Heat Shock Protein Chaperome Is a Multi-Faceted Vector for Tumor Cell Migratory Activity, Invasion, and Metastasis
Abstract
1. Introduction
- The maintenance of proliferation signals: One of the most important features of tumor cells is the ability to continuously proliferate uncontrollably. Cancer cells are capable of amplifying growth factor receptor genes, which allows them to enhance proliferative signals in comparison with normal cells [6,7].
- Reduction in the effect of growth inhibitors: Multiple mutations in tumor suppressor genes (p53 is amongst the most well known) lead to the disruption of their biological functions, which allow tumor cells to avoid the intracellular regulation of cell proliferation [8].
- The ability to avoid programmed cell death: Tumor cells develop various mechanisms to evade both external and internal inducers of apoptosis [9].
- Immortalization of the tumor clone: Acquired telomerase activity allows cancer cells to bypass the natural limitation in the number of cell divisions due to the length of telomeric sections of chromosomes.
- The induction of angiogenesis: Tumor cells are able to synthesize a number of molecular factors that allow the activation of angiogenesis in the tumor. The resulting vascular network has a number of specific characteristics that allow not only an increase in the metabolic supply of the tumor, but also contribute to the extravasation of tumor cells with their further metastasis [10,11].
- Invasion and metastasis: Metastasis is a complex multi-step process that allows tumor cells to spread throughout the body [12]. It depends on changes in the adhesive properties of tumor cells and also requires constant remodeling of the cytoskeleton.
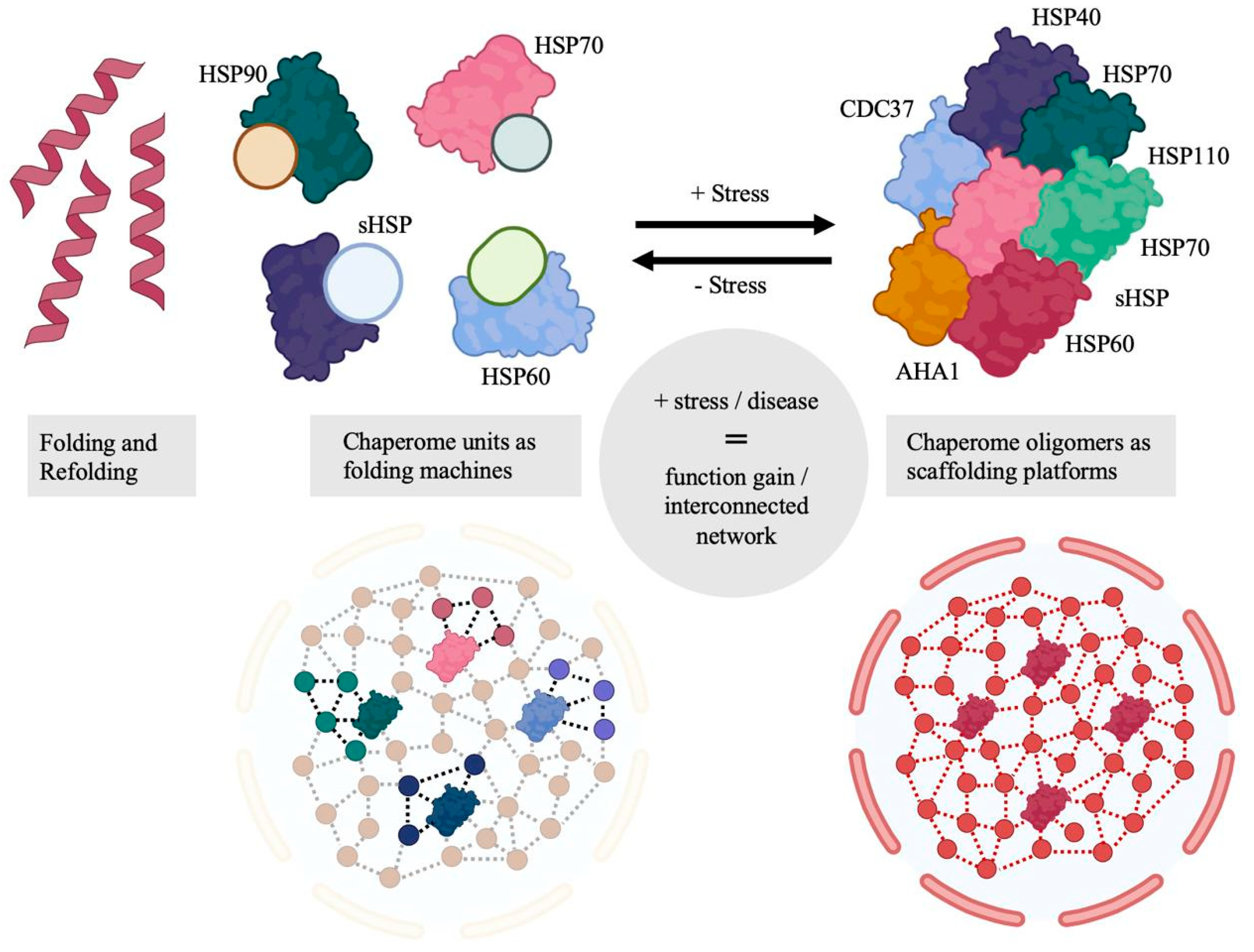
2. Molecular Chaperones
2.1. Chaperone Cycles
2.1.1. HSP70 Regulation
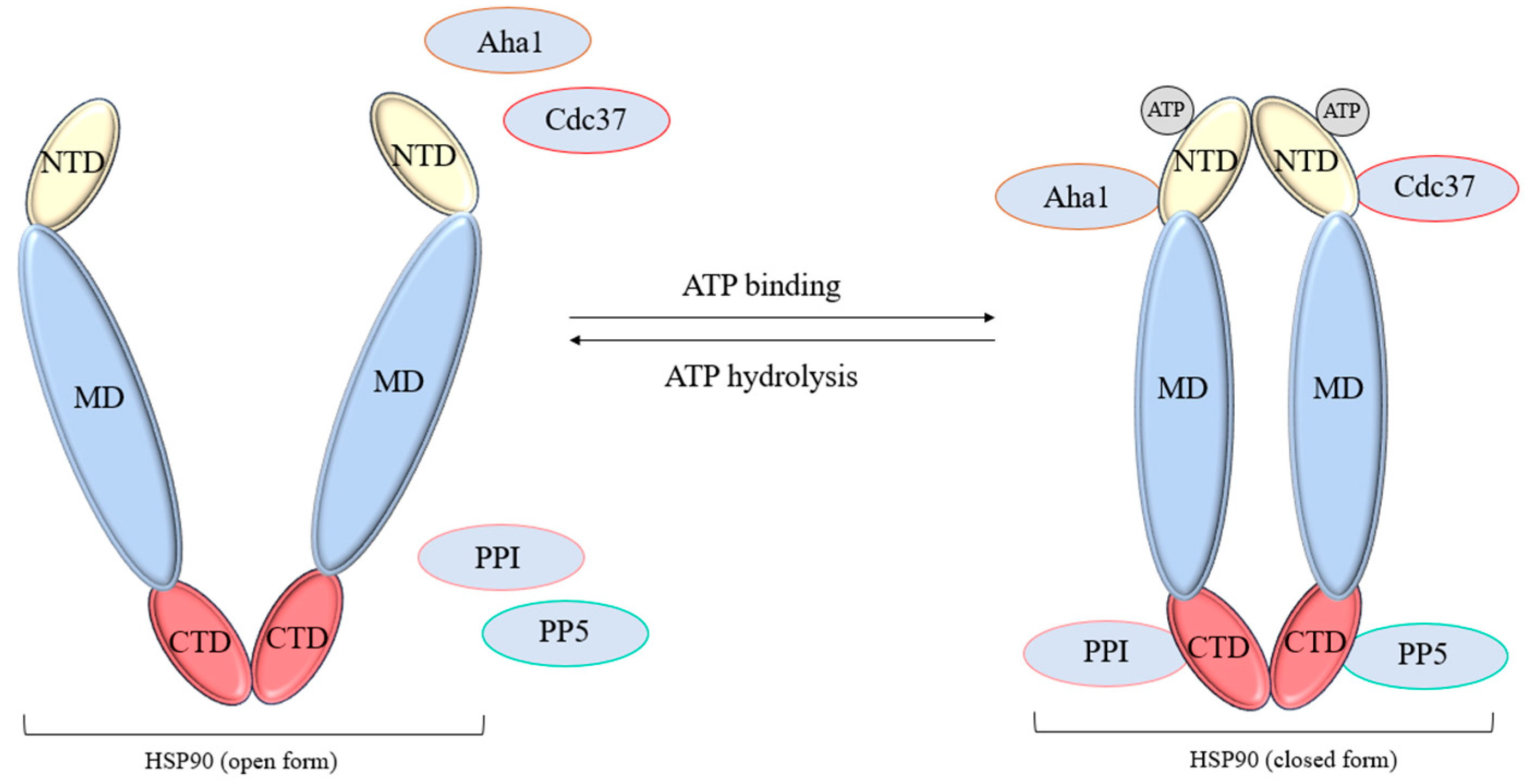
2.1.2. HSP90 Regulation
3. Tumor Cell Motility and Invasion
- Invasion. Cancer cells are capable of performing a pathological epithelial–mesenchymal transition (EMT). This process occurs due to an increase in the level of contractile activity of actomyosin and non-apoptotic blebbing activity, which allows the cell to move in the already-existing pores of collagen networks with minimal adhesion and matrix remodeling [107,108].
