The Stress Response Is Adaptive in a Context- and State-Dependent Manner
Abstract
1. Introduction
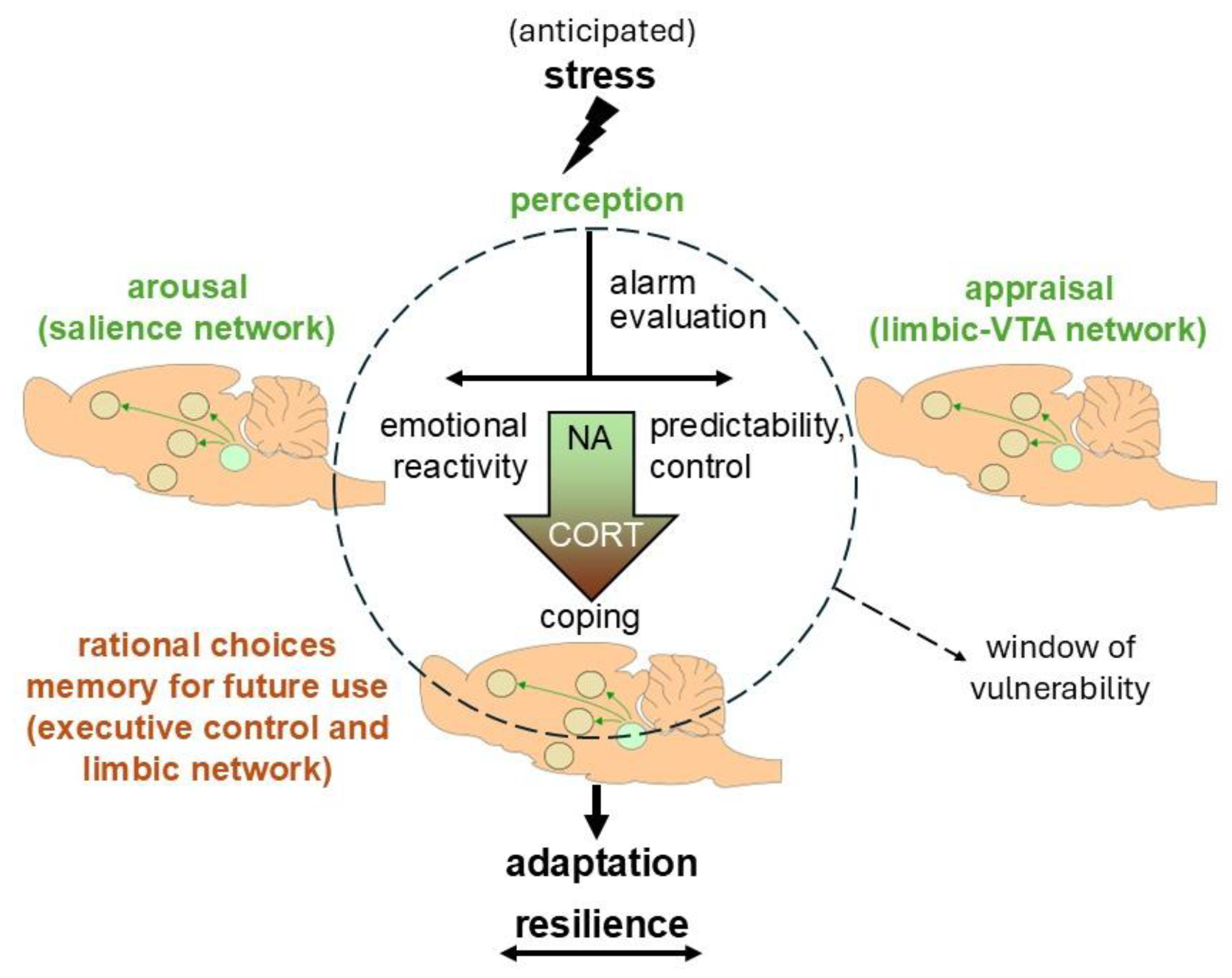
2. The Bimodal Stress Response

3. The Relevance of the Circumstances During Testing
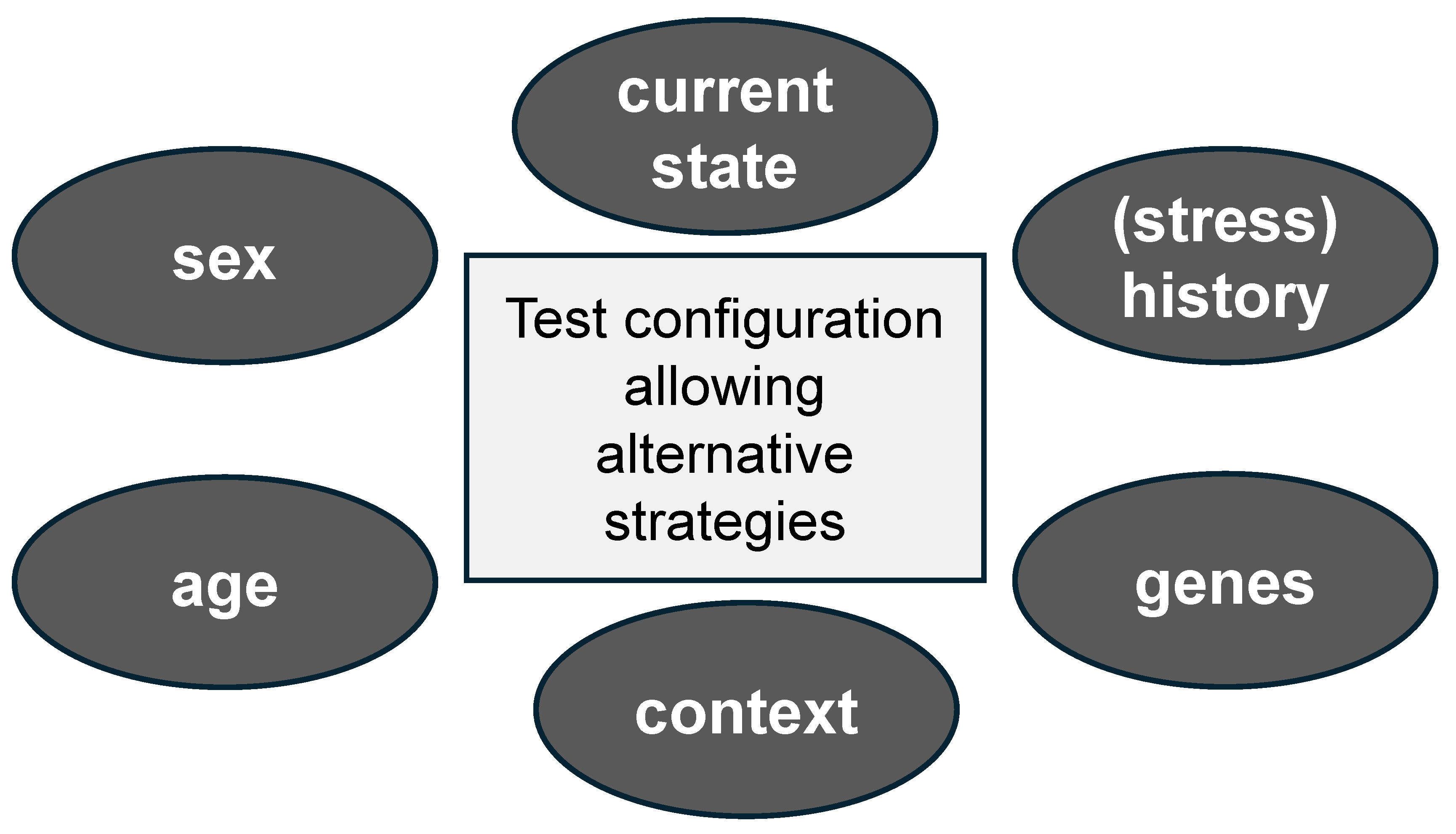
3.1. Relevance of Context and Experimental Design
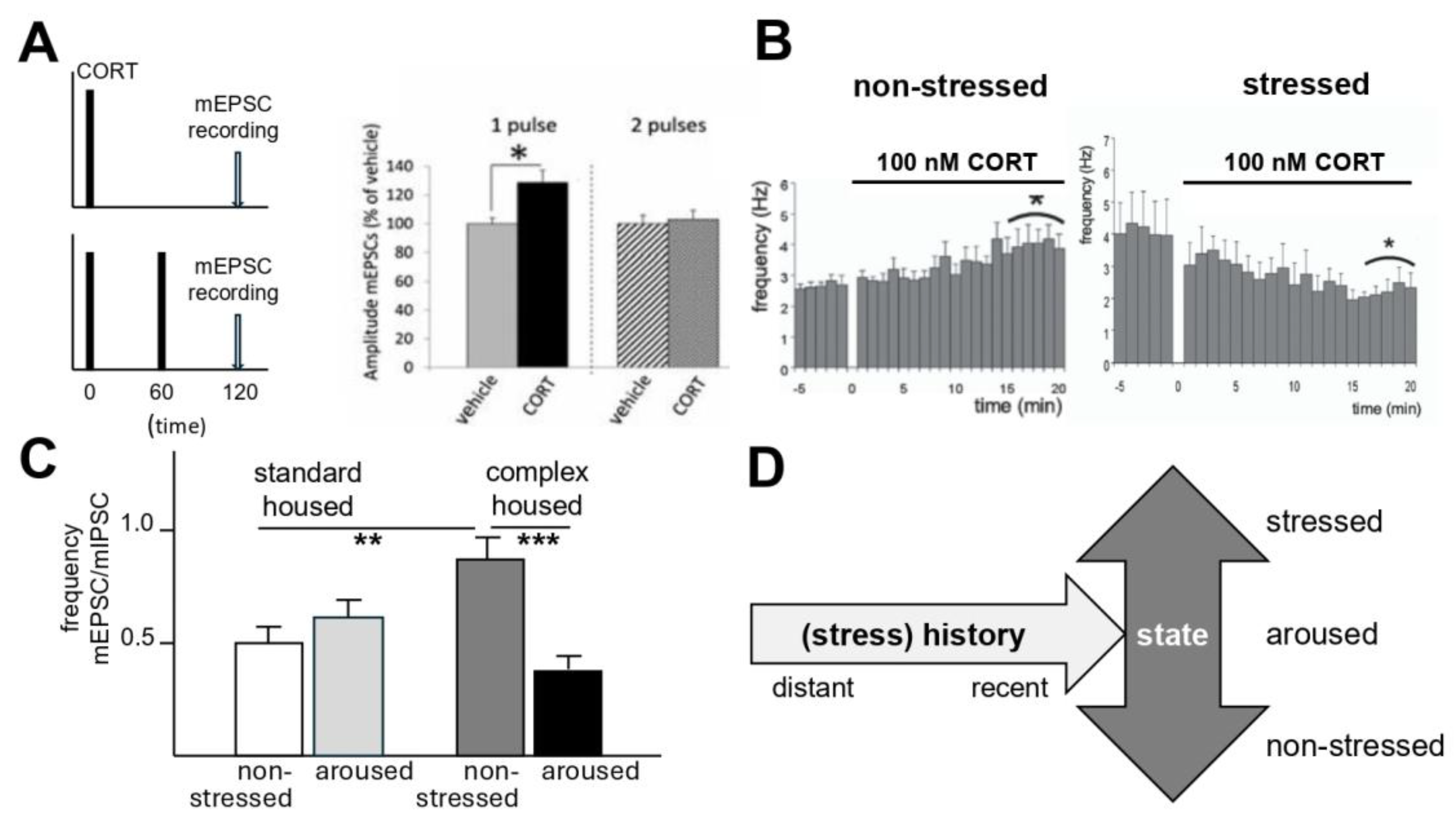
3.2. Current State of the Individual in Relation to Its Recent or More Distant History
4. Similarities at the Single-Cell and Network Level
4.1. Recent Stress Experience, Synaptic Plasticity, and Memory
4.2. Chronic Stress, Current State, and Excitability
4.3. Early Life Stress, Predicted Conditions, Synaptic Plasticity, and Learning
5. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joëls, M.; Baram, T.Z. The neuro-symphony of stress. Nat. Rev. Neurosci. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Joëls, M. The cortisol switch between vulnerability and resilience. Mol. Psychiatry 2024, 29, 20–34. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Cabib, S.; Campus, P.; Conversi, D.; Orsini, C.; Puglisi-Allegra, S. Functional and Dysfunctional Neuroplasticity in Learning to Cope with Stress. Brain Sci. 2020, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- De Boer, S.F.; Buwalda, B.; Koolhaas, J.M. Untangling the neurobiology of coping styles in rodents: Towards neural mechanisms underlying individual differences in disease susceptibility. Neurosci. Biobehav. Rev. 2017, 74, 401–422. [Google Scholar] [CrossRef] [PubMed]
- Joëls, M. Corticosteroids and the brain. J. Endocrinol. 2018, 238, R121–R130. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]
- Joëls, M.; Karst, H.; Tasker, J.G. The emerging role of rapid corticosteroid actions on excitatory and inhibitory synaptic signaling in the brain. Front. Neuroendocrinol. 2024, 74, 101146. [Google Scholar] [CrossRef]
- Di, S.; Malcher-Lopes, R.; Halmos, K.C.; Tasker, J.G. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. J. Neurosci. 2003, 23, 4850–4857. [Google Scholar] [CrossRef]
- Tasker, J.G.; Di, S.; Malcher-Lopes, R. Minireview: Rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology 2006, 147, 5549–5556. [Google Scholar] [CrossRef] [PubMed]
- Keller-Wood, M.E.; Dallman, M.F. Corticosteroid inhibition of ACTH secretion. Endocr. Rev. 1984, 5, 1–24. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Karst, H.; Berger, S.; Turiault, M.; Tronche, F.; Schütz, G.; Joëls, M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc. Natl. Acad. Sci. USA 2005, 102, 19204–19207. [Google Scholar] [CrossRef] [PubMed]
- Groc, L.; Choquet, D.; Chaouloff, F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat. Neurosci. 2008, 11, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Karst, H.; Joëls, M. Corticosterone slowly enhances miniature excitatory postsynaptic current amplitude in mice CA1 hippocampal cells. J. Neurophysiol. 2005, 94, 3479–3486. [Google Scholar] [CrossRef]
- Martin, S.; Henley, J.M.; Holman, D.; Zhou, M.; Wiegert, O.; van Spronsen, M.; Joëls, M.; Hoogenraad, C.C.; Krugers, H.J. Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PLoS ONE 2009, 4, e4714. [Google Scholar] [CrossRef]
- Xiong, H.; Cassé, F.; Zhou, Y.; Zhou, M.; Xiong, Z.Q.; Joëls, M.; Martin, S.; Krugers, H.J. mTOR is essential for corticosteroid effects on hippocampal AMPA receptor function and fear memory. Learn. Mem. 2015, 22, 577–583. [Google Scholar] [CrossRef]
