High-Density Lipoproteins from Coronary Artery Disease and Aortic Valve Stenosis Patients Differentially Regulate Gene Expression in a Model of Cardiac Adipocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory Assessment
2.3. Isolation of HDL
2.4. Analysis of HDL Subclasses
2.5. Cell Culture
2.6. Differentiation from Stromal Cells to Adipocytes
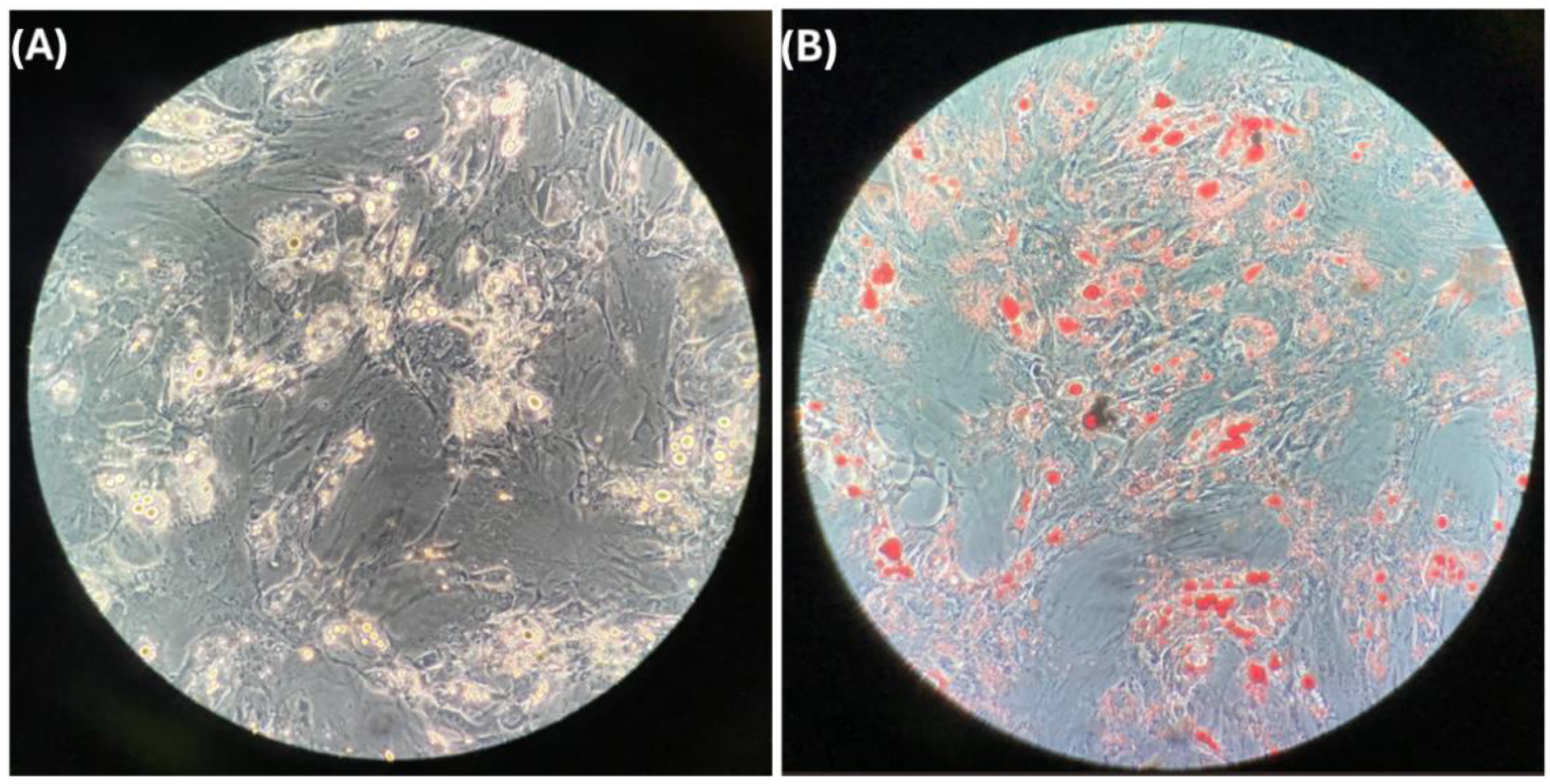
2.7. Oil Red Stain
2.8. Stimulation of Differentiated Adipocytes with HDL
2.9. Gene Expression Assay
2.10. Synthesis of Reconstituted HDL (rHDL)
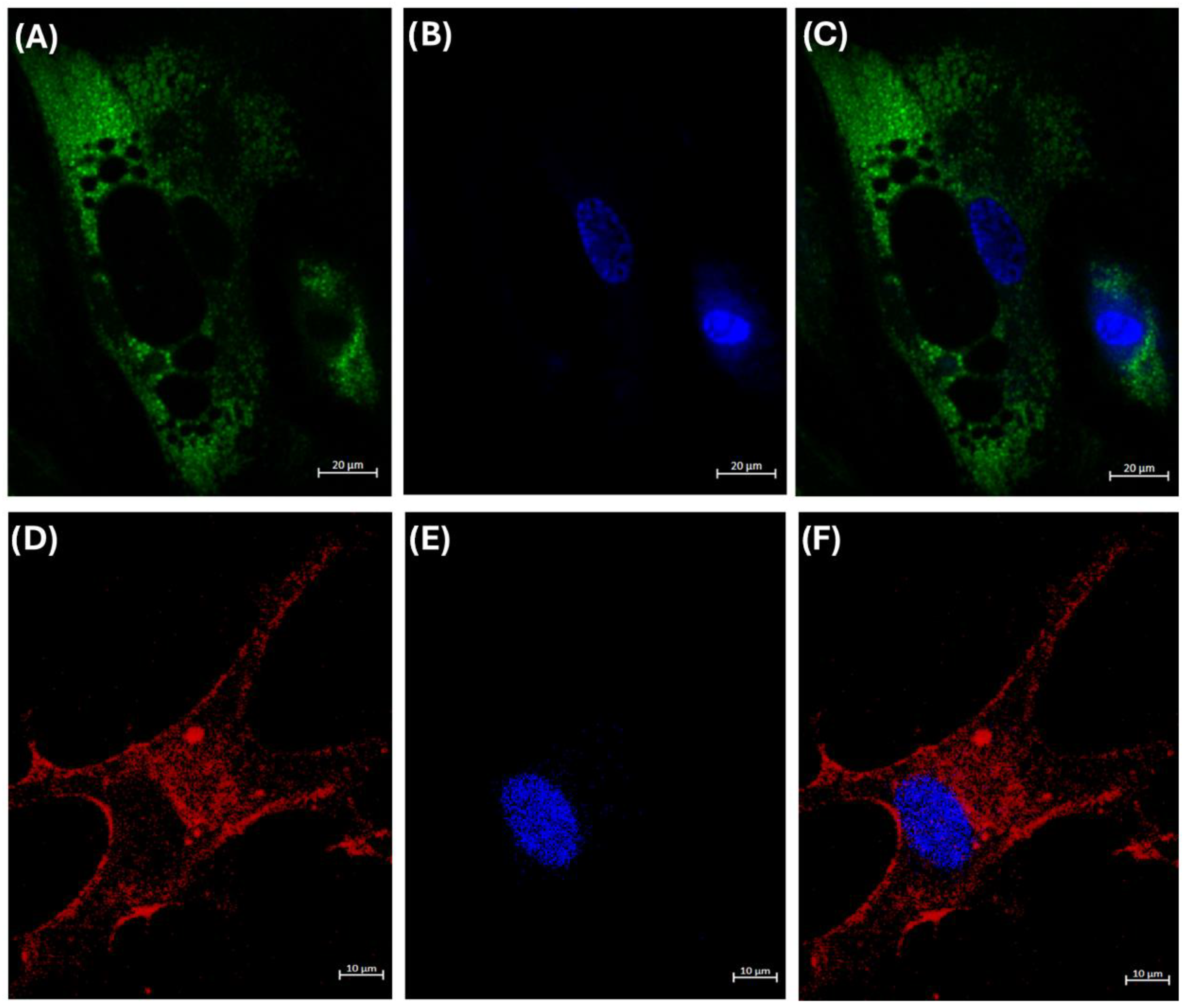
2.11. Internalization Assay of HDL
2.12. Statistical Analysis
3. Results
3.1. Patients’ Clinical Characteristics
3.2. Differentiated Adipocytes
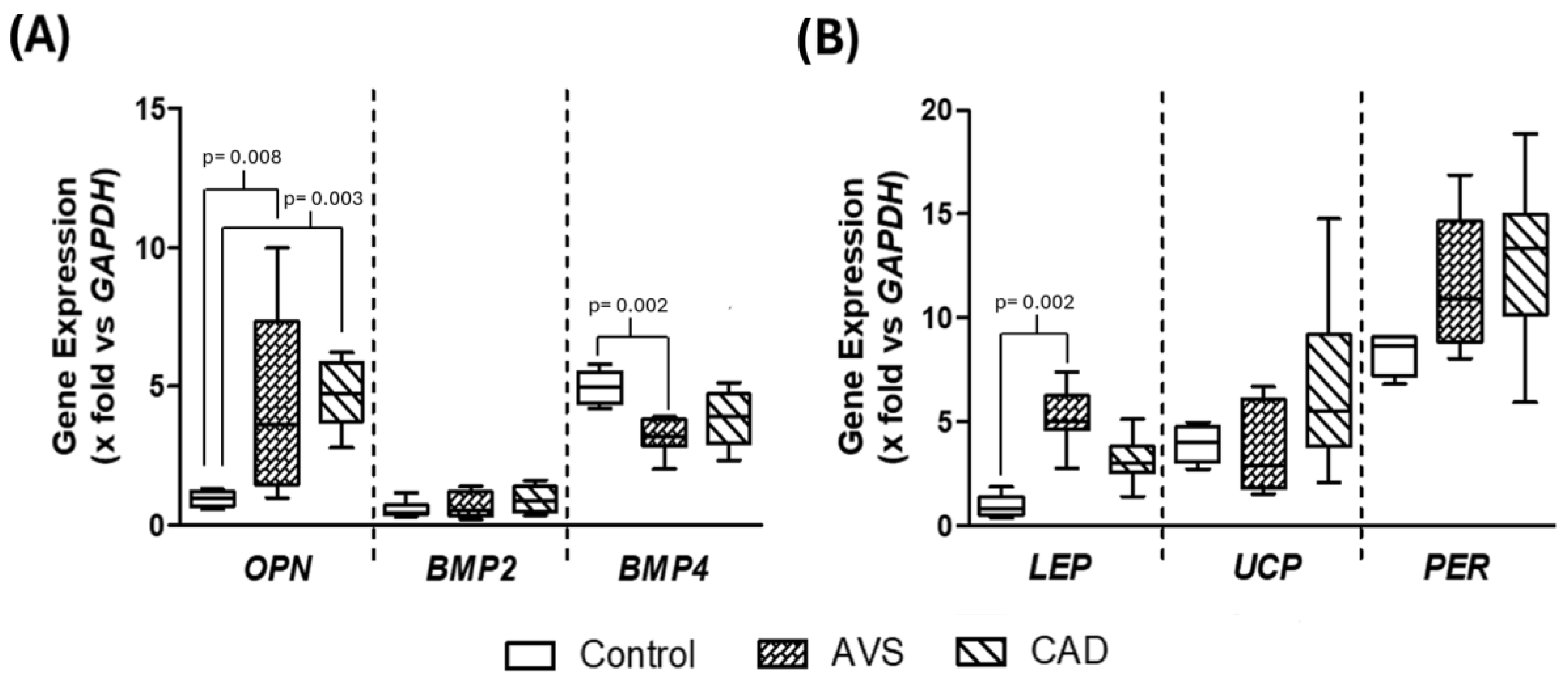
3.3. Gene Expression in Differentiated Adipocytes Stimulated with HDL
3.4. Internalization of HDL Cholesterol
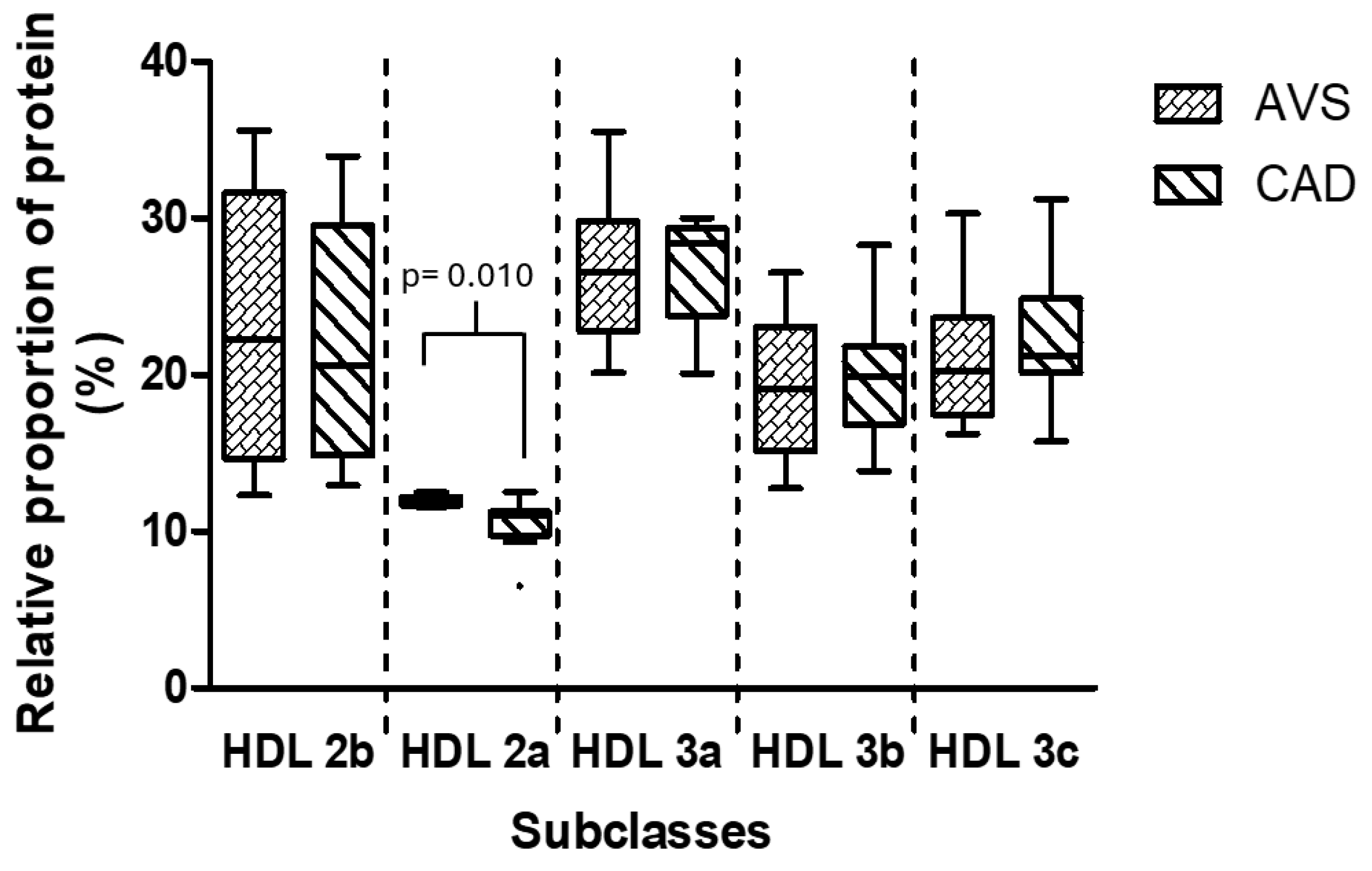
3.5. Composition and Relative Proportion of HDL Subclasses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Méndez, Ó.; Pacheco, H.G.; Martínez-Sánchez, C.; Franco, M. HDL-cholesterol in coronary artery disease risk: Function or structure? Clin. Chim. Acta 2014, 429, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Benkhoff, M.; Polzin, A. Lipoprotection in cardiovascular diseases. Pharmacol. Ther. 2024, 264, 108747. [Google Scholar] [CrossRef] [PubMed]
- Vaisar, T.; Babenko, I.; Horvath, K.V.; Niisuke, K.; Asztalos, B.F. Relationships between HDL subpopulation proteome and HDL function in overweight/obese people with and without coronary heart disease. Atherosclerosis 2024, 397, 118565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; van der Vorst, E.P.C. High-Density Lipoprotein Modifications: Causes and Functional Consequences in Type 2 Diabetes Mellitus. Cells 2024, 13, 1113. [Google Scholar] [CrossRef]
- Bhale, A.S.; Meilhac, O.; d’Hellencourt, C.L.; Vijayalakshmi, M.A.; Venkataraman, K. Cholesterol transport and beyond: Illuminating the versatile functions of HDL apolipoproteins through structural insights and functional implications. Biofactors 2024, 50, 922–956. [Google Scholar] [CrossRef]
- Gordon, T.; Castelli, W.P.; Hjortland, M.C.; Kannel, W.B.; Dawber, T.R. High density lipoprotein as a protective factor against coronary heart disease: The Framingham Study. Am. J. Med. 1977, 62, 707–714. [Google Scholar] [CrossRef]