- Intravasation. A process in which single tumor cells or groups of them disrupt the integrity of the basement membrane of a vessel, thereby penetrating into its lumen. It is important to note that the vascular network, the development of which was induced by the tumor, has a number of features contributing to the process of intravasation, namely, the presence of accessible points for the penetration of tumor cells into the vascular wall, sufficient vessel lumen for the passage of both individual cells and their groups, and sufficient blood flow velocity, contributing to the removal of intravasated cells [109,110].
4. GTPase and Motility Effectors Within Chaperone Network
5. Chaperome Members in Tumor Vesicle Trafficking
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HSPs | Heat Shock Proteins |
| ECM | Extracellular Matrix |
| HUGO | Human Genome Organization |
| DAMP | Damage-Associated Molecular Pattern |
| NBD | Nucleotide-Binding Domain |
| SBD | Substrate-Binding Domain |
| MD | Middle Domain |
| CTD | C-terminal Domain |
| TPR | Tetratricopeptide Repeat |
| NEFs | Nucleotide Exchange Factors |
| GRP | Glucose-regulated Protein |
| ER | Endoplasmic Reticulum |
| HOP | Hsp-Organizing Protein |
| STIP | Stress-induced Protein |
| JDPs | J-Domain Proteins |
| EMT | Epithelial–mesenchymal transition |
| LET | Low Electric Treatment |
| cSCC | Squamous cell Carcinoma |
| GR | Glucocorticoid Receptor |
| PPI | Peptidyl-prolyl isomerase |
| GEF | Guanine nucleotide Exchange Factor |
| GAP | GTPase-activating Protein |
| FKBP | FK506-binding Protein |
| GC | Gastric Cancer |
| NSCLC | Non-small cell Lung Cancer |
| CRPC | Castration-resistant prostate cancer |
| EGF | Epithelial growth factor |
| HSF | Heat shock factor |
| GDI | Guanine nucleotide Dissociation Inhibitor |
| ESCRT | Endosomal Sorting Complex Required for Transport |
| MMP | Matrix metalloproteinase |
References
- World Health Organization. Cancer over Time. Available online: https://gco.iarc.fr/overtime (accessed on 3 October 2025).
- World Cancer Research Fund. Available online: https://www.wcrf.org/preventing-cancer/cancer-statistics/global-cancer-data-by-country/ (accessed on 3 October 2025).
- Cruz, J.V.; Batista, C.; Afonso, B.D.; Alexandre-Moreira, M.S.; Dubois, L.G.; Pontes, B.; Moura Neto, V.; Mendes, F.D. Obstacles to Glioblastoma Treatment Two Decades after Temozolomide. Cancers 2022, 14, 3203. [Google Scholar] [CrossRef]
- Wu, J.-S.; Jiang, J.; Chen, B.-J.; Wang, K.; Tang, Y.-L.; Liang, X.-H. Plasticity of cancer cell invasion: Patterns and mechanisms. Transl. Oncol. 2021, 14, 100899. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Katoh, M.; Nakagama, H. FGF Receptors: Cancer Biology and Therapeutics. Med. Res. Rev. 2014, 34, 280–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Wang, Y.; Lin, C.; Zhang, D.; Chen, J.; Ouyang, L.; Wu, F.; Zhang, J.; Chen, L. Recent progress on vascular endothelial growth factor receptor inhibitors with dual targeting capabilities for tumor therapy. J. Hematol. Oncol. 2022, 15, 89. [Google Scholar] [CrossRef]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef]
- Abbasi-Malati, Z.; Khanicheragh, P.; Narmi, M.T.; Mardi, N.; Khosrowshahi, N.D.; Hiradfar, A.; Rezabakhsh, A.; Sadeghsoltani, F.; Rashidi, S.; Chegeni, S.A.; et al. Tumoroids, a valid preclinical screening platform for monitoring cancer angiogenesis. Stem Cell Res. Ther. 2024, 15, 267. [Google Scholar] [CrossRef] [PubMed]
- Hoff, P.M.; Machado, K.K. Role of angiogenesis in the pathogenesis of cancer. Cancer Treat. Rev. 2012, 38, 825–833. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Amissah, H.A.; Combs, S.E.; Shevtsov, M. Tumor Dormancy and Reactivation: The Role of Heat Shock Proteins. Cells 2024, 13, 1087. [Google Scholar] [CrossRef]
- De la Fuente, I.M.; López, J.I. Cell Motility and Cancer. Cancers 2020, 12, 2177. [Google Scholar] [CrossRef]
- Stuelten, C.H.; Parent, C.A.; Montell, D.J. Cell motility in cancer invasion and metastasis: Insights from simple model organisms. Nat. Rev. Cancer 2018, 18, 296–312. [Google Scholar] [CrossRef]
- Shevtsov, M.; Yudintceva, N.; Bobkov, D.; Likhomanova, R.; Nechaeva, A.; Lukacheva, A.; Mikhailova, N.; Fedorov, V.; Oganesyan, E.; Rozanov, O.; et al. Abstract 57: Membrane-bound heat shock protein Hsp70 as a new target in oncology: From theory to practice. Cancer Res. 2025, 85, 57. [Google Scholar] [CrossRef]
- Ambrose, A.J.; Chapman, E. Function, Therapeutic Potential, and Inhibition of Hsp70 Chaperones. J. Med. Chem. 2021, 64, 7060–7082. [Google Scholar] [CrossRef]
- Rastogi, S.; Joshi, A.; Sato, N.; Lee, S.; Lee, M.-J.; Trepel, J.B.; Neckers, L. An update on the status of HSP90 inhibitors in cancer clinical trials. Cell Stress Chaperones 2024, 29, 519–539. [Google Scholar] [CrossRef]
- Freilich, R.; Arhar, T.; Abrams, J.L.; Gestwicki, J.E. Protein–Protein Interactions in the Molecular Chaperone Network. Acc. Chem. Res. 2018, 51, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J. The general concept of molecular chaperones. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1993, 339, 257–261. [Google Scholar] [CrossRef]
- Hartl, F.U. Molecular chaperones in cellular protein folding. Nature 1996, 381, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, J.; Ellis, R.J. The Rubisco large subunit binding protein. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1986, 313, 419–428. [Google Scholar]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Broadley, S.A.; Hartl, F.U. The role of molecular chaperones in human misfolding diseases. FEBS Lett. 2009, 583, 2647–2653. [Google Scholar] [CrossRef]
- Regev-Rudzki, N.; Gabriel, K.; Bursać, D. The Evolution and Function of Co-Chaperones in Mitochondria. In The Networking of Chaperones by Co-Chaperones: Control of Cellular Protein Homeostasis; Blatch, G.L., Edkins, A.L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 201–217. [Google Scholar]
- Young, J.C.; Barral, J.M.; Ulrich Hartl, F. More than folding: Localized functions of cytosolic chaperones. Trends Biochem. Sci. 2003, 28, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Agarwal, A.; Uplap, S. HSP: Evolved and conserved proteins, structure and sequence studies. Int. J. Bioinform. Res. 2010, 2, 67–87. [Google Scholar] [CrossRef]
