CRISPR as a Tool to Uncover Gene Function in Polycystic Ovary Syndrome: A Literature Review of Experimental Models Targeting Ovarian and Metabolic Genes
Highlights
- CRISPR models reveal gene-specific contributions to ovarian and metabolic PCOS phenotypes, including hyperandrogenism and insulin resistance.
- Single-gene models show species-specific effects, highlighting the need for dual-gene and integrated models.
- CRISPR studies identify potential diagnostic markers and clarify gene-hormone interactions in PCOS.
- Combined gene editing and hormonal models provide a platform for studying the polygenic, multifactorial nature of PCOS.
Abstract
1. Introduction
Genetic Landscape of PCOS
2. Overview of CRISPR Technology and Its Applications in Endocrine Research
3. CRISPR in PCOS-Related Ovarian Gene Function
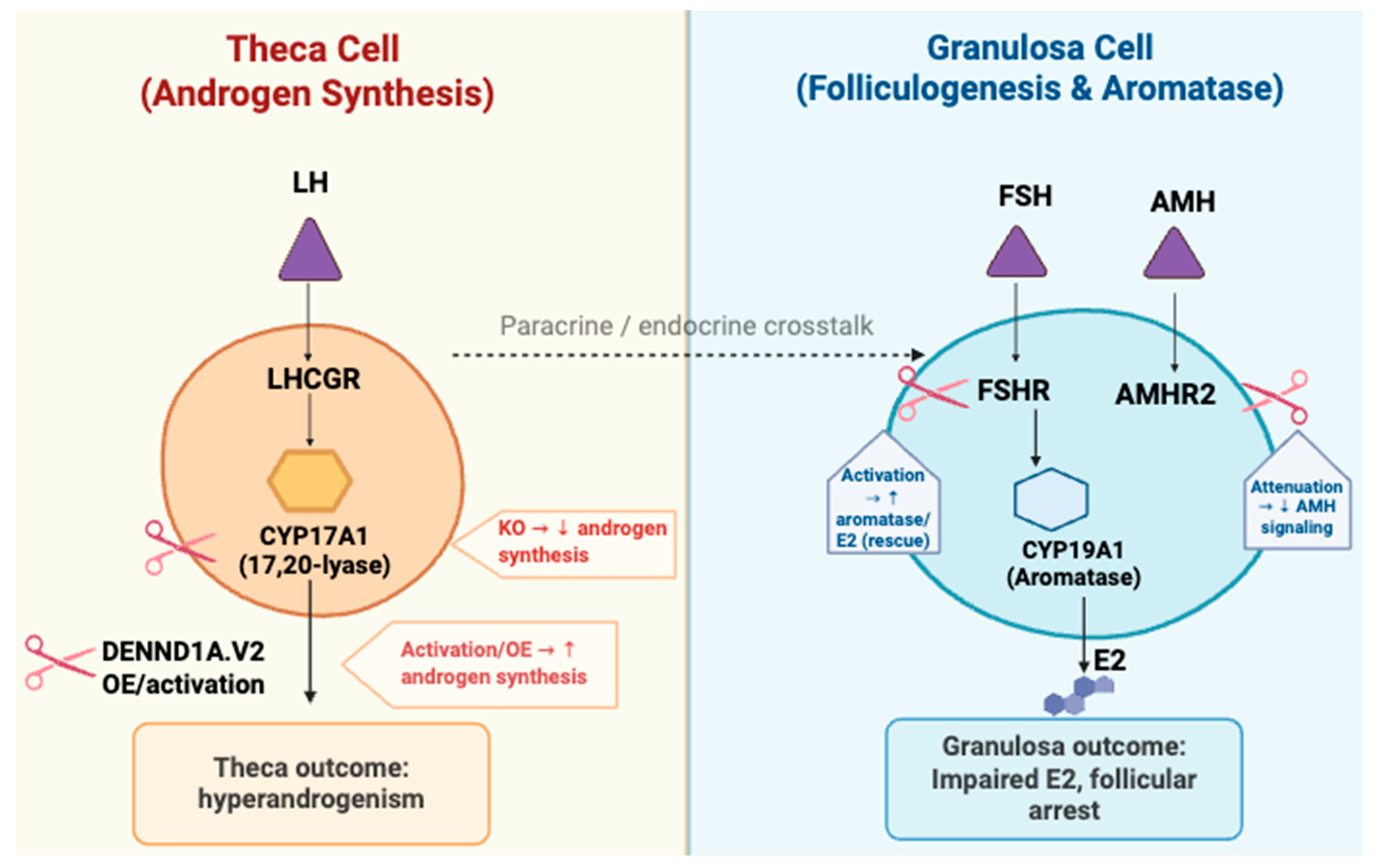
3.1. Theca Cell-Specific Genes
3.2. Granulosa Cell-Related Genes
3.3. Folliculogenesis and Ovulatory Dysfunction
4. CRISPR in Metabolic Gene Function in PCOS
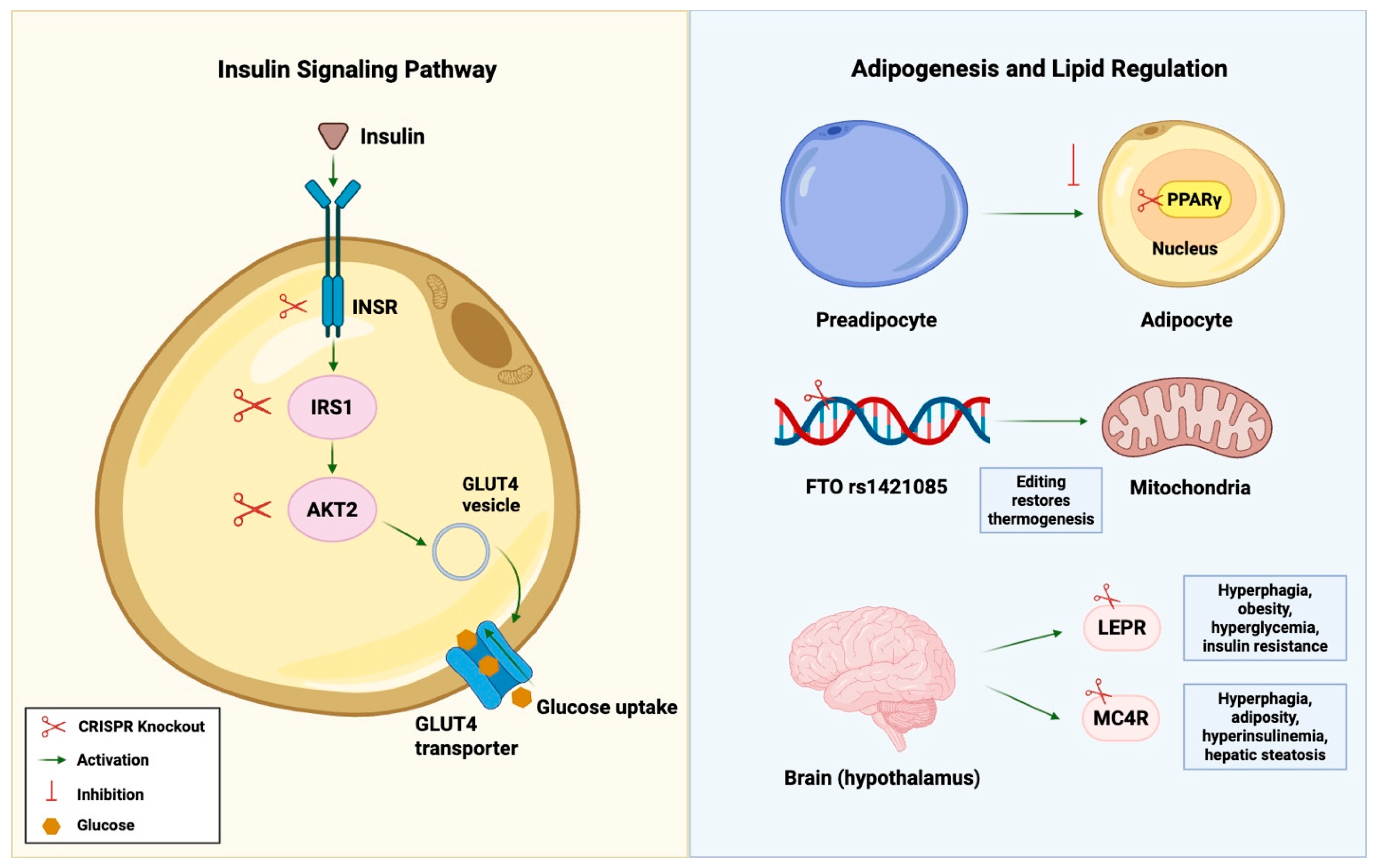
4.1. Insulin Signaling Pathways
4.2. Adipogenesis and Lipid Regulation
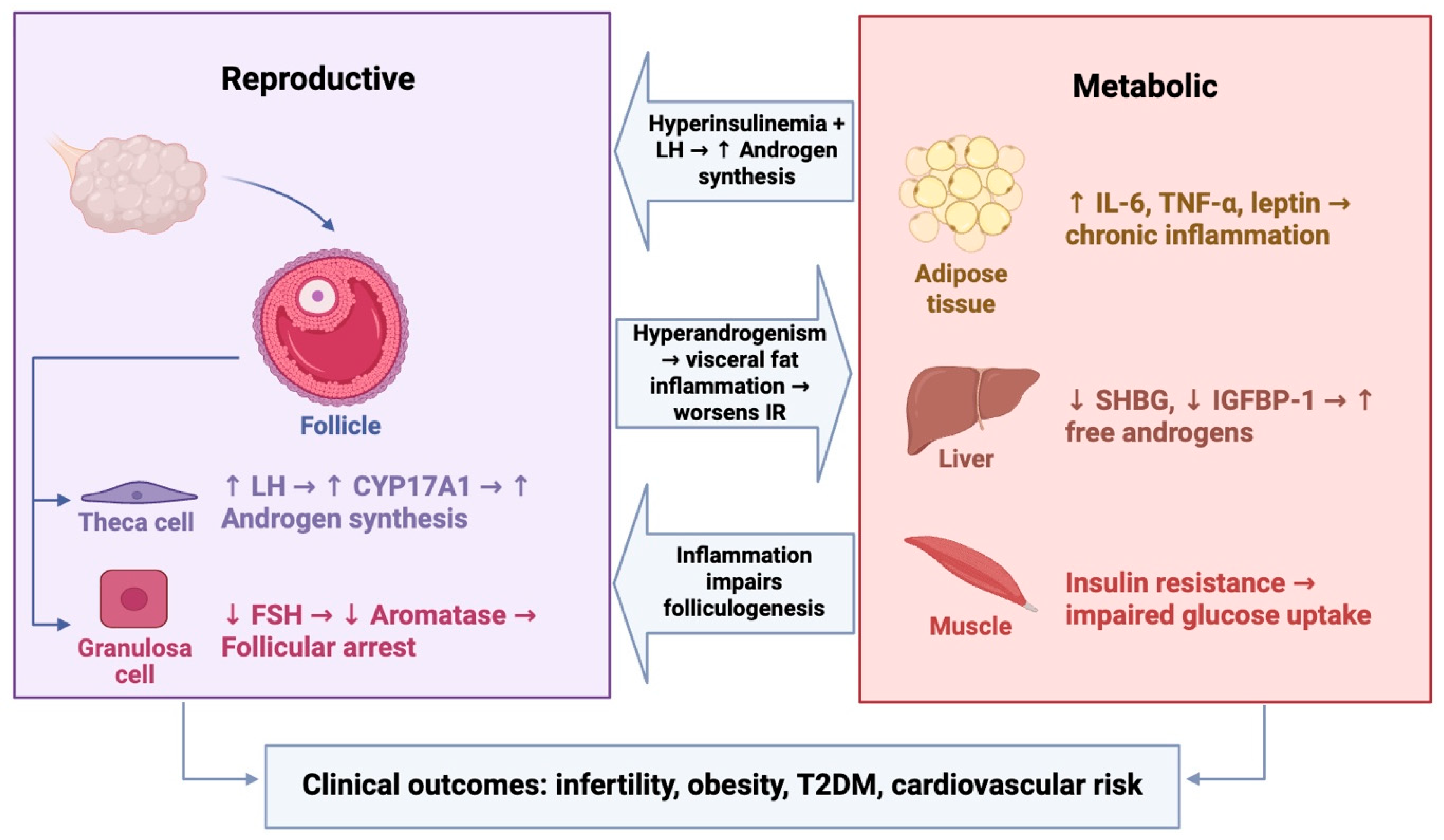
5. Combined Models: Reproductive and Metabolic Phenotypes
5.1. Dual Gene Models
5.2. Integration with Hormonal Induction Models
6. Insights from CRISPR Models
6.1. Validation of Candidate Gene Roles
6.2. Novel Discoveries from Loss-of-Function or Gain-of-Function Studies
7. Future Directions and Clinical Translation
7.1. Integrating CRISPR with Multi-Omic Frameworks
7.2. Precision Editing in PCOS
7.3. Safety, Delivery and Ethical Considerations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siddiqui, S.; Mateen, S.; Ahmad, R.; Moin, S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J. Assist. Reprod. Genet. 2022, 39, 2439–2473. [Google Scholar] [CrossRef]
- Sidra, S.; Tariq, M.H.; Farrukh, M.J.; Mohsin, M. Evaluation of clinical manifestations, health risks, and quality of life among women with polycystic ovary syndrome. PLoS ONE 2019, 14, e0223329. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, L.; Ge, Z.; Pan, Y.; Xie, L. Key Genes Associated With Non-Alcoholic Fatty Liver Disease and Polycystic Ovary Syndrome. Front. Mol. Biosci. 2022, 9, 888194. [Google Scholar] [CrossRef]
- Hoeger, K.M.; Dokras, A.; Piltonen, T. Update on PCOS: Consequences, Challenges, and Guiding Treatment. J. Clin. Endocrinol. Metab. 2020, 106, e1071–e1083. [Google Scholar] [CrossRef]
- Joshi, A. PCOS stratification for precision diagnostics and treatment. Front. Cell Dev. Biol. 2024, 12, 1358755. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, S.; Punjala-Patel, A.; Gavrilova-Jordan, L. Hypothalamic-Pituitary-Ovarian Axis Disorders Impacting Female Fertility. Biomedicines 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Christian, C.A.; Moenter, S.M. The Neurobiology of Preovulatory and Estradiol-Induced Gonadotropin-Releasing Hormone Surges. Endocr. Rev. 2010, 31, 544–577. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.E.; McCourt, B.; Martin, K.A.; Anderson, E.J.; Adams, J.M.; Schoenfeld, D.; Hall, J.E. Determinants of Abnormal Gonadotropin Secretion in Clinically Defined Women with Polycystic Ovary Syndrome1. J. Clin. Endocrinol. Metab. 1997, 82, 2248–2256. [Google Scholar] [CrossRef]
- Zhong, Y.; Ding, J.; Xia, F. DNA methylation and its impact on ovarian steroidogenesis in women with polycystic ovary syndrome: Insights from human and animal models. Arch. Gynecol. Obstet. 2025, 312, 363–374. [Google Scholar] [CrossRef]