- Yuen, E.Y.; Liu, W.; Karatsoreos, I.N.; Feng, J.; McEwen, B.S.; Yan, Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc. Natl. Acad. Sci. USA 2009, 106, 14075–14079. [Google Scholar] [CrossRef]
- Joëls, M.; de Kloet, E.R. Effects of glucocorticoids and norepinephrine on the excitability in the hippocampus. Science 1989, 245, 1502–1505. [Google Scholar] [CrossRef]
- Kerr, D.S.; Campbell, L.W.; Hao, S.Y.; Landfield, P.W. Corticosteroid modulation of hippocampal potentials: Increased effect with aging. Science 1989, 245, 1505–1509. [Google Scholar] [CrossRef]
- Mikasova, L.; Xiong, H.; Kerkhofs, A.; Bouchet, D.; Krugers, H.J.; Groc, L. Stress hormone rapidly tunes synaptic NMDA receptor through membrane dynamics and mineralocorticoid signalling. Sci. Rep. 2017, 7, 8053. [Google Scholar] [CrossRef]
- Conboy, L.; Sandi, C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacology 2010, 35, 674–685. [Google Scholar] [CrossRef] [PubMed]
- De Quervain, D.; Schwabe, L.; Roozendaal, B. Stress, glucocorticoids and memory: Implications for treating fear-related disorders. Nat. Rev. Neurosci. 2017, 18, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology 2000, 25, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, B.; McEwen, B.S.; Chattarji, S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009, 10, 423–433. [Google Scholar] [CrossRef]
- Sandi, C.; Rose, S.P. Corticosterone enhances long-term retention in one-day-old chicks trained in a weak passive avoidance learning paradigm. Brain Res. 1994, 647, 106–112. [Google Scholar] [CrossRef]
- Moser, E.I.; Krobert, K.A.; Moser, M.B.; Morris, R.G. Impaired spatial learning after saturation of long-term potentiation. Science 1998, 281, 2038–2042. [Google Scholar] [CrossRef]
- Whitlock, J.R.; Heynen, A.J.; Shuler, M.G.; Bear, M.F. Learning induces long-term potentiation in the hip-pocampus. Science 2006, 313, 1093–1097. [Google Scholar] [CrossRef]
- Karst, H.; Berger, S.; Erdmann, G.; Schütz, G.; Joëls, M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc. Natl. Acad. Sci. USA 2010, 107, 14449–14454. [Google Scholar] [CrossRef]
- Di, S.; Itoga, C.A.; Fisher, M.O.; Solomonow, J.; Roltsch, E.A.; Gilpin, N.W.; Tasker, J.G. Acute Stress Suppresses Synaptic Inhibition and Increases Anxiety via Endocannabinoid Release in the Basolateral Amygdala. J. Neurosci. 2016, 36, 8461–8470. [Google Scholar] [CrossRef]
- Karst, H.; Joëls, M. Corticosterone rapidly reduces glutamatergic but not GABAergic transmission in the infralimbic prefrontal cortex of male mice. Steroids 2023, 198, 109283. [Google Scholar] [CrossRef]
- Yuen, E.Y.; Liu, W.; Karatsoreos, I.N.; Ren, Y.; Feng, J.; McEwen, B.S.; Yan, Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol. Psychiatry 2011, 16, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Cheng, S.; Liu, W.; Ma, J.; Sun, W.; Xiao, W.; Liu, J.; Thai, T.T.; Al Shawi, A.F.; Zhang, D.; et al. Gender differences in the associations of adverse childhood experiences with depression and anxiety: A systematic review and meta-analysis. J. Affect. Disord. 2025, 378, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Abdulla, A.; Farooq, M.; Ishikawa, Y.; Liu, S.J. Emotional stress increases GluA2 expression and potentiates fear memory via adenylyl cyclase 5. Cell Rep. 2025, 44, 115180. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Real, E.; Takamiya, K.; Kang, M.G.; Ledoux, J.; Huganir, R.L.; Malinow, R. Emotion enhances learning via norepi-nephrine regulation of AMPA-receptor trafficking. Cell 2007, 131, 160–173. [Google Scholar] [CrossRef]
