Mesenchymal Stem-Cell-Derived Exosomes as Novel Drug Carriers in Anti-Cancer Treatment: A Myth or Reality?
Abstract
1. Introduction
2. MSC-Exos: Small Extracellular Vesicles with Huge Therapeutic Potential
3. MSC-Exos: Novel, Nano-Sized Carriers of Anti-Tumor Drugs
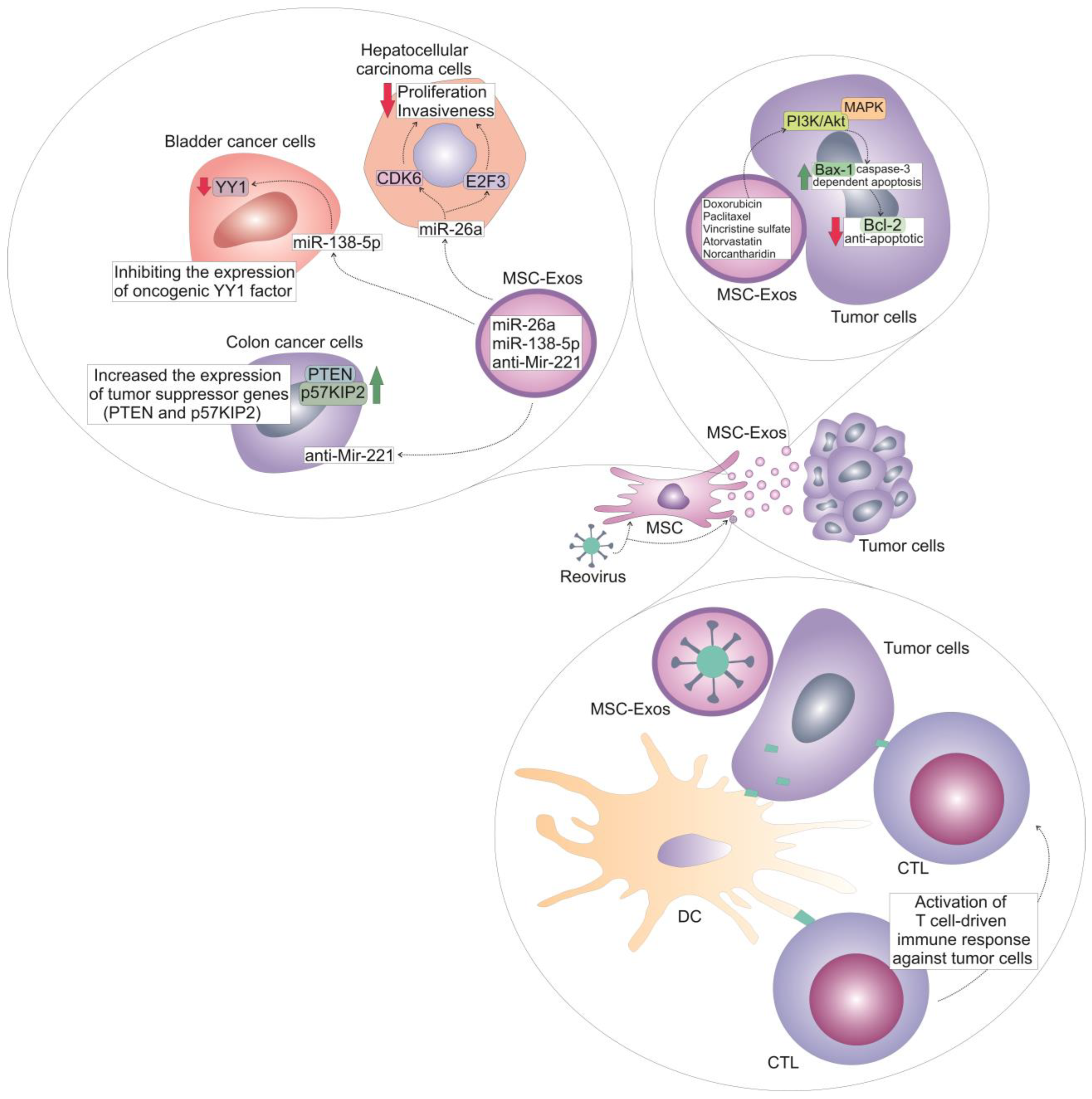
| Tissue Source of MSC-Exos | Incorporated Drug or Bioactive Molecule | Loading Technique, Delivery Method or Genetic Modification of MSC-Exos | Target Tumor Cells | Biological Effects | Ref. No. |
|---|---|---|---|---|---|
| MSC-exos-mediated delivery of chemotherapeutics | |||||
| Bone marrow | Doxorubicin | Electroporation | HER2/neu-overexpressing TUBO breast cancer cells | Increased toxicity of doxorubicin against cancer cells; Enhanced accumulation in the tumor tissue | [29] |
| Skin | KrasG12D—specific siRNAs ad shRNAs | Electroporation | Pancreatic ductal adenocarcinoma cells | Suppressed growth and progression of cancer cells; increased survival of MSC-Exos-treated tumor-bearing animals | [30,31] |
| Wharton’s Jelly | STAT-3 inhibitor (S3I-201) | Electroporation | Triple-negative breast cancer cells | Increased apoptosis of tumor cells; Reduced tumor growth and progression | [32] |
| Wharton’s Jelly | Paclitaxel | Electroporation | Hela cervical cancer cells | Increased apoptosis, attenuated EMT and invasiveness of cancer cells | [33] |
| Bone marrow | Vincristine sulfate | Sonicaton | Breast cancer cells | MSC-Exos improved targeted intra-tumor delivery of vincristine sulfate and lessen its side effects | [36] |
| Endometrium | Atorvastatin | Electroporation | Glioblastoma cells | Increased apoptosis of tumor cells | [37] |
| Bone marrow | Norcantharidin | Electroporation | Hepatocellular carcinoma cells | Increased cell-cycle arrest and apoptosis of cancer cells; Attenuated growth and progression of liver cancer in experimental animals | [40] |
| MSC-Exos-based delivery of oncolytic viruses | |||||
| Umbilical cord | Reovirus | Clathrin-mediated endocytosis and macropinocytosis | Acute myeloid leukemia cells | Direct oncolytic effect against tumor cells; enhanced anti-tumor immune response | [41,42] |
| MSC-Exos-dependent delivery of miRNAs | |||||
| Bone marrow | MiR-29b | MiR-29b-integrating recombinant lentiviral vector | Gastric cancer cells | Reduced number of peritoneal metastasis in experimental animals | [43] |
| Bone marrow | Antisense microRNA oligonucleotide | Electroporation | Glioblastoma cells | Attenuated tumor growth and progression in experimental animals | [44] |
| Adipose tissue | MiR-138-5p | MiR-138-5p-integrating recombinant lentiviral vector | Bladder cancer cells | Impaired proliferation, migration, and invasiveness of tumor cells; Reduced tumor growth in experimental animals | [45] |
| Umbilical cord blood | MiR-26a | Overexpression of anti-Glypican 3 single-stranded variable fragment and electroporation of miR-26a | Hepatocellular carcinoma cells | Attenuated proliferation and invasiveness of tumor cells; reduced growth and progression of liver cancer in experimental animals | [46] |
