Dietary Interventions Modulate Cell Competition and Locomotor Decline in an Alzheimer’s Disease Drosophila Model
Highlights
- Cell competition timing and efficiency, together with locomotion, can be modulated by dietary regimen, in a Drosophila Alzheimer’s Disease model
- Yeast-based diet potentiates Amyloid-β42 accumulation, triggering cell competition and locomotion decline
- Tissue fitness and diet can synergistically modulate the strength and profile of neuronal cell competition
- Targeting neuronal cell competition through nutritional interventions may offer new avenues to modulate the course of neurodegeneration
- Conserved fitness-sensing pathways like Flower can provide tractable biomarkers and therapeutic approaches for neurodegenerative diseases
Abstract
1. Introduction
2. Methods
2.1. Drosophila Husbandry and Stocks
2.2. Generation of hFWE Constructs
2.3. Experimental Protocols
2.3.1. Fly Brain Dissection
2.3.2. TUNEL Staining
2.3.3. Diets Protocol
2.3.4. Locomotion Assay—Buridan’s Paradigm
2.4. Image Quantification
2.5. Statistical Analysis
3. Results
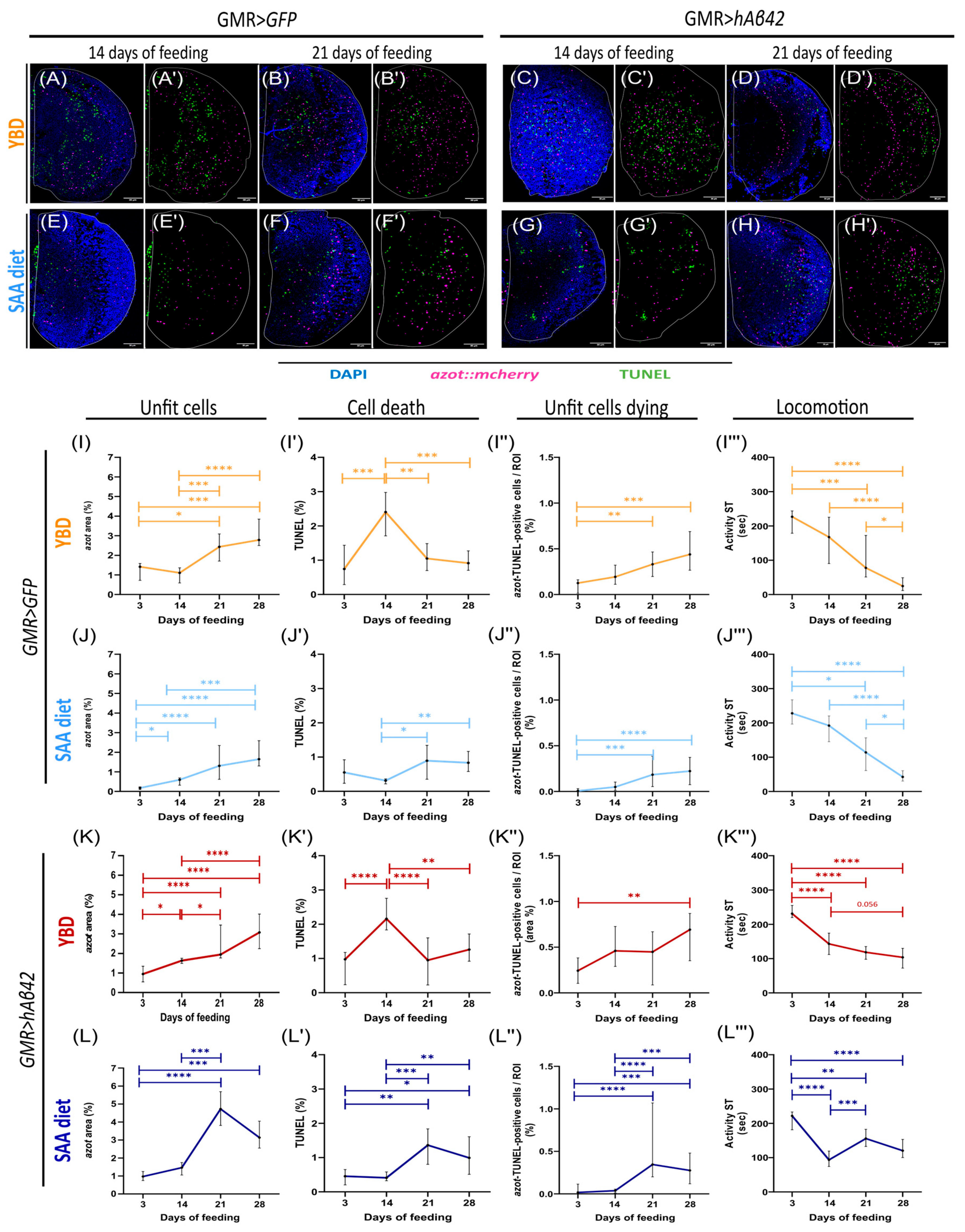
3.1. Yeast-Based Diet Hinders Cell Competition in AD Model, Leading to Locomotion Decline
3.2. Synthetic Diet Delays Cell Competition and Limits Locomotion Decline in the AD Model
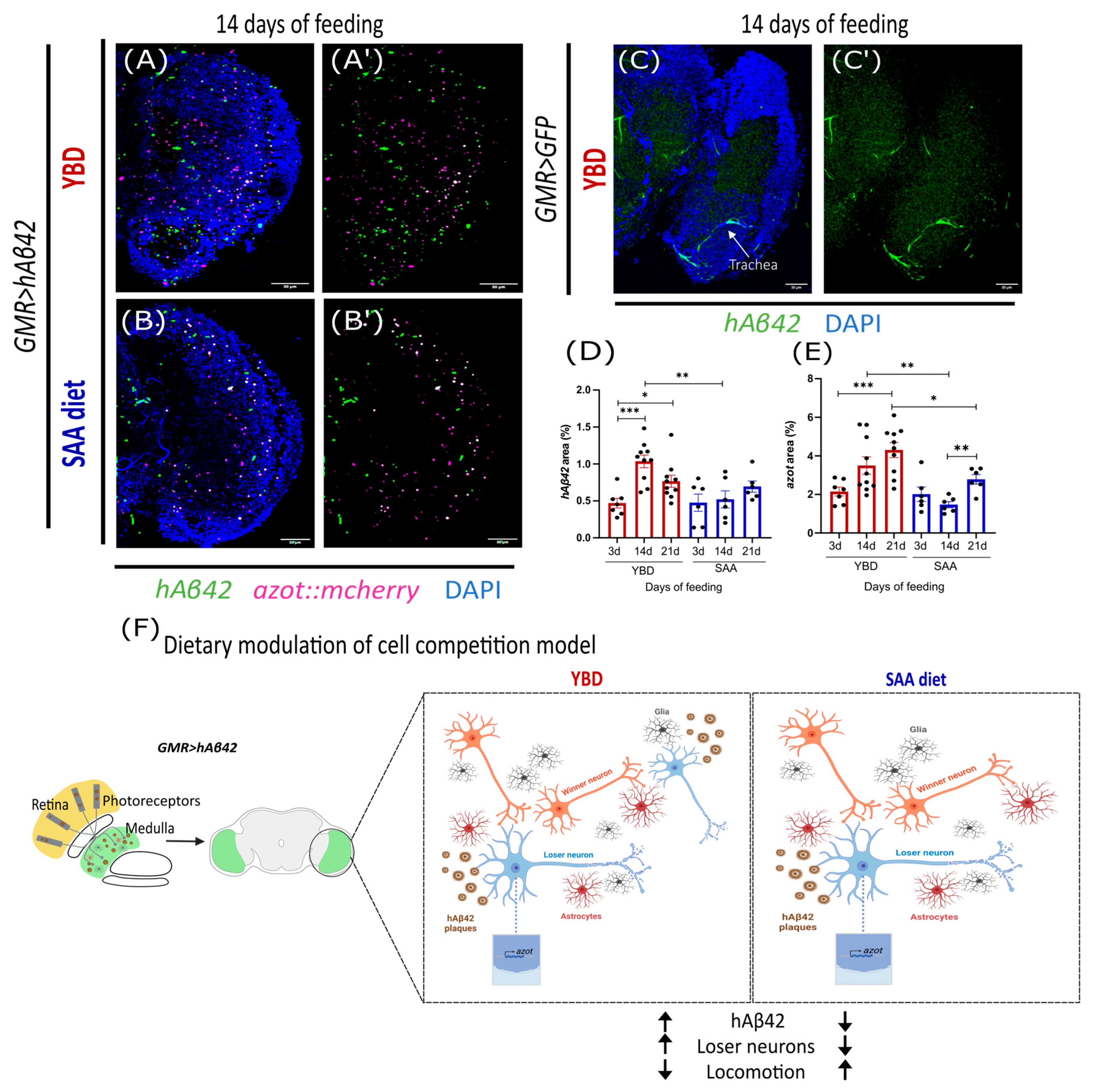
3.3. Synthetic Diet Delays the Accumulation of hAβ42
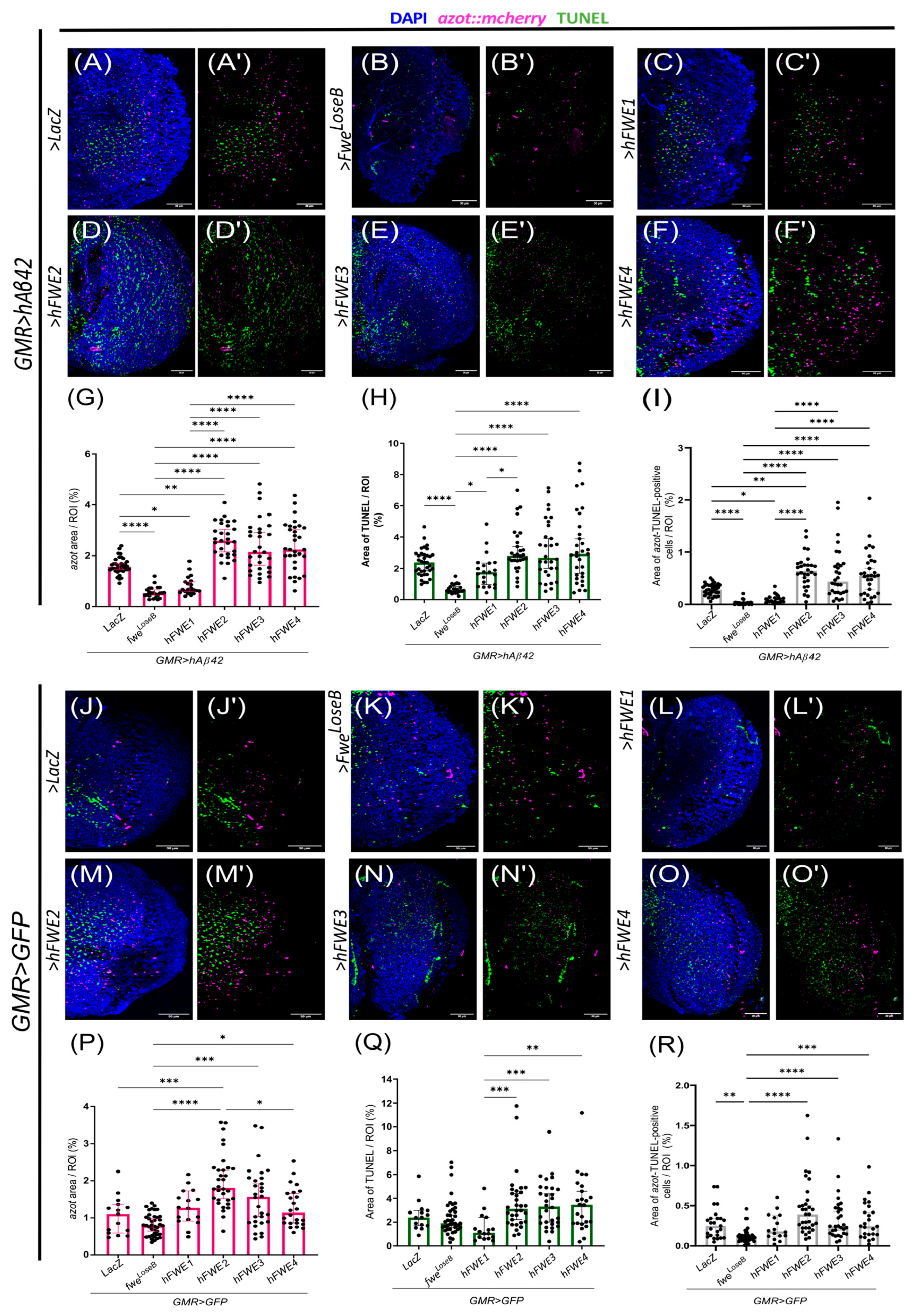
3.4. hFWE Isoforms Are Functionally Conserved in Drosophila Adult Neuronal Tissue
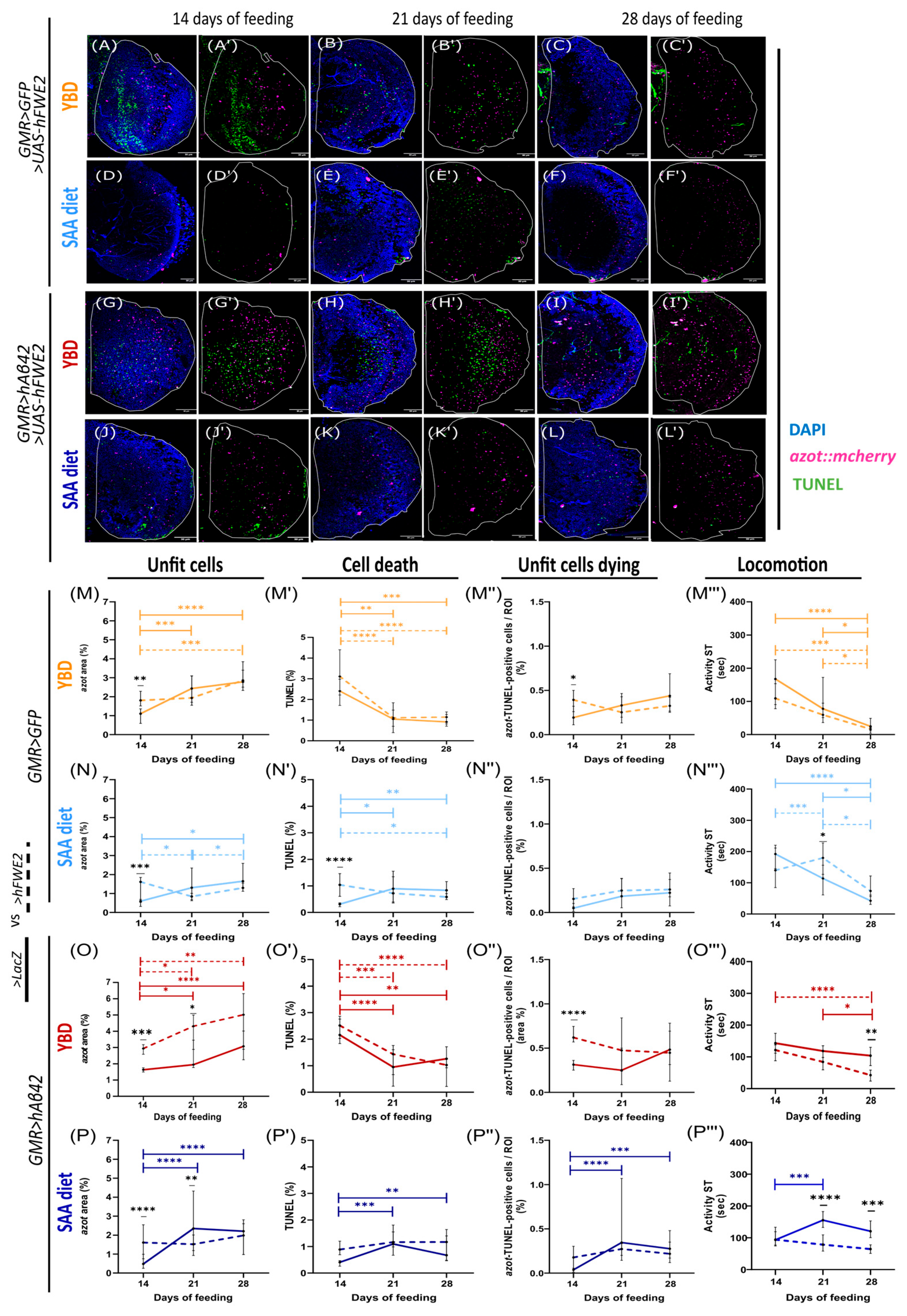
3.5. The Effect of hFWE2 Is Diet- and Context-Dependent
3.6. Foxo and Akt Seem to Be Involved in the Regulation of Azot Expression in the AD Model
4. Discussion
4.1. Yeast-Based Diet Leads to Locomotion Decline Despite Cell Competition Activation
