Fascin Drives Breast Cancer Cell Proliferation Partly by Modulating the Cell Cycle Checkpoint Regulators of the G1-S Phase
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Fascin Induction
2.3. RT-qPCR
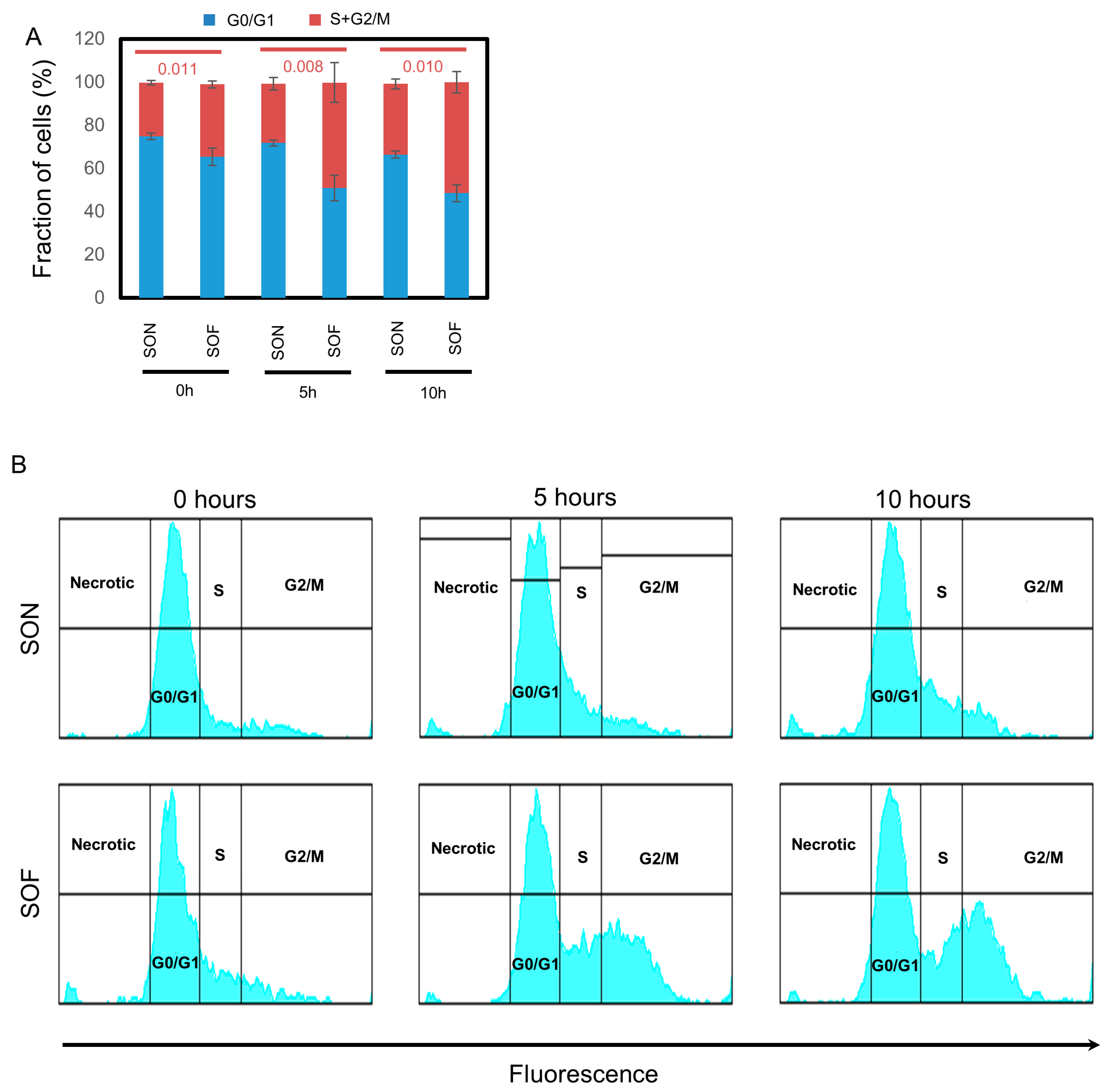
2.4. Flow Cytometry
2.5. RTCA
2.6. Quantitative Immunofluorescence
2.7. Western Blot
2.8. Expression Analysis of Human Breast Tumor Microarray Datasets
2.9. Immunohistochemistry
3. Results
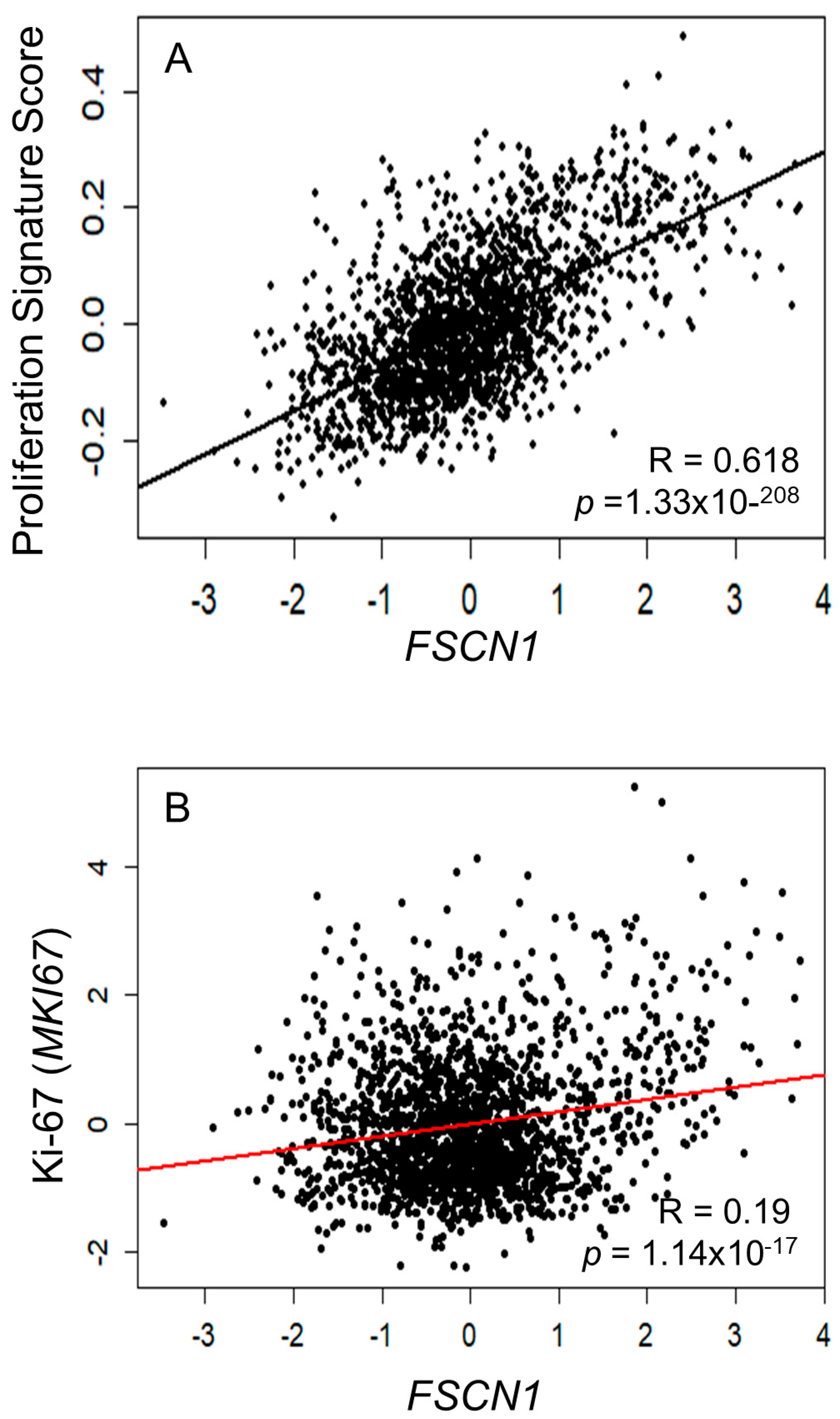
3.1. Bioinformatics Analysis Reveals a Correlation Between FSCN1 Expression and Proliferation Markers in BC
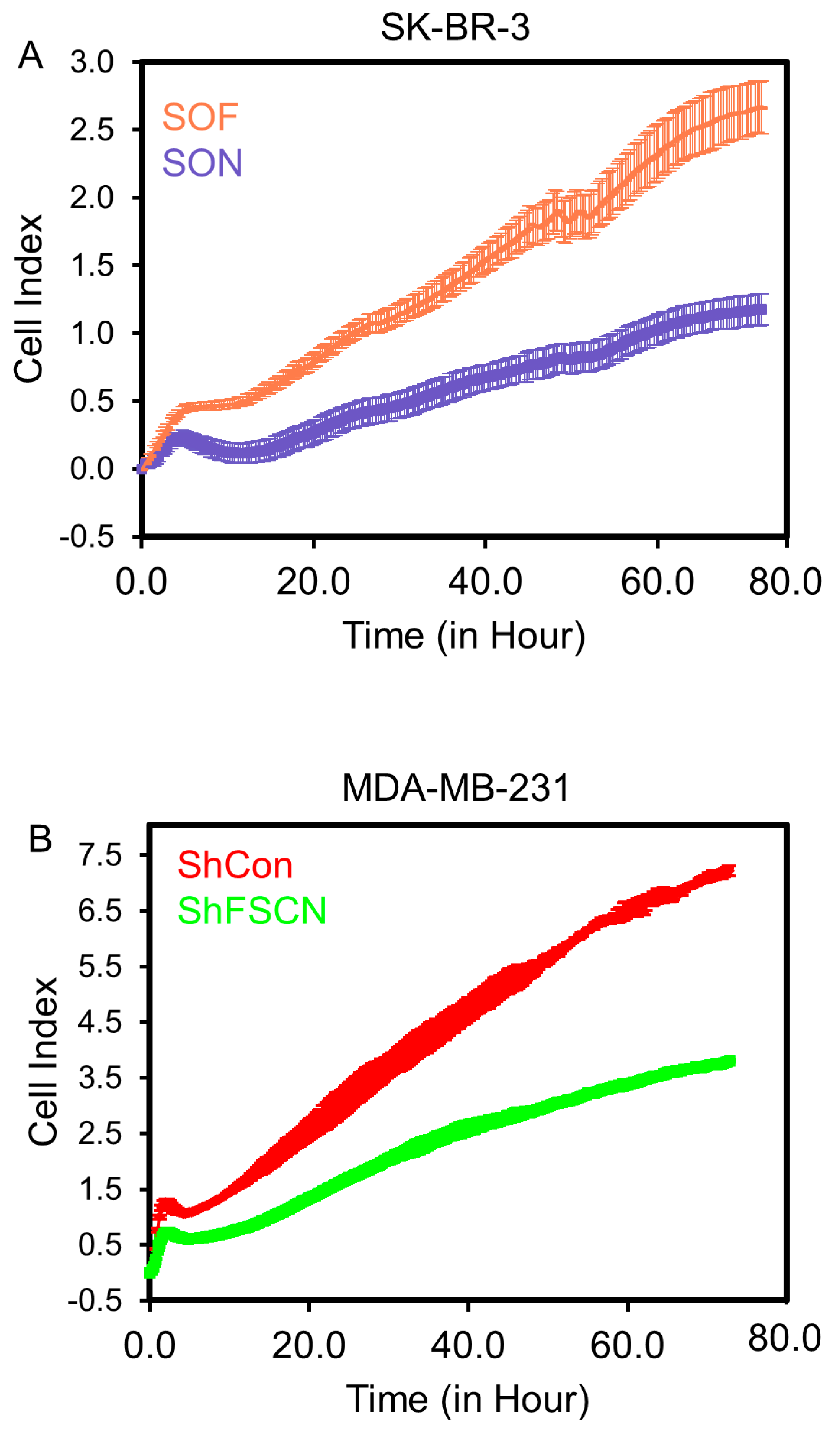
3.2. Fascin Expression Drives the Proliferation of BC Cells In Vitro
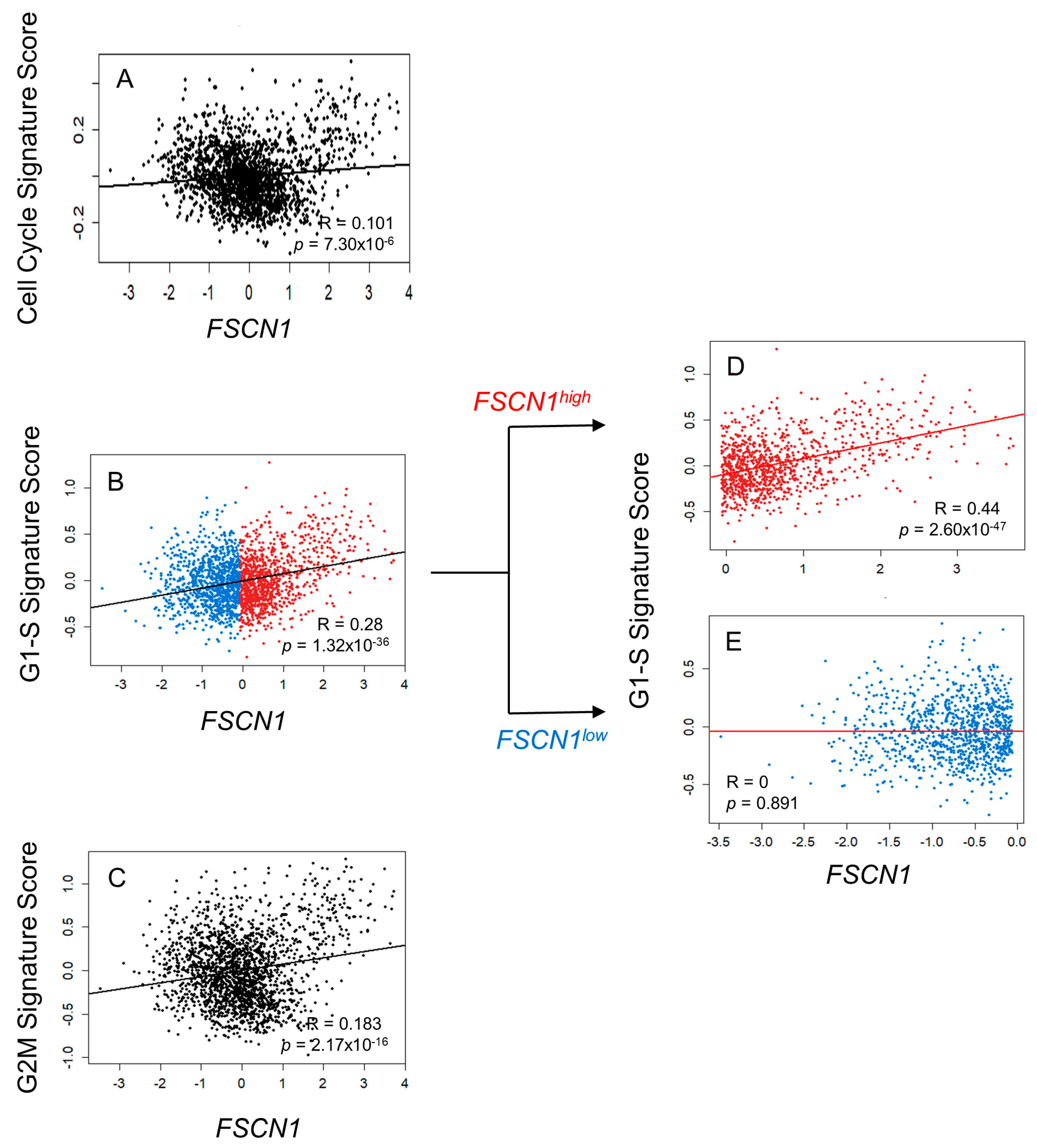
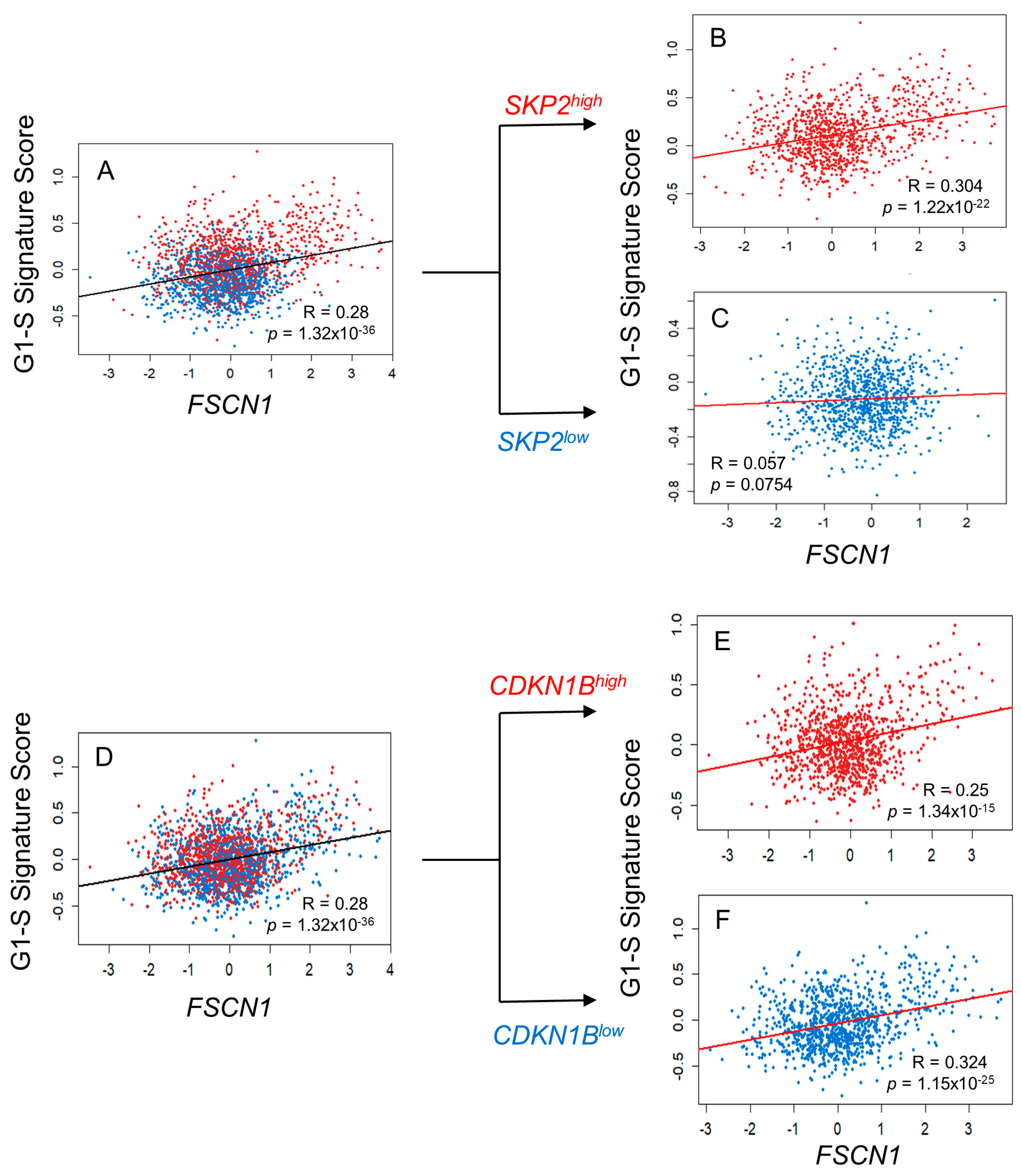
3.3. FSCN1 Expression in BC Patient Samples Correlates with G1-S Signature Score
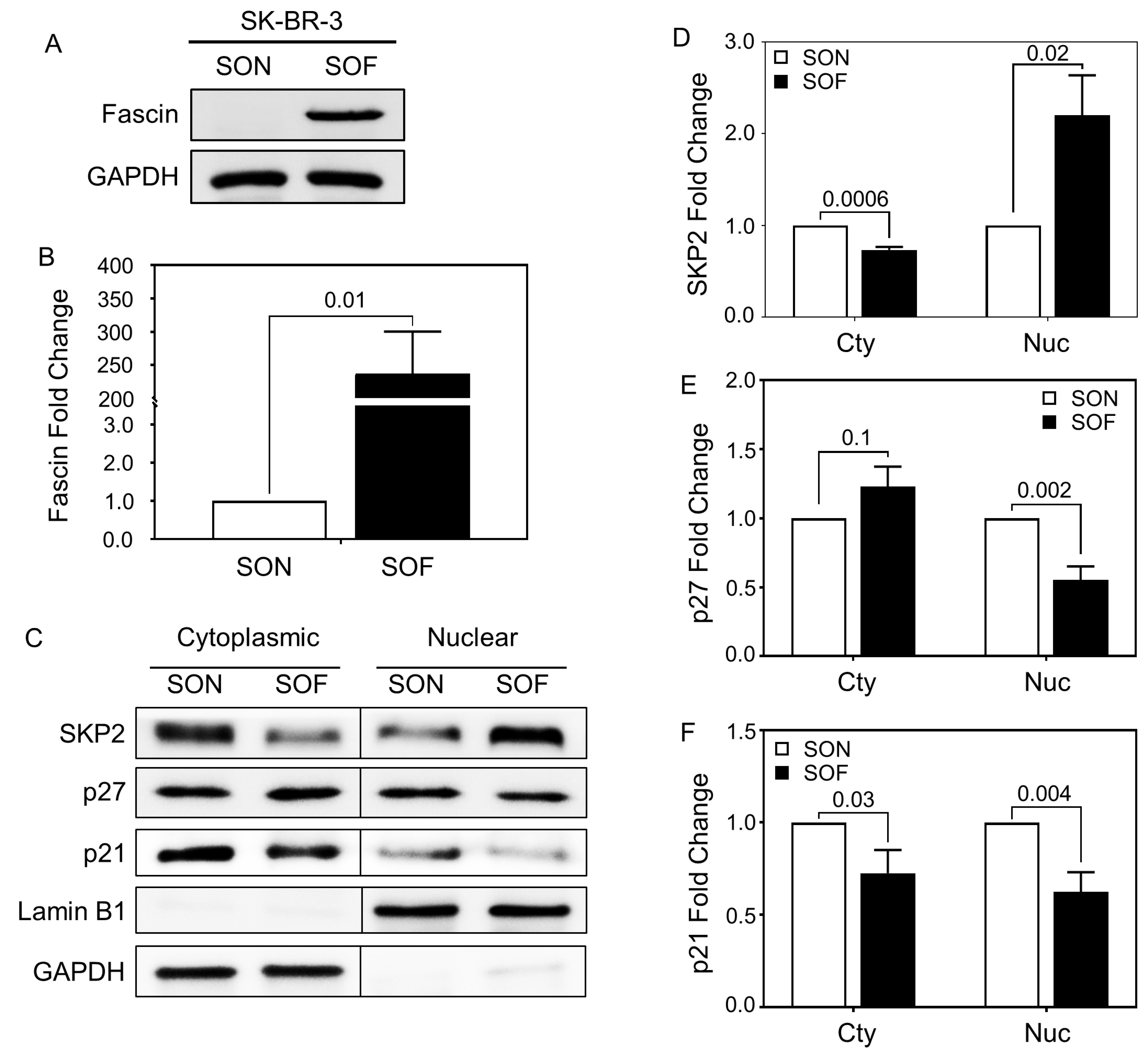
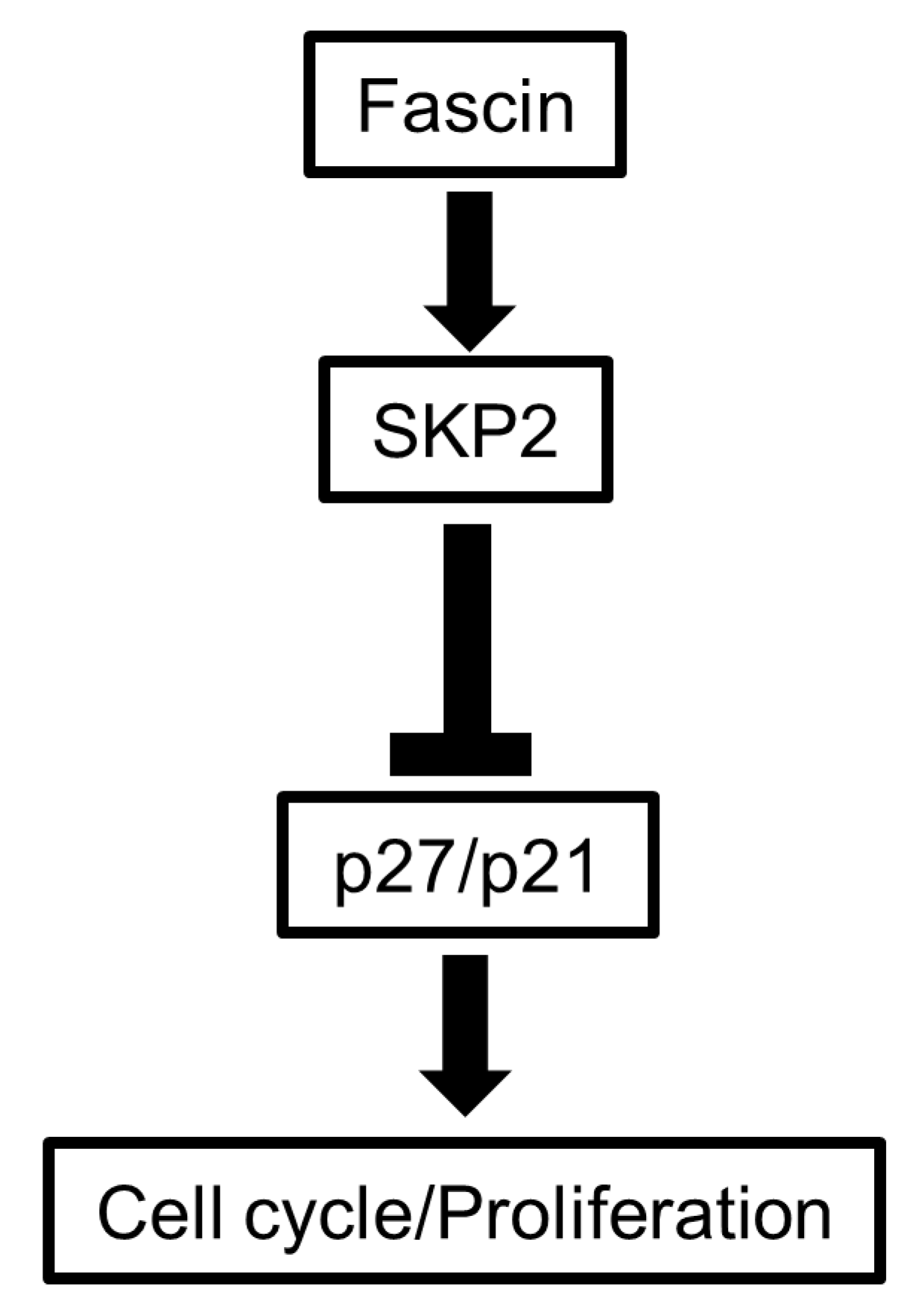
3.4. FSCN1 Modulates the Expression of SKP2-p27 Cell Cycle Checkpoint Regulators
3.5. Fascin Expression in BC Patient Tissues Correlates with Proliferation Markers and Poor Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast Cancer |
| EMT | Epithelial-to-Mesenchymal Transition |
| FAK | Focal Adhesion Kinase |
References
- Hashimoto, Y.; Kim, D.J.; Adams, J.C. The roles of fascins in health and disease. J. Pathol. 2011, 224, 289–300. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Li, L.; Cao, J.; Guo, Y.; Wu, Y.; Gao, W. Fascin actin-bundling protein 1 in human cancer: Promising biomarker or therapeutic target? Mol. Ther. Oncolytics 2021, 20, 240–264. [Google Scholar] [CrossRef]