| Umbilical cord | Anti-Mir221 | Overexpression of iRGD-Lamp2b | Colon cancer cells | Suppressed growth and progression of colon cancer in experimental animals | [47] |
4. MSC-Exos-Mediated Delivery of Oncolytic Viruses
5. MSC-Exo-Dependent Delivery of Anti-Cancer miRNAs in Malignant Cells
6. Current Challenges and Future Perspectives in MSC-Exo-Dependent Delivery of Anti-Cancer Agents
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zawrzykraj, M.; Deptuła, M.; Kondej, K.; Tymińska, A.; Pikuła, M. The effect of chemotherapy and radiotherapy on stem cells and wound healing. Current perspectives and challenges for cell-based therapies. Biomed. Pharmacother. 2023, 168, 115781. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Barazzuol, L.; Coppes, R.P.; van Luijk, P. Prevention and treatment of radiotherapy-induced side effects. Mol. Oncol. 2020, 14, 1538–1554. [Google Scholar] [CrossRef]
- Wu, Q.; Qian, W.; Sun, X.; Jiang, S. Small-molecule inhibitors, immune checkpoint inhibitors, and more: FDA-approved novel therapeutic drugs for solid tumors from 1991 to 2021. J. Hematol. Oncol. 2022, 15, 143. [Google Scholar]
- Li, X.F.; Liu, C.F.; Rao, G.W. Monoclonal Antibodies, Small Molecule Inhibitors and Antibody-drug Conjugates as HER2 Inhibitors. Curr. Med. Chem. 2021, 28, 3339–3360. [Google Scholar] [CrossRef]
- Abbasi, R.; Mesgin, R.M.; Nazari-Khanamiri, F.; Abdyazdani, N.; Imani, Z.; Talatapeh, S.P.; Nourmohammadi, A.; Nejati, V.; Rezaie, J. Mesenchymal stem cells-derived exosomes: Novel carriers for nanoparticle to combat cancer. Eur. J. Med. Res. 2023, 28, 579. [Google Scholar] [CrossRef]
- Liu, H.; Deng, S.; Han, L.; Ren, Y.; Gu, J.; He, L.; Liu, T.; Yuan, Z.X. Mesenchymal stem cells, exosomes and exosome-mimics as smart drug carriers for targeted cancer therapy. Colloids Surf. B Biointerfaces 2022, 209 Pt 1, 112163. [Google Scholar] [CrossRef]
- Zhao, W.; Li, K.; Li, L.; Wang, R.; Lei, Y.; Yang, H.; Sun, L. Mesenchymal Stem Cell-Derived Exosomes as Drug Delivery Vehicles in Disease Therapy. Int. J. Mol. Sci. 2024, 25, 7715. [Google Scholar] [CrossRef]
- Araldi, R.P.; Delvalle, D.A.; da Costa, V.R.; Alievi, A.L.; Teixeira, M.R.; Dias Pinto, J.R.; Kerkis, I. Exosomes as a Nano-Carrier for Chemotherapeutics: A New Era of Oncology. Cells 2023, 12, 2144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Guo, J.; Zhang, Z.; Qian, Y.; Wang, G.; Duan, M.; Zhao, H.; Yang, Z.; Jiang, X. Mesenchymal stem cell-derived exosome: A tumor regulator and carrier for targeted tumor therapy. Cancer Lett. 2022, 526, 29–40. [Google Scholar] [CrossRef]
- Xie, M.; Tao, L.; Zhang, Z.; Wang, W. Mesenchymal Stem Cells Mediated Drug Delivery in Tumor-Targeted Therapy. Curr. Drug Deliv. 2021, 18, 876–891. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, J.; Nejati, V.; Mahmoodi, M.; Ahmadi, M. Mesenchymal stem cells derived extracellular vesicles: A promising nanomedicine for drug delivery system. Biochem. Pharmacol. 2022, 203, 115167. [Google Scholar] [CrossRef]
