Lipid–Protein Interplay in the Regulation of Receptor Tyrosine Kinases
Highlights
- 1.
- Specific lipid–protein interactions tightly regulate RTKs’ activity and function in physiological and pathological contexts.
- 2.
- Plasma membrane lipid heterogeneity spatially and mechanically controls RTK trafficking, clustering, and downstream signaling.
- 1.
- A deeper understanding of lipid–RTK crosstalk is required to resolve the spatiotemporal complexity of this regulatory mechanism.
- 2.
- Lipid-dependent modulation of RTKs offers promising therapeutic approaches.
Abstract
1. Introduction
2. Cell Membrane
2.1. Membrane Lipid Composition
2.2. Membrane Lipid Structure and Organization
2.3. Membrane Lipid Functions
3. Receptor Tyrosine Kinases
RTKs Structure and Activation
4. Role of Lipids in the Maturation, Activation, and Recycling of RTKs
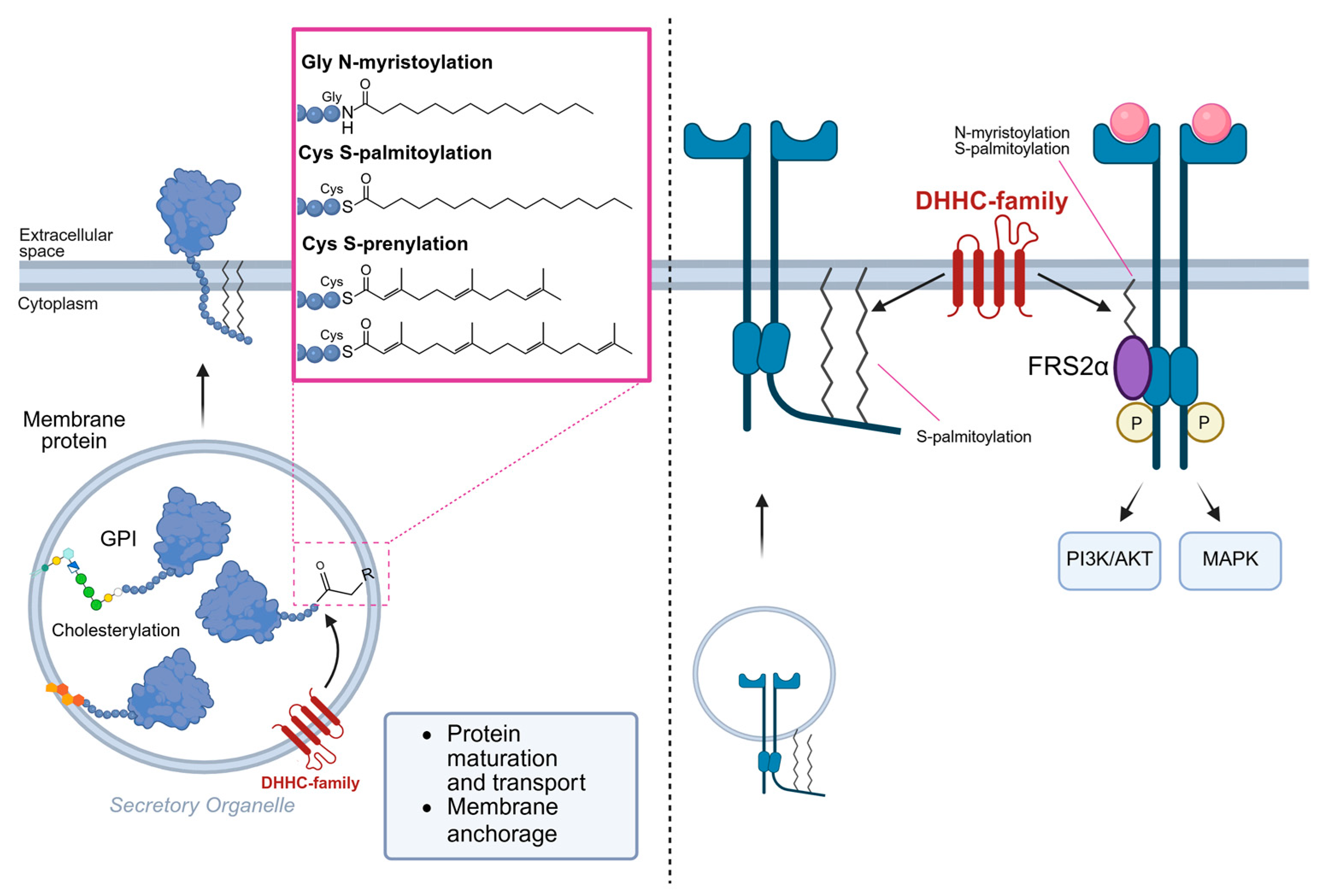
4.1. RTK Lipidation
4.2. Lipid Regulation of RTK Activation and Intracellular Signaling
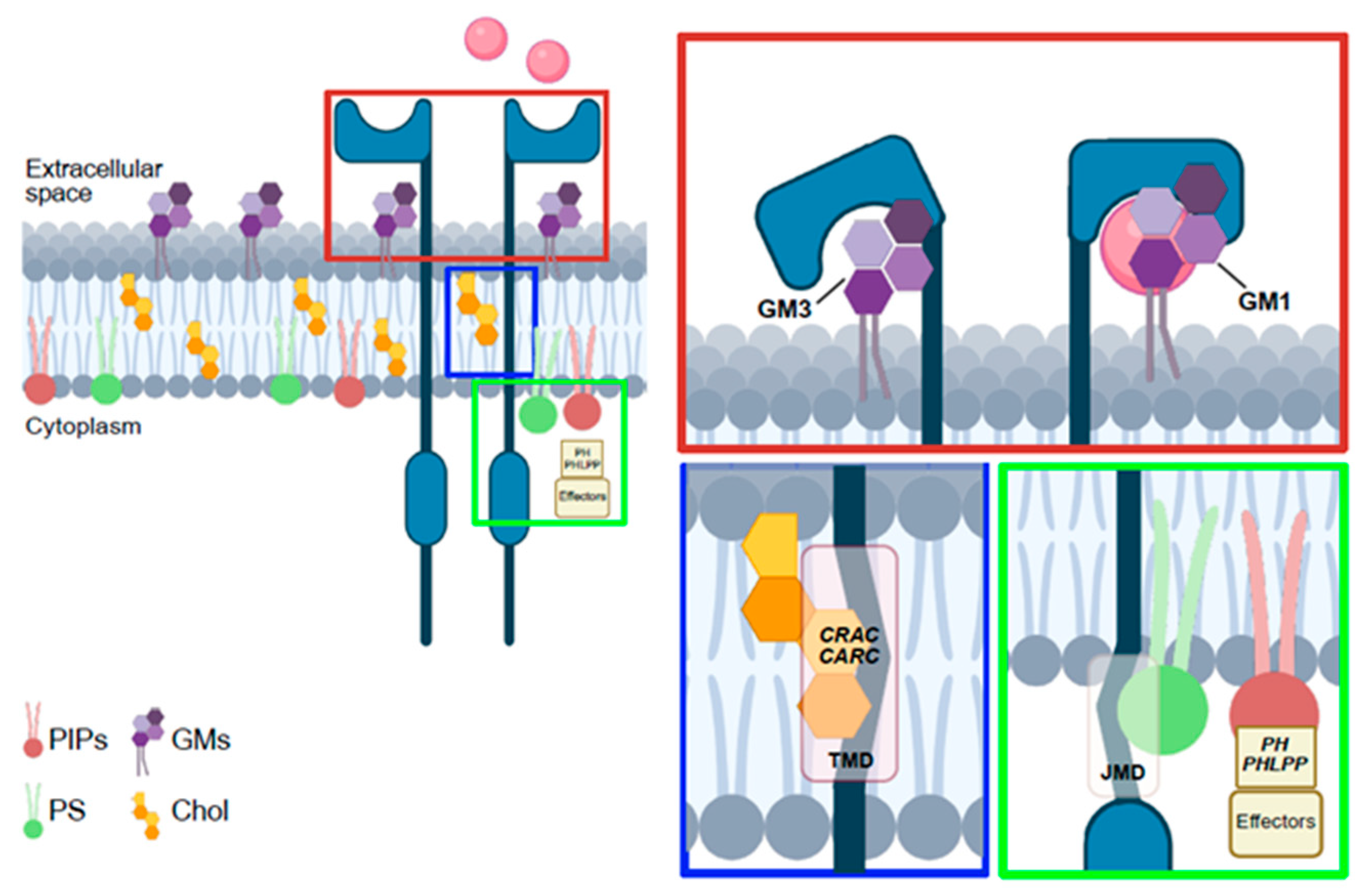
4.3. Lipids Spatially Regulate RTKs
5. Lipid-Targeted Therapeutic Strategies
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhang, N.; Li, Y. Receptor tyrosine kinases: Biological functions and anticancer targeted therapy. MedComm 2023, 4, e446. [Google Scholar] [CrossRef] [PubMed]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.P. Bilayer membrane interactions with nanofabricated scaffolds. Chem. Phys. Lipids 2015, 192, 75–86. [Google Scholar] [CrossRef]
- Horn, A.; Jaiswal, J.K. Structural and signaling role of lipids in plasma membrane repair. Curr. Top Membr. 2019, 84, 67–98. [Google Scholar] [CrossRef] [PubMed]
- Ces, O.; Mulet, X. Physical coupling between lipids and proteins: A paradigm for cellular control. Signal Transduct. 2006, 6, 112–132. [Google Scholar] [CrossRef]
- Dymond, M.K. Lipid monolayer spontaneous curvatures: A collection of published values. Chem. Phys. Lipids 2021, 239, 105117. [Google Scholar] [CrossRef]
- Murru, E.; Manca, C.; Carta, G.; Banni, S. Impact of Dietary Palmitic Acid on Lipid Metabolism. Front Nutr. 2022, 9, 861664. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Kumar, A.; Baycin-Hizal, D.; Zhang, Y.; Bowen, M.A.; Betenbaugh, M.J. Cellular traffic cops: The interplay between lipids and proteins regulates vesicular formation, trafficking, and signaling in mammalian cells. Curr. Opin Biotechnol. 2015, 36, 215–221. [Google Scholar] [CrossRef]
- Levental, I.; Veatch, S. The Continuing Mystery of Lipid Rafts. J. Mol. Biol. 2016, 428, 4749–4764. [Google Scholar] [CrossRef]
- Li, B.; Qin, Y.; Yu, X.; Xu, X.; Yu, W. Lipid raft involvement in signal transduction in cancer cell survival, cell death and metastasis. Cell Prolif. 2022, 55, e13167. [Google Scholar] [CrossRef]
- Briand, N.; Prado, C.; Mabilleau, G.; Lasnier, F.; Le Lièpvre, X.; Covington, J.D.; Ravussin, E.; Le Lay, S.; Dugail, I. Caveolin-1 expression and cavin stability regulate caveolae dynamics in adipocyte lipid store fluctuation. Diabetes 2014, 63, 4032–4044. [Google Scholar] [CrossRef]
- Le, P.U.; Nabi, I.R. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J. Cell Sci. 2003, 116, 1059–1071. [Google Scholar] [CrossRef]
- Mangiarotti, A.; Sabri, E.; Schmidt, K.V.; Hoffmann, C.; Milovanovic, D.; Lipowsky, R.; Dimova, R. Lipid packing and cholesterol content regulate membrane wetting and remodeling by biomolecular condensates. Nat. Commun. 2025, 16, 2756. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D.; Keller, S.L. Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc. Natl. Acad. Sci. USA 2008, 105, 124–128. [Google Scholar] [CrossRef]
