Purinergic-Mediated Calcium Signaling in Quiescent and Activated Hepatic Stellate Cells: Evidence That P2Y1 Receptor Delays Activation
Highlights
- A variety of Gq-coupled P2Y receptors are functional in qHSC and MFB.
- P2Y1 receptor is first described in qHSC and its stimulation regulates the transdifferentiation process.
- The study of purinergic signaling in HSC has great potential to gain more understanding about liver fibrogenesis.
- Specifically, P2Y1 receptor might be a molecular target to fibrosis onset.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Use Statement
2.2. Hepatic Stellate Cells Isolation
2.3. Reverse Transcription and Polymerase Chain Reaction
2.4. Intracellular Calcium Measurement by Fluorescent Microscopy
2.5. Immunofluorescence
2.6. Analysis of P2RY1 Transcript Expression in Public Databases
2.7. Biostatistics
3. Results
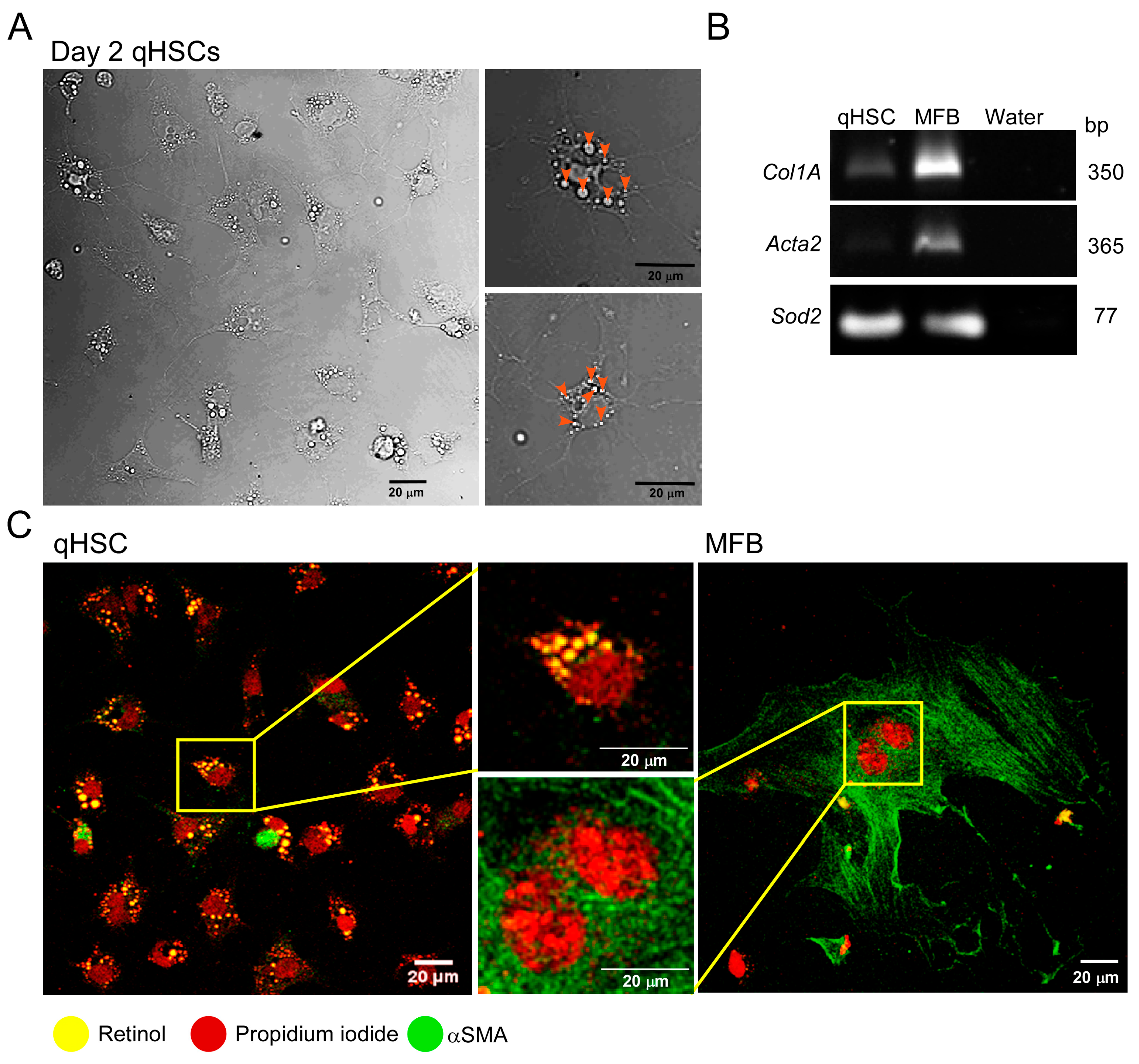
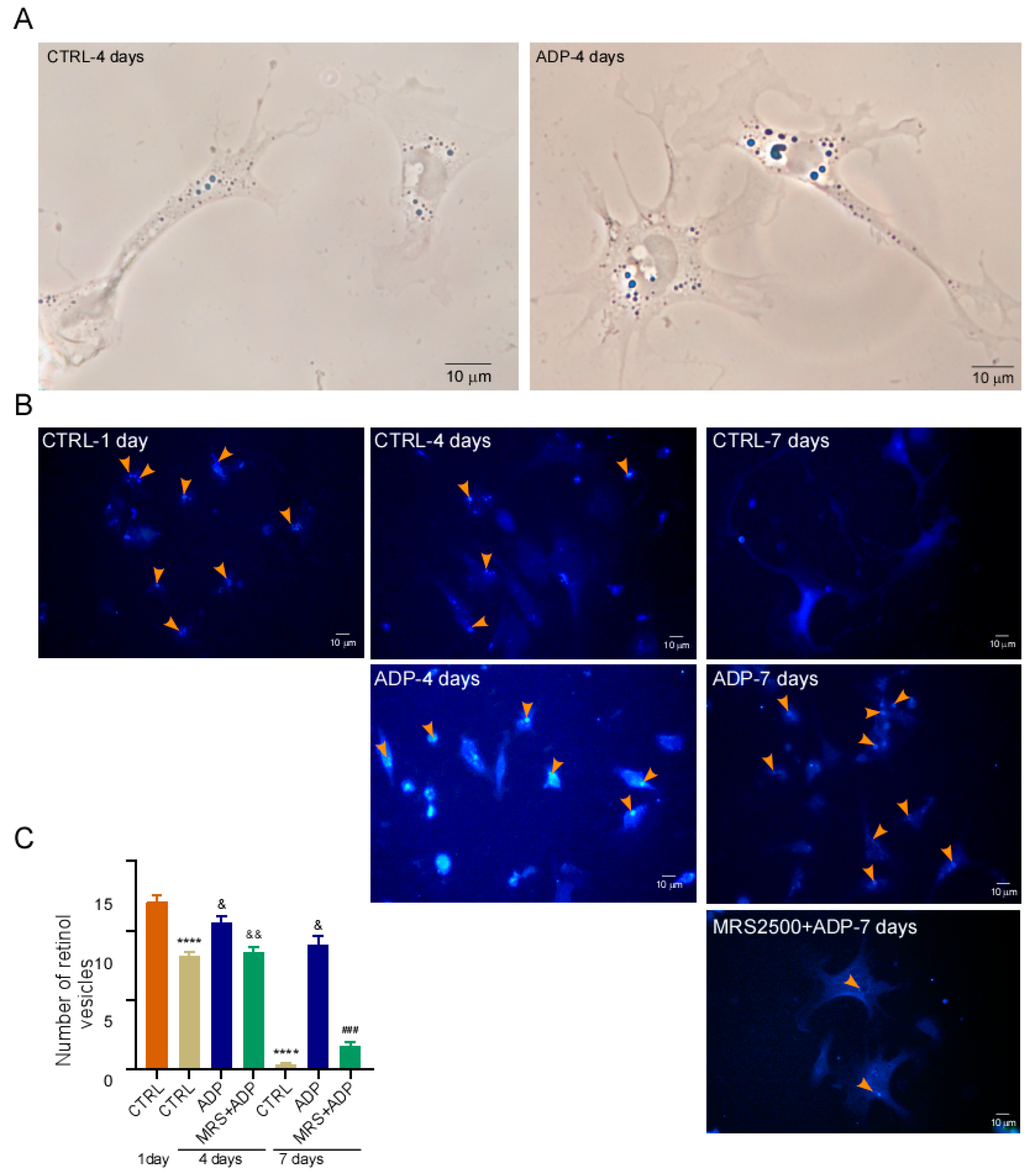
3.1. Primary Cultured qHSC Transdifferentiate to MFB
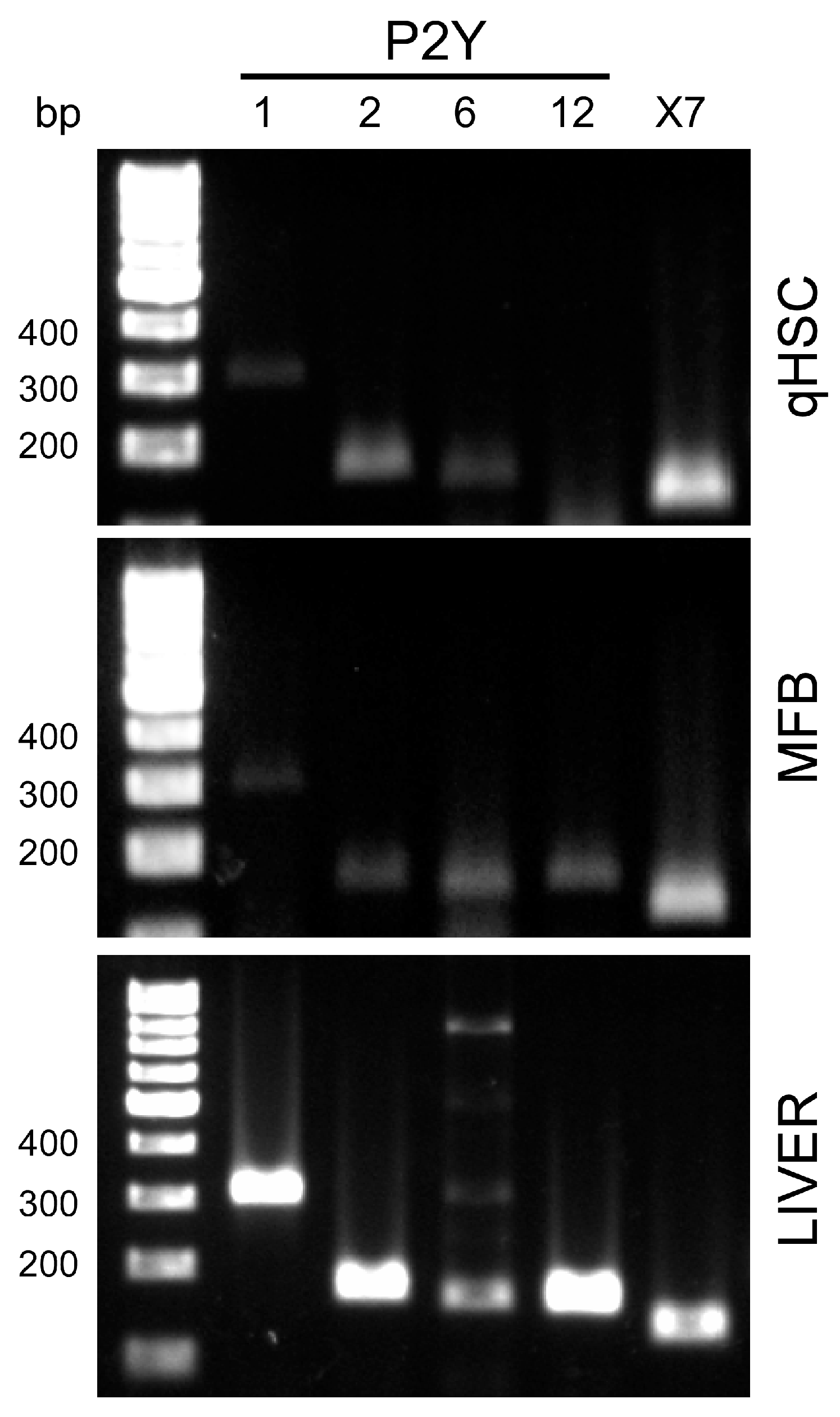
3.2. MFB and qHSC Express P2Y Receptors
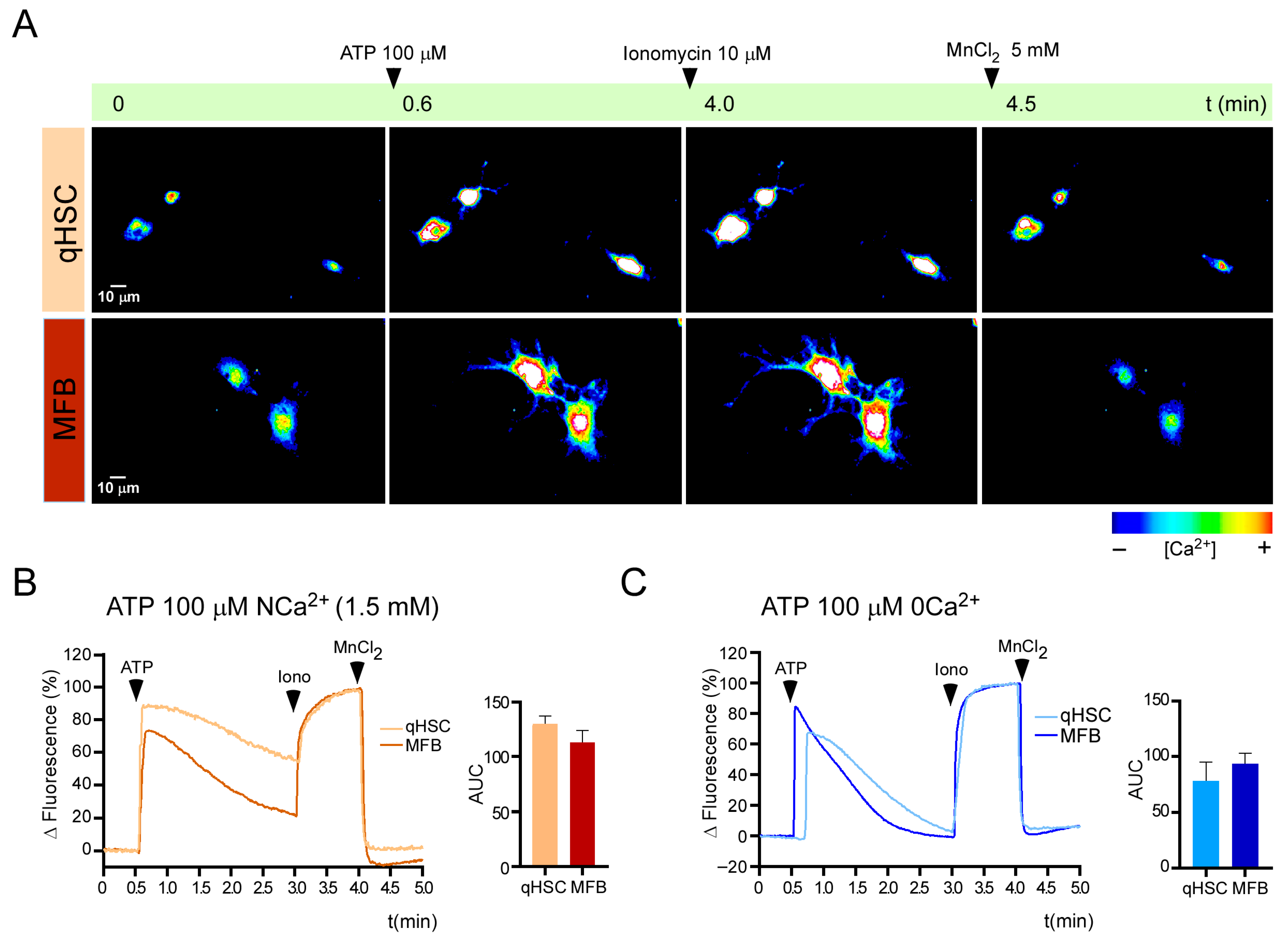
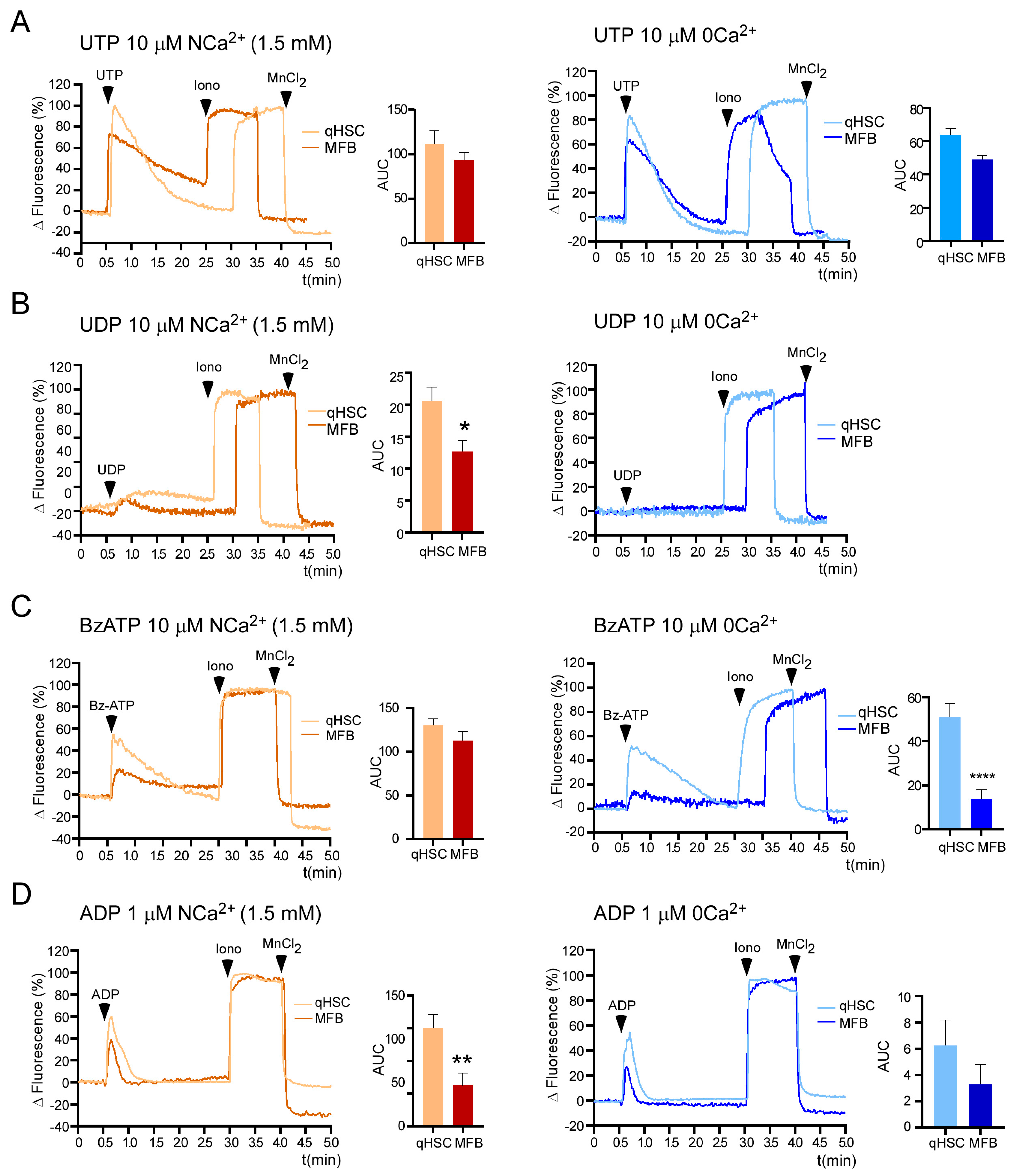
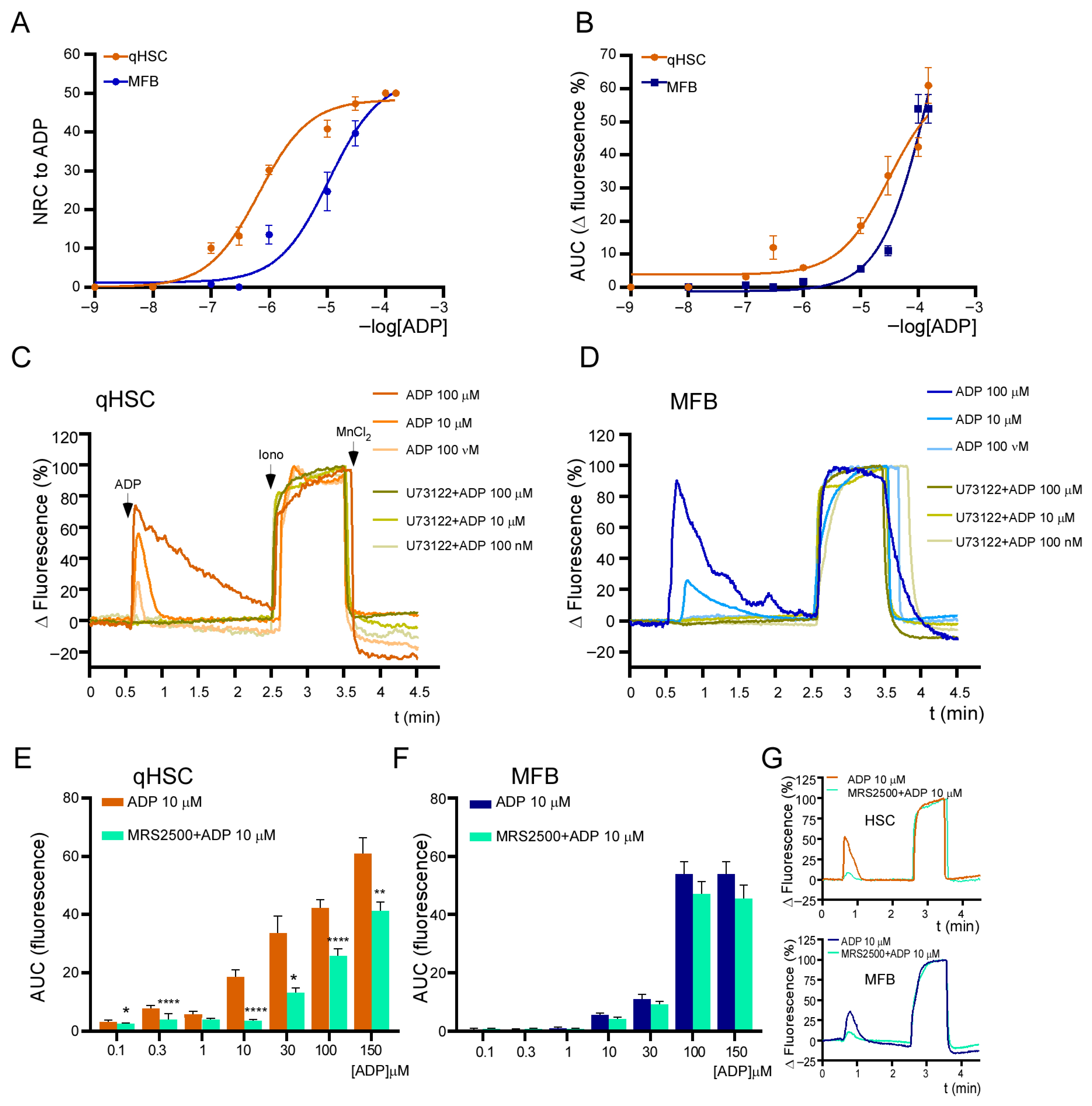
3.3. Calcium Responses Elicited by Purinergic Agonists in qHSC and MFB
3.4. ADP-Induced Responses Are Mediated by the P2Y1 Receptor and Depend Exclusively on Release from Intracellular Ca2+ Stores
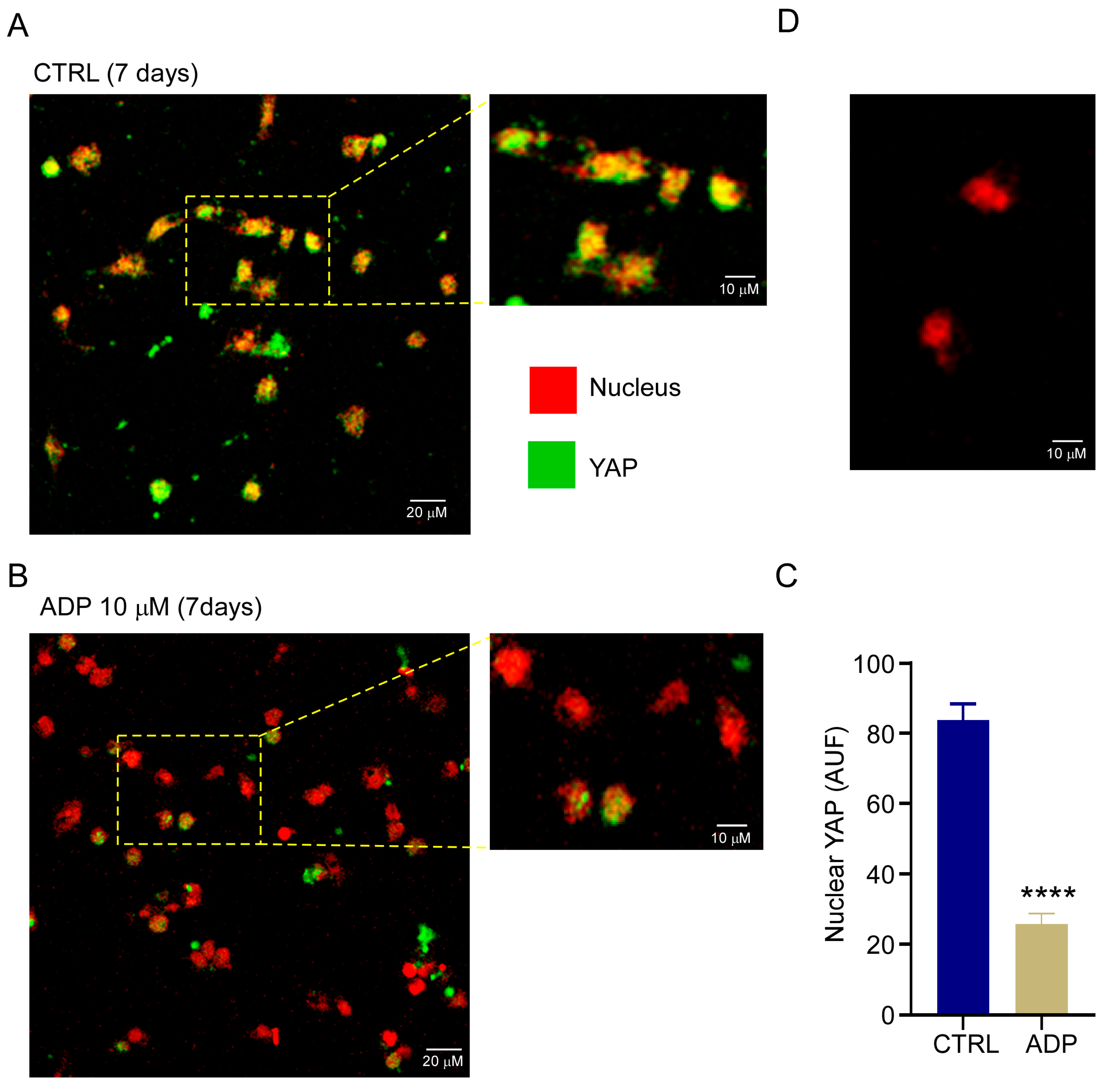
3.5. P2Y1 Receptor Agonism Delays qHSC Activation
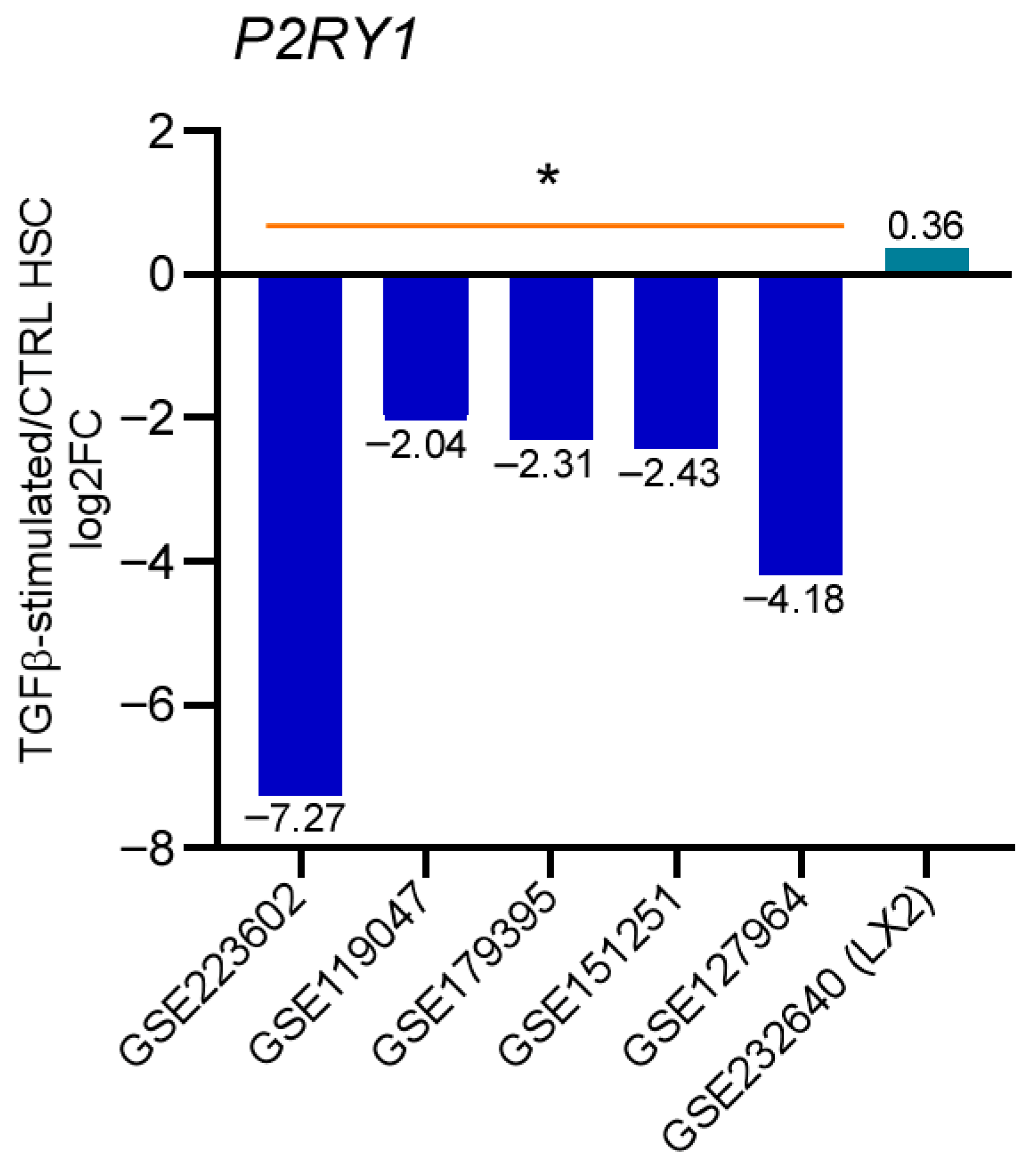