- Sharrett, A.R.; Ballantyne, C.M.; Coady, S.A.; Heiss, G.; Sorlie, P.D.; Catellier, D.; Patsch, W.; Atherosclerosis Risk in Communities Study Group. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2001, 104, 1108–1113. [Google Scholar] [CrossRef]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
- Luna-Luna, M.; Niesor, E.; Pérez-Méndez, Ó. HDL as Bidirectional Lipid Vectors: Time for New Paradigms. Biomedicines 2022, 10, 1180. [Google Scholar] [CrossRef]
- Toth, P.P.; Barter, P.J.; Rosenson, R.S.; Boden, W.E.; Chapman, M.J.; Cuchel, M.; D’Agostino, R.B., Sr.; Davidson, M.H.; Davidson, W.S.; Heinecke, J.W.; et al. High-density lipoproteins: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 484–525. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Calabresi, L.; Franceschini, G.; Baldassarre, D.; Amato, M.; Johansson, J.; Salvetti, M.; Monteduro, C.; Zulli, R.; Muiesan, M.L.; et al. Cardiovascular status of carriers of the apolipoprotein A-I(Milano) mutant: The Limone sul Garda study. Circulation 2001, 103, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Lebherz, C.; Sanmiguel, J.; Wilson, J.M.; Rader, D.J. Gene transfer of wild-type apoA-I and apoA-I Milano reduce atherosclerosis to a similar extent. Cardiovasc. Diabetol. 2007, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Reddy, S.T.; Van Lenten, B.J.; Fogelman, A.M. HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 2011, 8, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Luna-Luna, M.; Cruz-Robles, D.; Ávila-Vanzzini, N.; Herrera-Alarcón, V.; Martínez-Reding, J.; Criales-Vera, S.; Sandoval-Zárate, J.; Vargas-Barrón, J.; Martínez-Sánchez, C.; Tovar-Palacio, A.R.; et al. Differential expression of osteopontin, and osteoprotegerin mRNA in epicardial adipose tissue between patients with severe coronary artery disease and aortic valvular stenosis: Association with HDL subclasses. Lipids Health Dis. 2017, 16, 156. [Google Scholar] [CrossRef]
- Muñoz-Vega, M.; Massó, F.; Páez, A.; Vargas-Alarcón, G.; Coral-Vázquez, R.; Mas-Oliva, J.; Carreón-Torres, E.; Pérez-Méndez, Ó. HDL-Mediated Lipid Influx to Endothelial Cells Contributes to Regulating Intercellular Adhesion Molecule (ICAM)-1 Expression and eNOS Phosphorylation. Int. J. Mol. Sci. 2018, 19, 3394. [Google Scholar] [CrossRef]
- van der Vorst, E.P.C.; Theodorou, K.; Wu, Y.; Hoeksema, M.A.; Goossens, P.; Bursill, C.A.; Aliyev, T.; Huitema, L.F.A.; Tas, S.W.; Wolfs, I.M.J.; et al. High-Density Lipoproteins Exert Pro-inflammatory Effects on Macrophages via Passive Cholesterol Depletion and PKC-NF-κB/STAT1-IRF1 Signaling. Cell Metab. 2017, 25, 197–207. [Google Scholar] [CrossRef]