4.2. Synthetic Diet Delays hAβ42 Formation and Cell Competition Activation in AD Model
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauthier, S.; Rosa-Neto, P.; Morais, J.; Webster, C. World Alzheimer Report 2021: Journey Through the Diagnosis of Dementia; Alzheimer’s Disease International: London, UK, 2021. [Google Scholar]
- Bedse, G.; Di Domenico, F.; Serviddio, G.; Cassano, T. Aberrant insulin signaling in Alzheimer’s disease: Current knowledge. Front. Neurosci. 2015, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef]
- Huang, Y.; Mucke, L. Alzheimer Mechanisms and Therapeutic Strategies. Cell 2012, 148, 1204–1222. [Google Scholar] [CrossRef]
- Gómez-Isla, T.; Price, J.L.; McKeel, D.W., Jr.; Morris, J.C.; Growdon, J.H.; Hyman, B.T. Profound Loss of Layer II Entorhinal Cortex Neurons Occurs in Very Mild Alzheimer’s Disease. J. Neurosci. 1996, 16, 4491–4500. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Jeon, Y.; Lee, J.H.; Choi, B.; Won, S.Y.; Cho, K.S. Genetic Dissection of Alzheimer’s Disease Using Drosophila Models. Int. J. Mol. Sci. 2020, 21, 884. [Google Scholar] [CrossRef]
- Mcgurk, L.; Berson, A.; Bonini, N.M. Drosophila as an In Vivo Model for Human Neurodegenerative Disease. Genetics 2015, 201, 377–402. [Google Scholar] [CrossRef]
- Ambegaokar, S.S.; Roy, B.; Jackson, G.R. Neurodegenerative models in Drosophila: Polyglutamine disorders, Parkinson disease, and amyotrophic lateral sclerosis. Neurobiol. Dis. 2010, 40, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Costa-Rodrigues, C.; Couceiro, J.; Moreno, E. Cell competition from development to neurodegeneration. DMM Dis. Model. Mech. 2021, 14, dmm048926. [Google Scholar] [CrossRef] [PubMed]