- Zheng, M.; Andersen, C.Y.; Rasmussen, F.R.; Cadenas, J.; Christensen, S.T.; Mamsen, L.S. Expression of genes and enzymes involved in ovarian steroidogenesis in relation to human follicular development. Front. Endocrinol. 2023, 14, 1268248. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Panghiyangani, R.; Soeharso, P.; Andrijono; Suryandari, D.A.; Wiweko, B.; Kurniati, M.; Pujianto, D.A. CYP19A1 gene expression in patients with polycystic ovarian syndrome. J. Hum. Reprod. Sci. 2020, 13, 100–103. [Google Scholar] [CrossRef]
- Azhary, J.M.K.; Harada, M.; Kunitomi, C.; Kusamoto, A.; Takahashi, N.; Nose, E.; Oi, N.; Wada-Hiraike, O.; Urata, Y.; Hirata, T.; et al. Androgens Increase Accumulation of Advanced Glycation End Products in Granulosa Cells by Activating ER Stress in PCOS. Endocrinology 2020, 161, bqaa015. [Google Scholar] [CrossRef]
- Liao, B.; Qi, X.; Yun, C.; Qiao, J.; Pang, Y. Effects of Androgen Excess-Related Metabolic Disturbances on Granulosa Cell Function and Follicular Development. Front. Endocrinol. 2022, 13, 815968. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Liao, X.; Wang, Z.; Li, R.; Zou, S.; Jiang, T.; Zheng, B.; Duan, P.; Xiao, J. Diabetes Induces Abnormal Ovarian Function via Triggering Apoptosis of Granulosa Cells and Suppressing Ovarian Angiogenesis. Int. J. Biol. Sci. 2017, 13, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Nose, E.; Takahashi, N.; Hirota, Y.; Hirata, T.; Yoshino, O.; Koga, K.; Fujii, T.; Osuga, Y. Evidence of the activation of unfolded protein response in granulosa and cumulus cells during follicular growth and maturation. Gynecol. Endocrinol. 2015, 31, 783–787. [Google Scholar] [CrossRef]
- Park, H.; Park, J.; Kim, J.; Yang, S.; Jung, J.; Kim, M.; Kang, M.; Cho, Y.H.; Wee, G.; Yang, H.; et al. Melatonin improves the meiotic maturation of porcine oocytes by reducing endoplasmic reticulum stress during in vitro maturation. J. Pineal Res. 2017, 64, e12458. [Google Scholar] [CrossRef]
- Sørensen, A.E.; Udesen, P.B.; Wissing, M.L.; Englund, A.L.M.; Dalgaard, L.T. MicroRNAs related to androgen metabolism and polycystic ovary syndrome. Chem. Interact. 2016, 259, 8–16. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Cara, J.F.; Rosenfield, R.L. Insulin-Like Growth Factor I and Insulin Potentiate Luteinizing Hormone-Induced Androgen Synthesis by Rat Ovarian Thecal-Interstitial Cells. Endocrinology 1988, 123, 733–739. [Google Scholar] [CrossRef]
- Zhang, G.; Garmey, J.C.; Veldhuis, J.D. Interactive Stimulation by Luteinizing Hormone and Insulin of the Steroidogenic Acute Regulatory (StAR) Protein and 17α-Hydroxylase/17, 20-Lyase (CYP17) Genes in Porcine Theca Cells. Endocrinology 2000, 141, 2735–2742. [Google Scholar] [CrossRef]
- Toprak, S.; Yönem, A.; Çakır, B.; Güler, S.; Azal, Ö.; Özata, M.; Çorakçı, A. Insulin Resistance in Nonobese Patients with Polycystic Ovary Syndrome. Horm. Res. Paediatr. 2001, 55, 65–70. [Google Scholar] [CrossRef]
- Bulsara, J.; Patel, P.; Soni, A.; Acharya, S. A review: Brief insight into Polycystic Ovarian syndrome. Endocr. Metab. Sci. 2021, 3, 100085. [Google Scholar] [CrossRef]
- Goodarzi, M.O.; Dumesic, D.A.; Chazenbalk, G.; Azziz, R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 2011, 7, 219–231. [Google Scholar] [CrossRef]
- Rojas, J.; Chávez, M.; Olivar, L.; Rojas, M.; Morillo, J.; Mejías, J.; Calvo, M.; Bermúdez, V. Polycystic Ovary Syndrome, Insulin Resistance, and Obesity: Navigating the Pathophysiologic Labyrinth. Int. J. Reprod. Med. 2014, 2014, 719050. [Google Scholar] [CrossRef] [PubMed]
- Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome: Mechanism and Implications for Pathogenesis. Endocr. Rev. 1997, 18, 774–800. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Fertil. Steril. 2023, 120, 767–793. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, J.; Zhang, F.; Zhang, S.; Chen, X.; Liang, W.; Xie, Q. Resistance to the Insulin and Elevated Level of Androgen: A Major Cause of Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12, 741764. [Google Scholar] [CrossRef]
- Marshall, J.C.; Dunaif, A. Should all women with PCOS be treated for insulin resistance? Fertil. Steril. 2012, 97, 18–22. [Google Scholar] [CrossRef]
- De Leo, V.; la Marca, A.; Petraglia, F. Insulin-Lowering Agents in the Management of Polycystic Ovary Syndrome. Endocr. Rev. 2003, 24, 633–667. [Google Scholar] [CrossRef]
- Kelly, C.C.J.; Lyall, H.; Petrie, J.R.; Gould, G.W.; Connell, J.M.C.; Sattar, N. Low Grade Chronic Inflammation in Women with Polycystic Ovarian Syndrome. J. Clin. Endocrinol. Metab. 2001, 86, 2453–2455. [Google Scholar] [CrossRef]
- Orio, F.; Palomba, S.; Cascella, T.; Di Biase, S.; Manguso, F.; Tauchmanovà, L.; Nardo, L.G.; Labella, D.; Savastano, S.; Russo, T.; et al. The Increase of Leukocytes as a New Putative Marker of Low-Grade Chronic Inflammation and Early Cardiovascular Risk in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Phelan, N.; O’COnnor, A.; Tun, T.K.; Correia, N.; Boran, G.; Roche, H.M.; Gibney, J. Leucocytosis in women with polycystic ovary syndrome (PCOS) is incompletely explained by obesity and insulin resistance. Clin. Endocrinol. 2012, 78, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Botella-Carretero, J.I.; Villuendas, G.; Sancho, J.; Millán, J.L.S. Serum Interleukin-18 Concentrations Are Increased in the Polycystic Ovary Syndrome: Relationship to Insulin Resistance and to Obesity. J. Clin. Endocrinol. Metab. 2004, 89, 806–811. [Google Scholar] [CrossRef]
- Thathapudi, S.; Kodati, V.; Erukkambattu, J.; Katragadda, A.; Addepally, U.; Hasan, Q. Tumor Necrosis Factor-Alpha and Polycystic Ovarian Syndrome: A Clinical, Biochemical, and Molecular Genetic Study. Genet. Test. Mol. Biomark. 2014, 18, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Fadaei, R.; Moradi, N.; Fouani, F.Z.; Roozbehkia, M.; Zandieh, Z.; Ansaripour, S.; Vatannejad, A.; Doustimotlagh, A.H. Circulating levels of C1q/TNF-α-related protein 6 (CTRP6) in polycystic ovary syndrome. IUBMB Life 2020, 72, 1449–1459. [Google Scholar] [CrossRef]
- Yan, S.; Ding, J.; Zhang, Y.; Wang, J.; Zhang, S.; Yin, T.; Yang, J. C1QTNF6 participates in the pathogenesis of PCOS by affecting the inflammatory response of granulosa cells. Biol. Reprod. 2021, 105, 427–438. [Google Scholar] [CrossRef]
- Gonzalez, F.; Thusu, K.; Abdel-Rahman, E.; Prabhala, A.; Tomani, M.; Dandona, P. Elevated serum levels of tumor necrosis factor alpha in normal-weight women with polycystic ovary syndrome. Metabolism 1999, 48, 437–441. [Google Scholar] [CrossRef]
- Sayin, N.C.; Gücer, F.; Balkanli-Kaplan, P.; Yüce, M.A.; Ciftci, S.; Kücük, M.; Yardim, T. Elevated serum TNF-alpha levels in normal-weight women with polycystic ovaries or the polycystic ovary syndrome. J. Reprod. Med. 2003, 48, 165–170. [Google Scholar]
- Amato, G.; Conte, M.; Mazziotti, G.; Lalli, E.; Vitolo, G.; Tucker, A.T.; Bellastella, A.; Carella, C.; Izzo, A. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet. Gynecol. 2003, 101, 1177–1182. [Google Scholar] [CrossRef]