- Emert, B.; Hasin-Brumshtein, Y.; Springstead, J.R.; Vakili, L.; Berliner, J.A.; Lusis, A.J. HDL inhibits the effects of oxidized phospholipids on endothelial cell gene expression via multiple mechanisms. J. Lipid Res. 2014, 55, 1678–1692. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Myasoedova, V.A.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Calcifying Matrix Vesicles and Atherosclerosis. Biomed. Res. Int. 2017, 7463590. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Gárcia-Sánchez, C.; Torres-Tamayo, M.; Juárez-Meaveapeña, M.; López-Osorio, C.; Toledo-Ibelles, P.; Monter-Garrido, M.; Cruz-Robles, D.; Carreón-Torres, E.; Vargas-Alarcón, G.; Pérez-Méndez, O. Lipid plasma concentrations of hdl subclasses determined by enzymatic staining on polyacrylamide electrophoresis gels in children with metabolic syndrome. Clin. Chim. Acta 2011, 412, 292–298. [Google Scholar] [CrossRef]
- Kaczmarek, I.; Suchý, T.; Strnadová, M.; Thor, D. Qualitative and quantitative analysis of lipid droplets in mature 3T3-L1 adipocytes using oil red O. STAR Protoc. 2024, 5, 102977. [Google Scholar] [CrossRef] [PubMed]
- Bruedigam, C.; Driel, M.; Koedam, M.; Peppel, J.; van der Eerden, B.C.; Eijken, M.; van Leeuwen, J.P. Basic techniques in human mesenchymal stem cell cultures: Differentiation into osteogenic and adipogenic lineages, genetic perturbations, and phenotypic analyses. Curr. Protoc. Stem Cell Biol. 2011, 17, 1H.3.1–1H.3.20. [Google Scholar] [CrossRef]
- Fan, J.; Chen, Y.; Yan, H.; Liu, B.; Wang, Y.; Zhang, J.; Chen, Y.E.; Liu, E.; Liang, J. Genomic and Transcriptomic Analysis of Hypercholesterolemic Rabbits: Progress and Perspectives. Int. J. Mol. Sci. 2018, 19, 3512. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Dhore, C.R.; Cleutjens, J.P.; Lutgens, E.; Cleutjens, K.B.; Geusens, P.P.; Kitslaar, P.J.; Tordoir, J.H.; Spronk, H.M.; Vermeer, C.; Daemen, M.J. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1998–2003. [Google Scholar] [CrossRef]
- Wolak, T. Osteopontin—A multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis 2014, 236, 327–337. [Google Scholar] [CrossRef]
- Pérez-Ocampo, J.; Vergara-Serpa, O.; Velásquez-Franco, C.J.; Taborda, N.A.; Yassin, L.M.; Hernandez, J.C. Assessment of the role of high-density lipoproteins and their immunomodulatory activity in systemic lupus erythematosus immunopathology. Lupus Sci. Med. 2024, 11, e001242. [Google Scholar] [CrossRef]