- Xie, X.; Wu, H.; Li, M.; Chen, X.; Xu, X.; Ni, W.; Lu, C.; Ni, R.; Bao, B.; Xiao, M. Progress in the application of exosomes as therapeutic vectors in tumor-targeted therapy. Cytotherapy 2019, 21, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, V.; Sarkar, F.H.; Ramasamy, T.S. The multifaceted role of exosomes in cancer progression: Diagnostic and therapeutic implications [corrected]. Cell. Oncol. 2018, 41, 223–252. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Gazdic, M.; Simovic Markovic, B.; Jovicic, N.; Djonov, V.; Arsenijevic, N. Mesenchymal stem cell-derived factors: Immuno-modulatory effects and therapeutic potential. Biofactors 2017, 43, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Gazdic, M.; Volarevic, V.; Arsenijevic, N.; Stojkovic, M. Mesenchymal stem cells: A friend or foe in immune-mediated diseases. Stem Cell Rev. Rep. 2015, 11, 280–287. [Google Scholar] [CrossRef]
- Miloradovic, D.; Miloradovic, D.; Markovic, B.S.; Acovic, A.; Harrell, C.R.; Djonov, V.; Arsenijevic, N.; Volarevic, V. The Effects of Mesenchymal Stem Cells on Antimelanoma Immunity Depend on the Timing of Their Administration. Stem Cells Int. 2020, 2020, 8842659. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.H.; Kim, H.K.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef]
- Shen, Z.; Huang, W.; Liu, J.; Tian, J.; Wang, S.; Rui, K. Effects of Mesenchymal Stem Cell-Derived Exosomes on Autoimmune Diseases. Front. Immunol. 2021, 12, 749192. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.Q.; Luo, Y.Y.; Chang, J.; Song, J.J.; Hao, N.; Zhao, L. Immunomodulation: The next target of mesenchymal stem cell-derived exosomes in the context of ischemic stroke. World J. Stem Cells 2023, 15, 52–70. [Google Scholar] [CrossRef]
- Harrell, C.R.; Pavlovic, D.; Miloradovic, D.; Stojanovic, M.D.; Djonov, V.; Volarevic, V. “Derived Multiple Allogeneic Protein Paracrine Signaling (d-MAPPS)” Enhances T Cell-Driven Immune Response to Murine Mammary Carcinoma. Anal. Cell. Pathol. 2022, 2022, 3655595. [Google Scholar] [CrossRef]
- Fatima, F.; Nawaz, M. Stem cell-derived exosomes: Roles in stromal remodeling, tumor progression, and cancer immunotherapy. Chin. J. Cancer 2015, 34, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tan, X.; Tan, Y.; Li, Q.; Ma, J.; Wang, G. Mesenchymal Stem Cell Derived Exosomes in Cancer Progression, Metastasis and Drug Delivery: A Comprehensive Review. J. Cancer 2018, 9, 3129–3137. [Google Scholar] [CrossRef]
- Abid, A.I.; Conzatti, G.; Toti, F.; Anton, N.; Vandamme, T. Mesenchymal stem cell-derived exosomes as cell free nanotherapeutics and nanocarriers. Nanomedicine 2024, 61, 102769. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Y.; Xu, Y.; Li, G.; Li, Z.; Liu, T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. Mol. Cancer 2022, 21, 179. [Google Scholar] [CrossRef]