- Lee, B.; Xing, X.; Hammes, E.A.; Zeng, Z.; Estrada-Tobar, Z.M.; Kim, K.; Ireton, K.E.; Mimi Man, K.N.; Jacobi, A.A.; Berumen, R.A.; et al. Signaling by intracellular β2-adrenergic receptors regulates AMPA receptor trafficking and synaptic plasticity. Cell Rep. 2025, 44, 116011. [Google Scholar] [CrossRef]
- Zhou, M.; Hoogenraad, C.C.; Joëls, M.; Krugers, H.J. Combined β-adrenergic and corticosteroid receptor activation regulates AMPA receptor function in hippocampal neurons. J. Psychopharmacol. 2012, 26, 516–524. [Google Scholar] [CrossRef]
- Roozendaal, B.; Okuda, S.; Van der Zee, E.A.; McGaugh, J.L. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc. Natl. Acad. Sci. USA 2006, 103, 6741–6746. [Google Scholar] [CrossRef]
- Bonapersona, V.; Schuler, H.; Damsteegt, R.; Adolfs, Y.; Pasterkamp, R.J.; van den Heuvel, M.P.; Joëls, M.; Sarabdjitsingh, R.A. The mouse brain after foot shock in four dimensions: Temporal dynamics at a single-cell resolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2114002119. [Google Scholar] [CrossRef]
- Hermans, E.J.; Henckens, M.J.; Joëls, M.; Fernández, G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014, 37, 304–314. [Google Scholar] [CrossRef]
- Schwabe, L. Memory Under Stress: From Adaptation to Disorder. Biol. Psychiatry 2025, 97, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Schächinger, H.; de Kloet, E.R.; Oitzl, M.S. Stress impairs spatial but not early stimulus-response learning. Behav. Brain Res. 2010, 213, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Margittai, Z.; Strombach, T.; van Wingerden, M.; Joëls, M.; Schwabe, L.; Kalenscher, T. A friend in need: Time-dependent effects of stress on social discounting in men. Horm. Behav. 2015, 73, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Hermans, E.J.; Joëls, M.; Roozendaal, B. Mechanisms of memory under stress. Neuron 2022, 110, 1450–1467. [Google Scholar] [CrossRef]
- Silva, B.A.; Gräff, J. Face your fears: Attenuating remote fear memories by reconsolidation-updating. Trends Cogn. Sci. 2023, 4, 404–416. [Google Scholar] [CrossRef]
- Lee, J.L.C.; Nader, K.; Schiller, D. An Update on Memory Reconsolidation Updating. Trends Cogn. Sci. 2017, 21, 531–545. [Google Scholar] [CrossRef]
- Hackman, D.A.; Farah, M.J.; Meaney, M.J. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2010, 11, 651–659. [Google Scholar] [CrossRef]
- Jespersen, A.; Madden, R.A.; Whalley, H.C.; Reynolds, R.M.; Lawrie, S.M.; McIntosh, A.M.; Iveson, M.H. Socioeconomic Status and Depression—A Systematic Review. Epidemiol. Rev. 2025, 47, mxaf011. [Google Scholar] [CrossRef]
- Novais, A.; Monteiro, S.; Roque, S.; Correia-Neves, M.; Sousa, N. How age, sex and genotype shape the stress response. Neurobiol. Stress 2016, 6, 44–56. [Google Scholar] [CrossRef]
- Ventura, R.; Cabib, S.; Babicola, L.; Andolina, D.; Di Segni, M.; Orsini, C. Interactions Between Experience, Geno-type and Sex in the Development of Individual Coping Strategies. Front. Behav. Neurosci. 2021, 15, 785739. [Google Scholar] [CrossRef]