- Jeibmann, A.; Paulus, W. Drosophila melanogaster as a model organism of brain diseases. Int. J. Mol. Sci. 2009, 10, 407–440. [Google Scholar] [CrossRef]
- Coelho, D.S.; Schwartz, S.; Merino, M.M.; Hauert, B.; Topfel, B.; Tieche, C.; Rhiner, C.; Moreno, E. Culling Less Fit Neurons Protects against Amyloid-β-Induced Brain Damage and Cognitive and Motor Decline. Cell Rep. 2018, 25, 3661–3673.e3. [Google Scholar] [CrossRef]
- Palimariciuc, M.; Balmus, I.M.; Gireadă, B.; Ciobica, A.; Chiriță, R.; Iordache, A.C.; Apostu, M.; Dobrin, R.P. The Quest for Neurodegenerative Disease Treatment—Focusing on Alzheimer’s Disease Personalised Diets. Curr. Issues Mol. Biol. 2023, 45, 1519–1535. [Google Scholar] [CrossRef]
- Khandekar, A.; Ellis, S.J. An expanded view of cell competition. Development 2024, 151, dev204212. [Google Scholar] [CrossRef] [PubMed]
- Merino, M.M.; Levayer, R.; Moreno, E. Survival of the Fittest: Essential Roles of Cell Competition in Development, Aging, and Cancer. Trends Cell Biol. 2016, 26, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.; Lubatti, G.; Burgstaller, J.; Hu, D.; Green, A.P.; Di Gregorio, A.; Zawadzki, T.; Pernaute, B.; Mahammadov, E.; Perez-Montero, S.; et al. Cell competition acts as a purifying selection to eliminate cells with mitochondrial defects during early mouse development. Nat. Metab. 2022, 3, 1091–1108. [Google Scholar] [CrossRef]
- Baker, N.E. Emerging mechanisms of cell competition. Nat. Rev. Genet. 2020, 21, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Martínez, L.; Torres, M. Metabolic regulation of cell competition. Dev. Biol. 2021, 475, 30–36. [Google Scholar] [CrossRef]
- Cong, B.; Cagan, R.L. Cell competition and cancer from Drosophila to mammals. Oncogenesis 2024, 13, 1. [Google Scholar] [CrossRef]