- Subra, C.; Laulagnier, K.; Perret, B.; Record, M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 2007, 89, 205–212. [Google Scholar] [CrossRef]
- Miller, W.T. Tyrosine kinase signaling and the emergence of multicellularity. Biochim. Biophys. Acta 2012, 1823, 1053–1057. [Google Scholar] [CrossRef]
- Haglund, K.; Rusten, T.E.; Stenmark, H. Aberrant receptor signaling and trafficking as mechanisms in oncogenesis. Crit. Rev. Oncog. 2007, 13, 39–74. [Google Scholar] [CrossRef]
- Tomuleasa, C.; Tigu, A.B.; Munteanu, R.; Moldovan, C.S.; Kegyes, D.; Onaciu, A.; Gulei, D.; Ghiaur, G.; Einsele, H.; Croce, C.M. Therapeutic advances of targeting receptor tyrosine kinases in cancer. Signal Transduct. Target Ther. 2024, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Hristova, K. Receptor tyrosine kinase transmembrane domains: Function, dimer structure and dimerization energetics. Cell Adh. Migr. 2010, 4, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Vaparanta, K.; Jokilammi, A.; Tamirat, M.; Merilahti, J.A.M.; Salokas, K.; Varjosalo, M.; Ivaska, J.; Johnson, M.S.; Elenius, K. An extracellular receptor tyrosine kinase motif orchestrating intracellular STAT activation. Nat. Commun. 2022, 13, 6953. [Google Scholar] [CrossRef] [PubMed]
- Arter, C.; Trask, L.; Ward, S.; Yeoh, S.; Bayliss, R. Structural features of the protein kinase domain and targeted binding by small-molecule inhibitors. J. Biol. Chem. 2022, 298, 102247. [Google Scholar] [CrossRef]
- Ung, P.M.; Rahman, R.; Schlessinger, A. Redefining the Protein Kinase Conformational Space with Machine Learning. Cell Chem. Biol. 2018, 25, 916–924.e912. [Google Scholar] [CrossRef] [PubMed]
- Lew, E.D.; Furdui, C.M.; Anderson, K.S.; Schlessinger, J. The precise sequence of FGF receptor autophosphorylation is kinetically driven and is disrupted by oncogenic mutations. Sci. Signal 2009, 2, ra6. [Google Scholar] [CrossRef]
- Ravelli, C.; Grillo, E.; Corsini, M.; Coltrini, D.; Presta, M.; Mitola, S. β3 Integrin Promotes Long-Lasting Activation and Polarization of Vascular Endothelial Growth Factor Receptor 2 by Immobilized Ligand. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2161–2171. [Google Scholar] [CrossRef]
- Ravelli, C.; Corsini, M.; Bresciani, R.; Rizzo, A.M.; Zammataro, L.; Corsetto, P.A.; Grillo, E.; Mitola, S. Cancer-associated VEGFR2. Neoplasia 2025, 67, 101195. [Google Scholar] [CrossRef]
- Baldanzi, G.; Mitola, S.; Cutrupi, S.; Filigheddu, N.; van Blitterswijk, W.J.; Sinigaglia, F.; Bussolino, F.; Graziani, A. Activation of diacylglycerol kinase alpha is required for VEGF-induced angiogenic signaling in vitro. Oncogene 2004, 23, 4828–4838. [Google Scholar] [CrossRef]
- Hifdi, N.; Vaucourt, M.; Hnia, K.; Panasyuk, G.; Vandromme, M. Phosphoinositide signaling in the nucleus: Impacts on chromatin and transcription regulation. Biol. Cell 2025, 117, e2400096. [Google Scholar] [CrossRef]
- Yin, H.L.; Janmey, P.A. Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 2003, 65, 761–789. [Google Scholar] [CrossRef]
- Casaletto, J.B.; McClatchey, A.I. Spatial regulation of receptor tyrosine kinases in development and cancer. Nat. Rev. Cancer 2012, 12, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Grillo, E.; Ravelli, C.; Corsini, M.; Zammataro, L.; Mitola, S. Protein domain-based approaches for the identification and prioritization of therapeutically actionable cancer variants. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188614. [Google Scholar] [CrossRef]