3.6. P2Y1 Receptor Expression Is Downregulated During the Activation of TGF-Stimulated Human qHSC
4. Discussion
4.1. In Vitro qHSC Transdifferentiation
4.2. Purinergic Signaling in HSCs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamm, D.R.; McCommis, K.S. Hepatic stellate cells in physiology and pathology. J. Physiol. 2022, 600, 1825–1837. [Google Scholar] [CrossRef]
- Giampieri, M.P.; Jezequel, A.M.; Orlandi, F. The lipocytes in normal human liver. A quantitative study. Digestion 1981, 22, 165–169. [Google Scholar] [CrossRef]
- Wang, S.-S.; Tang, X.T.; Lin, M.; Yuan, J.; Peng, Y.J.; Yin, X.; Shang, G.; Ge, G.; Ren, Z.; Zhou, B.O. Perivenous Stellate Cells Are the Main Source of Myofibroblasts and Cancer-Associated Fibroblasts Formed After Chronic Liver Injuries. Hepatology 2021, 74, 1578–1594. [Google Scholar] [CrossRef]
- Yang, W.; He, H.; Wang, T.; Su, N.; Zhang, F.; Jiang, K.; Zhu, J.; Zhang, C.; Niu, K.; Wang, L.; et al. Single-Cell Transcriptomic Analysis Reveals a Hepatic Stellate Cell-Activation Roadmap and Myofibroblast Origin During Liver Fibrosis in Mice. Hepatology 2021, 74, 2774–2790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yuan, W.; Dong, T.; Qi, W.; Feng, Z.; Li, C.; Sun, Y. Increased matrix stiffness promotes fibrogenesis of hepatic stellate cells through AP-1-induced chromatin priming. Commun. Biol. 2025, 8, 920. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Cong, M.; Paik, Y.; Scholten, D.; Jiang, C.; Benner, C.; Iwaisako, K.; Moore-Morris, T.; Scott, B.; Tsukamoto, H.; et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 9448–9453. [Google Scholar] [CrossRef] [PubMed]