- Vabulas, R.M.; Raychaudhuri, S.; Hayer-Hartl, M.; Hartl, F.U. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb. Perspect. Biol. 2010, 2, a004390. [Google Scholar] [CrossRef]
- Hartl, F.U.; Hayer-Hartl, M. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 2009, 16, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Balchin, D.; Hayer-Hartl, M.; Hartl, F.U. Recent advances in understanding catalysis of protein folding by molecular chaperones. FEBS Lett. 2020, 594, 2770–2781. [Google Scholar] [CrossRef]
- Arispe, N.; Doh, M.; De Maio, A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones 2002, 7, 330. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef]
- Modrzejewska, M.; Zdanowska, O. The Role of Heat Shock Protein 70 (HSP70) in the Pathogenesis of Ocular Diseases—Current Literature Review. J. Clin. Med. 2024, 13, 3851. [Google Scholar] [CrossRef]
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. HSP70 Multi-Functionality in Cancer. Cells 2020, 9, 587. [Google Scholar] [CrossRef]
- Shevtsov, M.; Balogi, Z.; Khachatryan, W.; Gao, H.; Vígh, L.; Multhoff, G. Membrane-Associated Heat Shock Proteins in Oncology: From Basic Research to New Theranostic Targets. Cells 2020, 9, 1263. [Google Scholar] [CrossRef]
- Rérole, A.-L.; Gobbo, J.; De Thonel, A.; Schmitt, E.; Pais de Barros, J.P.; Hammann, A.; Lanneau, D.; Fourmaux, E.; Deminov, O.; Micheau, O.; et al. Peptides and Aptamers Targeting HSP70: A Novel Approach for Anticancer Chemotherapy. Cancer Res. 2011, 71, 484–495. [Google Scholar] [CrossRef]
- Nylandsted, J.; Rohde, M.; Brand, K.; Bastholm, L.; Elling, F.; Jäättelä, M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc. Natl. Acad. Sci. USA 2000, 97, 7871–7876. [Google Scholar] [CrossRef]
- Elmallah, M.I.Y.; Cordonnier, M.; Vautrot, V.; Chanteloup, G.; Garrido, C.; Gobbo, J. Membrane-anchored heat-shock protein 70 (Hsp70) in cancer. Cancer Lett. 2020, 469, 134–141. [Google Scholar] [CrossRef]
- Chalmin, F.; Ladoire, S.; Mignot, G.; Vincent, J.; Bruchard, M.; Remy-Martin, J.-P.; Boireau, W.; Rouleau, A.; Simon, B.; Lanneau, D.; et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Investig. 2010, 120, 457–471. [Google Scholar] [CrossRef] [PubMed]
- PD Hatfield, M.; Lovas, S. Role of Hsp70 in cancer growth and survival. Protein Pept. Lett. 2012, 19, 616–624. [Google Scholar] [CrossRef]
- Gobbo, J.; Marcion, G.; Cordonnier, M.; Dias, A.M.; Pernet, N.; Hammann, A.; Richaud, S.; Mjahed, H.; Isambert, N.; Clausse, V. Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer. J. Natl. Cancer Inst. 2016, 108, djv330. [Google Scholar] [CrossRef] [PubMed]
- Park, S.L.; Chung, T.-W.; Kim, S.; Hwang, B.; Kim, J.M.; Lee, H.M.; Cha, H.-J.; Seo, Y.; Choe, S.Y.; Ha, K.-T. HSP70-1 is required for interleukin-5-induced angiogenic responses through eNOS pathway. Sci. Rep. 2017, 7, 44687. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-H.; Hwang, B.; Park, S.L.; Kim, H.; Jung, S.; Choi, C.; Lee, H.M.; Yun, S.-J.; Choi, Y.H.; Cha, E.-J. IL-28A/IL-10Rβ axis promotes angiogenesis via eNOS/AKT signaling and AP-1/NF-κB/MMP-2 network by regulating HSP70-1 expression. J. Adv. Res. 2025, 73, 247–263. [Google Scholar] [CrossRef]
- Vos, M.J.; Hageman, J.; Carra, S.; Kampinga, H.H. Structural and Functional Diversities between Members of the Human HSPB, HSPH, HSPA, and DNAJ Chaperone Families. Biochemistry 2008, 47, 7001–7011. [Google Scholar] [CrossRef]
- Clerico, E.M.; Meng, W.; Pozhidaeva, A.; Bhasne, K.; Petridis, C.; Gierasch, L.M. Hsp70 molecular chaperones: Multifunctional allosteric holding and unfolding machines. Biochem. J. 2019, 476, 1653–1677. [Google Scholar] [CrossRef] [PubMed]
- English, C.A.; Sherman, W.; Meng, W.; Gierasch, L.M. The Hsp70 interdomain linker is a dynamic switch that enables allosteric communication between two structured domains. J. Biol. Chem. 2017, 292, 14765–14774. [Google Scholar] [CrossRef]
- Tagaeva, R.; Efimova, S.; Ischenko, A.; Zhakhov, A.; Shevtsov, M.; Ostroumova, O. A new look at Hsp70 activity in phosphatidylserine-enriched membranes: Chaperone-induced quasi-interdigitated lipid phase. Sci. Rep. 2023, 13, 19233. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Stangl, S.; Salvermoser, L.; Schwab, M.; Shevtsov, M.; Xanthopoulos, A.; Wang, F.; Dezfouli, A.B.; Thölke, D.; Ostheimer, C.; et al. Hsp70 in Liquid Biopsies—A Tumor-Specific Biomarker for Detection and Response Monitoring in Cancer. Cancers 2021, 13, 3706. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, G.; Zhao, K.; Zhang, G. Diversity of extracellular HSP70 in cancer: Advancing from a molecular biomarker to a novel therapeutic target. Front. Oncol. 2024, 14, 1388999. [Google Scholar] [CrossRef]
- Rodina, A.; Wang, T.; Yan, P.; Gomes, E.D.; Dunphy, M.P.S.; Pillarsetty, N.; Koren, J.; Gerecitano, J.F.; Taldone, T.; Zong, H.; et al. The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature 2016, 538, 397–401. [Google Scholar] [CrossRef]
- Shevtsov, M.; Bobkov, D.; Yudintceva, N.; Likhomanova, R.; Kim, A.; Fedorov, E.; Fedorov, V.; Mikhailova, N.; Oganesyan, E.; Shabelnikov, S.; et al. Membrane-bound Heat Shock Protein mHsp70 Is Required for Migration and Invasion of Brain Tumors. Cancer Res. Commun. 2024, 4, 2025–2044. [Google Scholar] [CrossRef]
- Amissah, H.A.; Antwi, M.H.; Amissah, T.A.; Combs, S.E.; Shevtsov, M. More than Just Protein Folding: The Epichaperome, Mastermind of the Cancer Cell. Cells 2025, 14, 204. [Google Scholar] [CrossRef]
- Wang, T.; Rodina, A.; Dunphy, M.P.; Corben, A.; Modi, S.; Guzman, M.L.; Gewirth, D.T.; Chiosis, G. Chaperome heterogeneity and its implications for cancer study and treatment. J. Biol. Chem. 2019, 294, 2162–2179. [Google Scholar] [CrossRef]
- Melnyk, A.; Rieger, H.; Zimmermann, R. Co-chaperones of the Mammalian Endoplasmic Reticulum. In The Networking of Chaperones by Co-Chaperones: Control of Cellular Protein Homeostasis; Blatch, G.L., Edkins, A.L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 179–200. [Google Scholar]
- Voisine, C.; Brehme, M. HSP90 et al.: Chaperome and Proteostasis Deregulation in Human Disease. In Heat Shock Protein 90 in Human Diseases and Disorders; Asea, A.A.A., Kaur, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 591–603. [Google Scholar]