- Al-Alwan, M.; Olabi, S.; Ghebeh, H.; Barhoush, E.; Tulbah, A.; Al-Tweigeri, T.; Ajarim, D.; Adra, C. Fascin is a key regulator of breast cancer invasion that acts via the modification of metastasis-associated molecules. PLoS ONE 2011, 6, e27339. [Google Scholar] [CrossRef]
- Ghebeh, H.; Al-Khaldi, S.; Olabi, S.; Al-Dhfyan, A.; Al-Mohanna, F.; Barnawi, R.; Tulbah, A.; Al-Tweigeri, T.; Ajarim, D.; Al-Alwan, M. Fascin is involved in the chemotherapeutic resistance of breast cancer cells predominantly via the PI3K/Akt pathway. Br. J. Cancer 2014, 111, 1552–1561. [Google Scholar] [CrossRef]
- Barnawi, R.; Al-Khaldi, S.; Colak, D.; Tulbah, A.; Al-Tweigeri, T.; Fallatah, M.; Monies, D.; Ghebeh, H.; Al-Alwan, M. beta1 Integrin is essential for fascin-mediated breast cancer stem cell function and disease progression. Int. J. Cancer 2019, 145, 830–841. [Google Scholar] [CrossRef]
- Barnawi, R.; Al-Khaldi, S.; Bakheet, T.; Fallatah, M.; Alaiya, A.; Ghebeh, H.; Al-Alwan, M. Fascin Activates beta-Catenin Signaling and Promotes Breast Cancer Stem Cell Function Mainly Through Focal Adhesion Kinase (FAK): Relation With Disease Progression. Front. Oncol. 2020, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.J.; Xu, L.Y.; Wu, J.Y.; Shen, Z.Y.; Zhao, Q.; Du, Z.P.; Lv, Z.; Gu, W.; Pan, F.; Xu, X.E.; et al. Involvement of CYR61 and CTGF in the fascin-mediated proliferation and invasiveness of esophageal squamous cell carcinomas cells. Am. J. Pathol. 2010, 176, 939–951. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, Y.; Shen, Z.; Teng, X.; Li, X.; Li, C.; Wu, W.; Zhou, Z.; Wang, Z. Fascin 1 promoted the growth and migration of non-small cell lung cancer cells by activating YAP/TEAD signaling. Tumour Biol. 2016, 37, 10909–10915. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, T.; Hou, X.M.; Ling, X.L. Knockdown of fascin-1 expression suppresses cell migration and invasion of non-small cell lung cancer by regulating the MAPK pathway. Biochem. Biophys. Res. Commun. 2018, 497, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wang, J.; Yao, Z.; Hu, Y.; Ma, S.; Fan, Q.; Gao, F.; Sun, Y.; Sun, J. Fascin induces melanoma tumorigenesis and stemness through regulating the Hippo pathway. Cell Commun. Signal. 2018, 16, 37. [Google Scholar] [CrossRef]
- Elfoly, M.; Mirza, J.Y.; Alaiya, A.; Al-Hazzani, A.A.; Tulbah, A.; Al-Alwan, M.; Ghebeh, H. PD-L1 intrinsically promotes the proliferation of breast cancer cells through the SKP2-p27/p21 axis. Cancer Cell Int. 2024, 24, 161. [Google Scholar] [CrossRef]
- Ravaioli, A.; Monti, F.; Regan, M.M.; Maffini, F.; Mastropasqua, M.G.; Spataro, V.; Castiglione-Gertsch, M.; Panzini, I.; Gianni, L.; Goldhirsch, A.; et al. p27 and Skp2 immunoreactivity and its clinical significance with endocrine and chemo-endocrine treatments in node-negative early breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol./ESMO 2008, 19, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Bustreo, S.; Osella-Abate, S.; Cassoni, P.; Donadio, M.; Airoldi, M.; Pedani, F.; Papotti, M.; Sapino, A.; Castellano, I. Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: A large case series study with a long-term follow-up. Breast Cancer Res. Treat. 2016, 157, 363–371. [Google Scholar] [CrossRef]