- Hilton, K.L.F.; Manwani, C.; Boles, J.E.; White, L.J.; Ozturk, S.; Garrett, M.D.; Hiscock, J.R. The phospholipid membrane compositions of bacterial cells, cancer cell lines and biological samples from cancer patients. Chem. Sci. 2021, 12, 13273–13282. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, P.; Li, J.; Zhao, Q.; Chang, Y.; He, X. Protein lipidation in health and disease: Molecular basis, physiological function and pathological implication. Signal Transduct. Target. Ther. 2024, 9, 60. [Google Scholar] [CrossRef]
- Wang, R.; Chen, Y.Q. Protein Lipidation Types: Current Strategies for Enrichment and Characterization. Int. J. Mol. Sci. 2022, 23, 2365. [Google Scholar] [CrossRef]
- Kim, D.H.; Triet, H.M.; Ryu, S.H. Regulation of EGFR activation and signaling by lipids on the plasma membrane. Prog. Lipid. Res. 2021, 83, 101115. [Google Scholar] [CrossRef]
- Kadry, Y.A.; Lee, J.Y.; Witze, E.S. Regulation of EGFR signalling by palmitoylation and its role in tumorigenesis. Open Biol. 2021, 11, 210033. [Google Scholar] [CrossRef]
- Coleman, D.T.; Gray, A.L.; Kridel, S.J.; Cardelli, J.A. Palmitoylation regulates the intracellular trafficking and stability of c-Met. Oncotarget 2016, 7, 32664–32677. [Google Scholar] [CrossRef]
- Adachi, N.; Hess, D.T.; McLaughlin, P.; Stamler, J.S. S-Palmitoylation of a Novel Site in the β2-Adrenergic Receptor Associated with a Novel Intracellular Itinerary. J. Biol. Chem. 2016, 291, 20232–20246. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, X.; Chen, X.; Aramsangtienchai, P.; Tong, Z.; Lin, H. Protein Lipidation: Occurrence, Mechanisms, Biological Functions, and Enabling Technologies. Chem. Rev. 2018, 118, 919–988. [Google Scholar] [CrossRef] [PubMed]
- Barylko, B.; Chen, Y.J.; Hennen, J.; Angert, I.; Chen, Y.; Mueller, J.D.; Sun, H.Q.; Taylor, C.A.; Liou, J.; Yin, H.; et al. Myristoylation-Dependent Palmitoylation of the Receptor Tyrosine Kinase Adaptor FRS2α. Biochemistry 2019, 58, 2809–2813, Erratum in Biochemistry 2019, 58, 5098. [Google Scholar] [CrossRef] [PubMed]
- Bocharov, E.V.; Sharonov, G.V.; Bocharova, O.V.; Pavlov, K.V. Conformational transitions and interactions underlying the function of membrane embedded receptor protein kinases. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1417–1429. [Google Scholar] [CrossRef]
- Hedger, G.; Sansom, M.S.; Koldsø, H. The juxtamembrane regions of human receptor tyrosine kinases exhibit conserved interaction sites with anionic lipids. Sci. Rep. 2015, 5, 9198. [Google Scholar] [CrossRef]
- Michailidis, I.E.; Rusinova, R.; Georgakopoulos, A.; Chen, Y.; Iyengar, R.; Robakis, N.K.; Logothetis, D.E.; Baki, L. Phosphatidylinositol-4,5-bisphosphate regulates epidermal growth factor receptor activation. Pflugers Arch. 2011, 461, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Rybak, J.A.; Schuck, R.J.; Sahoo, A.R.; Buck, M.; Barrera, F.N.; Smith, A.W. Phosphatidylinositol 4,5-bisphosphate drives the formation of EGFR and EphA2 complexes. Sci. Adv. 2024, 10, eadl0649. [Google Scholar] [CrossRef]
- Julien, S.; Bobowski, M.; Steenackers, A.; Le Bourhis, X.; Delannoy, P. How Do Gangliosides Regulate RTKs Signaling? Cells 2013, 2, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Coskun, Ü.; Grzybek, M.; Drechsel, D.; Simons, K. Regulation of human EGF receptor by lipids. Proc. Natl. Acad. Sci. USA 2011, 108, 9044–9048. [Google Scholar] [CrossRef] [PubMed]
- Meuillet, E.J.; Mania-Farnell, B.; George, D.; Inokuchi, J.I.; Bremer, E.G. Modulation of EGF receptor activity by changes in the GM3 content in a human epidermoid carcinoma cell line, A431. Exp. Cell Res. 2000, 256, 74–82. [Google Scholar] [CrossRef]