- Perez-Riba, A.; Itzhaki, L.S. The tetratricopeptide-repeat motif is a versatile platform that enables diverse modes of molecular recognition. Curr. Opin. Struct. Biol. 2019, 54, 43–49. [Google Scholar] [CrossRef]
- Joshi, S.; Wang, T.; Araujo, T.L.S.; Sharma, S.; Brodsky, J.L.; Chiosis, G. Adapting to stress—Chaperome networks in cancer. Nat. Rev. Cancer 2018, 18, 562–575. [Google Scholar] [CrossRef]
- Stricher, F.; Macri, C.; Ruff, M.; Muller, S. HSPA8/HSC70 chaperone protein. Autophagy 2013, 9, 1937–1954. [Google Scholar] [CrossRef]
- Qu, B.; Jia, Y.; Liu, Y.; Wang, H.; Ren, G.; Wang, H. The detection and role of heat shock protein 70 in various nondisease conditions and disease conditions: A literature review. Cell Stress Chaperones 2015, 20, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Polier, S.; Dragovic, Z.; Hartl, F.U.; Bracher, A. Structural Basis for the Cooperation of Hsp70 and Hsp110 Chaperones in Protein Folding. Cell 2008, 133, 1068–1079. [Google Scholar] [CrossRef]
- Kityk, R.; Vogel, M.; Schlecht, R.; Bukau, B.; Mayer, M.P. Pathways of allosteric regulation in Hsp70 chaperones. Nat. Commun. 2015, 6, 8308. [Google Scholar] [CrossRef] [PubMed]
- Melero, R.; Moro, F.; Pérez-Calvo, M.Á.; Perales-Calvo, J.; Quintana-Gallardo, L.; Llorca, O.; Muga, A.; Valpuesta, J.M. Modulation of the Chaperone DnaK Allosterism by the Nucleotide Exchange Factor GrpE. J. Biol. Chem. 2015, 290, 10083–10092. [Google Scholar] [CrossRef]
- Tripathi, A.; Del Galdo, S.; Chandramouli, B.; Kumar, N. Distinct dynamical features of plasmodial and human HSP70-HSP110 highlight the divergence in their chaperone-assisted protein folding. Biochim. Biophys. Acta-Proteins Proteom. 2023, 1871, 140942. [Google Scholar] [CrossRef]
- Andréasson, C.; Fiaux, J.; Rampelt, H.; Druffel-Augustin, S.; Bukau, B. Insights into the structural dynamics of the Hsp110–Hsp70 interaction reveal the mechanism for nucleotide exchange activity. Proc. Natl. Acad. Sci. USA 2008, 105, 16519–16524. [Google Scholar] [CrossRef] [PubMed]
- Rauch, J.N.; Tse, E.; Freilich, R.; Mok, S.-A.; Makley, L.N.; Southworth, D.R.; Gestwicki, J.E. BAG3 Is a Modular, Scaffolding Protein that physically Links Heat Shock Protein 70 (Hsp70) to the Small Heat Shock Proteins. J. Mol. Biol. 2017, 429, 128–141. [Google Scholar] [CrossRef]
- Tomiczek, B.; Delewski, W.; Nierzwicki, L.; Stolarska, M.; Grochowina, I.; Schilke, B.; Dutkiewicz, R.; Uzarska, M.A.; Ciesielski, S.J.; Czub, J.; et al. Two-step mechanism of J-domain action in driving Hsp70 function. PLOS Comput. Biol. 2020, 16, e1007913. [Google Scholar] [CrossRef]
- Kityk, R.; Kopp, J.; Mayer, M.P. Molecular Mechanism of J-Domain-Triggered ATP Hydrolysis by Hsp70 Chaperones. Mol. Cell 2018, 69, 227–237.e224. [Google Scholar] [CrossRef]
- Marszalek, J.; Craig, E.A. Interaction of client—The scaffold on which FeS clusters are build—With J-domain protein Hsc20 and its evolving Hsp70 partners. Front. Mol. Biosci. 2022, 9, 1034453. [Google Scholar] [CrossRef]
- Misselwitz, B.; Staeck, O.; Rapoport, T.A. J Proteins Catalytically Activate Hsp70 Molecules to Trap a Wide Range of Peptide Sequences. Mol. Cell 1998, 2, 593–603. [Google Scholar] [CrossRef]
- Ciesielski, S.J.; Young, C.; Ciesielska, E.J.; Ciesielski, G.L. Chapter Nine-The Hsp70 and JDP proteins: Structure-function perspective on molecular chaperone activity. In The Enzymes; Kaguni, L.S., Tamanoi, F., Eds.; Academic Press: New York, NY, USA, 2023; Volume 54, pp. 221–245. [Google Scholar]
- Malinverni, D.; Zamuner, S.; Rebeaud, M.E.; Barducci, A.; Nillegoda, N.B.; De Los Rios, P. Data-driven large-scale genomic analysis reveals an intricate phylogenetic and functional landscape in J-domain proteins. Proc. Natl. Acad. Sci. USA 2023, 120, e2218217120. [Google Scholar] [CrossRef]
- Perales-Calvo, J.; Muga, A.; Moro, F. Role of DnaJ G/F-rich Domain in Conformational Recognition and Binding of Protein Substrates. J. Biol. Chem. 2010, 285, 34231–34239. [Google Scholar] [CrossRef] [PubMed]
- Jelen, M.; Grochowina, I.; Grabinska-Rogala, A.; Ciesielski, S.J.; Dabrowska, K.; Tomiczek, B.; Nierzwicki, L.; Delewski, W.; Schilke, B.; Czub, J.; et al. Analysis of Reconstituted Tripartite Complex Supports Avidity-based Recruitment of Hsp70 by Substrate Bound J-domain Protein. J. Mol. Biol. 2023, 435, 168283. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Carneros, L.; Cuéllar, J.; Dublang, L.; Santiago, C.; Maréchal, J.-D.; Martín-Benito, J.; Maestro, M.; Fernández-Higuero, J.Á.; Orozco, N.; Moro, F.; et al. The self-association equilibrium of DNAJA2 regulates its interaction with unfolded substrate proteins and with Hsc70. Nat. Commun. 2023, 14, 5436. [Google Scholar] [CrossRef]
- Baaklini, I.; Wong, M.J.H.; Hantouche, C.; Patel, Y.; Shrier, A.; Young, J.C. The DNAJA2 Substrate Release Mechanism Is Essential for Chaperone-mediated Folding. J. Biol. Chem. 2012, 287, 41939–41954. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Gallardo, L.; Martín-Benito, J.; Marcilla, M.; Espadas, G.; Sabidó, E.; Valpuesta, J.M. The cochaperone CHIP marks Hsp70- and Hsp90-bound substrates for degradation through a very flexible mechanism. Sci. Rep. 2019, 9, 5102. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, Q.; Xia, H.; Xu, H.; Bi, F. PKM2 compensates for proteasome dysfunction by mediating the formation of the CHIP-HSP70-BAG3 complex and the aggregation of ubiquitinated proteins. FASEB J. 2022, 36, e22121. [Google Scholar] [CrossRef]
- Tawo, R.; Pokrzywa, W.; Kevei, É.; Akyuz, M.E.; Balaji, V.; Adrian, S.; Höhfeld, J.; Hoppe, T. The Ubiquitin Ligase CHIP Integrates Proteostasis and Aging by Regulation of Insulin Receptor Turnover. Cell 2017, 169, 470–482.e413. [Google Scholar] [CrossRef]
- Graner, M.W. Chapter Eight-HSP90 and Immune Modulation in Cancer. In Advances in Cancer Research; Isaacs, J., Whitesell, L., Eds.; Academic Press: New York, NY, USA, 2016; Volume 129, pp. 191–224. [Google Scholar]
- Altieri, D.C.; Stein, G.S.; Lian, J.B.; Languino, L.R. TRAP-1, the mitochondrial Hsp90. Biochim. Biophys. Acta-Mol. Cell Res. 2012, 1823, 767–773. [Google Scholar] [CrossRef]
- Barrott, J.J.; Haystead, T.A.J. Hsp90, an unlikely ally in the war on cancer. FEBS J. 2013, 280, 1381–1396. [Google Scholar] [CrossRef]