- Schoumacher, M.; El-Marjou, F.; Lae, M.; Kambou, N.; Louvard, D.; Robine, S.; Vignjevic, D.M. Conditional expression of fascin increases tumor progression in a mouse model of intestinal cancer. Eur. J. Cell Biol. 2014, 93, 388–395. [Google Scholar] [CrossRef]
- Ma, Y.; Li, A.; Faller, W.J.; Libertini, S.; Fiorito, F.; Gillespie, D.A.; Sansom, O.J.; Yamashiro, S.; Machesky, L.M. Fascin 1 is transiently expressed in mouse melanoblasts during development and promotes migration and proliferation. Development 2013, 140, 2203–2211. [Google Scholar] [CrossRef]
- Xing, P.; Li, J.G.; Jin, F.; Zhao, T.T.; Liu, Q.; Dong, H.T.; Wei, X.L. Fascin, an actin-bundling protein, promotes breast cancer progression in vitro. Cell Biochem. Funct. 2011, 29, 303–310. [Google Scholar] [CrossRef]
- Heinz, L.S.; Muhs, S.; Schiewek, J.; Grub, S.; Nalaskowski, M.; Lin, Y.N.; Wikman, H.; Oliveira-Ferrer, L.; Lange, T.; Wellbrock, J.; et al. Strong fascin expression promotes metastasis independent of its F-actin bundling activity. Oncotarget 2017, 8, 110077–110091. [Google Scholar] [CrossRef]
- Uxa, S.; Castillo-Binder, P.; Kohler, R.; Stangner, K.; Muller, G.A.; Engeland, K. Ki-67 gene expression. Cell Death Differ. 2021, 28, 3357–3370. [Google Scholar] [CrossRef] [PubMed]