- Chen, K.L.; Chou, R.H.; Chang, C.C.; Kuo, C.S.; Wei, J.H.; Huang, P.H.; Lin, S.J. The high-density lipoprotein cholesterol (HDL-C)-concentration-dependent association between anti-inflammatory capacity and sepsis: A single-center cross-sectional study. PLoS ONE 2024, 19, e0296863. [Google Scholar] [CrossRef]
- Luna-Luna, M.; Zentella-Dehesa, A.; Pérez-Méndez, Ó. Epicardial Adipose Tissue in the Progression and Calcification of the Coronary Artery Disease. In Biochemistry of Cardiovascular Dysfunction in Obesity. Advances in Biochemistry in Health and Disease; Tappia, P.S., Bhullar, S.K., Dhalla, N.S., Eds.; Springer: Cham, Switzerland, 2020; Volume 20, pp. 195–213. [Google Scholar] [CrossRef]
- Villasante Fricke, A.C.; Iacobellis, G. Epicardial Adipose Tissue: Clinical Biomarker of Cardio-Metabolic Risk. Int. J. Mol. Sci. 2019, 20, 5989. [Google Scholar] [CrossRef]
- Rabkin, S.W. Epicardial fat: Properties, function and relationship to obesity. Obes. Rev. 2007, 8, 253–261. [Google Scholar] [CrossRef]
- Toro, R.; Mangas, A.; Gómez, F. Enfermedad de la válvula aórtica calcificada. Su asociación con la arteriosclerosis [Calcified aortic valve disease: Association with atherosclerosis]. Med. Clin. 2011, 136, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Luna-Luna, M.; Criales-Vera, S.; Medina-Leyte, D.; Díaz-Zamudio, M.; Flores-Zapata, A.; Cruz-Robles, D.; López-Meneses, M.; Olvera-Cruz, S.; Ramírez-Marroquín, S.; Flores-Castillo, C.; et al. Bone Morphogenetic Protein-2 and Osteopontin Gene Expression in Epicardial Adipose Tissue from Patients with Coronary Artery Disease Is Associated with the Presence of Calcified Atherosclerotic Plaques. Diabetes Metab. Syndr. Obes. 2020, 13, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Sibouakaz, D.; Othmani-Mecif, K.; Fernane, A.; Taghlit, A.; Benazzoug, Y. Biochemical and Ultrastructural Cardiac Changes Induced by High-Fat Diet in Female and Male Prepubertal Rabbits. Anal. Cell Pathol. 2018, 2018, 6430696. [Google Scholar] [CrossRef] [PubMed]
- Sabitha, P.; Vasudevan, D.M.; Kamath, P. Effect of high fat diet without cholesterol supplementation on oxidative stress and lipid peroxidation in New Zealand white rabbits. J. Atheroscler. Thromb. 2010, 17, 213–218. [Google Scholar] [CrossRef][Green Version]
