, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Chai, A.C.; Robinson, A.L.; Chai, K.X.; Chen, L.M. Ibuprofen regulates the expression and function of membrane-associated serine proteases prostasin and matriptase. BMC Cancer 2015, 15, 1025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tseng, I.C.; Chou, F.P.; Su, S.F.; Oberst, M.; Madayiputhiya, N.; Lee, M.S.; Wang, J.K.; Sloane, D.E.; Johnson, M.; Lin, C.Y. Purification from human milk of matriptase complexes with secreted serpins: Mechanism for inhibition of matriptase other than HAI-1. Am. J. Physiol. Cell Physiol. 2008, 295, C423–C431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, J.; Scott, C.A.; Ho, Y.N.; Mahabaleshwar, H.; Marsay, K.S.; Zhang, C.; Teow, C.K.; Ng, S.S.; Zhang, W.; Tergaonkar, V.; et al. Matriptase activation of Gq drives epithelial disruption and inflammation via RSK and DUOX. Elife 2021, 10, e66596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaymon, D.O.; Barndt, R.; Stires, H.; Riggins, R.B.; Johnson, M.D. ROS is a master regulator of in vitro matriptase activation. PLoS ONE 2023, 18, e0267492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magri, Z.; Jetton, D.; Muendlein, H.I.; Connolly, W.M.; Russell, H.; Smirnova, I.; Sharma, S.; Bunnell, S.; Poltorak, A. CD14 is a decision-maker between Fas-mediated death and inflammation. Cell Rep. 2024, 43, 114685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uchimura, K.; Hayata, M.; Mizumoto, T.; Miyasato, Y.; Kakizoe, Y.; Morinaga, J.; Onoue, T.; Yamazoe, R.; Ueda, M.; Adachi, M.; et al. The serine protease prostasin regulates hepatic insulin sensitivity by modulating TLR4 signalling. Nat. Commun. 2014, 5, 3428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sugitani, Y.; Nishida, A.; Inatomi, O.; Ohno, M.; Imai, T.; Kawahara, M.; Kitamura, K.; Andoh, A. Sodium absorption stimulator prostasin (PRSS8) has an anti-inflammatory effect via downregulation of TLR4 signaling in inflammatory bowel disease. J. Gastroenterol. 2020, 55, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Takizawa, S.; Uchimura, K.; Miyazaki, A.; Tsuchiya, K. Liver-Specific Overexpression of Prostasin Attenuates High-Fat Diet-Induced Metabolic Dysregulation in Mice. Int. J. Mol. Sci. 2021, 22, 8314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, L.M.; Wang, C.; Chen, M.; Marcello, M.R.; Chao, J.; Chao, L.; Chai, K.X. Prostasin attenuates inducible nitric oxide synthase expression in lipopolysaccharide-induced urinary bladder inflammation. Am. J. Physiol. Renal Physiol. 2006, 291, F567–F577. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Hatfield, M.L.; Fu, Y.Y.; Chai, K.X. Prostasin regulates iNOS and cyclin D1 expression by modulating protease-activated receptor-2 signaling in prostate epithelial cells. Prostate 2009, 69, 1790–1801. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, S.M.; Szabo, R.; Lee, M.; Kirchhofer, D.; Craik, C.S.; Bugge, T.H.; Camerer, E. Matriptase activation connects tissue factor-dependent coagulation initiation to epithelial proteolysis and signaling. Blood 2016, 127, 3260–3269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleischer, M.I.; Röhrig, N.; Raker, V.K.; Springer, J.; Becker, D.; Ritz, S.; Bros, M.; Stege, H.; Haist, M.; Grabbe, S.; et al. Protease- and cell type-specific activation of protease-activated receptor 2 in cutaneous inflammation. J. Thromb. Haemost. 2022, 20, 2823–2836. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, A.T.; Lyon De Ana, C.; Arafa, E.I.; Salwig, I.; Barker, K.A.; Korkmaz, F.T.; Ramanujan, A.; Etesami, N.S.; Soucy, A.M.; Martin, I.M.C.; et al. Antigen presentation by lung epithelial cells directs CD4+ TRM cell function and regulates barrier immunity. Nat. Commun. 2021, 12, 5834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hewitt, R.J.; Lloyd, C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Grossman, R.L.; Heath, A.P.; Ferretti, V.; Varmus, H.E.; Lowy, D.R.; Kibbe, W.A.; Staudt, L.M. Toward a Shared Vision for Cancer Genomic Data. N. Engl. J. Med. 2016, 375, 1109–1112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-M.; Chai, K.X. Autologous Paracrine Prostasin–Matriptase Serine Protease Interaction in Lymphoid Cancer Cells. Cells 2025, 14, 247. https://doi.org/10.3390/cells14040247
Chen L-M, Chai KX. Autologous Paracrine Prostasin–Matriptase Serine Protease Interaction in Lymphoid Cancer Cells. Cells. 2025; 14(4):247. https://doi.org/10.3390/cells14040247
Chicago/Turabian StyleChen, Li-Mei, and Karl X. Chai. 2025. "Autologous Paracrine Prostasin–Matriptase Serine Protease Interaction in Lymphoid Cancer Cells" Cells 14, no. 4: 247. https://doi.org/10.3390/cells14040247
APA StyleChen, L.-M., & Chai, K. X. (2025). Autologous Paracrine Prostasin–Matriptase Serine Protease Interaction in Lymphoid Cancer Cells. Cells, 14(4), 247. https://doi.org/10.3390/cells14040247