- Volpi, A.; Bacci, F.; Paradiso, A.; Saragoni, L.; Scarpi, E.; Ricci, M.; Aldi, M.; Bianchi, S.; Muretto, P.; Nuzzo, F.; et al. Prognostic relevance of histological grade and its components in node-negative breast cancer patients. Mod. Pathol. 2004, 17, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Carrano, A.C.; Eytan, E.; Hershko, A.; Pagano, M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1999, 1, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Sutterluty, H.; Chatelain, E.; Marti, A.; Wirbelauer, C.; Senften, M.; Muller, U.; Krek, W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell Biol. 1999, 1, 207–214. [Google Scholar] [CrossRef]
- Tsvetkov, L.M.; Yeh, K.H.; Lee, S.J.; Sun, H.; Zhang, H. p27Kip1 ubiquitination and degradation is regulated by the SCFSkp2 complex through phosphorylated Thr187 in p27. Curr. Biol. 1999, 9, 661–664. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, J.; Wu, Q.; Cai, Y.; Zhu, L.; Lu, X.; Chen, S.; Chen, C.; Wang, Z. Acquisition of epithelial-mesenchymal transition is associated with Skp2 expression in paclitaxel-resistant breast cancer cells. Br. J. Cancer 2014, 110, 1958–1967. [Google Scholar] [CrossRef]
- Heldt, F.S.; Barr, A.R.; Cooper, S.; Bakal, C.; Novak, B. A comprehensive model for the proliferation-quiescence decision in response to endogenous DNA damage in human cells. Proc. Natl. Acad. Sci. USA 2018, 115, 2532–2537. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef]