- Clavería, C.; Torres, M. Cell Competition: Mechanisms and Physiological Roles. Annu. Rev. Cell Dev. Biol 2016, 32, 411–439. [Google Scholar] [CrossRef]
- Brás-Pereira, C.; Moreno, E. Mechanical cell competition. Curr. Opin. Cell Biol. 2018, 51, 15–21. [Google Scholar] [CrossRef]
- Portela, M.; Casas-Tinto, S.; Rhiner, C.; López-Gay, J.M.; Domínguez, O.; Soldini, D.; Moreno, E. Drosophila SPARC is a self-protective signal expressed by loser cells during cell competition. Dev. Cell 2010, 19, 562–573. [Google Scholar] [CrossRef]
- Rhiner, C.; López-Gay, J.M.; Soldini, D.; Casas-Tinto, S.; Martín, F.A.; Lombardía, L.; Moreno, E. Flower Forms an Extracellular Code that Reveals the Fitness of a Cell to its Neighbors in Drosophila. Dev. Cell 2010, 18, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Merino, M.M.; Rhiner, C.; Portela, M.; Moreno, E. “Fitness fingerprints” mediate physiological culling of unwanted neurons in drosophila. Curr. Biol. 2013, 23, 1300–1309. [Google Scholar] [CrossRef]
- Merino, M.M.; Rhiner, C.; Lopez-Gay, J.M.; Buechel, D.; Hauert, B.; Moreno, E. Elimination of Unfit Cells Maintains Tissue Health and Prolongs Lifespan. Cell 2015, 160, 461–476. [Google Scholar] [CrossRef]
- Merino, M.M. Azot expression in the Drosophila gut modulates organismal lifespan. Commun. Integr. Biol. 2023, 16, 2156735. [Google Scholar] [CrossRef]
- Marques-reis, M.; Hauert, B.; Moreno, E. Longitudinal analysis of Flower-dependent cell competition fitness markers in Drosophila melanogaster. bioRxiv 2024. [Google Scholar] [CrossRef]
- Casas-Tinto, S.; Zhang, Y.; Sanchez-Garcia, J.; Gomez-Velazquez, M.; Rincon-Limas, D.E.; Fernandez-Funez, P. The ER stress factor XBP1s prevents amyloid-b neurotoxicity. Hum. Mol. Genet. 2011, 20, 2144–2160. [Google Scholar] [CrossRef] [PubMed]