- Mueller, P.A.; Bergstrom, P.; Rosario, S.; Heard, M.; Pamir, N. Fish Oil Supplementation Modifies the Proteome, Lipidome, and Function of High-Density Lipoprotein: Findings from a Trial in Young Healthy Adults. J. Nutr. 2024, 154, 1130–1140. [Google Scholar] [CrossRef]
- Lund, J.; Lähteenmäki, E.; Eklund, T.; Bakke, H.G.; Thoresen, G.H.; Pirinen, E.; Jauhiainen, M.; Rustan, A.C.; Lehti, M. Human HDL subclasses modulate energy metabolism in skeletal muscle cells. J. Lipid Res. 2024, 65, 100481. [Google Scholar] [CrossRef]
- Cheng, A.M.; Handa, P.; Tateya, S.; Schwartz, J.; Tang, C.; Mitra, P.; Oram, J.F.; Chait, A.; Kim, F. Apolipoprotein A-I attenuates palmitate-mediated NF-κB activation by reducing Toll-like receptor-4 recruitment into lipid rafts. PLoS ONE 2012, 7, e33917. [Google Scholar] [CrossRef]
- Yamada, H.; Umemoto, T.; Kawano, M.; Kawakami, M.; Kakei, M.; Momomura, S.I.; Ishikawa, S.E.; Hara, K. High-density lipoprotein and apolipoprotein A-I inhibit palmitate-induced translocation of toll-like receptor 4 into lipid rafts and inflammatory cytokines in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2017, 484, 403–408. [Google Scholar] [CrossRef]
- Umemoto, T.; Han, C.Y.; Mitra, P.; Averill, M.M.; Tang, C.; Goodspeed, L.; Omer, M.; Subramanian, S.; Wang, S.; Den Hartigh, L.J.; et al. Apolipoprotein AI and high-density lipoprotein have anti-inflammatory effects on adipocytes via cholesterol transporters: ATP-binding cassette A-1, ATP-binding cassette G-1, and scavenger receptor B-1. Circ. Res. 2013, 112, 1345–1354. [Google Scholar] [CrossRef]
- Tekavec, S.; Sorčan, T.; Giacca, M.; Režen, T. VLDL and HDL attenuate endoplasmic reticulum and metabolic stress in HL-1 cardiomyocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158713. [Google Scholar] [CrossRef]
- Pétremand, J.; Puyal, J.; Chatton, J.Y.; Duprez, J.; Allagnat, F.; Frias, M.; James, R.W.; Waeber, G.; Jonas, J.C.; Widmann, C. HDLs protect pancreatic β-cells against ER stress by restoring protein folding and trafficking. Diabetes 2012, 61, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Back, S.H.; Hur, J.; Lin, Y.H.; Gildersleeve, R.; Shan, J.; Yuan, C.L.; Krokowski, D.; Wang, S.; Hatzoglou, M.; et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013, 15, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Backlund, M.; Paukku, K.; Kontula, K.K.; Lehtonen, J.Y. Endoplasmic reticulum stress increases AT1R mRNA expression via TIA-1-dependent mechanism. Nucleic Acids Res. 2016, 44, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Hadi, T.; Ramseyer, C.; Gautier, T.; Bellaye, P.S.; Lopez, T.; Schmitt, A.; Foley, S.; Yesylevskyy, S.; Minervini, T.; Douhard, R.; et al. Lipoproteins LDL versus HDL as nanocarriers to target either cancer cells or macrophages. JCI Insight 2020, 5, e140280. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Yang, S.; Li, W.; Liang, B.; Chen, H.; Ye, Y.; Wang, F.; Liu, X.; Jiang, Y.; et al. Causal relationship between gut microbiota, plasma metabolites, inflammatory cytokines and abdominal aortic aneurysm: A Mendelian randomization study. Clin. Exp. Hypertens. 2024, 46, 2390419. [Google Scholar] [CrossRef]
- Brown, R.J.; Rader, D.J. When HDL gets fat. Circ. Res. 2008, 103, 131–132. [Google Scholar] [CrossRef]
- Mousa, H.; Al Saei, A.; Razali, R.M.; Zughaier, S.M. Vitamin D status affects proteomic profile of HDL-associated proteins and inflammatory mediators in dyslipidemia. J. Nutr. Biochem. 2024, 123, 109472. [Google Scholar] [CrossRef]
- Lepedda, A.J.; Nieddu, G.; Zinellu, E.; Muro, P.D.; Piredda, F.; Guarino, A.; Spirito, R.; Carta, F.; Turrini, F.; Formato, M. Proteomic analysis of plasma-purified VLDL, LDL, and HDL fractions from atherosclerotic patients undergoing carotid endarterectomy: Identification of serum amyloid a as a potential marker. Oxid. Med. Cell Longev. 2013, 2013, 385214. [Google Scholar] [CrossRef]
- Yan, L.R.; Wang, D.X.; Liu, H.; Zhang, X.X.; Zhao, H.; Hua, L.; Xu, P.; Li, Y.S. A pro-atherogenic HDL profile in coronary heart disease patients: An iTRAQ labelling-based proteomic approach. PLoS ONE 2014, 9, e98368. [Google Scholar] [CrossRef]
- Santana, M.F.M.; Sawada, M.I.B.A.C.; Junior, D.R.S.; Giacaglia, M.B.; Reis, M.; Xavier, J.; Côrrea-Giannella, M.L.; Soriano, F.G.; Gebrim, L.H.; Ronsein, G.E.; et al. Proteomic Profiling of HDL in Newly Diagnosed Breast Cancer Based on Tumor Molecular Classification and Clinical Stage of Disease. Cells 2024, 13, 1327. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Charles-Schoeman, C.; Miao, Y.; Elashoff, D.; Lee, Y.Y.; Katselis, G.; Lee, T.D.; Reddy, S.T. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: Association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis Rheum. 2012, 64, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, V.A.; Bertolini, F.; Valerio, V.; Moschetta, D.; Massaiu, I.; Rusconi, V.; De Giorgi, D.; Ciccarelli, M.; Parisi, V.; Poggio, P. The Role of Adiponectin and Leptin in Fibro-Calcific Aortic Valve Disease: A Systematic Review and Meta-Analysis. Biomedicines 2024, 12, 1977. [Google Scholar] [CrossRef] [PubMed]