- Suzuki, M.; Nagane, M.; Kato, K.; Yamauchi, A.; Shimizu, T.; Yamashita, H.; Aihara, N.; Kamiie, J.; Kawashima, N.; Naito, S.; et al. Endothelial ganglioside GM3 regulates angiogenesis in solid tumors. Biochem. Biophys. Res. Commun. 2021, 569, 10–16. [Google Scholar] [CrossRef]
- Chung, T.W.; Kim, S.J.; Choi, H.J.; Kim, K.J.; Kim, M.J.; Kim, S.H.; Lee, H.J.; Ko, J.H.; Lee, Y.C.; Suzuki, A.; et al. Ganglioside GM3 inhibits VEGF/VEGFR-2-mediated angiogenesis: Direct interaction of GM3 with VEGFR-2. Glycobiology 2009, 19, 229–239. [Google Scholar] [CrossRef]
- Mukherjee, P.; Faber, A.C.; Shelton, L.M.; Baek, R.C.; Chiles, T.C.; Seyfried, T.N. Thematic review series: Sphingolipids. Ganglioside GM3 suppresses the proangiogenic effects of vascular endothelial growth factor and ganglioside GD1a. J. Lipid Res. 2008, 49, 929–938. [Google Scholar] [CrossRef]
- Lunghi, G.; Fazzari, M.; Ciampa, M.G.; Mauri, L.; Di Biase, E.; Chiricozzi, E.; Sonnino, S. Regulation of signal transduction by gangliosides in lipid rafts: Focus on GM3-IR and GM1-TrkA interactions. FEBS Lett. 2022, 596, 3124–3132. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Ledeen, R. The Key Role of GM1 Ganglioside in Parkinson’s Disease. Biomolecules 2022, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Rusnati, M.; Urbinati, C.; Tanghetti, E.; Dell’Era, P.; Lortat-Jacob, H.; Presta, M. Cell membrane GM1 ganglioside is a functional coreceptor for fibroblast growth factor 2. Proc. Natl. Acad. Sci. USA 2002, 99, 4367–4372. [Google Scholar] [CrossRef]
- Cannarozzo, C.; Fred, S.M.; Girych, M.; Biojone, C.; Enkavi, G.; Róg, T.; Vattulainen, I.; Casarotto, P.C.; Castrén, E. Cholesterol-recognition motifs in the transmembrane domain of the tyrosine kinase receptor family: The case of TRKB. Eur. J. Neurosci. 2021, 53, 3311–3322. [Google Scholar] [CrossRef] [PubMed]
- Girych, M.; Kulig, W.; Enkavi, G.; Vattulainen, I. How Neuromembrane Lipids Modulate Membrane Proteins: Insights from G-Protein-Coupled Receptors (GPCRs) and Receptor Tyrosine Kinases (RTKs). Cold Spring Harb. Perspect. Biol. 2023, 15, a041419. [Google Scholar] [CrossRef]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug. Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Reyes, G.; Niederst, M.; Cohen-Katsenelson, K.; Stender, J.D.; Kunkel, M.T.; Chen, M.; Brognard, J.; Sierecki, E.; Gao, T.; Nowak, D.G.; et al. Pleckstrin homology domain leucine-rich repeat protein phosphatases set the amplitude of receptor tyrosine kinase output. Proc. Natl. Acad. Sci. USA 2014, 111, E3957–E3965. [Google Scholar] [CrossRef]
- Gao, T.; Furnari, F.; Newton, A.C. PHLPP: A phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol. Cell 2005, 18, 13–24. [Google Scholar] [CrossRef]
- Huang, B.X.; Akbar, M.; Kevala, K.; Kim, H.Y. Phosphatidylserine is a critical modulator for Akt activation. J. Cell Biol. 2011, 192, 979–992. [Google Scholar] [CrossRef]
- McKay, M.M.; Morrison, D.K. Integrating signals from RTKs to ERK/MAPK. Oncogene 2007, 26, 3113–3121. [Google Scholar] [CrossRef]
- Anderson, D.H. Role of lipids in the MAPK signaling pathway. Prog. Lipid Res. 2006, 45, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Mangiarotti, A.; Chen, N.; Zhao, Z.; Lipowsky, R.; Dimova, R. Wetting and complex remodeling of membranes by biomolecular condensates. Nat. Commun. 2023, 14, 2809. [Google Scholar] [CrossRef] [PubMed]
- Rohwedder, A.; Knipp, S.; Roberts, L.D.; Ladbury, J.E. Composition of receptor tyrosine kinase-mediated lipid micro-domains controlled by adaptor protein interaction. Sci. Rep. 2021, 11, 6160. [Google Scholar] [CrossRef]