- Ellouze, I.; Sheffler, J.; Nagpal, R.; Arjmandi, B. Dietary Patterns and Alzheimer’s Disease: An Updated Review Linking Nutrition to Neuroscience. Nutrients 2023, 15, 3204. [Google Scholar] [CrossRef]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 2021, 24, 810–834. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Nagatake, T.; Egami, R.; Gu, G.; Takigawa, I.; Ikeda, W.; Nakatani, T.; Kunisawa, J.; Fujita, Y. Obesity Suppresses Cell-Competition-Mediated Apical Elimination of RasV12-Transformed Cells from Epithelial Tissues. Cell Rep. 2018, 23, 974–982. [Google Scholar] [CrossRef]
- Hirabayashi, S.; Baranski, T.J.; Cagan, R.L. Transformed drosophila cells evade diet-mediated insulin resistance through wingless signaling. Cell 2013, 154, 664–675. [Google Scholar] [CrossRef]
- Hamann, J.C.; Surcel, A.; Chen, R.; Teragawa, C.; Albeck, J.G.; Robinson, D.N.; Hamann, J.C.; Surcel, A.; Chen, R.; Teragawa, C.; et al. Entosis Is Induced by Glucose Starvation. Cell Rep. 2017, 20, 201–210. [Google Scholar] [CrossRef]
- Bowling, S.; Di Gregorio, A.; Sancho, M.; Pozzi, S.; Aarts, M.; Signore, M.; Schneider, M.D.; Barbera, J.P.M.; Gil, J.; Rodríguez, T.A. P53 and mTOR signalling determine fitness selection through cell competition during early mouse embryonic development. Nat. Commun. 2018, 9, 1763. [Google Scholar] [CrossRef]
- Sanaki, Y.; Nagata, R.; Kizawa, D.; Léopold, P.; Igaki, T. Hyperinsulinemia Drives Epithelial Tumorigenesis by Abrogating Cell Competition. Dev. Cell 2020, 53, 379–389.e5. [Google Scholar] [CrossRef]
- Madan, E.; Pelham, C.J.; Nagane, M.; Parker, T.M.; Canas-Marques, R.; Fazio, K.; Shaik, K.; Yuan, Y.; Henriques, V.; Galzerano, A.; et al. Flower isoforms promote competitive growth in cancer. Nature 2019, 572, 260–264. [Google Scholar] [CrossRef]