- Takemura, S.; Kawada, N.; Hirohashi, K.; Kinoshita, H.; Inoue, M. Nucleotide receptors in hepatic stellate cells of the rat. FEBS Lett. 1994, 354, 53–56. [Google Scholar] [CrossRef]
- Dranoff, J.A.; Ogawa, M.; Kruglov, E.A.; Gaça, M.D.A.; Sévigny, J.; Robson, S.C.; Wells, R.G. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G417–G424. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Wang, S.; Xu, R.; Lv, X. Purinergic P2X7 receptor mediates acetaldehyde-induced hepatic stellate cells activation via PKC-dependent GSK3β pathway. Int. Immunopharmacol. 2017, 43, 164–171. [Google Scholar] [CrossRef]
- Li, Z.X.; Sheng, X.D.; Wang, Y.L.; Lv, X.W. Blocking P2X4 purinergic receptor attenuates alcohol-related liver fibrosis by inhibiting hepatic stellate cell activation through PI3K/AKT signaling pathway. Int. Immunopharmacol. 2022, 113, 109326. [Google Scholar] [CrossRef]
- Mederacke, I.; Filliol, A.; Affo, S.; Nair, A.; Hernandez, C.; Sun, Q.; Hamberger, F.; Brundu, F.; Chen, Y.; Ravichandra, A.; et al. The purinergic P2Y14 receptor links hepatocyte death to hepatic stellate cell activation and fibrogenesis in the liver. Sci. Transl. Med. 2022, 14, eabe5795. [Google Scholar] [CrossRef]
- NORMA Oficial Mexicana NOM-062-ZOO-1999; Especificaciones técnicas para la producción, Cuidado y uso de los animales de laboratorio. Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación de México. Diario Oficial de la Federación de México: Mexico City, Mexico, 2001.