- López, A.; Elimelech, A.R.; Klimm, K.; Sattler, M. The Charged Linker Modulates the Conformations and Molecular Interactions of Hsp90. ChemBioChem 2021, 22, 1084–1092. [Google Scholar] [CrossRef]
- Goode, K.M.; Petrov, D.P.; Vickman, R.E.; Crist, S.A.; Pascuzzi, P.E.; Ratliff, T.L.; Davisson, V.J.; Hazbun, T.R. Targeting the Hsp90 C-terminal domain to induce allosteric inhibition and selective client downregulation. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 1992–2006. [Google Scholar] [CrossRef]
- Onuoha, S.C.; Coulstock, E.T.; Grossmann, J.G.; Jackson, S.E. Structural Studies on the Co-chaperone Hop and Its Complexes with Hsp90. J. Mol. Biol. 2008, 379, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Lott, A.; Oroz, J.; Zweckstetter, M. Molecular basis of the interaction of Hsp90 with its co-chaperone Hop. Protein Sci. 2020, 29, 2422–2432. [Google Scholar] [CrossRef] [PubMed]
- Castelli, M.; Bhattacharya, K.; Abboud, E.; Serapian, S.A.; Picard, D.; Colombo, G. Phosphorylation of the Hsp90 Co-Chaperone Hop Changes its Conformational Dynamics and Biological Function. J. Mol. Biol. 2023, 435, 167931. [Google Scholar] [CrossRef]
- Sahasrabudhe, P.; Rohrberg, J.; Biebl, M.M.; Rutz, D.A.; Buchner, J. The Plasticity of the Hsp90 Co-chaperone System. Mol. Cell 2017, 67, 947–961.e945. [Google Scholar] [CrossRef] [PubMed]
- Ebong, I.-o.; Beilsten-Edmands, V.; Patel, N.A.; Morgner, N.; Robinson, C.V. The interchange of immunophilins leads to parallel pathways and different intermediates in the assembly of Hsp90 glucocorticoid receptor complexes. Cell Discov. 2016, 2, 16002. [Google Scholar] [CrossRef]
- Zgajnar, N.R.; De Leo, S.A.; Lotufo, C.M.; Erlejman, A.G.; Piwien-Pilipuk, G.; Galigniana, M.D. Biological Actions of the Hsp90-binding Immunophilins FKBP51 and FKBP52. Biomolecules 2019, 9, 52. [Google Scholar] [CrossRef]
- Ernst, K.; Schnell, L.; Barth, H. Host Cell Chaperones Hsp70/Hsp90 and Peptidyl-Prolyl Cis/Trans Isomerases Are Required for the Membrane Translocation of Bacterial ADP-Ribosylating Toxins. In Uptake and Trafficking of Protein Toxins; Barth, H., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 163–198. [Google Scholar]
- Storer, C.L.; Dickey, C.A.; Galigniana, M.D.; Rein, T.; Cox, M.B. FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol. Metab. 2011, 22, 481–490. [Google Scholar] [CrossRef]
- Guy, N.C.; Garcia, Y.A.; Sivils, J.C.; Galigniana, M.D.; Cox, M.B. Functions of the Hsp90-Binding FKBP Immunophilins. In The Networking of Chaperones by Co-Chaperones: Control of Cellular Protein Homeostasis; Blatch, G.L., Edkins, A.L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 35–68. [Google Scholar]
- Mazaira, G.I.; Erlejman, A.G.; Zgajnar, N.R.; Piwien-Pilipuk, G.; Galigniana, M.D. The transportosome system as a model for the retrotransport of soluble proteins. Mol. Cell. Endocrinol. 2023, 577, 112047. [Google Scholar] [CrossRef] [PubMed]
- Haslbeck, V.; Eckl, J.M.; Drazic, A.; Rutz, D.A.; Lorenz, O.R.; Zimmermann, K.; Kriehuber, T.; Lindemann, C.; Madl, T.; Richter, K. The activity of protein phosphatase 5 towards native clients is modulated by the middle- and C-terminal domains of Hsp90. Sci. Rep. 2015, 5, 17058. [Google Scholar] [CrossRef]
- Jaime-Garza, M.; Nowotny, C.A.; Coutandin, D.; Wang, F.; Tabios, M.; Agard, D.A. Hsp90 provides a platform for kinase dephosphorylation by PP5. Nat. Commun. 2023, 14, 2197. [Google Scholar] [CrossRef]
- Oroz, J.; Blair, L.J.; Zweckstetter, M. Dynamic Aha1 co-chaperone binding to human Hsp90. Protein Sci. 2019, 28, 1545–1551. [Google Scholar] [CrossRef]
- Wortmann, P.; Götz, M.; Hugel, T. Cooperative Nucleotide Binding in Hsp90 and Its Regulation by Aha1. Biophys. J. 2017, 113, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Willhoft, O.; Kerr, R.; Patel, D.; Zhang, W.; Al-Jassar, C.; Daviter, T.; Millson, S.H.; Thalassinos, K.; Vaughan, C.K. The crystal structure of the Sgt1-Skp1 complex: The link between Hsp90 and both SCF E3 ubiquitin ligases and kinetochores. Sci. Rep. 2017, 7, 41626. [Google Scholar] [CrossRef] [PubMed]
- Eisele, F.; Eisele-Bürger, A.M.; Hao, X.; Berglund, L.L.; Höög, J.L.; Liu, B.; Nyström, T. An Hsp90 co-chaperone links protein folding and degradation and is part of a conserved protein quality control. Cell Rep. 2021, 35, 109328. [Google Scholar] [CrossRef]
- Calderwood, S.K. Cdc37 as a Co-chaperone to Hsp90. In The Networking of Chaperones by Co-Chaperones: Control of Cellular Protein Homeostasis; Blatch, G.L., Edkins, A.L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 103–112. [Google Scholar]
- Keramisanou, D.; Vasantha Kumar, M.V.; Boose, N.; Abzalimov, R.R.; Gelis, I. Assembly mechanism of early Hsp90-Cdc37-kinase complexes. Sci. Adv. 2022, 8, eabm9294. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, L.; Jiang, J.; Zheng, Z.; Shang, J.; Wang, C.; Chen, W.; Bao, Q.; Xu, X.; et al. Small-molecule inhibitor targeting the Hsp90-Cdc37 protein-protein interaction in colorectal cancer. Sci. Adv. 2019, 5, eaax2277. [Google Scholar] [CrossRef] [PubMed]
- Likhomanova, R.; Oganesyan, E.; Yudintceva, N.; Fofanov, G.; Nechaeva, A.; Ulitin, A.; Kim, A.; Aksenov, N.; Shatrova, A.; Ziganshin, R.; et al. Glioblastoma cell motility and invasion is regulated by membrane-associated heat shock protein Hsp70. J. Neuro-Oncol. 2025, 175, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Buffone, A.; Hammer, D.A.; Kim, S.H.J.; Anderson, N.R.; Mochida, A.; Lee, D.-H.; Guin, S. Not all (cells) who wander are lost: Upstream migration as a pervasive mode of amoeboid cell motility. Front. Cell Dev. Biol. 2023, 11, 1291201. [Google Scholar] [CrossRef]
- Vollmann-Zwerenz, A.; Leidgens, V.; Feliciello, G.; Klein, C.A.; Hau, P. Tumor Cell Invasion in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 1932. [Google Scholar] [CrossRef]
- Seker-Polat, F.; Pinarbasi Degirmenci, N.; Solaroglu, I.; Bagci-Onder, T. Tumor Cell Infiltration into the Brain in Glioblastoma: From Mechanisms to Clinical Perspectives. Cancers 2022, 14, 443. [Google Scholar] [CrossRef]
- Borriello, L.; Karagiannis, G.S.; Duran, C.L.; Coste, A.; Oktay, M.H.; Entenberg, D.; Condeelis, J.S. The role of the tumor microenvironment in tumor cell intravasation and dissemination. Eur. J. Cell Biol. 2020, 99, 151098. [Google Scholar] [CrossRef]