- Piper, M.D.W.; Soultoukis, G.A.; Blanc, E.; Mesaros, A.; Herbert, S.L.; Juricic, P.; He, X.; Atanassov, I.; Salmonowicz, H.; Yang, M.; et al. Matching Dietary Amino Acid Balance to the In Silico-Translated Exome Optimizes Growth and Reproduction without Cost to Lifespan. Cell Metab. 2017, 25, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Colomb, J.; Reiter, L.; Blaszkiewicz, J.; Wessnitzer, J.; Brembs, B. Open source tracking and analysis of adult Drosophila locomotion in Buridan’s paradigm with and without visual targets. PLoS ONE 2012, 7, e42247. [Google Scholar] [CrossRef]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. Ilastik: Interactive Machine Learning for (Bio)Image Analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Wei, T.M.; Tseng, S.C.; Lo, C.C. Characterizing approach behavior of Drosophila melanogaster in Buridan’s paradigm. PLoS ONE 2021, 16, e0245990. [Google Scholar] [CrossRef]
- Simon, A.F.; Liang, D.T.; Krantz, D.E. Differential decline in behavioral performance of Drosophila melanogaster with age. Mech. Ageing Dev. 2006, 127, 647–651. [Google Scholar] [CrossRef]
- Hay, N. Interplay between FOXO, TOR, and Akt. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 1965–1970. [Google Scholar] [CrossRef]
- Kramer, J.M.; Davidge, J.T.; Lockyer, J.M.; Staveley, B.E. Expression of Drosophila FOXO regulates growth and can phenocopy starvation. BMC Dev. Biol. 2003, 3, 5. [Google Scholar] [CrossRef]
- Levayer, R.; Hauert, B.; Moreno, E. Cell mixing induced by myc is required for competitive tissue invasion and destruction. Nature 2015, 524, 476–480. [Google Scholar] [CrossRef]