- Gomari, H.; Forouzandeh Moghadam, M.; Soleimani, M.; Ghavami, M.; Khodashenas, S. Targeted delivery of doxorubicin to HER2 positive tumor models. Int. J. Nanomed. 2019, 14, 5679–5690. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Bannoura, S.F.; Uddin, M.H.; Nagasaka, M.; Fazili, F.; Al-Hallak, M.N.; Philip, P.A.; El-Rayes, B.; Azmi, A.S. Targeting KRAS in pancreatic cancer: New drugs on the horizon. Cancer Metastasis Rev. 2021, 40, 819–835. [Google Scholar] [CrossRef]
- Hosseini, M.; Ezzeddini, R.; Hashemi, S.M.; Soudi, S.; Salek Farrokhi, A. Enhanced anti-tumor efficacy of S3I-201 in breast cancer mouse model through Wharton jelly- exosome. Cancer Cell Int. 2024, 24, 318. [Google Scholar] [CrossRef] [PubMed]
- Abas, B.I.; Demirbolat, G.M.; Cevik, O. Wharton jelly-derived mesenchymal stem cell exosomes induce apoptosis and suppress EMT signaling in cervical cancer cells as an effective drug carrier system of paclitaxel. PLoS ONE 2022, 17, e0274607. [Google Scholar] [CrossRef]
- Nishi, H.; Ono, M.; Ohno, S.; Yamanaka, Z.; Sasaki, T.; Ohyashiki, K.; Ohyashiki, J.H.; Kuroda, M. Hypoxia-induced paclitaxel resistance in cervical cancer modulated by miR-100 targeting of USP15. Gynecol. Oncol. Rep. 2023, 45, 101138. [Google Scholar] [CrossRef]
- Schulmeister, L. Preventing vincristine sulfate medication errors. Oncol. Nurs. Forum 2004, 31, E90–E98. [Google Scholar] [CrossRef]
- Farouk, A.H.; Aref, A.; Fathy, B.A.; Abdallah, A.N. Stem cells derived exosomes as biological nano carriers for VCR sulfate for treating breast cancer stem cells. Sci. Rep. 2024, 14, 10964. [Google Scholar] [CrossRef] [PubMed]
- Valipour, E.; Ranjbar, F.E.; Mousavi, M.; Ai, J.; Malekshahi, Z.V.; Mokhberian, N.; Taghdiri-Nooshabadi, Z.; Khanmohammadi, M.; Nooshabadi, V.T. The anti-angiogenic effect of atorvastatin loaded exosomes on glioblastoma tumor cells: An in vitro 3D culture model. Microvasc. Res. 2022, 143, 104385. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.M.; Chen, Y.J.; Bao, Y.X. Pharmacological mechanisms of norcantharidin against hepatocellular carcinoma. Am. J. Cancer Res. 2023, 13, 5024–5038. [Google Scholar]
- Zhou, J.; Ren, Y.; Tan, L.; Song, X.; Wang, M.; Li, Y.; Cao, Z.; Guo, C. Norcantharidin: Research advances in pharmaceutical activities and derivatives in recent years. Biomed. Pharmacother. 2020, 131, 110755. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhao, L.; Wang, Y.; Wang, Y. Treatment for Hepatocellular Carcinoma Is Enhanced When Norcantharidin Is Encapsulated in Exosomes Derived from Bone Marrow Mesenchymal Stem Cells. Mol. Pharm. 2021, 18, 1003–1013. [Google Scholar] [CrossRef]
- Gao, X.; Liu, J.; Sun, R.; Zhang, J.; Cao, X.; Zhang, Y.; Zhao, M. Alliance between titans: Combination strategies of CAR-T cell therapy and oncolytic virus for the treatment of hematological malignancies. Ann. Hematol. 2024, 103, 2569–2589. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Wang, X.; Jin, L.; Luo, H.; Yang, Z.; Yang, N.; Lin, X.; Yang, Y.; Zhao, X.; He, Z. Human umbilical cord mesenchymal stem cell exosomes deliver potent oncolytic reovirus to acute myeloid leukemia cells. Virology 2024, 598, 110171. [Google Scholar] [CrossRef]