- Sznurkowska, M.K.; Aceto, N. The gate to metastasis: Key players in cancer cell intravasation. FEBS J. 2022, 289, 4336–4354. [Google Scholar] [CrossRef]
- Perez, V.M.; Kearney, J.F.; Yeh, J.J. The PDAC Extracellular Matrix: A Review of the ECM Protein Composition, Tumor Cell Interaction, and Therapeutic Strategies. Front. Oncol. 2021, 11, 91–98. [Google Scholar] [CrossRef]
- Crosas-Molist, E.; Samain, R.; Kohlhammer, L.; Orgaz, J.L.; George, S.L.; Maiques, O.; Barcelo, J.; Sanz-Moreno, V. Rho GTPase signaling in cancer progression and dissemination. Physiol. Rev. 2021, 102, 455–510. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, R.; Pedersen, E.D.; Wang, Z.; Brakebusch, C. Rho GTPase function in tumorigenesis. Biochim. Biophys. Acta-Rev. Cancer 2009, 1796, 91–98. [Google Scholar] [CrossRef]
- Svensmark, J.H.; Brakebusch, C. Rho GTPases in cancer: Friend or foe? Oncogene 2019, 38, 7447–7456. [Google Scholar] [CrossRef]
- Bobkov, D.E.; Lukacheva, A.V.; Gorb, A.I.; Poljanskaya, G.G. Role of Rho Family Small GTPases in the Regulation of Normal and Pathological Processes. Cell Tissue Biol. 2024, 18, 229–243. [Google Scholar] [CrossRef]
- Parri, M.; Chiarugi, P. Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal. 2010, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Lukacheva, A.V.; Bogachev, M.I.; Musorina, A.S.; Kriger, D.V.; Poljanskaya, G.G.; Bobkov, D.E. It’s Not Just About Speed: Single-Cell Tracking Reveals Changes in MSC Motility Associated with Replicative Senescence. Stem Cell Rev. Rep. 2025, 21, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.R.; Geny, B. Multifaceted role of Rho, Rac, Cdc42 and Ras in intercellular junctions, lessons from toxins. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 797–812. [Google Scholar] [CrossRef]
- Narumiya, S.; Thumkeo, D. Rho signaling research: History, current status and future directions. FEBS Lett. 2018, 592, 1763–1776. [Google Scholar] [CrossRef]
- Al-Koussa, H.; Atat, O.E.; Jaafar, L.; Tashjian, H.; El-Sibai, M. The Role of Rho GTPases in Motility and Invasion of Glioblastoma Cells. Anal. Cell. Pathol. 2020, 2020, 9274016. [Google Scholar] [CrossRef]
- Mehidi, A.; Rossier, O.; Schaks, M.; Chazeau, A.; Binamé, F.; Remorino, A.; Coppey, M.; Karatas, Z.; Sibarita, J.-B.; Rottner, K.; et al. Transient Activations of Rac1 at the Lamellipodium Tip Trigger Membrane Protrusion. Curr. Biol. 2019, 29, 2852–2866.e2855. [Google Scholar] [CrossRef] [PubMed]
- Bendezú, F.O.; Vincenzetti, V.; Vavylonis, D.; Wyss, R.; Vogel, H.; Martin, S.G. Spontaneous Cdc42 Polarization Independent of GDI-Mediated Extraction and Actin-Based Trafficking. PLoS Biol. 2015, 13, e1002097. [Google Scholar] [CrossRef] [PubMed]
- Yamao, M.; Naoki, H.; Kunida, K.; Aoki, K.; Matsuda, M.; Ishii, S. Distinct predictive performance of Rac1 and Cdc42 in cell migration. Sci. Rep. 2015, 5, 17527. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Park, J.K.; Shin, S.C.; Lee, J.-J.; Hong, S.K.; Song, I.-K.; Kim, B.; Song, E.J.; Lee, K.-J.; Kim, E.E. The complex of Fas-associated factor 1 with Hsp70 stabilizes the adherens junction integrity by suppressing RhoA activation. J. Mol. Cell Biol. 2022, 14, mjac037. [Google Scholar] [CrossRef]
- Nickelsen, A.; Götz, C.; Lenz, F.; Niefind, K.; König, S.; Jose, J. Analyzing the interactome of human CK2β in prostate carcinoma cells reveals HSP70-1 and Rho guanin nucleotide exchange factor 12 as novel interaction partners. FASEB BioAdv. 2023, 5, 114–130. [Google Scholar] [CrossRef]
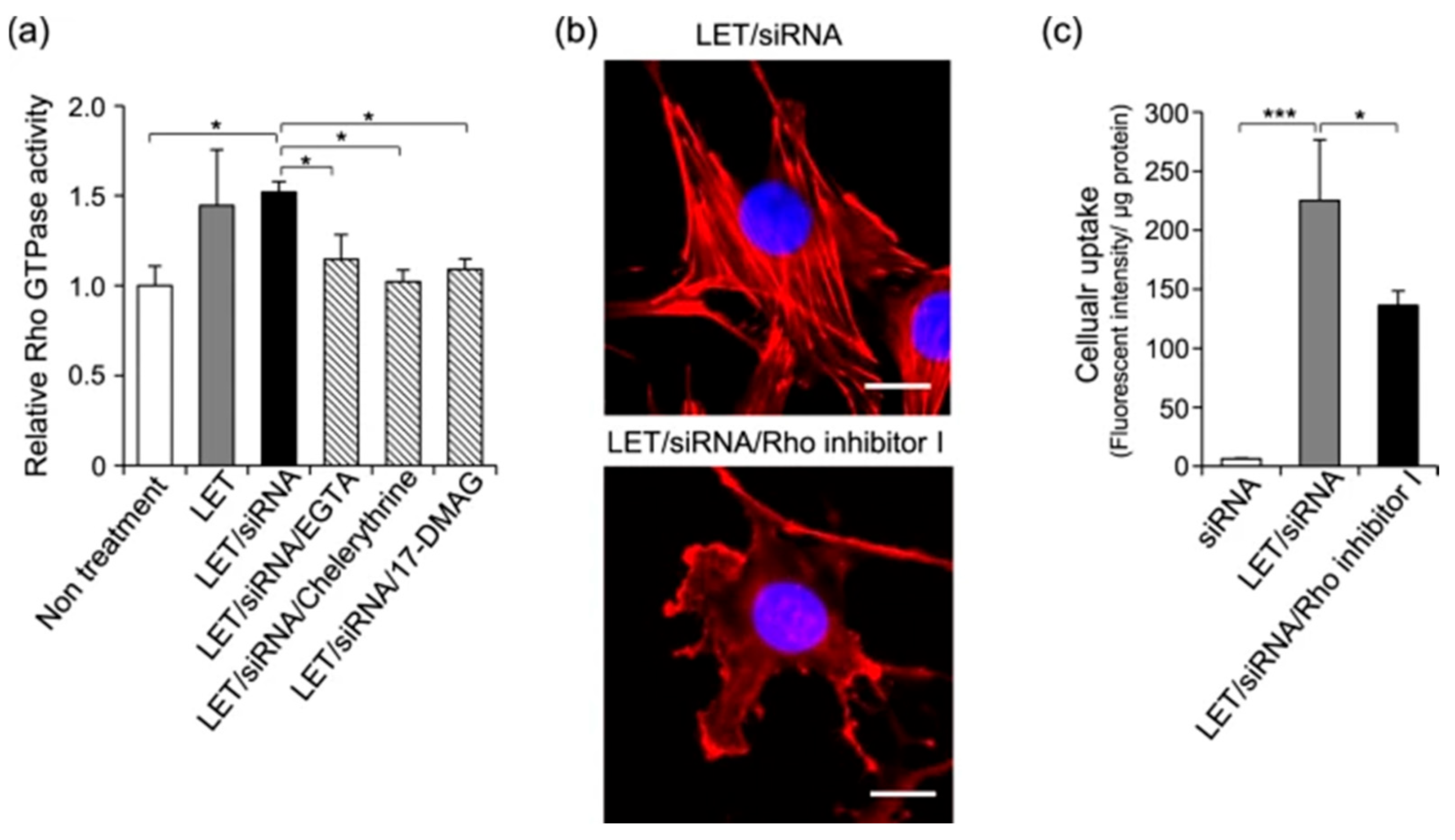
- Hasan, M.; Hama, S.; Kogure, K. Low Electric Treatment activates Rho GTPase via Heat Shock Protein 90 and Protein Kinase C for Intracellular Delivery of siRNA. Sci. Rep. 2019, 9, 4114. [Google Scholar] [CrossRef]
- Lyu, C.; Vaddi, P.K.; Elshafae, S.; Pradeep, A.; Ma, D.; Chen, S. Unveiling RACK1: A key regulator of the PI3K/AKT pathway in prostate cancer development. Oncogene 2025, 44, 322–335. [Google Scholar] [CrossRef]
- Tian, R.; Tian, J.; Zuo, X.; Ren, S.; Zhang, H.; Liu, H.; Wang, Z.; Cui, Y.; Niu, R.; Zhang, F. RACK1 facilitates breast cancer progression by competitively inhibiting the binding of β-catenin to PSMD2 and enhancing the stability of β-catenin. Cell Death Dis. 2023, 14, 685. [Google Scholar] [CrossRef]
- Derry, M.M.; Somasagara, R.R.; Raina, K.; Kumar, S.; Gomez, J.; Patel, M.; Agarwal, R.; Agarwal, C. Target Identification of Grape Seed Extract in Colorectal Cancer Using Drug Affinity Responsive Target Stability (DARTS) Technique: Role of Endoplasmic Reticulum Stress Response Proteins. Curr. Cancer Drug Targets 2014, 14, 323–336. [Google Scholar] [CrossRef]
- Tang, J.; Fang, K.; Li, C.; Chang, X. ARHGEF10L Promotes Cervical Tumorigenesis via RhoA-Mediated Signaling. Evid.-Based Complement. Altern. Med. 2021, 2021, 6683264. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, P.; Zhu, X.-H.; Zhou, S.-Q.; Ye, M.-L.; Yang, X.-J.; Gong, S.; Huang, S.-Y.; Tan, X.-R.; He, S.-W.; et al. DNAJA4 suppresses epithelial-mesenchymal transition and metastasis in nasopharyngeal carcinoma via PSMD2-mediated MYH9 degradation. Cell Death Dis. 2023, 14, 697. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Pu, L.; Hu, J.; Fang, L.; Zhou, F.; Zhang, H.; Yang, Y.; Rong, X.; Deng, S.; et al. DNAJB4 suppresses breast cancer progression and promotes tumor immunity by regulating the Hippo signaling pathway. Discov. Oncol. 2023, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Kaida, A.; Yamamoto, S.; Parrales, A.; Young, E.D.; Ranjan, A.; Alalem, M.A.; Morita, K.-i.; Oikawa, Y.; Harada, H.; Ikeda, T.; et al. DNAJA1 promotes cancer metastasis through interaction with mutant p53. Oncogene 2021, 40, 5013–5025. [Google Scholar] [CrossRef] [PubMed]
- Meshalkina, D.A.; Shevtsov, M.A.; Dobrodumov, A.V.; Komarova, E.Y.; Voronkina, I.V.; Lazarev, V.F.; Margulis, B.A.; Guzhova, I.V. Knock-down of Hdj2/DNAJA1 co-chaperone results in an unexpected burst of tumorigenicity of C6 glioblastoma cells. Oncotarget 2016, 7, 22050–22063. [Google Scholar] [CrossRef]
- Jang, J.; Lee, S.-H.; Kang, D.-H.; Sim, D.-W.; Ryu, K.-S.; Jo, K.-S.; Lee, J.; Ryu, H.; Kim, E.-H.; Won, H.-S. Structural resemblance of the DNAJA-family protein, Tid1, to the DNAJB-family Hsp40. BMB Rep. 2022, 55, 488. [Google Scholar] [CrossRef]