- Knebel, B.; Janssen, O.; Hahn, S.; Jacob, S.; Gleich, J.; Kotzka, J.; Muller-Wieland, D. Increased Low Grade Inflammatory Serum Markers in Patients with Polycystic Ovary Syndrome (PCOS) and their Relationship to PPARγ Gene Variants. Exp. Clin. Endocrinol. Diabetes 2008, 116, 481–486. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Dewailly, D.; Lujan, M.E.; Carmina, E.; Cedars, M.I.; Laven, J.; Norman, R.J.; Escobar-Morreale, H.F. Definition and significance of polycystic ovarian morphology: A task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum. Reprod. Updat. 2013, 20, 334–352. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Su, P.; Chen, C.; Sun, Y. Physiopathology of polycystic ovary syndrome in endocrinology, metabolism and inflammation. J. Ovarian Res. 2025, 18, 1–10. [Google Scholar] [CrossRef]
- Azhary, J.M.K.; Harada, M.; Takahashi, N.; Nose, E.; Kunitomi, C.; Koike, H.; Hirata, T.; Hirota, Y.; Koga, K.; Wada-Hiraike, O.; et al. Endoplasmic Reticulum Stress Activated by Androgen Enhances Apoptosis of Granulosa Cells via Induction of Death Receptor 5 in PCOS. Endocrinology 2018, 160, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Phillip, J.; Cartwright, S.; Scott, A. The size and morphology of T.G.E. and vomiting and wasting disease viruses of pigs. Vet. Rec. 1971, 88, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, A.; Maftoohi, M.; Samarin, M.E.; Barani, S.; Banimohammad, M.; Samie, R. The last update on polycystic ovary syndrome(PCOS), diagnosis criteria, and novel treatment. Endocr. Metab. Sci. 2025, 17, 100228. [Google Scholar] [CrossRef]
- Mahoney, A.; D’angelo, A. Treatment Options for Managing Anovulation in Women with PCOS: An Extensive Literature Review of Evidence-Based Recommendations for Future Directions. Life 2025, 15, 863. [Google Scholar] [CrossRef]
- Szczesnowicz, A.; Szeliga, A.; Niwczyk, O.; Bala, G.; Meczekalski, B. Do GLP-1 Analogs Have a Place in the Treatment of PCOS? New Insights and Promising Therapies. J. Clin. Med. 2023, 12, 5915. [Google Scholar] [CrossRef]
- Fitz, V.; Graca, S.; Mahalingaiah, S.; Liu, J.; Lai, L.; Butt, A.; Armour, M.; Rao, V.; Naidoo, D.; Maunder, A.; et al. Inositol for Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis to Inform the 2023 Update of the International Evidence-based PCOS Guidelines. J. Clin. Endocrinol. Metab. 2024, 109, 1630–1655. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, L.; Huang, X. Genome modification by CRISPR/Cas9. FEBS J. 2014, 281, 5186–5193. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, G.; Chen, J.; Wang, C.; Dong, X.; Chang, H.-M.; Yuan, S.; Zhao, Y.; Mu, L. Genetic and Epigenetic Landscape for Drug Development in Polycystic Ovary Syndrome. Endocr. Rev. 2024, 45, 437–459. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E. The Role of Gene Alterations in the Pathogenesis of Polycystic Ovary Syndrome. J. Clin. Med. 2025, 14, 3347. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.G.; Urbanek, M.; Ehrmann, D.A.; Armstrong, L.L.; Lee, J.Y.; Sisk, R.; Karaderi, T.; Barber, T.M.; McCarthy, M.I.; Franks, S.; et al. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat. Commun. 2015, 6, 7502. [Google Scholar] [CrossRef] [PubMed]
- Dapas, M.; Lin, F.T.J.; Nadkarni, G.N.; Sisk, R.; Legro, R.S.; Urbanek, M.; Hayes, M.G.; Dunaif, A. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. 2020, 17, e1003132. [Google Scholar] [CrossRef]
- McAllister, J.M.; Modi, B.; Miller, B.A.; Biegler, J.; Bruggeman, R.; Legro, R.S.; Strauss, J.F. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc. Natl. Acad. Sci. USA 2014, 111, E1519–E1527. [Google Scholar] [CrossRef]
