The Expression of Neuroendocrine Markers in a Small Subset of Ameloblastoma with Implications of Clusterin
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Immunohistochemical Analyses
2.3. Transmission Electron Microscopic Analysis
2.4. RNA Isolation and Reverse Transcriptase-Polymerase Chain Reaction
2.5. Statistical Analysis
3. Results
3.1. Clinical and Histological Summaries
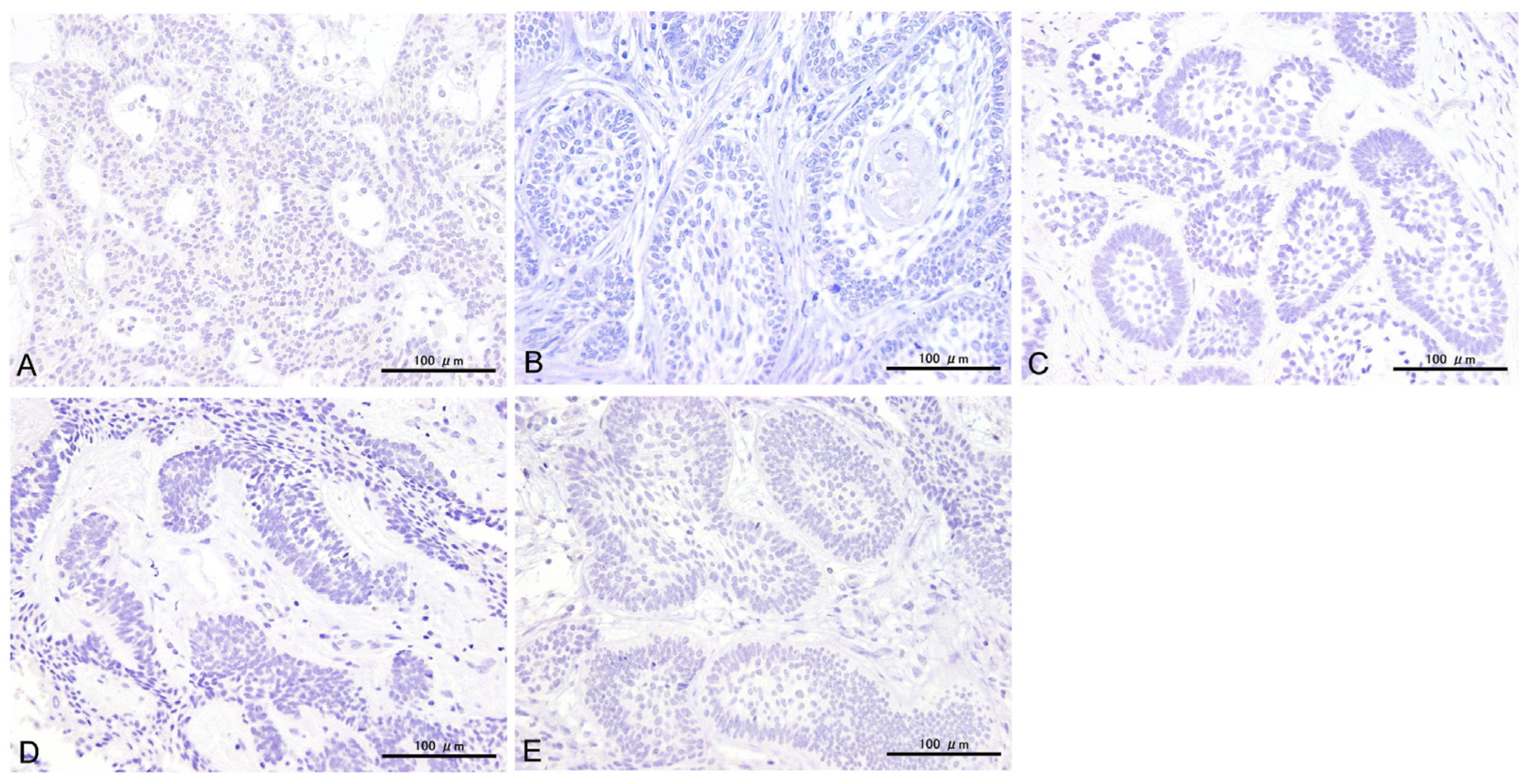
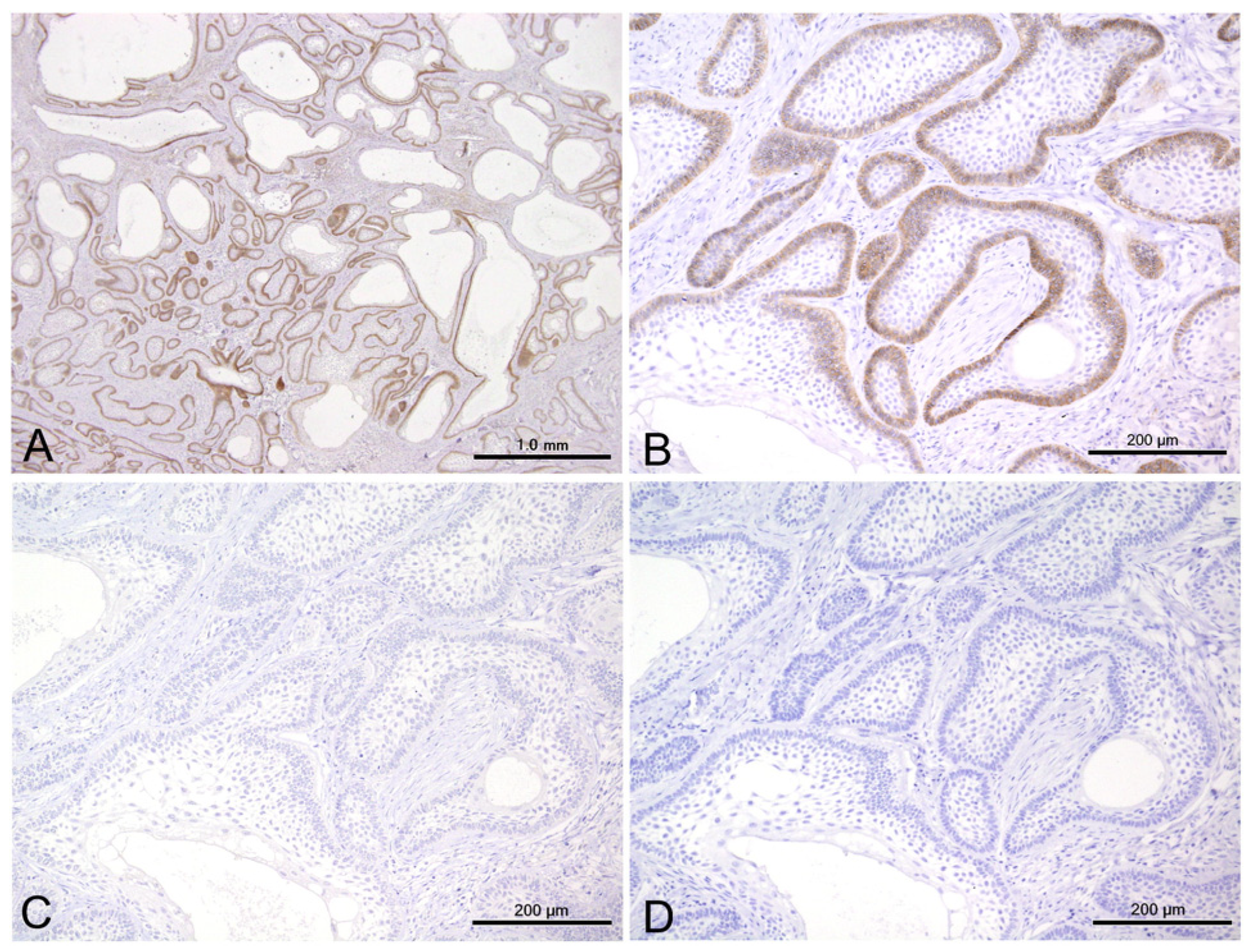
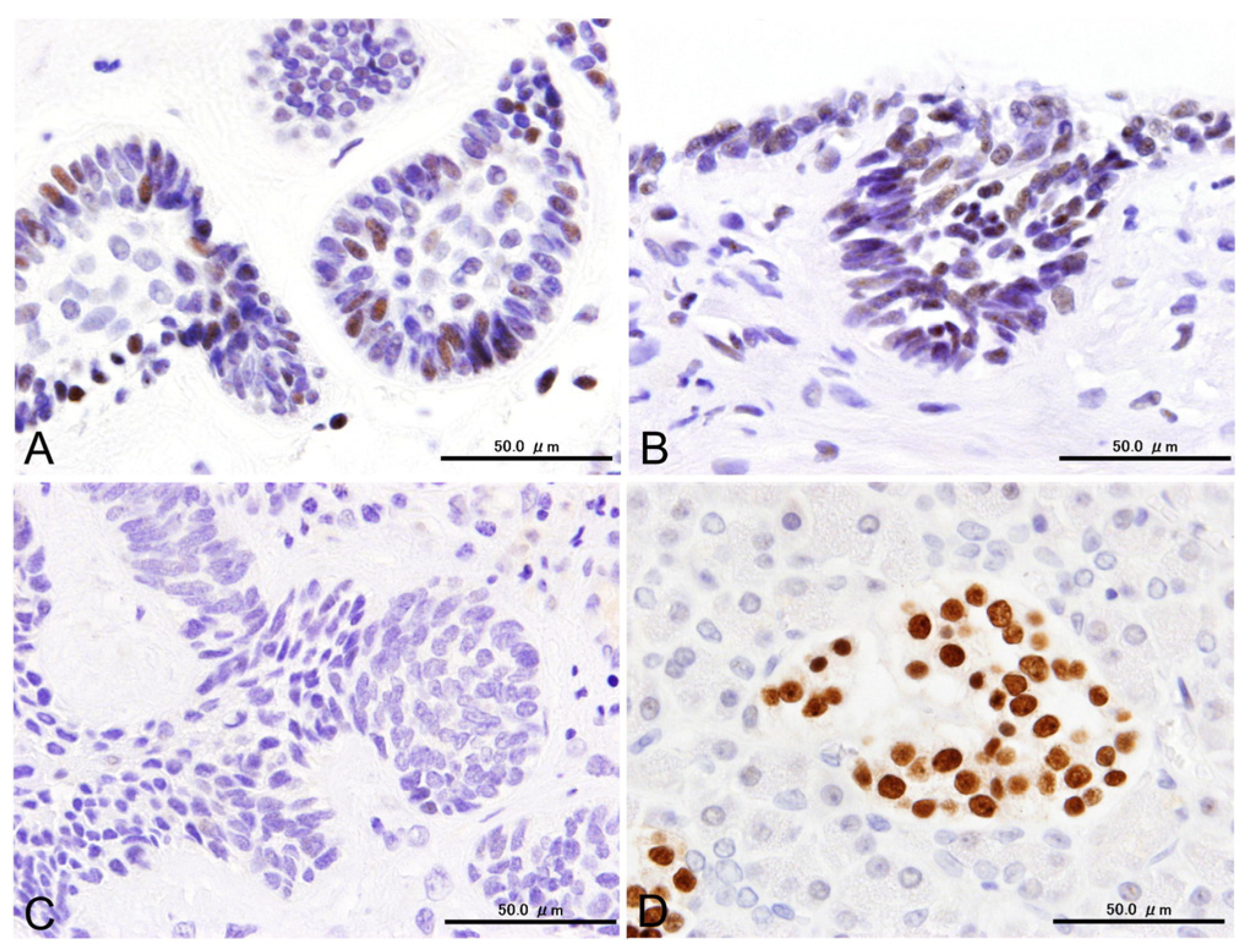