- Pugh, C.R.; Tremblay, D.; Fleshner, M.; Rudy, J.W. A selective role for corticosterone in contextual-fear conditioning. Behav. Neurosci. 1997, 111, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Sep, M.S.C.; van Ast, V.A.; Gorter, R.; Joëls, M.; Geuze, E. Time-dependent effects of psychosocial stress on the contextualization of neutral memories. Psychoneuroendocrinology 2019, 108, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Gulmez Karaca, K.; Bahtiyar, S.; van Dongen, L.; Wolf, O.T.; Hermans, E.J.; Henckens, M.J.A.G.; Roozendaal, B. Posttraining noradrenergic stimulation maintains hippocampal engram reactivation and episodic-like specificity of re-mote memory. Neuropsychopharmacology 2025, 50, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Atucha, E.; Pais, M.; Ronzoni, G.; Schoenmaker, C.; Atsak, P.; Roura, D.; Lohkamp, K.J.; Schelling, G.; McGaugh, J.L.; Glennon, J.C.; et al. Noradrenergic activation of the basolateral amygdala facilitates memory specificity for similar events experienced close in time. Nat. Neurosci. 2025, 28, 1910–1918. [Google Scholar] [CrossRef]
- Bahtiyar, S.; Gulmez Karaca, K.; Henckens, M.J.A.G.; Roozendaal, B. Norepinephrine and glucocorticoid effects on the brain mechanisms underlying memory accuracy and generalization. Mol. Cell. Neurosci. 2020, 108, 103537. [Google Scholar] [CrossRef]
- Lesuis, S.L.; Brosens, N.; Immerzeel, N.; van der Loo, R.J.; Mitrić, M.; Bielefeld, P.; Fitzsimons, C.P.; Lucassen, P.J.; Kushner, S.A.; van den Oever, M.C.; et al. Glucocorticoids Promote Fear Generalization by Increasing the Size of a Dentate Gyrus Engram Cell Population. Biol. Psychiatry 2021, 90, 494–504. [Google Scholar] [CrossRef]
- Lesuis, S.L.; Park, S.; Hoorn, A.; Rashid, A.J.; Mocle, A.J.; Salter, E.W.; Vislavski, S.; Gray, M.T.; Torelli, A.M.; DeCristofaro, A.; et al. Stress disrupts engram ensembles in lateral amygdala to generalize threat memory in mice. Cell 2025, 188, 121–140.e20. [Google Scholar] [CrossRef]
- Roozendaal, B.; Mirone, G. Opposite effects of noradrenergic and glucocorticoid activation on accuracy of an episodic-like memory. Psychoneuroendocrinology 2020, 114, 104588. [Google Scholar] [CrossRef]
- Schwabe, L.; Dalm, S.; Schächinger, H.; Oitzl, M.S. Chronic stress modulates the use of spatial and stimu-lus-response learning strategies in mice and man. Neurobiol. Learn. Mem. 2008, 90, 495–503. [Google Scholar] [CrossRef]
- Schwabe, L.; Schächinger, H.H.; de Kloet, E.R.; Oitzl, M.S. Corticosteroids operate as a switch between memory systems. J. Cogn. Neurosci. 2010, 2, 1362–1372. [Google Scholar] [CrossRef]
- Schwabe, L.; Wolf, O.T.; Oitzl, M.S. Memory formation under stress: Quantity and quality. Neurosci. Biobehav. Rev. 2010, 34, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Tegenthoff, M.; Höffken, O.; Wolf, O.T. Mineralocorticoid receptor blockade pre-vents stress-induced modulation of multiple memory systems in the human brain. Biol. Psychiatry 2013, 74, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Klumpers, F.; Schröder, T.N.; Oplaat, K.T.; Krugers, H.J.; Oitzl, M.S.; Joëls, M.; Doeller, C.F.; Fernández, G. Stress Induces a Shift Towards Striatum-Dependent Stimulus-Response Learning via the Mineralocorticoid Receptor. Neuropsychopharmacology 2017, 42, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Wirz, L.; Reuter, M.; Wacker, J.; Felten, A.; Schwabe, L. A Haplotype Associated with Enhanced Mineralocorticoid Receptor Expression Facilitates the Stress-Induced Shift from “Cognitive” to “Habit” Learning. eNeuro 2017, 4, ENEURO.0359-17.2017. [Google Scholar] [CrossRef]
- Dias-Ferreira, E.; Sousa, J.C.; Melo, I.; Morgado, P.; Mesquita, A.R.; Cerqueira, J.J.; Costa, R.M.; Sousa, N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science 2009, 325, 621–625. [Google Scholar] [CrossRef]