- Zeadin, M.G.; Butcher, M.K.; Shaughnessy, S.G.; Werstuck, G.H. Leptin promotes osteoblast differentiation and mineralization of primary cultures of vascular smooth muscle cells by inhibiting glycogen synthase kinase (GSK)-3β. Biochem. Biophys. Res. Commun. 2012, 425, 924–930. [Google Scholar] [CrossRef]
- Zeadin, M.; Butcher, M.; Werstuck, G.; Khan, M.; Yee, C.K.; Shaughnessy, S.G. Effect of leptin on vascular calcification in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2069–2075. [Google Scholar] [CrossRef]
- Mu, W.J.; Song, Y.J.; Yang, L.J.; Qian, S.W.; Yang, Q.Q.; Liu, Y.; Tang, Q.Q.; Tang, Y. Bone morphogenetic protein 4 in perivascular adipose tissue ameliorates hypertension through regulation of angiotensinogen. Front. Cardiovasc. Med. 2022, 9, 1038176. [Google Scholar] [CrossRef]
- Mu, W.; Qian, S.; Song, Y.; Yang, L.; Song, S.; Yang, Q.; Liu, H.; Liu, Y.; Pan, D.; Tang, Y.; et al. BMP4-mediated browning of perivascular adipose tissue governs an anti-inflammatory program and prevents atherosclerosis. Redox Biol. 2021, 43, 101979. [Google Scholar] [CrossRef]
- Morris, T.G.; Borland, S.J.; Clarke, C.J.; Wilson, C.; Hannun, Y.A.; Ohanian, V.; Canfield, A.E.; Ohanian, J. Sphingosine 1-phosphate activation of ERM contributes to vascular calcification. J. Lipid Res. 2018, 59, 69–78. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Chester, A.; Yacoub, M. Cellular regulation of the structure and function of aortic valves. J. Adv. Res. 2010, 1, 5–12. [Google Scholar] [CrossRef]

| Parameter | AVS (n = 10) | CAD (n = 11) |
|---|---|---|
| Age (years) | 59 (46–69) | 67 (60–71) |
| Gender (M) | 8 (80%) | 11 (100%) |
| BMI (kg/m2) | 25.8 (23.0–29.3) | 24.7 (24.0–26.5) |
| SBP (mm Hg) | 115.0 (105.3–120.0) | 128.5 (117.5–142.5) * |
| DBP (mm Hg) | 67.5 (60.0–73.3) | 77.0 (67.5–83.3) |
| Ca2+ (mg/dL) | 9.2 (8.6–9.5) | 9.4 (8.7–9.6) |
| Alkaline phosphatase (U/L) | 101.7 (71.8–129.6) | 74.8 (67.5–98.9) |
| Glucose (mg/dL) | 84.2 (73.6–147.8) | 91.2 (72.3–100.3) |
| Cholesterol (mg/dL) | 119.6 (88.7–152.8) | 119.0 (99.4–132.0) |
| TG (mg/dL) | 97.4 (72.7–120.4) | 119.2 (89.2–142.7) |
| LDL-C | 73.0 (41.0–92.8) | 61.7 (39.3–73.6) |
| HDL-C (mg/dL) | 33.9 (26.0–34.8) | 30.5 (24.3–37.5) |
| HDL-TG (mg/dL) | 17.0 (11.7–21.1) | 19.6 (17.2–22.1) |
| HDL-PH (mg/dL) | 54.8 (48.5–65.0) | 68.3 (59.9–74-8) |
| Statins treatment | 5 (50%) | 8 (73%) |