3.2. Immunohistochemical Results
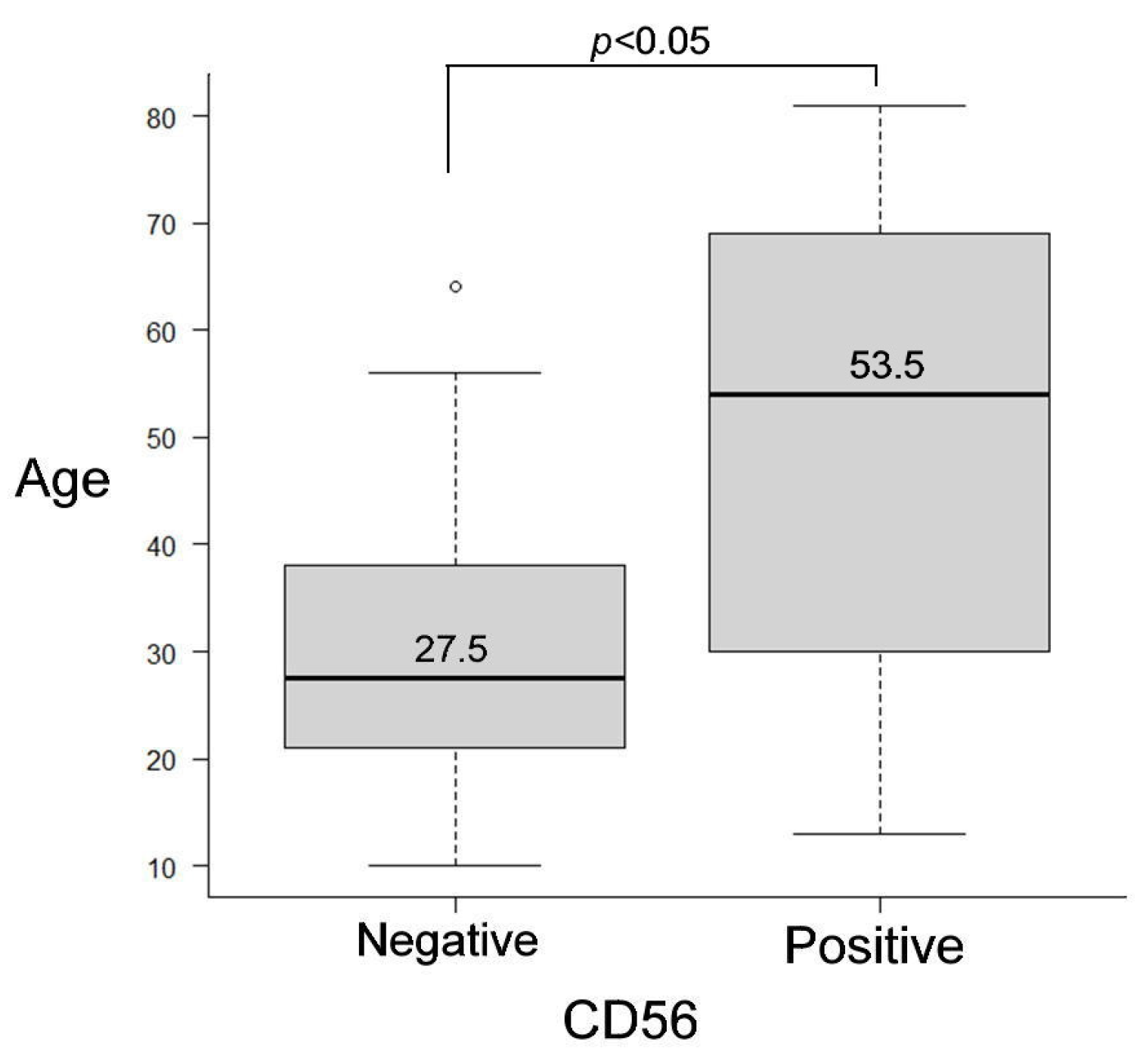
3.3. Statistical Results for Immunohistochemistry
3.4. Transmission Electron Microscopic (TEM) Findings
3.5. mRNA Expressions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. Head and Neck Tumors, 5th ed.; WHO classification of tumors series; International Agency for Research on Cancer: Lyon, France, 2023; Volume 9, Available online: https://tumourclassification.iarc.who.int/chapters/52 (accessed on 29 October 2024).