- Belletti, B.; Baldassarre, G. New light on p27kip1 in breast cancer. Cell Cycle 2012, 11, 3701–3702. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Tarpey, P.S.; Davies, H.; Van Loo, P.; Greenman, C.; Wedge, D.C.; Nik-Zainal, S.; Martin, S.; Varela, I.; Bignell, G.R.; et al. The landscape of cancer genes and mutational processes in breast cancer. Nature 2012, 486, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Dehan, E.; Pagano, M. Skp2, the FoxO1 hunter. Cancer Cell 2005, 7, 209–210. [Google Scholar] [CrossRef]
- Rossig, L.; Jadidi, A.S.; Urbich, C.; Badorff, C.; Zeiher, A.M.; Dimmeler, S. Akt-dependent phosphorylation of p21Cip1 regulates PCNA binding and proliferation of endothelial cells. Mol. Cell. Biol. 2001, 21, 5644–5657. [Google Scholar] [CrossRef]
- Zhou, B.P.; Liao, Y.; Xia, W.; Spohn, B.; Lee, M.H.; Hung, M.C. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 2001, 3, 245–252. [Google Scholar] [CrossRef]
- Jung, Y.S.; Qian, Y.; Chen, X. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell. Signal. 2010, 22, 1003–1012. [Google Scholar] [CrossRef]
- Chan, C.H.; Morrow, J.K.; Li, C.F.; Gao, Y.; Jin, G.; Moten, A.; Stagg, L.J.; Ladbury, J.E.; Cai, Z.; Xu, D.; et al. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell 2013, 154, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Uyama, N.; Iimuro, Y.; Kawada, N.; Reynaert, H.; Suzumura, K.; Hirano, T.; Kuroda, N.; Fujimoto, J. Fascin, a novel marker of human hepatic stellate cells, may regulate their proliferation, migration, and collagen gene expression through the FAK-PI3K-Akt pathway. Lab. Investig. 