- Zeggini, E.; Scott, L.J.; Saxena, R.; Voight, B.F.; Marchini, J.L.; Hu, T.; de Bakker, P.I.; Abecasis, G.R.; Almgren, P.; Andersen, G.; et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat. Genet. 2008, 40, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Moraru, A.; Cakan-Akdogan, G.; Strassburger, K.; Males, M.; Mueller, S.; Jabs, M.; Muelleder, M.; Frejno, M.; Braeckman, B.P.; Ralser, M.; et al. THADA Regulates the Organismal Balance between Energy Storage and Heat Production. Dev. Cell 2017, 41, 72–81.e6. [Google Scholar] [CrossRef]
- Rasool, S.U.A.; Ashraf, S.; Nabi, M.; Masoodi, S.R.; Fazili, K.M.; Amin, S. Clinical Manifestations of Hyperandrogenism and Ovulatory Dysfunction Are Not Associated with His1058 C/T SNP (rs1799817) Polymorphism of Insulin Receptor Gene Tyrosine Kinase Domain in Kashmiri Women with PCOS. Int. J. Endocrinol. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Singh, R.; Kaur, S.; Yadav, S.; Bhatia, S. Gonadotropins as pharmacological agents in assisted reproductive technology and polycystic ovary syndrome. Trends Endocrinol. Metab. 2023, 34, 194–215. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, O.; Uitterlinden, A.; Piersma, D.; Hofman, A.; Themmen, A.; de Jong, F.; Fauser, B.; Laven, J. Genetic polymorphisms of GnRH and gonadotrophic hormone receptors affect the phenotype of polycystic ovary syndrome. Hum. Reprod. 2009, 24, 2014–2022. [Google Scholar] [CrossRef]
- Laven, J.S.; Mulders, A.G.; A Suryandari, D.; Gromoll, J.; Nieschlag, E.; Fauser, B.C.; Simoni, M. Follicle-stimulating hormone receptor polymorphisms in women with normogonadotropic anovulatory infertility. Fertil. Steril. 2003, 80, 986–992. [Google Scholar] [CrossRef]
- Farsimadan, M.; Ghosi, F.M.; Takamoli, S.; Vaziri, H. Association analysis of KISS1 polymorphisms and haplotypes with polycystic ovary syndrome. Br. J. Biomed. Sci. 2021, 78, 201–205. [Google Scholar] [CrossRef]
- Albalawi, F.S.; Daghestani, M.H.; Daghestani, M.H.; Eldali, A.; Warsy, A.S. rs4889 polymorphism in KISS1 gene, its effect on polycystic ovary syndrome development and anthropometric and hormonal parameters in Saudi women. J. Biomed. Sci. 2018, 25, 50. [Google Scholar] [CrossRef]
- Porter, D.T.; Moore, A.M.; A Cobern, J.; Padmanabhan, V.; Goodman, R.L.; Coolen, L.M.; Lehman, M.N. Prenatal Testosterone Exposure Alters GABAergic Synaptic Inputs to GnRH and KNDy Neurons in a Sheep Model of Polycystic Ovarian Syndrome. Endocrinology 2019, 160, 2529–2542. [Google Scholar] [CrossRef]
- Abbara, A.; Eng, P.C.; Phylactou, M.; Clarke, S.A.; Richardson, R.; Sykes, C.M.; Phumsatitpong, C.; Mills, E.; Modi, M.; Izzi-Engbeaya, C.; et al. Kisspeptin receptor agonist has therapeutic potential for female reproductive disorders. J. Clin. Investig. 2020, 130, 6739–6753. [Google Scholar] [CrossRef]
- Abbara, A.; Ufer, M.; Voors-Pette, C.; Berman, L.; Ezzati, M.; Wu, R.; Lee, T.-Y.; Ferreira, J.C.A.; Migoya, E.; Dhillo, W.S. Endocrine profile of the kisspeptin receptor agonist MVT-602 in healthy premenopausal women with and without ovarian stimulation: Results from 2 randomized, placebo-controlled clinical tricals. Fertil. Steril. 2023, 121, 95–106. [Google Scholar] [CrossRef]