- Bordes, J.; Miranda, L.; Reinhardt, M.; Narayan, S.; Hartmann, J.; Newman, E.L.; Brix, L.M.; van Doeselaar, L.; Engelhardt, C.; Dillmann, L.; et al. Automatically annotated motion tracking identifies a distinct social behavioral profile following chronic social defeat stress. Nat. Commun. 2023, 14, 4319. [Google Scholar] [CrossRef]
- Dolensek, N.; Gehrlach, D.A.; Klein, A.S.; Gogolla, N. Facial expressions of emotion states and their neuronal corre-lates in mice. Science 2020, 368, c89–c94. [Google Scholar] [CrossRef] [PubMed]
- Sanguino-Gómez, J.; Güçlü, U.; Krugers, H.J.; Lozano, A. Coping strategy dynamics and resilience profiles after early life stress revealed by behavioral sequencing. bioRxiv 2025. [Google Scholar] [CrossRef]
- Shemesh, Y.; Sztainberg, Y.; Forkosh, O.; Shlapobersky, T.; Chen, A.; Schneidman, E. High-order social interactions in groups of mice. Elife 2013, 2, e00759. [Google Scholar] [CrossRef] [PubMed]
- von Ziegler, L.M.; Roessler, F.K.; Sturman, O.; Waag, R.; Privitera, M.; Duss, S.N.; O’Connor, E.C.; Bohacek, J. Analysis of behavioral flow resolves latent phenotypes. Nat. Methods 2024, 21, 2376–2387. [Google Scholar] [CrossRef]
- Sandi, C.; Loscertales, M.; Guaza, C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur. J. Neurosci. 1997, 9, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Sarabdjitsingh, R.A.; Jezequel, J.; Pasricha, N.; Mikasova, L.; Kerkhofs, A.; Karst, H.; Groc, L.; Joëls, M. Ultradian corti-costerone pulses balance glutamatergic transmission and synaptic plasticity. Proc. Natl. Acad. Sci. USA 2014, 111, 14265–14270. [Google Scholar] [CrossRef] [PubMed]
- De Quervain, D.J.; Roozendaal, B.; McGaugh, J.L. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 1998, 394, 787–790. [Google Scholar] [CrossRef] [PubMed]
- De Quervain, D.J.; Roozendaal, B.; Nitsch, R.M.; McGaugh, J.L.; Hock, C. Acute cortisone administration impairs re-trieval of long-term declarative memory in humans. Nat. Neurosci. 2000, 3, 313–314. [Google Scholar] [CrossRef]
- Maier, S.F.; Seligman, M.E. Learned helplessness at fifty: Insights from neuroscience. Psychol. Rev. 2016, 123, 349–367. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Molendijk, M.L. Coping Neurobiology. Biol. Psychiatry 2021, 90, e19–e21. [Google Scholar] [CrossRef]
- Ashokan, A.; Sivasubramanian, M.; Mitra, R. Seeding Stress Resilience through Inoculation. Neural Plast. 2016, 2016, 4928081. [Google Scholar] [CrossRef]
- Dai, Q.; Smith, G.D. Resilience to depression: Implication for psychological vaccination. Front. Psychiatry 2023, 14, 1071859. [Google Scholar] [CrossRef]
- Baram, T.Z.; Birnie, M.T. Enduring memory consequences of early-life stress/adversity: Structural, synaptic, molecular and epigenetic mechanisms. Neurobiol. Stress 2024, 33, 100669. [Google Scholar] [CrossRef] [PubMed]
- Meaney, M.J.; Szyf, M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci. 2005, 7, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Molet, J.; Heins, K.; Zhuo, X.; Mei, Y.T.; Regev, L.; Baram, T.Z.; Stern, H. Fragmentation and high entropy of neonatal ex-perience predict adolescent emotional outcome. Transl. Psychiatry 2016, 6, e702. [Google Scholar] [CrossRef]
- Champagne, F.A.; Francis, D.D.; Mar, A.; Meaney, M.J. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 2003, 79, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Champagne, D.L.; Bagot, R.C.; van Hasselt, F.; Ramakers, G.; Meaney, M.J.; de Kloet, E.R.; Joëls, M.; Krugers, H. Maternal care and hippocampal plasticity: Evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 2008, 28, 6037–6045. [Google Scholar] [CrossRef] [PubMed]