- Lanier, L.L.; Testi, R.; Bindl, J.; Phillips, J.H. Identity of Leu-19 (CD56) Leukocyte Differentiation Antigen and Neural Cell Adhesion Molecule. J. Exp. Med. 1989, 169, 2233–2238. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Fukada, S.; Yamamoto, N.; Ikemoto-Uezumi, M.; Nakatani, M.; Morita, M.; Yamaguchi, A.; Yamada, H.; Nishino, I.; Hamada, Y.; et al. Identification and Characterization of PDGFRα+ Mesenchymal Progenitors in Human Skeletal Muscle. Cell Death Dis. 2014, 5, e1186. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Yu, L.; Zheng, C.; Liang, Q.; Suye, S.; Yang, X.; Yin, H.; Ren, Z.; Shi, L.; Zhang, Z.; et al. Diagnostic Value of Four Neuroendocrine Markers in Small Cell Neuroendocrine Carcinomas of the Cervix: A Meta-Analysis. Sci. Rep. 2020, 10, 14975. [Google Scholar] [CrossRef]
- Pyo, J.-S.; Kim, D.-H.; Yang, J. Diagnostic Value of CD56 Immunohistochemistry in Thyroid Lesions. Int. J. Biol. Markers 2018, 33, 161–167. [Google Scholar] [CrossRef]
- Kusafuka, K.; Hirobe, K.; Wato, M.; Tanaka, A.; Nakajima, T. CD56 Expression Is Associated with Neuroectodermal Differentiation in Ameloblastomas: An Immunohistochemical Evaluation in Comparison with Odontogenic Cystic Lesions. Med. Mol. Morphol. 2011, 44, 79–85. [Google Scholar] [CrossRef]
- Cairns, L.; Naidu, A.; Robinson, C.M.; Sloan, P.; Wright, J.M.; Hunter, K.D. CD56 (NCAM) Expression in Ameloblastomas and Other Odontogenic Lesions. Histopathology 2010, 57, 544–548. [Google Scholar] [CrossRef]
- Jaafari-Ashkavandi, Z.; Dehghani-Nazhvani, A.; Razmjouyi, F. CD56 Expression in Odontogenic Cysts and Tumors. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 240–245. [Google Scholar] [CrossRef]
- Megyesfalvi, Z.; Barany, N.; Lantos, A.; Valko, Z.; Pipek, O.; Lang, C.; Schwendenwein, A.; Oberndorfer, F.; Paku, S.; Ferencz, B.; et al. Expression Patterns and Prognostic Relevance of Subtype-Specific Transcription Factors in Surgically Resected Small-Cell Lung Cancer: An International Multicenter Study. J. Pathol. 2022, 257, 674–686. [Google Scholar] [CrossRef]
- Baine, M.K.; Hsieh, M.-S.; Lai, W.V.; Egger, J.V.; Jungbluth, A.A.; Daneshbod, Y.; Beras, A.; Spencer, R.; Lopardo, J.; Bodd, F.; et al. SCLC Subtypes Defined by ASCL1, NEUROD1, POU2F3, and YAP1: A Comprehensive Immunohistochemical and Histopathologic Characterization. J. Thorac. Oncol. 2020, 15, 1823–1835. [Google Scholar] [CrossRef]
- Perez-Ordoñez, B. Neuroendocrine Carcinomas of the Larynx and Head and Neck: Challenges in Classification and Grading. Head. Neck Pathol. 2018, 12, 1–8. [Google Scholar] [CrossRef]
- Mete, O.; Wenig, B.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Overview of the 2022 WHO Classification of Head and Neck Neuroendocrine Neoplasms. Head Neck Pathol 2022, 16, 123–142. [Google Scholar] [CrossRef]