| CAC (AU) | 0 (0–0) | 412.3 (60.4–1484) |
| Lipid (mg/dL) | Group | HDL 2b | HDL 2a | HDL 3a | HDL 3b | HDL 3c |
|---|---|---|---|---|---|---|
| Cholesterol | AVS | 11.8 (11.5–16.5) | 4.5 (3.8–5.7) | 6.9 (5.7–9.1) | 2.8 (2.3–4.5) | 2.5 (1.8–3.6) |
| CAD | 12.7 (10.2–17.3) | 4.6 (3.8–6.2) | 6.9 (5.3–9.3) | 3.1 (2.3–3.5) | 2.8 (2.3–3.7) | |
| Triglycerides | AVS | 7.4 (3.6–9.5) | 2.8 (1.4–3.7) | 4.0 (2.9–4.8) | 1.6 (1.30–1.8) | 1.4 (1.0–1.6) |
| CAD | 7.5 (4.4–10.3) | 2.8 (2.4–3.9) | 5.0 (4.4–5.7) * | 2.4 (1.6–3.3) * | 2.7 (1.5–3.0) * | |
| Phospholipids | AVS | 22.7 (18.4–26.0) | 8.3 (7.5–9.5) | 13.8 (10.5–15.4) | 6.6 (3.1–7.2) | 5.9 (2.7–7.1) |
| CAD | 28.7 (25.0–29.6) * | 10.8 (8.7–11.6) * | 15.6 (13.5–16.15) | 6.4 (5.5–7.6) | 6.1 (4.2–9.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Luna, M.; Páez, A.; Massó, F.; López-Marure, R.; Zozaya-García, J.M.; Vargas-Castillo, A.; Gómez-Pineda, D.; Tovar, A.R.; Magaña, J.J.; Fragoso, J.M.; et al. High-Density Lipoproteins from Coronary Artery Disease and Aortic Valve Stenosis Patients Differentially Regulate Gene Expression in a Model of Cardiac Adipocytes. Cells 2025, 14, 205. https://doi.org/10.3390/cells14030205
Luna-Luna M, Páez A, Massó F, López-Marure R, Zozaya-García JM, Vargas-Castillo A, Gómez-Pineda D, Tovar AR, Magaña JJ, Fragoso JM, et al. High-Density Lipoproteins from Coronary Artery Disease and Aortic Valve Stenosis Patients Differentially Regulate Gene Expression in a Model of Cardiac Adipocytes. Cells. 2025; 14(3):205. https://doi.org/10.3390/cells14030205
Chicago/Turabian StyleLuna-Luna, María, Araceli Páez, Felipe Massó, Rebeca López-Marure, Jorge Moisés Zozaya-García, Ariana Vargas-Castillo, Daniel Gómez-Pineda, Armando R. Tovar, Jonathan J. Magaña, José Manuel Fragoso, and et al. 2025. "High-Density Lipoproteins from Coronary Artery Disease and Aortic Valve Stenosis Patients Differentially Regulate Gene Expression in a Model of Cardiac Adipocytes" Cells 14, no. 3: 205. https://doi.org/10.3390/cells14030205
APA StyleLuna-Luna, M., Páez, A., Massó, F., López-Marure, R., Zozaya-García, J. M., Vargas-Castillo, A., Gómez-Pineda, D., Tovar, A. R., Magaña, J. J., Fragoso, J. M., Gutiérrez-Saldaña, M., Téllez-Osorio, Z., & Pérez-Méndez, Ó. (2025). High-Density Lipoproteins from Coronary Artery Disease and Aortic Valve Stenosis Patients Differentially Regulate Gene Expression in a Model of Cardiac Adipocytes. Cells, 14(3), 205. https://doi.org/10.3390/cells14030205