- Nederhof, E.; Schmidt, M.V. Mismatch or cumulative stress: Toward an integrated hypothesis of programming effects. Physiol. Behav. 2012, 106, 691–700. [Google Scholar] [CrossRef]
- Santarelli, S.; Zimmermann, C.; Kalideris, G.; Lesuis, S.L.; Arloth, J.; Uribe, A.; Dournes, C.; Balsevich, G.; Hartmann, J.; Masana, M.; et al. An adverse early life environment can enhance stress resilience in adulthood. Psychoneuroendocrinology 2017, 78, 213–221. [Google Scholar] [CrossRef]
- Bonapersona, V.; Kentrop, J.; Van Lissa, C.J.; van der Veen, R.; Joëls, M.; Sarabdjitsingh, R.A. The behavioral phenotype of early life adversity: A 3-level meta-analysis of rodent studies. Neurosci. Biobehav. Rev. 2019, 102, 299–307. [Google Scholar] [CrossRef]
- Rocha, M.; Wang, D.; Avila-Quintero, V.; Bloch, M.H.; Kaffman, A. Deficits in hippocampal-dependent memory across different rodent models of early life stress: Systematic review and meta-analysis. Transl. Psychiatry 2021, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Malave, L.; van Dijk, M.T.; Anacker, C. Early life adversity shapes neural circuit function during sensitive postnatal developmental periods. Transl. Psychiatry 2022, 12, 306. [Google Scholar] [CrossRef]
- Gerlicher, A.M.V.; Verweij, S.A.; Kindt, M. Better, worse, or different than expected: On the role of value and identi-ty prediction errors in fear memory reactivation. Sci. Rep. 2022, 12, 5862. [Google Scholar] [CrossRef]
- Karst, H.; Riera Llobet, A.; Joëls, M.; van der Veen, R. Complex housing in adulthood state-dependently affects the excitation-inhibition balance in the infralimbic prefrontal cortex of male C57Bl/6 mice. Behav. Brain Res. 2025, 476, 115233. [Google Scholar] [CrossRef]
- McKlveen, J.M.; Morano, R.L.; Fitzgerald, M.; Zoubovsky, S.; Cassella, S.N.; Scheimann, J.R.; Ghosal, S.; Mahbod, P.; Packard, B.A.; Myers, B.; et al. Chronic stress increases prefrontal inhibition: A mechanism for stress-induced prefrontal dysfunction. Biol. Psychiatry 2026, 80, 754–764. [Google Scholar] [CrossRef]
- Rodrigues, D.; Santa, C.; Manadas, B.; Monteiro, P. Chronic Stress Alters Synaptic Inhibition/Excitation Balance of Pyramidal Neurons But Not PV Interneurons in the Infralimbic and Prelimbic Cortices of C57BL/6J Mice. eNeuro 2024, 11, ENEURO.0053-24.2024. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.B.; Bagot, R.C.; Diorio, J.; Wong, T.P.; Meaney, M.J. Maternal care differentially affects neuronal excitability and synaptic plasticity in the dorsal and ventral hippocampus. Neuropsychopharmacology 2015, 40, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.B.; Parent, C.; Tse, Y.C.; Wong, T.P.; Meaney, M.J. Generalization of Conditioned Auditory Fear is Regulated by Maternal Effects on Ventral Hippocampal Synaptic Plasticity. Neuropsychopharmacology 2018, 43, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Brunson, K.L.; Kramár, E.; Lin, B.; Chen, Y.; Colgin, L.L.; Yanagihara, T.K.; Lynch, G.; Baram, T.Z. Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 2005, 25, 9328–9338. [Google Scholar] [CrossRef]
- Wang, X.D.; Rammes, G.; Kraev, I.; Wolf, M.; Liebl, C.; Scharf, S.H.; Rice, C.J.; Wurst, W.; Holsboer, F.; Deussing, J.M.; et al. Forebrain CRF1 modulates early-life stress-programmed cognitive deficits. J. Neurosci. 2011, 31, 13625–13634. [Google Scholar] [CrossRef]