- La Rosa, S.; Sessa, F.; Uccella, S. Mixed Neuroendocrine-Non-neuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms. Endocr. Pathol. 2016, 27, 284–311. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Juhlin, C.C. Second-Generation Neuroendocrine Immunohistochemical Markers: Reflections from Clinical Implementation. Biology 2021, 10, 874. [Google Scholar] [CrossRef]
- Yirmibeş, S.; Adım, Ş.B.; Saraydaroğlu, Ö. CD56 and Smooth Muscle Actin Immunoreactivity in Basal Cell Carcinomas: Are They Indicators of Differentiation or Do They Hold a Diagnostic Use? J. Cutan. Pathol. 2023, 50, 56–61. [Google Scholar] [CrossRef]
- Collins, A.P.; Mubarak, N.; Hemaidan, H.S.; Hemaidan, S.M.; Hemaidan, A. Malignant Ameloblastoma with Hepatic Metastasis in a 38-Year-Old Haitian Woman. Am. J. Case Rep. 2021, 22, e929422. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Mohan, K.; Gasparoni, G.; Salhab, A.; Orlich, M.M.; Geffers, R.; Hoffmann, S.; Adams, R.H.; Walter, J.; Nordheim, A. Age-Associated Changes in Endothelial Transcriptome and Epigenetic Landscapes Correlate With Elevated Risk of Cerebral Microbleeds. J. Am. Heart Assoc. 2023, 12, e031044. [Google Scholar] [CrossRef]
- Matthiessen, M.E.; Vedtofte, P.; Rømert, P. Morphology of a Simple Ameloblastoma Related to the Human Enamel Organ. Scand. J. Dent. Res. 1980, 88, 181–186. [Google Scholar] [CrossRef]
- Tsutsumi, Y. Electron Microscopic Study Using Formalin-Fixed, Paraffin-Embedded Material, with Special Reference to Observation of Microbial Organisms and Endocrine Granules. Acta Histochem. Cytochem. 2018, 51, 63–71. [Google Scholar] [CrossRef]
- Capella, C.; Usellini, L.; Papotti, M.; Macrì, L.; Finzi, G.; Eusebi, V.; Bussolati, G. Ultrastructural Features of Neuroendocrine Differentiated Carcinomas of the Breast. Ultrastruct. Pathol. 1990, 14, 321–334. [Google Scholar] [CrossRef]
- Panneer Selvam, S.; Ponniah, I. Expression of Ameloblastin in the Human Tooth Germ and Ameloblastoma. Oral. Dis. 2018, 24, 1538–1544. [Google Scholar] [CrossRef]
- Crivelini, M.M.; Felipini, R.C.; Miyahara, G.I.; de Sousa, S.C.O.M. Expression of Odontogenic Ameloblast-Associated Protein, Amelotin, Ameloblastin, and Amelogenin in Odontogenic Tumors: Immunohistochemical Analysis and Pathogenetic Considerations. J. Oral. Pathol. Med. 2012, 41, 272–280. [Google Scholar] [CrossRef]