- Wang, S.-F.; Huang, K.-H.; Tseng, W.-C.; Lo, J.-F.; Li, A.F.-Y.; Fang, W.-L.; Chen, C.-F.; Yeh, T.-S.; Chang, Y.-L.; Chou, Y.-C.; et al. DNAJA3/Tid1 Is Required for Mitochondrial DNA Maintenance and Regulates Migration and Invasion of Human Gastric Cancer Cells. Cancers 2020, 12, 3463. [Google Scholar] [CrossRef]
- Lauer, S.M.; Omar, M.H.; Golkowski, M.G.; Kenerson, H.L.; Lee, K.-S.; Pascual, B.C.; Lim, H.C.; Forbush, K.; Smith, F.D.; Gordan, J.D.; et al. Recruitment of BAG2 to DNAJ-PKAc scaffolds promotes cell survival and resistance to drug-induced apoptosis in fibrolamellar carcinoma. Cell Rep. 2024, 43, 113678. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, X.; Wang, Q.; Guo, Z.; Hu, L.; Dong, Z.; Hu, W. Non-canonical Small GTPase RBJ Promotes NSCLC Progression Through the Canonical MEK/ERK Signaling Pathway. Curr. Pharm. Des. 2022, 28, 3446–3455. [Google Scholar] [CrossRef]
- Paré, A.M.; Cheng-Boivin, Z.; Dabbaghizadeh, A.; Minotti, S.; Durham, H.D.; Gentil, B.J. Interactors of sacsin’s DNAJ domain identify function in organellar transport and membrane composition relevant to ARSACS pathogenesis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zhong, Y.; Shi, L.; Xu, Z.; Gao, J.; Ma, Q.; Gao, T.; Tang, J.; Xiong, M.; Xu, Y.; Dai, H.; et al. Benzbromarone interferes with the interaction between Hsp90 and Aha1 by interacting with both of them. Commun. Biol. 2025, 8, 761. [Google Scholar] [CrossRef]
- Liu, J.; Yan, G.; Chen, Q.; Zeng, Q.; Wang, X. Modified 5-aminolevulinic acid photodynamic therapy (M-PDT) inhibits cutaneous squamous cell carcinoma cell proliferation via targeting PP2A/PP5-mediated MAPK signaling pathway. Int. J. Biochem. Cell Biol. 2021, 137, 106036. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Zhang, M.; Jiang, Y.; Ng, A.S.; Bridges, E.; Zhang, W.; Zeng, X.; Luo, Q.; Liang, J.; et al. GTP Cyclohydrolase Drives Breast Cancer Development and Promotes EMT in an Enzyme-Independent Manner. Cancer Res. 2023, 83, 3400–3413. [Google Scholar] [CrossRef] [PubMed]
- Seclì, L.; Avalle, L.; Poggio, P.; Fragale, G.; Cannata, C.; Conti, L.; Iannucci, A.; Carrà, G.; Rubinetto, C.; Miniscalco, B.; et al. Targeting the Extracellular HSP90 Co-Chaperone Morgana Inhibits Cancer Cell Migration and Promotes Anticancer Immunity. Cancer Res. 2021, 81, 4794–4807. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yin, L.; Ding, K.; Xia, Y.Y.; Wang, X.H.; Wu, J.Z.; He, X. Raf1 is a prognostic factor for progression in patients with non-small cell lung cancer after radiotherapy. Oncol. Rep. 2018, 39, 1966–1974. [Google Scholar] [CrossRef]
- Gayen, N.; Mitra, S.; Roy, S.; Mandal, A.K. Hsp70/Hsp90 organizing protein (HOP) maintains CRAF kinase activity and regulates MAPK signaling by enhancing Hsp90-CRAF association. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lin, L.; Wen, J.; Lin, B.; Xia, E.; Zheng, C.; Ye, L.; Wang, Y.; Wang, O.; Chen, Y. Stress-induced phosphoprotein 1 facilitates breast cancer cell progression and indicates poor prognosis for breast cancer patients. Hum. Cell 2021, 34, 901–917. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.L.; Zigmond, J.; Bergan, R. Abstract 2655: Novel stress-induced extracellular regulatory mechanism leads to decreased cellular invasion in prostate cancer. Cancer Res. 2025, 85, 2655. [Google Scholar] [CrossRef]
- García-Alonso, S.; Mesa, P.; Ovejero, L.d.l.P.; Aizpurua, G.; Lechuga, C.G.; Zarzuela, E.; Santiveri, C.M.; Sanclemente, M.; Muñoz, J.; Musteanu, M.; et al. Structure of the RAF1-HSP90-CDC37 complex reveals the basis of RAF1 regulation. Mol. Cell 2022, 82, 3438–3452.e3438. [Google Scholar] [CrossRef]
- Li, L.; Tao, X.; Li, Y.; Gao, Y.; Li, Q. CDC37L1 acts as a suppressor of migration and proliferation in gastric cancer by down-regulating CDK6. J. Cancer 2021, 12, 3145–3153. [Google Scholar] [CrossRef]
- Yu, Z.; Peng, Y.; Gao, J.; Zhou, M.; Shi, L.; Zhao, F.; Wang, C.; Tian, X.; Feng, L.; Huo, X.; et al. The p23 co-chaperone is a succinate-activated COX-2 transcription factor in lung adenocarcinoma tumorigenesis. Sci. Adv. 2023, 9, eade0387. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Graziano, F.; Rappa, F.; Marino Gammazza, A.; Logozzi, M.; Fais, S.; Maugeri, R.; Bucchieri, F.; Conway de Macario, E.; Macario, A.J.L.; et al. Exosomal Chaperones and miRNAs in Gliomagenesis: State-of-Art and Theranostics Perspectives. Int. J. Mol. Sci. 2018, 19, 2626. [Google Scholar] [CrossRef]
- Amissah, H.A.; Likhomanova, R.; Opoku, G.; Amissah, T.A.; Balogi, Z.; Török, Z.; Vigh, L.; Combs, S.E.; Shevtsov, M. Plasma Membrane Epichaperome–Lipid Interface: Regulating Dynamics and Trafficking. Cells 2025, 14, 1582. [Google Scholar] [CrossRef] [PubMed]
- Jay, D.; Luo, Y.; Li, W. Extracellular Heat Shock Protein-90 (eHsp90): Everything You Need to Know. Biomolecules 2022, 12, 911. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Ono, K.; Kawata, K.; Okamoto, K.; Calderwood, S.K. Regulatory Roles of HSP90-Rich Extracellular Vesicles. In Heat Shock Protein 90 in Human Diseases and Disorders; Asea, A.A.A., Kaur, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–17. [Google Scholar]
- Géminard, C.; de Gassart, A.; Blanc, L.; Vidal, M. Degradation of AP2 During Reticulocyte Maturation Enhances Binding of Hsc70 and Alix to a Common Site on TfR for Sorting into Exosomes. Traffic 2004, 5, 181–193. [Google Scholar] [CrossRef]
- Bhasne, K.; Bogoian-Mullen, A.; Clerico, E.M.; Gierasch, L.M. The Hsc70 system maintains the synaptic SNARE protein SNAP-25 in an assembly-competent state and delays its aggregation. J. Biol. Chem. 2024, 300, 108001. [Google Scholar] [CrossRef]
- Lauwers, E.; Wang, Y.-C.; Gallardo, R.; Van der Kant, R.; Michiels, E.; Swerts, J.; Baatsen, P.; Zaiter, S.S.; McAlpine, S.R.; Gounko, N.V.; et al. Hsp90 Mediates Membrane Deformation and Exosome Release. Mol. Cell 2018, 71, 689–702.e689. [Google Scholar] [CrossRef]