- Bagot, R.C.; Tse, Y.C.; Nguyen, H.B.; Wong, A.S.; Meaney, M.J.; Wong, T.P. Maternal care influences hippocampal N-methyl-D-aspartate receptor function and dynamic regulation by corticosterone in adulthood. Biol. Psychiatry 2012, 72, 491–498. [Google Scholar] [CrossRef]
- Rodenas-Ruano, A.; Chávez, A.E.; Cossio, M.J.; Castillo, P.E.; Zukin, R.S. REST-dependent epigenetic remodeling pro-motes the developmental switch in synaptic NMDA receptors. Nat. Neurosci. 2012, 10, 1382–1390. [Google Scholar] [CrossRef]
- Pillai, A.G.; Arp, M.; Velzing, E.; Lesuis, S.L.; Schmidt, M.V.; Holsboer, F.; Joëls, M.; Krugers, H.J. Early life stress determines the effects of glucocorticoids and stress on hippocampal function: Electrophysiological and behavioral evidence respectively. Neuropharmacology 2018, 133, 307–318. [Google Scholar] [CrossRef]
- Diniz, C.R.A.F.; Crestani, A.P. The times they are a-changin’: A proposal on how brain flexibility goes beyond the obvious to include the concepts of “upward” and “downward” to neuroplasticity. Mol. Psychiatry 2023, 28, 977–992. [Google Scholar] [CrossRef]
- Caradonna, S.G.; Einhorn, N.R.; Saudagar, V.; Khalil, H.; Petty, G.H.; Lihagen, A.; LeFloch, C.; Lee, F.S.; Akil, H.; Guidotti, A.; et al. Corticosterone induces discrete epigenetic signatures in the dorsal and ventral hippocampus that depend upon sex and genotype: Focus on methylated Nr3c1 gene. Transl. Psychiatry 2022, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Domanico, M.J.; Stevens, S.; Wainston, I.; Khoo, E.; McCall, C.; Swack, B.D.; Sachs, B.D. Sub-chronic stress exerts partially distinct behavioral and epigenetic effects in male and female mice. Front. Behav. Neurosci. 2025, 19, 1649660. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Tanapat, P. Stress and hippocampal neurogenesis. Biol. Psychiatry 1999, 46, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Magariños, A.M.; Verdugo, J.M.; McEwen, B.S. Chronic stress alters synaptic terminal structure in hippocampus. Proc. Natl. Acad. Sci. USA 1997, 94, 14002–14008. [Google Scholar] [CrossRef] [PubMed]
- Surget, A.; Belzung, C. Adult hippocampal neurogenesis shapes adaptation and improves stress response: A mechanistic and integrative perspective. Mol. Psychiatry 2022, 27, 403–421. [Google Scholar] [CrossRef]
- Radley, J.J.; Herman, J.P. Preclinical Models of Chronic Stress: Adaptation or Pathology? Biol. Psychiatry 2023, 94, 194–202. [Google Scholar] [CrossRef]
- Kalisch, R.; Russo, S.J.; Müller, M.B. Neurobiology and systems biology of stress resilience. Physiol. Rev. 2024, 104, 1205–1263. [Google Scholar] [CrossRef]
- Li, Y.; He, Z.; Liu, Z.; Peng, N.; Xiao, Y.; Ye, Y.; Zhou, X. Adverse Childhood Experiences and the Diagnosis of ICD-11 Post-traumatic Stress Disorder or Complex Post-traumatic Stress Disorder: A Systematic Review and Three-level Meta-analysis. Trauma Violence Abus. 2025, in press. [Google Scholar] [CrossRef]
- Zhou, L.; Sommer, I.E.C.; Yang, P.; Sikirin, L.; van Os, J.; Bentall, R.P.; Varese, F.; Begemann, M.J.H. What Do Four Decades of Research Tell Us About the Association Between Childhood Adversity and Psychosis: An Updated and Ex-tended Multi-Level Meta-Analysis. Am. J. Psychiatry 2025, 182, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Hermans, E.J.; Hendler, T.; Kalisch, R. Building Resilience: The Stress Response as a Driving Force for Neuroplas-ticity and Adaptation. Biol. Psychiatry 2025, 97, 330–338. [Google Scholar] [CrossRef]