- Vafadar-Isfahani, B.; Ball, G.; Coveney, C.; Lemetre, C.; Boocock, D.; Minthon, L.; Hansson, O.; Miles, A.K.; Janciauskiene, S.M.; Warden, D.; et al. Identification of SPARC-like 1 protein as part of a biomarker panel for Alzheimer’s disease in cerebrospinal fluid. J. Alzheimer’s Dis. 2012, 28, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, M.; Du, Y.; Liu, S.; Li, D.; Zhang, S.; Ji, F.; Zhang, J.; Jiao, J. Compromised cell competition exhausts neural stem cells pool. Cell Prolif. 2024, 57, e13710. [Google Scholar] [CrossRef] [PubMed]
- Marques dos Reis, M. The Role of Neural Death in Alzheimer’s Disease Flower Interacting Candidates in an Amyloid-Beta Model and Exploring a Tau Model in a Cell Competition Context Biological Engineering Examination Committee. Master’s Thesis, University of Lisbon, Lisbon, Portugal, 2017. [Google Scholar]
- Kos, K.; Wilding, J.P.H. SPARC: A key player in the pathologies associated with obesity and diabetes. Nat. Rev. Endocrinol. 2010, 6, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.; Lubatti, G.; Burgstaller, J.; Hu, D.; Green, A.; Gregorio, A.D.; Zawadzki, T.; Pernaute, B.; Mahammadov, E.; Dore, M.; et al. Differences in mitochondrial activity trigger cell competition during early mouse development. bioRxiv 2020. [Google Scholar] [CrossRef]
- Petrova, E.; López-Gay, J.M.; Rhiner, C.; Moreno, E. Flower-deficient mice have reduced susceptibility to skin papilloma formation. Dis. Model. Mech. 2012, 5, 553–561. [Google Scholar] [CrossRef]
- Díaz, G.; Lengele, L.; Sourdet, S.; Soriano, G.; de Souto Barreto, P. Nutrients and amyloid β status in the brain: A narrative review. Ageing Res. Rev. 2022, 81, 101728. [Google Scholar] [CrossRef]