2012, 92, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Reichert, M.; Saur, D.; Hamacher, R.; Schmid, R.M.; Schneider, G. Phosphoinositide-3-kinase signaling controls S-phase kinase-associated protein 2 transcription via E2F1 in pancreatic ductal adenocarcinoma cells. Cancer Res. 2007, 67, 4149–4156. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhao, W.; Yang, D. Stat3 induces oncogenic Skp2 expression in human cervical carcinoma cells. Biochem. Biophys. Res. Commun. 2012, 418, 186–190. [Google Scholar] [CrossRef]
- Snyder, M.; Huang, J.; Huang, X.Y.; Zhang, J.J. A signal transducer and activator of transcription 3.Nuclear Factor kappaB (Stat3.NFkappaB) complex is necessary for the expression of fascin in metastatic breast cancer cells in response to interleukin (IL)-6 and tumor necrosis factor (TNF)-alpha. J. Biol. Chem. 2014, 289, 30082–30089. [Google Scholar] [CrossRef]
| Fascin | ||||
|---|---|---|---|---|
| − | + | * p | ||
| CP | Age | |||
| <40 years | 15 § (71) | 6 (29) | 0.591 | |
| ≥40 years | 30 (64) | 17 (36) | ||
| Histological Grade | ||||
| I&II | 28 (82) | 6 (18) | 0.0096 | |
| III | 17 (50) | 17 (50) | ||
| BC Subtype | ER Status | |||
| Negative | 7 (29) | 17 (71) | <0.0001 | |
| Positive | 38 (86) | 6 (14) | ||
| PR Status | ||||
| Negative | 18 (49) | 19 (51) | 0.0009 | |
| Positive | 27 (87) | 4 (13) | ||
| Her2/neu Status | ||||
| Negative | 31 (70) | 13 (30) | 0.04219 | |
| Positive | 14 (58) | 10 (42) | ||
| IE | PD-L1 | |||
| <5% | 41 (37) | 15 (27) | 0.0157 | |
| ≥5% | 4 (33) | 8 (67) | ||
| Proliferation | Ki-67 | |||
| <20% | 34 (85) | 6 (15) | 0.0002 | |
| ≥20% | 11 (39) | 17 (61) | ||
| SKP2 | ||||
| <10% | 37 (80) | 9 (20) | 0.0007 | |