- Pereira, D.B.; Chao, M.V. The tyrosine kinase Fyn determines the localization of TrkB receptors in lipid rafts. J. Neurosci. 2007, 27, 4859–4869. [Google Scholar] [CrossRef]
- Zabroski, I.O.; Nugent, M.A. Lipid Raft Association Stabilizes VEGF Receptor 2 in Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 798. [Google Scholar] [CrossRef]
- Laurenzana, A.; Fibbi, G.; Chillà, A.; Margheri, G.; Del Rosso, T.; Rovida, E.; Del Rosso, M.; Margheri, F. Lipid rafts: Integrated platforms for vascular organization offering therapeutic opportunities. Cell. Mol. Life Sci. 2015, 72, 1537–1557. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J.; Casey, L. Cholesterol levels modulate EGF receptor-mediated signaling by altering receptor function and trafficking. Biochemistry 2002, 41, 10315–10322. [Google Scholar] [CrossRef]
- Lu, Y.C.; Chen, H.C. Involvement of lipid rafts in adhesion-induced activation of Met and EGFR. J. Biomed. Sci. 2011, 18, 78. [Google Scholar] [CrossRef]
- Balbis, A.; Posner, B.I. Compartmentalization of EGFR in cellular membranes: Role of membrane rafts. J. Cell Biochem. 2010, 109, 1103–1108. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Brandan, E. A novel mechanism of sequestering fibroblast growth factor 2 by glypican in lipid rafts, allowing skeletal muscle differentiation. Mol. Cell Biol. 2010, 30, 1634–1649. [Google Scholar] [CrossRef]
- Dirkx, E.; Schwenk, R.W.; Glatz, J.F.; Luiken, J.J.; van Eys, G.J. High fat diet induced diabetic cardiomyopathy. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 219–225. [Google Scholar] [CrossRef]
- Martín-Segura, A.; Ahmed, T.; Casadomé-Perales, Á.; Palomares-Perez, I.; Palomer, E.; Kerstens, A.; Munck, S.; Balschun, D.; Dotti, C.G. Age-associated cholesterol reduction triggers brain insulin resistance by facilitating ligand-independent receptor activation and pathway desensitization. Aging Cell 2019, 18, e12932. [Google Scholar] [CrossRef]
- Egawa, J.; Pearn, M.L.; Lemkuil, B.P.; Patel, P.M.; Head, B.P. Membrane lipid rafts and neurobiology: Age-related changes in membrane lipids and loss of neuronal function. J. Physiol. 2016, 594, 4565–4579. [Google Scholar] [CrossRef] [PubMed]
- Sural-Fehr, T.; Singh, H.; Cantuti-Catelvetri, L.; Zhu, H.; Marshall, M.S.; Rebiai, R.; Jastrzebski, M.J.; Givogri, M.I.; Rasenick, M.M.; Bongarzone, E.R. Inhibition of the IGF-1-PI3K-Akt-mTORC2 pathway in lipid rafts increases neuronal vulnerability in a genetic lysosomal glycosphingolipidosis. Dis. Model Mech. 2019, 12, dmm036590. [Google Scholar] [CrossRef] [PubMed]
- Dolganiuc, A. Role of lipid rafts in liver health and disease. World J. Gastroenterol. 2011, 17, 2520–2535. [Google Scholar] [CrossRef] [PubMed]
- Kosti, A.; Chiou, J.; Guardia, G.D.A.; Lei, X.; Balinda, H.; Landry, T.; Lu, X.; Qiao, M.; Gilbert, A.; Brenner, A.; et al. ELF4 is a critical component of a miRNA-transcription factor network and is a bridge regulator of glioblastoma receptor signaling and lipid dynamics. Neuro-Oncol. 2023, 25, 459–470. [Google Scholar] [CrossRef]
- Codini, M.; Garcia-Gil, M.; Albi, E. Cholesterol and Sphingolipid Enriched Lipid Rafts as Therapeutic Targets in Cancer. Int. J. Mol. Sci. 2021, 22, 726. [Google Scholar] [CrossRef]
- Irwin, M.E.; Mueller, K.L.; Bohin, N.; Ge, Y.; Boerner, J.L. Lipid raft localization of EGFR alters the response of cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J. Cell. Physiol. 2011, 226, 2316–2328. [Google Scholar] [CrossRef]
- Sottocornola, E.; Misasi, R.; Mattei, V.; Ciarlo, L.; Gradini, R.; Garofalo, T.; Berra, B.; Colombo, I.; Sorice, M. Role of gangliosides in the association of ErbB2 with lipid rafts in mammary epithelial HC11 cells. FEBS J. 2006, 273, 1821–1830. [Google Scholar] [CrossRef]