- Tsujigiwa, H.; Nagatsuka, H.; Han, P.P.; Gunduz, M.; Siar, C.H.; Oida, S.; Nagai, N. Analysis of Amelogenin Gene (AMGX, AMGY) Expression in Ameloblastoma. Oral. Oncol. 2005, 41, 843–850. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Rooper, L.M.; Bishop, J.A.; Westra, W.H. INSM1 Is a Sensitive and Specific Marker of Neuroendocrine Differentiation in Head and Neck Tumors. Am. J. Surg. Pathol. 2018, 42, 665–671. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Dermawan, J.K.; Lanigan, C.P.; Farver, C.F. Insulinoma-Associated Protein 1 (INSM1) Is a Sensitive and Highly Specific Marker of Neuroendocrine Differentiation in Primary Lung Neoplasms: An Immunohistochemical Study of 345 Cases, Including 292 Whole-Tissue Sections. Mod. Pathol. 2019, 32, 100–109. [Google Scholar] [CrossRef]
- Wyatt, A.R.; Yerbury, J.J.; Wilson, M.R. Structural Characterization of Clusterin-Chaperone Client Protein Complexes. J. Biol. Chem. 2009, 284, 21920–21927. [Google Scholar] [CrossRef]
- Wyatt, A.R.; Yerbury, J.J.; Berghofer, P.; Greguric, I.; Katsifis, A.; Dobson, C.M.; Wilson, M.R. Clusterin Facilitates in Vivo Clearance of Extracellular Misfolded Proteins. Cell. Mol. Life Sci. 2011, 68, 3919–3931. [Google Scholar] [CrossRef]
- Rodríguez-Rivera, C.; Garcia, M.M.; Molina-Álvarez, M.; González-Martín, C.; Goicoechea, C. Clusterin: Always Protecting. Synthesis, Function and Potential Issues. Biomed. Pharmacother. 2021, 134, 111174. [Google Scholar] [CrossRef]
- Andersen, C.L.; Schepeler, T.; Thorsen, K.; Birkenkamp-Demtröder, K.; Mansilla, F.; Aaltonen, L.A.; Laurberg, S.; Ørntoft, T.F. Clusterin Expression in Normal Mucosa and Colorectal Cancer. Mol. Cell. Proteom. 2007, 6, 1039–1048. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Czeczok, T.W.; Stashek, K.M.; Maxwell, J.E.; O’Dorisio, T.M.; Howe, J.R.; Hornick, J.L.; Bellizzi, A.M. Clusterin in Neuroendocrine Epithelial Neoplasms: Absence of Expression in a Well-Differentiated Tumor Suggests a Jejunoileal Origin. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Wei, L.; Zha, D.-J.; Qiao, L.; Lu, L.-J.; Chen, F.-Q.; Qiu, J.-H. Exposure to Acoustic Stimuli Promotes the Development and Differentiation of Neural Stem Cells from the Cochlear Nuclei through the Clusterin Pathway. Int. J. Mol. Med. 2015, 35, 637–644. [Google Scholar] [CrossRef][Green Version]
- Landin, M.A.D.S.S.; Shabestari, M.; Babaie, E.; Reseland, J.E.; Osmundsen, H. Gene Expression Profiling during Murine Tooth Development. Front. Genet. 2012, 3, 139. [Google Scholar] [CrossRef]
- Osmundsen, H.; Landin, M.A.; From, S.H.; Kolltveit, K.M.; Risnes, S. Changes in Gene-Expression during Development of the Murine Molar Tooth Germ. Arch. Oral. Biol. 2007, 52, 803–813. [Google Scholar] [CrossRef][Green Version]
- Khan, Q.-E.-S.; Sehic, A.; Khuu, C.; Risnes, S.; Osmundsen, H. Expression of Clu and Tgfb1 during Murine Tooth Development: Effects of in-Vivo Transfection with Anti-miR-214. Eur. J. Oral. Sci. 2013, 121, 303–312. [Google Scholar] [CrossRef]