- Eguchi, T.; Sogawa, C.; Ono, K.; Matsumoto, M.; Tran, M.T.; Okusha, Y.; Lang, B.J.; Okamoto, K.; Calderwood, S.K. Cell Stress Induced Stressome Release Including Damaged Membrane Vesicles and Extracellular HSP90 by Prostate Cancer Cells. Cells 2020, 9, 755. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Sogawa, C.; Kawai, H.; Tran, M.T.; Taha, E.A.; Lu, Y.; Oo, M.W.; Okusha, Y.; Okamura, H.; Ibaragi, S.; et al. Triple knockdown of CDC37, HSP90-alpha and HSP90-beta diminishes extracellular vesicles-driven malignancy events and macrophage M2 polarization in oral cancer. J. Extracell. Vesicles 2020, 9, 1769373. [Google Scholar] [CrossRef]
- Ferreira, J.V.; Rosa Soares, A.; Ramalho, J.S.; Ribeiro-Rodrigues, T.; Máximo, C.; Zuzarte, M.; Girão, H.; Pereira, P. Exosomes and STUB1/CHIP cooperate to maintain intracellular proteostasis. PLoS ONE 2019, 14, e0223790. [Google Scholar] [CrossRef]
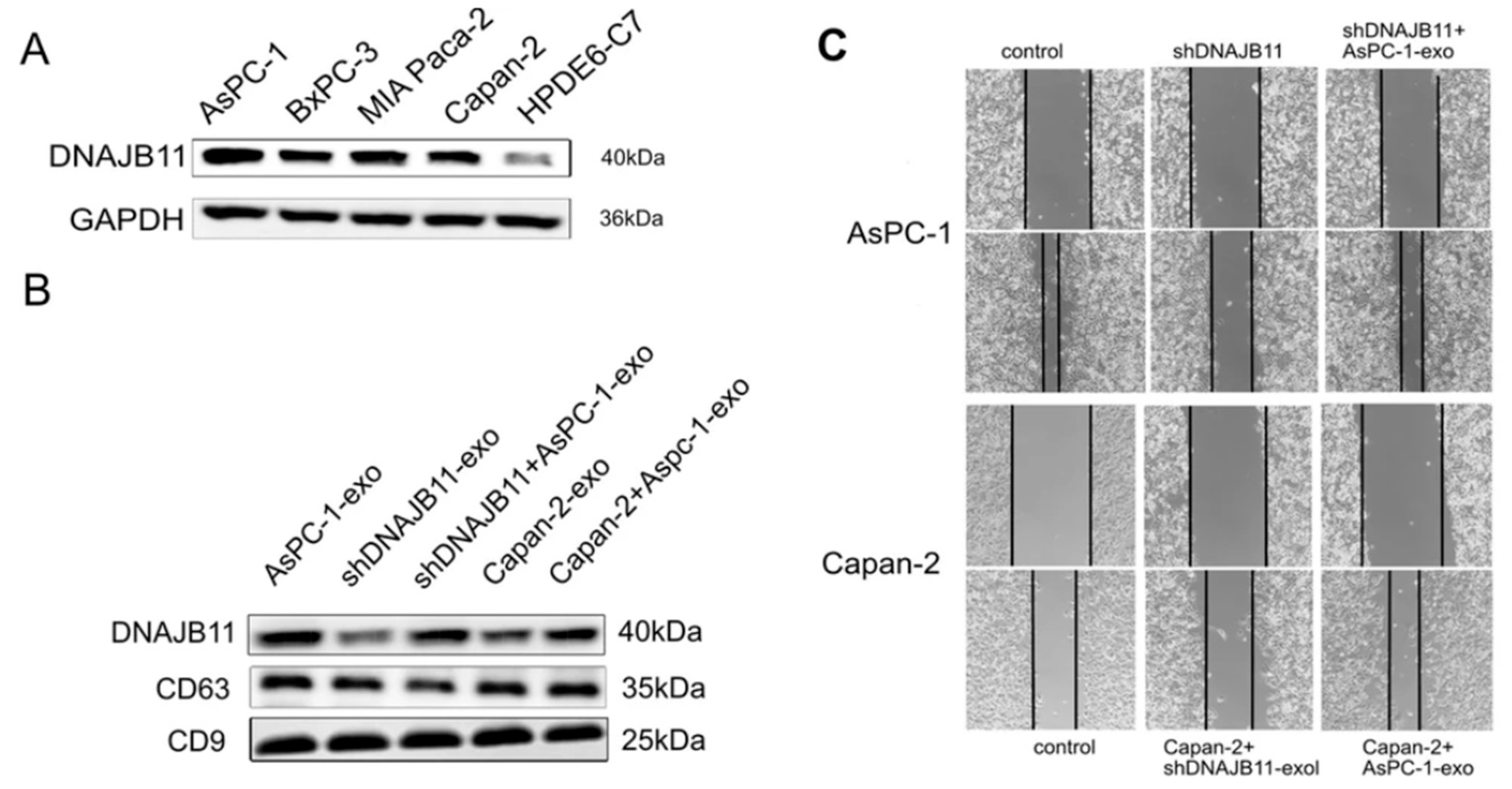
- Liu, P.; Zu, F.; Chen, H.; Yin, X.; Tan, X. Exosomal DNAJB11 promotes the development of pancreatic cancer by modulating the EGFR/MAPK pathway. Cell. Mol. Biol. Lett. 2022, 27, 87. [Google Scholar] [CrossRef]
- Joshi, B.S.; Garcia Romeu, H.; Aliyandi, A.; de Vries, M.P.; Zuhorn, I.S. DNAJB6-Containing Extracellular Vesicles as Chaperone Delivery Systems: A Proteomic Analysis. Pharmaceutics 2022, 14, 2485. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, X.; Miller-Little, W.; Wang, H.; Willard, B.; Bulek, K.; Zhao, J.; Li, X. The Ras GTPase-activating-like protein IQGAP1 bridges Gasdermin D to the ESCRT system to promote IL-1β release via exosomes. EMBO J. 2023, 42, e110780. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, X.; Greene, K.S.; Si, H.; Antonyak, M.A.; Druso, J.E.; Wilson, K.F.; Cerione, R.A.; Feng, Q.; Wang, H. Cdc42 functions as a regulatory node for tumour-derived microvesicle biogenesis. J. Extracell. Vesicles 2021, 10, e12051. [Google Scholar] [CrossRef]
- Baker-Williams, A.J.; Hashmi, F.; Budzyński, M.A.; Woodford, M.R.; Gleicher, S.; Himanen, S.V.; Makedon, A.M.; Friedman, D.; Cortes, S.; Namek, S.; et al. Co-chaperones TIMP2 and AHA1 Competitively Regulate Extracellular HSP90:Client MMP2 Activity and Matrix Proteolysis. Cell Rep. 2019, 28, 1894–1906.e1896. [Google Scholar] [CrossRef]
- Komarova, E.Y.; Suezov, R.V.; Nikotina, A.D.; Aksenov, N.D.; Garaeva, L.A.; Shtam, T.A.; Zhakhov, A.V.; Martynova, M.G.; Bystrova, O.A.; Istomina, M.S.; et al. Hsp70-containing extracellular vesicles are capable of activating of adaptive immunity in models of mouse melanoma and colon carcinoma. Sci. Rep. 2021, 11, 21314. [Google Scholar] [CrossRef] [PubMed]
- Duff, D.; Long, A. Roles for RACK1 in cancer cell migration and invasion. Cell. Signal. 2017, 35, 250–255. [Google Scholar] [CrossRef]
- Kaida, A.; Iwakuma, T. Regulation of p53 and Cancer Signaling by Heat Shock Protein 40/J-Domain Protein Family Members. Int. J. Mol. Sci. 2021, 22, 13527. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, W.; Zhang, L.; Zhang, J. Targeting mutant p53 stabilization for cancer therapy. Front. Pharmacol. 2023, 14, 1215995. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, J.; Du, Y.; Zhang, L.; Qin, X. Abnormally High Expression of DNAJB6 Accelerates Malignant Progression of Lung Adenocarcinoma. Biomedicines 2024, 12, 1981. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zhang, J.; Zeng, W.; Chen, X. DNAJB1 stabilizes MDM2 and contributes to cancer cell proliferation in a p53-dependent manner. Biochim. Biophys. Acta-Gene Regul. Mech. 2014, 1839, 62–69. [Google Scholar] [CrossRef]
- Huang, X.; Shi, D.; Zou, X.; Wu, X.; Huang, S.; Kong, L.; Yang, M.; Xiao, Y.; Chen, B.; Chen, X.; et al. BAG2 drives chemoresistance of breast cancer by exacerbating mutant p53 aggregate. Theranostics 2023, 13, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Q.; Wei, H.; Tang, B.; Tian, B.; Ma, Z.; Gu, Q.; Su, X.; Dong, Y.; Shi, W. Targeting the BAG2/CHIP Aix Promotes Gastric Cancer Apoptosis by Blocking Apoptosome Assembly. Front. Immunol. 2025, 16, 1578416. [Google Scholar]


| Research Task | Experimental Approach |
|---|---|
| Gene expression evaluation and co-occurrence evaluation | Flow cytometry |
| Transcriptomics | |
| Western blot assays | |
| Fluorescent staining | |
| Gene expression control | siRNA |
| Heat shock | |
| Oxidative stress | |
| Exogenous HSP and co-chaperones (vesicle or soluble) | |
| Low electric treatment | |
| Network profiling | Mass spectrometry |
| Western blot assay | |
| Inhibitory assays | |
| Cell motility assessment | Single cell tracking |
| Wound healing assay | |
| Inhibitory assays | |
| Transwell assay |
| HSP Node | Co-Chaperone/Interactor | Affected Node | Evidence Level | References |
|---|---|---|---|---|
| HSPA1A | RACK1 | AKT/P53 | In vivo | [127,128,167] |
| HSPA1A | DNAJA1,3 | P53, MMPs | In vivo | [133,134,136,168,169] |
| HSPA1A | DNAJB1,4,6 | P53, MMPs | In vitro/In vivo (DNAJB6) | [134,162,170,171] |
| HSPA1A | DNAJB11 (exosomal) | MAPK pathway | In vivo | [161] |
| HSPA1A | BAG2 | P53/PKA | In vivo | [137,172,173] |
| HSP90 | AHA1 | AKT/MMPs | In vitro | [140,165] |
| HSP90 | CDC37 | CDK6/RAF1 | In vitro | [149,158,159] |
| HSP90/70 | HOP | RAF1/MMPs | In vivo | [144,145,146,148] |
| HSP90/70 | CHIP | HSF | In vitro | [80,160,173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorov, V.; Kurkin, A.; Fofanov, G.; Kaneva, V.; Kondratenko, A.; Combs, S.E.; Shevtsov, M. Heat Shock Protein Chaperome Is a Multi-Faceted Vector for Tumor Cell Migratory Activity, Invasion, and Metastasis. Cells 2025, 14, 1837. https://doi.org/10.3390/cells14231837
Fedorov V, Kurkin A, Fofanov G, Kaneva V, Kondratenko A, Combs SE, Shevtsov M. Heat Shock Protein Chaperome Is a Multi-Faceted Vector for Tumor Cell Migratory Activity, Invasion, and Metastasis. Cells. 2025; 14(23):1837. https://doi.org/10.3390/cells14231837
Chicago/Turabian StyleFedorov, Viacheslav, Andrey Kurkin, Georgii Fofanov, Vitaliya Kaneva, Anna Kondratenko, Stephanie E. Combs, and Maxim Shevtsov. 2025. "Heat Shock Protein Chaperome Is a Multi-Faceted Vector for Tumor Cell Migratory Activity, Invasion, and Metastasis" Cells 14, no. 23: 1837. https://doi.org/10.3390/cells14231837
APA StyleFedorov, V., Kurkin, A., Fofanov, G., Kaneva, V., Kondratenko, A., Combs, S. E., & Shevtsov, M. (2025). Heat Shock Protein Chaperome Is a Multi-Faceted Vector for Tumor Cell Migratory Activity, Invasion, and Metastasis. Cells, 14(23), 1837. https://doi.org/10.3390/cells14231837