| ≥10% | 8 (36) | 14 (64) | ||
| Loss of p27 | ||||
| <50% | 29 (58) | 21 (42) | 0.0208 | |
| ≥50% | 16 (89) | 2 (11) | ||
| Loss of p21 | ||||
| <10% | 24 (60) | 16 (40) | 0.2977 | |
| ≥10% | 21 (75) | 7 (25) | ||
| Loss of (p27/p21) | ||||
| No | 15 (52) | 14 (48) | 0.0396 | |
| Yes | 30 (77) | 9 (23) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghebeh, H.; Al-Nasrallah, H.K.; Elfoly, M.; Aldossry, A.; Tulbah, A.; Al-Tweigeri, T.; Al-Alwan, M. Fascin Drives Breast Cancer Cell Proliferation Partly by Modulating the Cell Cycle Checkpoint Regulators of the G1-S Phase. Cells 2025, 14, 1839. https://doi.org/10.3390/cells14231839
Ghebeh H, Al-Nasrallah HK, Elfoly M, Aldossry A, Tulbah A, Al-Tweigeri T, Al-Alwan M. Fascin Drives Breast Cancer Cell Proliferation Partly by Modulating the Cell Cycle Checkpoint Regulators of the G1-S Phase. Cells. 2025; 14(23):1839. https://doi.org/10.3390/cells14231839
Chicago/Turabian StyleGhebeh, Hazem, Huda K. Al-Nasrallah, Marwa Elfoly, Alanoud Aldossry, Asma Tulbah, Taher Al-Tweigeri, and Monther Al-Alwan. 2025. "Fascin Drives Breast Cancer Cell Proliferation Partly by Modulating the Cell Cycle Checkpoint Regulators of the G1-S Phase" Cells 14, no. 23: 1839. https://doi.org/10.3390/cells14231839
APA StyleGhebeh, H., Al-Nasrallah, H. K., Elfoly, M., Aldossry, A., Tulbah, A., Al-Tweigeri, T., & Al-Alwan, M. (2025). Fascin Drives Breast Cancer Cell Proliferation Partly by Modulating the Cell Cycle Checkpoint Regulators of the G1-S Phase. Cells, 14(23), 1839. https://doi.org/10.3390/cells14231839