- Lim, J.; Ahn, H.; Min, S.; Hong, S.-D.; Lee, J.-I.; Hong, S.-P. Oligonucleotide Microarray Analysis of Ameloblastoma Compared with Dentigerous Cyst. J. Oral. Pathol. Med. 2006, 35, 278–285. [Google Scholar] [CrossRef]



| Antibody | Source | Clone | Code | Retrieval | Dilution |
|---|---|---|---|---|---|
| CD56 | Agilent | 123C3 | M7304 | HIER, pH6.0 | 1:100 |
| SYP | Agilent | M0776 | M7315 | HIER, pH6.0 | 1:20 |
| CgA | Leica | PA0515 | PA0515 | HIER, pH6.0 | 1:200 |
| INSM1 | Santa Cruz | A-8 | sc-271408 | HIER, pH9.0 | 1:100 |
| CLU | Biocare | 41D | CM218A | HIER, pH6.0 | 1:100 |
| Gene Name | Sequence | Size (bp) |
|---|---|---|
| NCAM1 | F: 5′-GTGTGGTTACAGGCGAGGAT-3′ | 191 |
| R: 5′-GATGACATCTCGGCCTTTGT-3′ | ||
| SYP | F: 5′-GGGCGTGACTTCAGACTCTC-3′ | 181 |
| R: 5′-CAAGACCACCTTGGGTCCTA-3′ | ||
| CHGA | F: 5′-CTACGCGCCTTGTCTCCTAC-3′ | 153 |
| R: 5′-AGTTGTGCCCAGTGGATAGG-3′ | ||
| GAPDH | Not disclosed (Commercial primer set; Takara Bio Inc., Primer set ID: HA067812) | 138 |
| Case | Type | Sex | Age | CD56 | SYP | CgA | INSM1 | CLU |
|---|---|---|---|---|---|---|---|---|
| 4 | Conventional | M | 33 | + | + | − | − | + |
| 25 | Unicystic | M | 57 | + | + | − | + | + |
| 30 | Conventional | M | 81 | + | + | − | + | + |
| 32 | Unicystic | F | 44 | + | + | − | + | + |
| 36 | Conventional | M | 70 | + | + | − | + | + |
| Conventional | Unicystic | p-Value | |
|---|---|---|---|
| CD56(−) | 8 | 2 | 0.142 |
| CD56 (+) | 13 | 13 | |
| SYP (−) | 18 | 13 | 1 |
| SYP (+) | 3 | 2 | |
| CLU (−) | 18 | 10 | 0.236 |
| CLU (+) | 3 | 5 |
| CLU (−) | CLU (+) | p-Value | |
|---|---|---|---|
| CD56 (−) | 10 | 0 | 0.0756 |
| CD56 (+) | 18 | 8 | |
| SYP (−) | 28 | 3 | <0.001 |
| SYP (+) | 0 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, H.; Ochiai, T.; Roy, R.R.; Shimada, K. The Expression of Neuroendocrine Markers in a Small Subset of Ameloblastoma with Implications of Clusterin. Cells 2025, 14, 224. https://doi.org/10.3390/cells14030224
Hasegawa H, Ochiai T, Roy RR, Shimada K. The Expression of Neuroendocrine Markers in a Small Subset of Ameloblastoma with Implications of Clusterin. Cells. 2025; 14(3):224. https://doi.org/10.3390/cells14030224
Chicago/Turabian StyleHasegawa, Hiromasa, Takanaga Ochiai, Rita R. Roy, and Katsumitsu Shimada. 2025. "The Expression of Neuroendocrine Markers in a Small Subset of Ameloblastoma with Implications of Clusterin" Cells 14, no. 3: 224. https://doi.org/10.3390/cells14030224
APA StyleHasegawa, H., Ochiai, T., Roy, R. R., & Shimada, K. (2025). The Expression of Neuroendocrine Markers in a Small Subset of Ameloblastoma with Implications of Clusterin. Cells, 14(3), 224. https://doi.org/10.3390/cells14030224