- Kimura, Y.; Ohzawa, H.; Miyato, H.; Kaneko, Y.; Kuchimaru, T.; Takahashi, R.; Yamaguchi, H.; Kurashina, K.; Saito, S.; Hosoya, Y.; et al. Intraperitoneal transfer of microRNA-29b-containing small extracellular vesicles can suppress peritoneal metastases of gastric cancer. Cancer Sci. 2023, 114, 2939–2950. [Google Scholar] [CrossRef]
- Kim, G.; Kim, M.; Lee, Y.; Byun, J.W.; Lee, M. Systemic delivery of microRNA 21 antisense oligonucleotides to the brain using T7 peptide decorated exosomes. J. Control. Release 2020, 317, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, T.; Zheng, Y.; Xu, X.; Sun, R.; Zhan, S.; Guo, X.; Zhao, Z.; Zhu, W.; Feng, B.; et al. Evaluating adipose-derived stem cell exosomes as miRNA drug delivery systems for the treatment of bladder cancer. Cancer Med. 2022, 11, 3687–3699. [Google Scholar] [CrossRef] [PubMed]
- Mahati, S.; Fu, X.; Ma, X.; Zhang, H.; Xiao, L. Delivery of miR-26a Using an Exosomes-Based Nanosystem Inhibited Proliferation of Hepatocellular Carcinoma. Front. Mol. Biosci. 2021, 8, 738219. [Google Scholar] [CrossRef]
- Han, S.; Li, G.; Jia, M.; Zhao, Y.; He, C.; Huang, M.; Jiang, L.; Wu, M.; Yang, J.; Ji, X.; et al. Delivery of Anti-miRNA-221 for Colorectal Carcinoma Therapy Using Modified Cord Blood Mesenchymal Stem Cells-Derived Exosomes. Front. Mol. Biosci. 2021, 8, 743013. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, J.; Tong, A.; Yang, H. Oncolytic Viruses for Cancer Therapy: Barriers and Recent Advances. Mol. Ther. Oncolytics 2019, 15, 234–247. [Google Scholar] [CrossRef]
- Parker Kerrigan, B.C.; Shimizu, Y.; Andreeff, M.; Lang, F.F. Mesenchymal stromal cells for the delivery of oncolytic viruses in gliomas. Cytotherapy 2017, 19, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Friesen, K.R.; Gupta, D.; Fisher, K.D.; Wood, M.; Seymour, L.W. Exosomes as a stromal-targeting extension of oncolytic virus therapeutics. Cytotherapy 2024, 26, 31–32. [Google Scholar] [CrossRef]
- Mirlekar, B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022, 10, 20503121211069012. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Darestani, N.; Gilmanova, A.I.; Al-Gazally, M.E.; Zekiy, A.O.; Ansari, M.J.; Zabibah, R.S.; Jawad, M.A.; Al-Shalah, S.A.J.; Rizaev, J.A.; Alnassar, Y.S.; et al. Mesenchymal stem cell-released oncolytic virus: An innovative strategy for cancer treatment. Cell Commun. Signal. 2023, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Oveili, E.; Vafaei, S.; Bazavar, H.; Eslami, Y.; Mamaghanizadeh, E.; Yasamineh, S.; Gholizadeh, O. The potential use of mesenchymal stem cells-derived exosomes as microRNAs delivery systems in different diseases. Cell Commun. Signal. 2023, 21, 20. [Google Scholar] [CrossRef]