- Dallel, M.; Douma, Z.; Finan, R.R.; Hachani, F.; Letaifa, D.B.; Mahjoub, T.; Almawi, W.Y. Contrasting association of Leptin receptor polymorphisms and haplotypes with polycystic ovary syndrome in Bahraini and Tunisian women: A case–control study. Biosci. Rep. 2021, 41, BSR20202726. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Cline, D.L.; Glavas, M.M.; Covey, S.D.; Kieffer, T.J. Tissue-Specific Effects of Leptin on Glucose and Lipid Metabolism. Endocr. Rev. 2021, 42, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Lan, J.; Li, M.; Wang, F. Associations of Leptin Receptor and Peroxisome Proliferator-Activated Receptor Gamma Polymorphisms with Polycystic Ovary Syndrome: A Meta-Analysis. Ann. Nutr. Metab. 2019, 75, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, H.; Bai, H.; Huang, W.; Zhang, R.; Tan, J.; Guan, L.; Fan, P. Association of SOD2 A16V and PON2 S311C polymorphisms with polycystic ovary syndrome in Chinese women. J. Endocrinol. Investig. 2019, 42, 909–921. [Google Scholar] [CrossRef]
- Bresciani, G.; Cruz, I.B.M.; de Paz, J.A.; Cuevas, M.J.; González-Gallego, J. The MnSOD Ala16Val SNP: Relevance to human diseases and interaction with environmental factors. Free Radic. Res. 2013, 47, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Jamnongjit, M.; Gill, A.; Hammes, S.R. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc. Natl. Acad. Sci. USA 2005, 102, 16257–16262. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, W.; Yang, P.; Tian, Y.; Su, S.; Zhang, C.; Chen, Z.-J.; Zhao, H. ERBB4 Confers Risk for Polycystic Ovary Syndrome in Han Chinese. Sci. Rep. 2017, 7, srep42000. [Google Scholar] [CrossRef]
- Day, F.; Karaderi, T.; Jones, M.R.; Meun, C.; He, C.; Drong, A.; Kraft, P.; Lin, N.; Huang, H.; Broer, L.; et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018, 14, e1007813. [Google Scholar] [CrossRef] [PubMed]
- Veikkolainen, V.; Ali, N.; Doroszko, M.; Kiviniemi, A.; Miinalainen, I.; Ohlsson, C.; Poutanen, M.; Rahman, N.; Elenius, K.; Vainio, S.J.; et al. Erbb4 regulates the oocyte microenvironment during folliculogenesis. Hum. Mol. Genet. 2020, 29, 2813–2830. [Google Scholar] [CrossRef]
- Clark, K.L.; George, J.W.; Przygrodzka, E.; Plewes, M.R.; Hua, G.; Wang, C.; Davis, J.S. Hippo Signaling in the Ovary: Emerging Roles in Development, Fertility, and Disease. Endocr. Rev. 2022, 43, 1074–1096. [Google Scholar] [CrossRef]
- Wang, C.; Jeong, K.; Jiang, H.; Guo, W.; Gu, C.; Lu, Y.; Liang, J. YAP/TAZ regulates the insulin signaling via IRS1/2 in endometrial cancer. Am. J. Cancer Res. 2016, 6, 996–1010. [Google Scholar]
- Tyrmi, J.S.; Arffman, R.K.; Pujol-Gualdo, N.; Kurra, V.; Morin-Papunen, L.; Sliz, E.; Piltonen, T.T.; Laisk, T.; Kettunen, J.; Laivuori, H. Leveraging Northern European population history: Novel low-frequency variants for polycystic ovary syndrome. Hum. Reprod. 2021, 37, 352–365. [Google Scholar] [CrossRef]
- Ruth, K.S.; Day, F.R.; Hussain, J.; Martínez-Marchal, A.; Aiken, C.E.; Azad, A.; Thompson, D.J.; Knoblochova, L.; Abe, H.; Tarry-Adkins, J.L.; et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature 2021, 596, 393–397. [Google Scholar] [CrossRef]
- Forslund, M.; Landin-Wilhelmsen, K.; Schmidt, J.; Brännström, M.; Trimpou, P.; Dahlgren, E. Higher menopausal age but no differences in parity in women with polycystic ovary syndrome compared with controls. Acta Obstet. Gynecol. Scand. 2018, 98, 320–326. [Google Scholar] [CrossRef]
- de Ziegler, D.; Pirtea, P.; Fanchin, R.; Ayoubi, J.M. Ovarian reserve in polycystic ovary syndrome: More, but for how long? Fertil. Steril. 2018, 109, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadi, A.M.; Sarray, S.; Arekat, M.R.; Saleh, L.R.; Mahmood, N.; Almawi, W.Y. The high-molecular weight multimer form of adiponectin is a useful marker of polycystic ovary syndrome in Bahraini Arab women. Clin. Nutr. ESPEN 2016, 13, e33–e38. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, X.; Xu, J.; Sun, Y.; Shi, Y.; Fang, S. Association between Adiponectin Receptor 1 Gene Polymorphism and Insulin Resistance in Chinese Patients with Polycystic Ovary Syndrome. Gynecol. Obstet. Investig. 2013, 77, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadi, A.M.; Babi, A.; Finan, R.R.; Atageldiyeva, K.; Shaimardanova, M.; Mustafa, F.E.; Mahmood, N.A.; Aimagambetova, G.; Almawi, W.Y. ADIPOQ gene polymorphisms and haplotypes linked to altered susceptibility to PCOS: A case–control study. Reprod. Biomed. Online 2022, 45, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Ezzidi, I.; Mtiraoui, N.; Ali, M.E.M.; Al Masoudi, A.; Abu Duhier, F. Adiponectin (ADIPOQ) gene variants and haplotypes in Saudi Arabian women with polycystic ovary syndrome (PCOS): A case–control study. Gynecol. Endocrinol. 2019, 36, 66–71. [Google Scholar] [CrossRef]
- A Alfaqih, M.; Khader, Y.S.; Al-Dwairi, A.N.; Alzoubi, A.; Othman, A.-S.; Hatim, A. Lower Levels of Serum Adiponectin and the T Allele of rs1501299 of the ADIPOQ Gene Are Protective against Polycystic Ovarian Syndrome in Jordan. Korean J. Fam. Med. 2018, 39, 108–113. [Google Scholar] [CrossRef]
- Singh, A.; Bora, P.; Krishna, A. Direct action of adiponectin ameliorates increased androgen synthesis and reduces insulin receptor expression in the polycystic ovary. Biochem. Biophys. Res. Commun. 2017, 488, 509–515. [Google Scholar] [CrossRef]
- Zhang, F.; Lupski, J.R. Non-coding genetic variants in human disease: Figure 1. Hum. Mol. Genet. 2015, 24, R102–R110. [Google Scholar] [CrossRef]
- Zhu, Y.; Tazearslan, C.; Suh, Y. Challenges and progress in interpretation of non-coding genetic variants associated with human disease. Exp. Biol. Med. 2017, 242, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Fitipaldi, H.; Franks, P.W. Ethnic, gender and other sociodemographic biases in genome-wide association studies for the most burdensome non-communicable diseases: 2005–2022. Hum. Mol. Genet. 2022, 32, 520–532. [Google Scholar] [CrossRef]
- Fatumo, S.; Chikowore, T.; Choudhury, A.; Ayub, M.; Martin, A.R.; Kuchenbaecker, K. A roadmap to increase diversity in genomic studies. Nat. Med. 2022, 28, 243–250. [Google Scholar] [CrossRef]
- Peterson, R.E.; Kuchenbaecker, K.; Walters, R.K.; Chen, C.-Y.; Popejoy, A.B.; Periyasamy, S.; Lam, M.; Iyegbe, C.; Strawbridge, R.J.; Brick, L.; et al. Genome-wide Association Studies in Ancestrally Diverse Populations: Opportunities, Methods, Pitfalls, and Recommendations. Cell 2019, 179, 589–603. [Google Scholar] [CrossRef]
- Zhang, Y.; Movva, V.C.; Williams, M.S.; Lee, M.T.M. Polycystic Ovary Syndrome Susceptibility Loci Inform Disease Etiological Heterogeneity. J. Clin. Med. 2021, 10, 2688. [Google Scholar] [CrossRef]
- Liu, P.; Huang, L.; Song, C.-Q. Editorial: Genome editing applications of CRISPR/Cas9 in metabolic diseases, hormonal system and cancer research. Front. Endocrinol. 2023, 14, 1256966. [Google Scholar] [CrossRef]
- Lidaka, L.; Bekere, L.; Lazdane, G.; Lazovska, M.; Dzivite-Krisane, I.; Gailite, L. Role of Single Nucleotide Variants in the YAP1 Gene in Adolescents with Polycystic Ovary Syndrome. Biomedicines 2022, 10, 1688. [Google Scholar] [CrossRef]
- Padder, K.A.; Yousuf, M.A.; Jahan, N.; Yousuf, S.D.; Ganie, M.A. Interplay between elevated RAB5B gene expression and insulin resistance among women with PCOS-insights from a case-control study. Endocrine 2024, 88, 323–329. [Google Scholar] [CrossRef]