- Rysman, E.; Brusselmans, K.; Scheys, K.; Timmermans, L.; Derua, R.; Munck, S.; Van Veldhoven, P.P.; Waltregny, D.; Daniëls, V.W.; Machiels, J.; et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010, 70, 8117–8126. [Google Scholar] [CrossRef]
- Soto-Guzman, A.; Robledo, T.; Lopez-Perez, M.; Salazar, E.P. Oleic acid induces ERK1/2 activation and AP-1 DNA binding activity through a mechanism involving Src kinase and EGFR transactivation in breast cancer cells. Mol. Cell. Endocrinol. 2008, 294, 81–91. [Google Scholar] [CrossRef]
- Pierchala, B.A.; Milbrandt, J.; Johnson, E.M. Glial cell line-derived neurotrophic factor-dependent recruitment of Ret into lipid rafts enhances signaling by partitioning Ret from proteasome-dependent degradation. J. Neurosci. 2006, 26, 2777–2787. [Google Scholar] [CrossRef]
- Liu, H.; Paddock, M.N.; Wang, H.; Murphy, C.J.; Geck, R.C.; Navarro, A.J.; Wulf, G.M.; Elemento, O.; Haucke, V.; Cantley, L.C.; et al. The INPP4B Tumor Suppressor Modulates EGFR Trafficking and Promotes Triple-Negative Breast Cancer. Cancer Discov. 2020, 10, 1226–1239. [Google Scholar] [CrossRef]
- Zeno, W.F.; Thatte, A.S.; Wang, L.; Snead, W.T.; Lafer, E.M.; Stachowiak, J.C. Molecular Mechanisms of Membrane Curvature Sensing by a Disordered Protein. J. Am. Chem. Soc. 2019, 141, 10361–10371. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Kondo, A.; Itoh, T. A curvature-dependent membrane binding by tyrosine kinase Fer involves an intrinsically disordered region. Biochem. Biophys. Res. Commun. 2018, 495, 1522–1527. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Somasundaram, P.; Karimi, I.; Lagunas-Rangel, F.A.; Alsehli, A.M.; Fredriksson, R.; Jonsson, J.; Schiöth, H.B. Statin drugs and lipid modulation: Mechanistic basis considering lipid rafts, kinase signaling, myopathy, and cancer. Pharmacol. Res. 2025, 220, 107912. [Google Scholar] [CrossRef] [PubMed]
- Polita, A.R.; Bagdonaitė, R.T.; Shivabalan, A.P.; Valinčius, G. Influence of Simvastatin and Pravastatin on the Biophysical Properties of Model Lipid Bilayers and Plasma Membranes of Live Cells. ACS Biomater. Sci. Eng. 2024, 10, 5714–5722. [Google Scholar] [CrossRef]
- Redondo-Morata, L.; Lea Sanford, R.; Andersen, O.S.; Scheuring, S. Effect of Statins on the Nanomechanical Properties of Supported Lipid Bilayers. Biophys. J. 2016, 111, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Valenza, M.; Carroll, J.B.; Leoni, V.; Bertram, L.N.; Björkhem, I.; Singaraja, R.R.; Di Donato, S.; Lutjohann, D.; Hayden, M.R.; Cattaneo, E. Cholesterol biosynthesis pathway is disturbed in YAC128 mice and is modulated by huntingtin mutation. Hum. Mol. Genet. 2007, 16, 2187–2198. [Google Scholar] [CrossRef]
- van der Luit, A.H.; Vink, S.R.; Klarenbeek, J.B.; Perrissoud, D.; Solary, E.; Verheij, M.; van Blitterswijk, W.J. A new class of anticancer alkylphospholipids uses lipid rafts as membrane gateways to induce apoptosis in lymphoma cells. Mol. Cancer Ther. 2007, 6, 2337–2345. [Google Scholar] [CrossRef]
- Dymond, M.; Attard, G.; Postle, A.D. Testing the hypothesis that amphiphilic antineoplastic lipid analogues act through reduction of membrane curvature elastic stress. J. R. Soc. Interface 2008, 5, 1371–1386. [Google Scholar] [CrossRef]
- Chen, X.; Resh, M.D. Cholesterol depletion from the plasma membrane triggers ligand-independent activation of the epidermal growth factor receptor. J. Biol. Chem. 2002, 277, 49631–49637. [Google Scholar] [CrossRef]