- Mederacke, I.; Dapito, D.H.; Affò, S.; Uchinami, H.; Schwabe, R.F. High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nat. Protoc. 2015, 10, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.L.; Bloomer, S.A.; Chan, E.P.; Gaça, M.D.A.; Georges, P.C.; Sackey, B.; Uemura, M.; Janmey, P.A.; Wells, R.G. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G110–G118. [Google Scholar] [CrossRef] [PubMed]
- Mata-Martínez, E.; González-Gallardo, A.; Díaz-Muñoz, M.; Vázquez-Cuevas, F.G. Purinergic Activation of Store-Operated Calcium Entry (SOCE) Regulates Cell Migration in Metastatic Ovarian Cancer Cells. Pharmaceuticals 2023, 16, 944. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Cuevas, F.G.; Cruz-Rico, A.; Garay, E.; García-Carrancá, A.; Pérez-Montiel, D.; Juárez, B.; Arellano, R.O. Differential expression of the P2X7 receptor in ovarian surface epithelium during the oestrous cycle in the mouse. Reprod. Fertil. Dev. 2013, 25, 971–984. [Google Scholar] [CrossRef]
- Clough, E.; Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; et al. NCBI GEO: Archive for gene expression and epigenomics data sets: 23-year update. Nucleic Acids Res. 2024, 52, D138–D144. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets--update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Bai, B.; Shaha, A.; He, X.; He, Y.; Ye, Z.; Shah, V.H.; Kang, N. Targeting Src SH3 domain-mediated glycolysis of HSC suppresses transcriptome, myofibroblastic activation, and colorectal liver metastasis. Hepatology 2024, 80, 578–594. [Google Scholar] [CrossRef]
- Fabre, T.; Molina, M.F.; Soucy, G.; Goulet, J.-P.; Willems, B.; Villeneuve, J.-P.; Bilodeau, M.; Shoukry, N.H. Type 3 cytokines IL-17A and IL-22 drive TGF-β-dependent liver fibrosis. Sci. Immunol. 2018, 3, eaar7754. [Google Scholar] [CrossRef]
- Gart, E.; van Duyvenvoorde, W.; Toet, K.; Caspers, M.P.M.; Verschuren, L.; Nielsen, M.J.; Leeming, D.J.; Souto Lima, E.; Menke, A.; Hanemaaijer, R.; et al. Butyrate Protects against Diet-Induced NASH and Liver Fibrosis and Suppresses Specific Non-Canonical TGF-β Signaling Pathways in Human Hepatic Stellate Cells. Biomedicines 2021, 9, 1954. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, K.; Liu, D.; Guo, L.; Chen, Y.; Li, Q.; Maiers, J.L.; Liu, Z.; Shah, V.H.; Dou, C.; et al. p300 Acetyltransferase Is a Cytoplasm-to-Nucleus Shuttle for SMAD2/3 and TAZ Nuclear Transport in Transforming Growth Factor β-Stimulated Hepatic Stellate Cells. Hepatology 2019, 70, 1409–1423. [Google Scholar] [CrossRef]
- Martin-Mateos, R.; De Assuncao, T.M.; Arab, J.P.; Jalan-Sakrikar, N.; Yaqoob, U.; Greuter, T.; Verma, V.K.; Mathison, A.J.; Cao, S.; Lomberk, G.; et al. Enhancer of Zeste Homologue 2 Inhibition Attenuates TGF-β Dependent Hepatic Stellate Cell Activation and Liver Fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 197–209. [Google Scholar] [CrossRef]
- Arumugam, S.; Li, B.; Boodapati, S.L.T.; Nathanson, M.H.; Sun, B.; Ouyang, X.; Mehal, W.Z. Mitochondrial DNA and the STING pathway are required for hepatic stellate cell activation. Hepatology 2023, 78, 1448–1461. [Google Scholar] [CrossRef]
- Kennedy, C. P2Y (11) Receptors: Properties, Distribution and Functions. Adv. Exp. Med. Biol. 2017, 1051, 107–122. [Google Scholar] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Donnelly-Roberts, D.L.; Namovic, M.T.; Han, P.; Jarvis, M.F. Mammalian P2X7 receptor pharmacology: Comparison of recombinant mouse, rat and human P2X7 receptors. Br. J. Pharmacol. 2009, 157, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.; Lecchi, A.; Ohno, M.; Joshi, B.V.; Besada, P.; Tchilibon, S.; Lombardi, R.; Bischofberger, N.; Harden, T.K.; Jacobson, K.A. Antiaggregatory activity in human platelets of potent antagonists of the P2Y 1 receptor. Biochem. Pharmacol. 2004, 68, 1995–2002. [Google Scholar] [CrossRef]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Mannaerts, I.; Leite, S.B.; Verhulst, S.; Claerhout, S.; Eysackers, N.; Thoen, L.F.; Hoorens, A.; Reynaert, H.; Halder, G.; van Grunsven, L.A. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J. Hepatol. 2015, 63, 679–688. [Google Scholar] [CrossRef]
- Li, D.; Friedman, S.L. Liver fibrogenesis and the role of hepatic stellate cells: New insights and prospects for therapy. J. Gastroenterol. Hepatol. 1999, 14, 618–633. [Google Scholar] [CrossRef]
- Foglia, B.; Cannito, S.; Bocca, C.; Parola, M.; Novo, E. ERK Pathway in Activated, Myofibroblast-Like, Hepatic Stellate Cells: A Critical Signaling Crossroad Sustaining Liver Fibrosis. Int. J. Mol. Sci. 2019, 20, 2700. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Bae, M.; Lee, Y.; Park, Y.-K.; Shin, D.-G.; Joshi, P.; Hong, S.-H.; Alder, N.; Koo, S.I.; Lee, J.-Y. Astaxanthin attenuates the increase in mitochondrial respiration during the activation of hepatic stellate cells. J. Nutr. Biochem. 2019, 71, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Z.; Tang, F.; Zhao, Y.; Feng, D.; Li, Y.; Hu, Y.; Wang, C.; Zhou, J.; Tian, X.; et al. Carnosol-mediated Sirtuin 1 activation inhibits Enhancer of Zeste Homolog 2 to attenuate liver fibrosis. Pharmacol. Res. 2018, 128, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.W.; Yuan, Y.P.; Sun, Q.S.; Zhan, X.D.; Jiang, Y.X.; Tang, X.N. Effects of Bulleyaconitine A on Extracellular Matrix Secretion and Expression of Related Proteins in Acetaldehyde-Activated Hepatic Stellate Cells. Bull. Exp. Biol. Med. 2024, 177, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Pelegrin, P. P2X7 receptor and the NLRP3 inflammasome: Partners in crime. Biochem. Pharmacol. 2021, 187, 114385. [Google Scholar] [CrossRef]