- Konrad, K.; Puetz, V.B. A context-dependent model of resilient functioning after childhood maltreatment-the case for flexible biobehavioral synchrony. Transl. Psychiatry 2024, 14, 388. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M. Resilience as a dynamic concept. Dev. Psychopathol. 2012, 24, 335–344. [Google Scholar] [CrossRef]
- Karatsoreos, I.N.; McEwen, B.S. Psychobiological allostasis: Resistance, resilience and vulnerability. Trends Cogn. Sci. 2011, 15, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.A.; Kaufmann, F.N.; Lavoie, O.; Menard, C. Central and peripheral stress-induced epigenetic mechanisms of resilience. Curr. Opin. Psychiatry 2021, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fallon, I.P.; Tanner, M.K.; Greenwood, B.N.; Baratta, M.V. Sex differences in resilience: Experiential factors and their mechanisms. Eur. J. Neurosci. 2020, 52, 2530–2547. [Google Scholar] [CrossRef]
- Nestler, E.J.; Russo, S.J. Neurobiological basis of stress resilience. Neuron 2024, 112, 1911–1929. [Google Scholar] [CrossRef]
- Van Hasselt, F.N.; Cornelisse, S.; Zhang, T.Y.; Meaney, M.J.; Velzing, E.H.; Krugers, H.J.; Joëls, M. Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus 2012, 22, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Belsky, J.; Bakermans-Kranenburg, M.J.; van IJzendoorn, M.H. For Better and For Worse: Differential Susceptibility to Environmental Influences. Curr. Dir. Psychol. Sci. 2007, 16, 300–304. [Google Scholar] [CrossRef]
- Bonapersona, V. Integrating Information in Stress Research. Ph.D. Thesis, University of Utrecht, Utrecht, The Netherlands, 2022. Chapter 9. Available online: https://dspace.library.uu.nl/handle/1874/422412 (accessed on 30 August 2022).
- Oitzl, M.S.; Workel, J.O.; Fluttert, M.; Frösch, F.; De Kloet, E.R. Maternal deprivation affects behaviour from youth to senescence: Amplification of individual differences in spatial learning and memory in senescent Brown Nor-way rats. Eur. J. Neurosci. 2000, 12, 3771–3780. [Google Scholar]
- Van Hasselt, F.N.; de Visser, L.; Tieskens, J.M.; Cornelisse, S.; Baars, A.M.; Lavrijsen, M.; Krugers, H.J.; van den Bos, R.; Joels, M. Individual variations in maternal care early in life correlate with later life decision-making and c-fos expression in prefrontal subregions of rats. PLoS ONE 2012, 7, e37820. [Google Scholar]
- Stupart, O.; Robbins, T.W.; Dalley, J.W. "The wrong tools for the right job": A critical meta-analysis of tradi-tional tests to assess behavioural impacts of maternal separation. Psychopharmacology 2023, 240, 2239–2256. [Google Scholar] [CrossRef]
- Torres-Berrío, A.; Bortolami, A.; Peña, C.J.; Nestler, E.J. Neurobiology of resilience to early life stress. Neuropsychopharmacology 2025, 51, 29–45. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krugers, H.J.; Joëls, M. The Stress Response Is Adaptive in a Context- and State-Dependent Manner. Cells 2025, 14, 1957. https://doi.org/10.3390/cells14241957
Krugers HJ, Joëls M. The Stress Response Is Adaptive in a Context- and State-Dependent Manner. Cells. 2025; 14(24):1957. https://doi.org/10.3390/cells14241957
Chicago/Turabian StyleKrugers, Harmen J., and Marian Joëls. 2025. "The Stress Response Is Adaptive in a Context- and State-Dependent Manner" Cells 14, no. 24: 1957. https://doi.org/10.3390/cells14241957
APA StyleKrugers, H. J., & Joëls, M. (2025). The Stress Response Is Adaptive in a Context- and State-Dependent Manner. Cells, 14(24), 1957. https://doi.org/10.3390/cells14241957