- Noghero, A.; Perino, A.; Seano, G.; Saglio, E.; Lo Sasso, G.; Veglio, F.; Primo, L.; Hirsch, E.; Bussolino, F.; Morello, F. Liver X receptor activation reduces angiogenesis by impairing lipid raft localization and signaling of vascular endothelial growth factor receptor-2. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2280–2288. [Google Scholar] [CrossRef]
- Sviridov, D.; Mukhamedova, N.; Miller, Y.I. Lipid rafts as a therapeutic target. J. Lipid Res. 2020, 61, 687–695. [Google Scholar] [CrossRef]
- Runkle, K.B.; Kharbanda, A.; Stypulkowski, E.; Cao, X.J.; Wang, W.; Garcia, B.A.; Witze, E.S. Inhibition of DHHC20-Mediated EGFR Palmitoylation Creates a Dependence on EGFR Signaling. Mol. Cell 2016, 62, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Richartz, J.; Yam, S.C.; Zhan, N.; Schepers, M.; Tiane, A.; Mulder, M.T.; Wens, I.; Vanmierlo, T. Liver X receptors: A therapeutic target in demyelinating disorders. Pharmacol. Res. 2025, 219, 107861. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Peiffer, C. Targeting ceramide synthesis to reverse insulin resistance. Diabetes 2010, 59, 2351–2353. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, A.; Stachowicz, A.; Kuś, K.; Ulatowska-Białas, M.; Totoń-Żurańska, J.; Kiepura, A.; Stachyra, K.; Suski, M.; Gajda, M.; Jawień, J.; et al. Inhibition of Atherosclerosis and Liver Steatosis by Agmatine in Western Diet-Fed apoE-Knockout Mice Is Associated with Decrease in Hepatic De Novo Lipogenesis and Reduction in Plasma Triglyceride/High-Density Lipoprotein Cholesterol Ratio. Int. J. Mol. Sci. 2021, 22, 10688. [Google Scholar] [CrossRef] [PubMed]
- Rodal, S.K.; Skretting, G.; Garred, O.; Vilhardt, F.; van Deurs, B.; Sandvig, K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 1999, 10, 961–974. [Google Scholar] [CrossRef]
- Grosse, P.Y.; Bressolle, F.; Pinguet, F. Antiproliferative effect of methyl-beta-cyclodextrin in vitro and in human tumour xenografted athymic nude mice. Br. J. Cancer 1998, 78, 1165–1169. [Google Scholar] [CrossRef]
- Argiris, A.; Cohen, E.; Karrison, T.; Esparaz, B.; Mauer, A.; Ansari, R.; Wong, S.; Lu, Y.; Pins, M.; Dancey, J.; et al. A phase II trial of perifosine, an oral alkylphospholipid, in recurrent or metastatic head and neck cancer. Cancer Biol. Ther. 2006, 5, 766–770. [Google Scholar] [CrossRef]
- Jiménez-López, J.M.; Ríos-Marco, P.; Marco, C.; Segovia, J.L.; Carrasco, M.P. Alterations in the homeostasis of phospholipids and cholesterol by antitumor alkylphospholipids. Lipids Health Dis. 2010, 9, 33. [Google Scholar] [CrossRef]
- Zhang, R.; Wuerch, E.; Yong, V.W.; Xue, M. LXR agonism for CNS diseases: Promises and challenges. J. Neuroinflamm. 2024, 21, 97. [Google Scholar] [CrossRef]
- Liu, S.; Cao, H.; Chen, D.; Yu, S.; Sha, H.; Wu, J.; Ma, R.; Wang, Z.; Jing, C.; Zhang, J.; et al. LXR ligands induce apoptosis of EGFR-TKI-resistant human lung cancer cells. Oncol. Lett. 2018, 15, 7168–7174. [Google Scholar] [CrossRef]
- Wajapeyee, N.; Beamon, T.C.; Gupta, R. Roles and therapeutic targeting of ceramide metabolism in cancer. Mol. Metab. 2024, 83, 101936. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Y.; Du, J.; Liu, F.; Wu, C.; Xiao, W.; Yu, G.; Chen, X.; Gale, R.P.; Zeng, H. Targeting ceramide transfer protein sensitizes AML to FLT3 inhibitors via a GRP78-ATF6-CHOP axis. Nat. Commun. 2025, 16, 1358. [Google Scholar] [CrossRef] [PubMed]
- Iturrioz-Rodríguez, N.; Bertorelli, R.; Ciofani, G. Lipid-Based Nanocarriers for The Treatment of Glioblastoma. Adv. Nanobiomed. Res. 2021, 1, 2000054. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhang, Y.; Shao, W.; Kankala, R.K.; Chen, A. β-Cyclodextrin-based nanoassemblies for the treatment of atherosclerosis. Regen. Biomater. 2024, 11, rbae071. [Google Scholar] [CrossRef]