- Nomura, S.; Wang, L.; Hasuzawa, N.; Nagayama, A.; Moriyama, S.; Ashida, K.; Moriyama, Y.; Nomura, M.; Yamamoto, K. Imeglimin attenuates liver fibrosis by inhibiting vesicular ATP release from hepatic stellate cells. FEBS Lett. 2025. advance online publication. [Google Scholar] [CrossRef]
- Ulrich, H.; Glaser, T.; Thomas, A.P. Purinergic signaling in liver disease: Calcium signaling and induction of inflammation. Purinergic Signal. 2025, 21, 69–81. [Google Scholar] [CrossRef]
- Emmett, D.S.; Feranchak, A.; Kilic, G.; Puljak, L.; Miller, B.; Dolovcak, S.; McWilliams, R.; Doctor, R.B.; Fitz, J.G. Characterization of ionotrophic purinergic receptors in hepatocytes. Hepatology 2008, 47, 698–705. [Google Scholar] [CrossRef]
- Vincenzi, F.; Pasquini, S.; Contri, C.; Cappello, M.; Nigro, M.; Travagli, A.; Merighi, S.; Gessi, S.; Borea, P.A.; Varani, K. Pharmacology of Adenosine Receptors: Recent Advancements. Biomolecules 2023, 13, 1387. [Google Scholar] [CrossRef]
- Dixon, C.J.; Cobbold, P.H.; Green, A.K. Actions of ADP, but not ATP, on cytosolic free Ca2+ in single rat hepatocytes mimicked by 2-methylthioATP. Br. J. Pharmacol. 1995, 116, 1979–1984. [Google Scholar] [CrossRef]
- Fausther, M.; Sheung, N.; Saiman, Y.; Bansal, M.B.; Dranoff, J.A. Activated hepatic stellate cells upregulate transcription of ecto-5′-nucleotidase/CD73 via specific SP1 and SMAD promoter elements. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G904–G914. [Google Scholar] [CrossRef]
- Andrade, C.M.; Wink, M.R.; Margis, R.; Borojevic, R.; Battastini, A.M.; Guma, F.C. Activity and expression of ecto-nucleotide pyrophosphate/phosphodiesterases in a hepatic stellate cell line. Mol. Cell Biochem. 2009, 325, 179–185. [Google Scholar] [CrossRef]
| Target Transcript | Forward | Reverse |
|---|---|---|
| Acta2 | CTGAGCGTGGCTATTCCTTC | CTTCTGCATCCTGTCAGCAA |
| Col1A1 | GAGCGGAGAGTACTGGATCG | CCTTCTTGAGGTTGCCAGTC |
| P2ry1 | TACCAGCCCTCATCTTCTAC | CATTGGACGTGGTGTCATAG |
| P2ry2 | ACCTGGAACCCTGGAATAG | AGGCGGCATAGGAAGATATAG |
| P2ry4 | CCTGGACTGGACTAAGGAA | TCAGAGGCAACAGGATGA |
| P2ry6 | TCTGGCACTTCCTCCTAAA | CTTGAAATCCTCACGGTAGAC |
| P2ry12 | CAGTCTGCAAGTTCCACTAAC | TGGGTGATCTTGTAGTCTCTG |
| P2rx7 | TGACGAAGTTAGGACACAGC | GGATACTCAGGACACAGCG |
| Sod2 | TGGACAAACCTGAGCCCTAA | GACCCAAAGTCACGCTTGATA |
| Accession Number | Experimental Conditions | Control | Reference |
|---|---|---|---|
| GSE223602 | 3× healthy derived donor TGFB (5 ng/mL) for 24 h | 3× healthy derived donor ctrl | [19] |
| GSE119047 | 3× healthy derived donor TGFB (2.5 ng/mL) for 24 h | 3× healthy derived donor ctrl | [20] |
| GSE179395 | 8× healthy derived donor TGFB (2 ng/mL) for 96 h | 6× healthy derived donor ctrl | [21] |
| GSE151251 | 3× healthy derived donor TGFB | 3× healthy derived donor ctrl | |
| GSE127964 | 3× healthy derived donor TGFB (5 ng/mL) for 24 h | 3× healthy derived donor ctrl | [22] |
| GSE119606 | 3× healthy derived donor TGFB (10 ng/mL) for 48 h | 3× healthy derived donor ctrl | [23] |
| GSE232640 | 4× LX2 cell line TGFB (5 ng/mL) for 16 h | 4× LX2 cell line ctrl | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mata-Martínez, E.; Juárez-Mercado, A.P.; González-Gallardo, A.; Núñez-Ríos, J.D.; Díaz-Muñoz, M.; Hernández-Muñoz, R.; Vázquez-Cuevas, F.G. Purinergic-Mediated Calcium Signaling in Quiescent and Activated Hepatic Stellate Cells: Evidence That P2Y1 Receptor Delays Activation. Cells 2025, 14, 1845. https://doi.org/10.3390/cells14231845
Mata-Martínez E, Juárez-Mercado AP, González-Gallardo A, Núñez-Ríos JD, Díaz-Muñoz M, Hernández-Muñoz R, Vázquez-Cuevas FG. Purinergic-Mediated Calcium Signaling in Quiescent and Activated Hepatic Stellate Cells: Evidence That P2Y1 Receptor Delays Activation. Cells. 2025; 14(23):1845. https://doi.org/10.3390/cells14231845
Chicago/Turabian StyleMata-Martínez, Esperanza, Ana Patricia Juárez-Mercado, Adriana González-Gallardo, José David Núñez-Ríos, Mauricio Díaz-Muñoz, Rolando Hernández-Muñoz, and Francisco G. Vázquez-Cuevas. 2025. "Purinergic-Mediated Calcium Signaling in Quiescent and Activated Hepatic Stellate Cells: Evidence That P2Y1 Receptor Delays Activation" Cells 14, no. 23: 1845. https://doi.org/10.3390/cells14231845
APA StyleMata-Martínez, E., Juárez-Mercado, A. P., González-Gallardo, A., Núñez-Ríos, J. D., Díaz-Muñoz, M., Hernández-Muñoz, R., & Vázquez-Cuevas, F. G. (2025). Purinergic-Mediated Calcium Signaling in Quiescent and Activated Hepatic Stellate Cells: Evidence That P2Y1 Receptor Delays Activation. Cells, 14(23), 1845. https://doi.org/10.3390/cells14231845