- Tsuda, M.; Kobayashi, T.; Matsuo, T.; Aigaki, T. Insulin-degrading enzyme antagonizes insulin-dependent tissue growth and Ab-induced neurotoxicity in Drosophila. FEBS Lett. 2010, 584, 2916–2920. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, S.E.; Shah, A.; Easton, A.N.; Hall, H.; Weake, V.M. Characterizing a gene expression toolkit for eye- and photoreceptor-specific expression in Drosophila. Fly 2021, 15, 73–88. [Google Scholar] [CrossRef]
- Kramer, J.M.; Staveley, B.E. GAL4 causes developmental defects and apoptosis when expressed in the developing eye of Drosophila melanogaster. Genet. Mol. Res. 2003, 2, 43–47. [Google Scholar] [PubMed]
- Currier, T.A.; Pang, M.M.; Clandinin, T.R. Visual processing in the fly, from photoreceptors to behavior. Genetics 2023, 224, iyad064. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa-Rodrigues, C.; Jacobs, J.R.; Couceiro, J.; Brás-Pereira, C.; Moreno, E. Dietary Interventions Modulate Cell Competition and Locomotor Decline in an Alzheimer’s Disease Drosophila Model. Cells 2025, 14, 2011. https://doi.org/10.3390/cells14242011
Costa-Rodrigues C, Jacobs JR, Couceiro J, Brás-Pereira C, Moreno E. Dietary Interventions Modulate Cell Competition and Locomotor Decline in an Alzheimer’s Disease Drosophila Model. Cells. 2025; 14(24):2011. https://doi.org/10.3390/cells14242011
Chicago/Turabian StyleCosta-Rodrigues, Carolina, Jovin R. Jacobs, Joana Couceiro, Catarina Brás-Pereira, and Eduardo Moreno. 2025. "Dietary Interventions Modulate Cell Competition and Locomotor Decline in an Alzheimer’s Disease Drosophila Model" Cells 14, no. 24: 2011. https://doi.org/10.3390/cells14242011
APA StyleCosta-Rodrigues, C., Jacobs, J. R., Couceiro, J., Brás-Pereira, C., & Moreno, E. (2025). Dietary Interventions Modulate Cell Competition and Locomotor Decline in an Alzheimer’s Disease Drosophila Model. Cells, 14(24), 2011. https://doi.org/10.3390/cells14242011