- Mall, J.; Haider, M.F.; Naseem, N.; Rahman, M.A.; Ahmad, I. Lipid-based nanocarriers in combination chemotherapy: A promising strategy for advanced skin cancer management. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 1–29. [Google Scholar] [CrossRef]
| Therapeutic Strategy | Mechanism of Action | Effect | References |
|---|---|---|---|
| Statins | Inhibition of Cholesterol biosynthesis (HMG-CoA reductase), lipids metabolism, and lipid–lipid interaction. Penetration of membrane bilayer. | Lipid rafts’ instability leads RTKs signaling disruption and higher sensitivity to TKIs. Modification of membrane mechanical properties lead to alteration of protein function. | [79,87,90] |
| Methyl-beta-cyclodextrin | Forms inclusion complexes with Cholesterol, extracting it from membranes. | Used as nanocarrier for hydrophobic drugs, impairs lipid-dependent RTK signaling, and can trigger apoptosis | [93,100,101] |
| AIBP, HDL, and mimetics | Targeting of Chol transporters like ABCA1. | Increased Chol efflux, destabilization of lipid rafts, and reduced RTKs signaling | [95] |
| Alkylphospholipids | Insert into plasma membranes, disrupting lipid metabolism, phosphatidylcholine synthesis, and raft-associated signaling. Penetration of membrane bilayer. | Induction of cancer cell apoptosis and regulation of receptor trafficking, regulation of membrane-associated proteins. | [102,103] |
| LXR agonists | Activate LXRs to upregulate Cholesterol efflux transporters (ABCA1, ABCG1), reducing intracellular Cholesterol pools. | Reduce lipid accumulation and membrane rigidity; potential to modulate RTK localization. | [97,104,105] |
| Ceramide inhibitors | Modulation of ceramide biosynthesis and membrane composition. | Restoration of insulin sensitivity. | [98,106,107] |
| DHHC enzymes inhibitors | Modulation of proteins’ lipidation events. | Higher sensitivity to anticancer therapies, increasing sensitivity to TKIs. | [36,96] |
| Diet modulation and metabolites intake | Regulation of fatty acids metabolism and mitochondrial stability. | Restore lipid homeostasis and membrane composition, indirectly influencing RTK activation and downstream signaling. | [99] |
| Lipid-based nanocarriers | Cross blood–brain barrier and metabolic active tissues. | Delivering of RTK-targeted drugs or RNA-based therapies | [108,109,110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domenichini, M.; Gogna, A.; Maggi, C.; Moreschi, E.; Ventura, A.; Codibue, M.; Grillo, E.; Corsini, M.; Mitola, S. Lipid–Protein Interplay in the Regulation of Receptor Tyrosine Kinases. Cells 2025, 14, 1836. https://doi.org/10.3390/cells14231836
Domenichini M, Gogna A, Maggi C, Moreschi E, Ventura A, Codibue M, Grillo E, Corsini M, Mitola S. Lipid–Protein Interplay in the Regulation of Receptor Tyrosine Kinases. Cells. 2025; 14(23):1836. https://doi.org/10.3390/cells14231836
Chicago/Turabian StyleDomenichini, Mattia, Anna Gogna, Camilla Maggi, Elisa Moreschi, Anna Ventura, Martina Codibue, Elisabetta Grillo, Michela Corsini, and Stefania Mitola. 2025. "Lipid–Protein Interplay in the Regulation of Receptor Tyrosine Kinases" Cells 14, no. 23: 1836. https://doi.org/10.3390/cells14231836
APA StyleDomenichini, M., Gogna, A., Maggi, C., Moreschi, E., Ventura, A., Codibue, M., Grillo, E., Corsini, M., & Mitola, S. (2025). Lipid–Protein Interplay in the Regulation of Receptor Tyrosine Kinases. Cells, 14(23), 1836. https://doi.org/10.3390/cells14231836