- Grassilli, S.; Bertagnolo, V.; Brugnoli, F. Mir-29b in Breast Cancer: A Promising Target for Therapeutic Approaches. Diagnostics 2022, 12, 2139. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, M.S.; Mehrabian, E.; Mirzaei, H. MiR-21: A key player in glioblastoma pathogenesis. J. Cell Biochem. 2018, 119, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, M.; Liang, H.; Guo, S.; Guo, X.; Yuan, M.; Lian, H.; Yan, X.; Zhang, S.; Chen, X.; et al. miR-138-5p contributes to cell proliferation and invasion by targeting Survivin in bladder cancer cells. Mol. Cancer 2016, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yang, Y.; Peng, F.; Liu, Y.; Fu, X.; Ji, B. Gold nanoparticles-loaded anti-miR221 enhances antitumor effect of sorafenib in hepatocellular carcinoma cells. Int. J. Med. Sci. 2019, 16, 1541–1548. [Google Scholar] [CrossRef]
- Ahn, S.H.; Ryu, S.W.; Choi, H.; You, S.; Park, J.; Choi, C. Manufacturing Therapeutic Exosomes: From Bench to Industry. Mol. Cells 2022, 45, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, J.; Zhang, Y.; He, J.; Wang, M.; Hei, Y.; Guo, S.; Xu, X.; Liu, Y. Different origin-derived exosomes and their clinical advantages in cancer therapy. Front. Immunol. 2024, 15, 1401852. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Li, L.; He, D.; Chi, J.; Li, Q.; Wu, Y.; Zhao, Y.; Zhang, S.; Wang, L.; et al. Engineered extracellular vesicles and their mimetics for cancer immunotherapy. J. Control. Release 2022, 349, 679–698. [Google Scholar] [CrossRef]
- Mishra, L.C.; Pandey, U.; Gupta, A.; Gupta, J.; Sharma, M.; Mishra, G. Alternating exosomes and their mimetics as an emergent strategy for targeted cancer therapy. Front. Mol. Biosci. 2022, 9, 939050. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Zhong, Y.; Shen, J.; An, W. Biomimetic Exosomes: A New Generation of Drug Delivery System. Front. Bioeng. Biotechnol. 2022, 10, 865682. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Jin, L.; Guo, P.; Zhang, Z.; Zhanghuang, C.; Tan, X.; Mi, T.; Liu, J.; Wu, X.; et al. Exosome mimetics derived from bone marrow mesenchymal stem cells deliver doxorubicin to osteosarcoma in vitro and in vivo. Drug Deliv. 2022, 29, 3291–3303. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrell, C.R.; Volarevic, A.; Djonov, V.; Volarevic, V. Mesenchymal Stem-Cell-Derived Exosomes as Novel Drug Carriers in Anti-Cancer Treatment: A Myth or Reality? Cells 2025, 14, 202. https://doi.org/10.3390/cells14030202
Harrell CR, Volarevic A, Djonov V, Volarevic V. Mesenchymal Stem-Cell-Derived Exosomes as Novel Drug Carriers in Anti-Cancer Treatment: A Myth or Reality? Cells. 2025; 14(3):202. https://doi.org/10.3390/cells14030202
Chicago/Turabian StyleHarrell, Carl Randall, Ana Volarevic, Valentin Djonov, and Vladislav Volarevic. 2025. "Mesenchymal Stem-Cell-Derived Exosomes as Novel Drug Carriers in Anti-Cancer Treatment: A Myth or Reality?" Cells 14, no. 3: 202. https://doi.org/10.3390/cells14030202
APA StyleHarrell, C. R., Volarevic, A., Djonov, V., & Volarevic, V. (2025). Mesenchymal Stem-Cell-Derived Exosomes as Novel Drug Carriers in Anti-Cancer Treatment: A Myth or Reality? Cells, 14(3), 202. https://doi.org/10.3390/cells14030202