- Hossain, M.A. CRISPR-Cas9: A fascinating journey from bacterial immune system to human gene editing. Prog. Mol. Biol. Transl. Sci. 2021, 178, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; La Russa, M.; Qi, L.S. CRISPR/Cas9 in Genome Editing and Beyond. Annu. Rev. Biochem. 2016, 85, 227–264. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.J.; Yuen, G.; Luo, J. Multiplexed CRISPR/Cas9 gene knockout with simple crRNA:tracrRNA co-transfection. Cell Biosci. 2019, 9, 41. [Google Scholar] [CrossRef]
- Kantor, A.; McClements, M.E.; MacLaren, R.E. CRISPR-Cas9 DNA Base-Editing and Prime-Editing. Int. J. Mol. Sci. 2020, 21, 6240. [Google Scholar] [CrossRef]
- Wang, S.-W.; Gao, C.; Zheng, Y.-M.; Yi, L.; Lu, J.-C.; Huang, X.-Y.; Cai, J.-B.; Zhang, P.-F.; Cui, Y.-H.; Ke, A.-W. Current applications and future perspective of CRISPR/Cas9 gene editing in cancer. Mol. Cancer 2022, 21, 57. [Google Scholar] [CrossRef]
- Reh, W.A.; Vasquez, K.M. Gene Targeting by Homologous Recombination. In eLS; Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470015902.a0005988.pub2 (accessed on 26 October 2025).
- Gupta, D.; Bhattacharjee, O.; Mandal, D.; Sen, M.K.; Dey, D.; Dasgupta, A.; Kazi, T.A.; Gupta, R.; Sinharoy, S.; Acharya, K.; et al. CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life Sci. 2019, 232, 116636. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Sahin, G.N.; Seli, E. Gene editing using CRISPR-Cas9 technology: Potential implications in assisted reproduction. Curr. Opin. Obstet. Gynecol. 2025, 37, 141–148. [Google Scholar] [CrossRef]
- Guller, A.S.; Kayabolen, G.N.S.; Soyler, G.; Comar, M.Y.; Duzgun, I.Y.; Sivaslioglu, A.; Karahuseyinoglu, S. O-104 The Impact of application of CRISPR /dCas9 systems for increasing the expression of FSH receptor in human granulosa cells. Hum. Reprod. 2023, 38, dead093.127. [Google Scholar] [CrossRef]
- Livadas, S.; Paparodis, R.; Anagnostis, P.; Gambineri, A.; Bjekić-Macut, J.; Petrović, T.; Yildiz, B.O.; Micić, D.; Mastorakos, G.; Macut, D. Assessment of Type 2 Diabetes Risk in Young Women with Polycystic Ovary Syndrome. Diagnostics 2023, 13, 2067. [Google Scholar] [CrossRef]
- Srivastav, A.K.; Mishra, M.K.; Lillard, J.W.; Singh, R. Transforming Pharmacogenomics and CRISPR Gene Editing with the Power of Artificial Intelligence for Precision Medicine. Pharmaceutics 2025, 17, 555. [Google Scholar] [CrossRef] [PubMed]
- Matboli, M.; Kamel, M.M.; Essawy, N.; Bekhit, M.M.; Abdulrahman, B.; Mohamed, G.F.; Eissa, S. Identification of Novel Insulin Resistance Related ceRNA Network in T2DM and Its Potential Editing by CRISPR/Cas9. Int. J. Mol. Sci. 2021, 22, 8129. [Google Scholar] [CrossRef] [PubMed]
- Magoffin, D.A. Ovarian theca cell. Int. J. Biochem. Cell Biol. 2005, 37, 1344–1349. [Google Scholar] [CrossRef]
- Richards, J.S.; A Ren, Y.; Candelaria, N.; E Adams, J.; Rajkovic, A. Ovarian Follicular Theca Cell Recruitment, Differentiation, and Impact on Fertility: 2017 Update. Endocr. Rev. 2017, 39, 1–20. [Google Scholar] [CrossRef]
- Dong, J.; Rees, D.A. Polycystic ovary syndrome: Pathophysiology and therapeutic opportunities. BMJ Med. 2023, 2, e000548. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Andrisse, S.; Chen, Y.; Childress, S.; Xue, P.; Wang, Z.; Jones, D.; Ko, C.; Divall, S.; Wu, S. Androgen Receptor in the Ovary Theca Cells Plays a Critical Role in Androgen-Induced Reproductive Dysfunction. Endocrinology 2016, 158, 98–108. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Yang, Y.; Chen, Z.; Zhou, Y.; Fei, W.; Zhang, X.; Zheng, Y. Extracellular matrix dysregulation in PCOS: Pathogenesis, therapeutic strategies, and innovative technologies. J. Biol. Eng. 2025, 19, 61. [Google Scholar] [CrossRef]
- Waterbury, J.S.; E Teves, M.; Gaynor, A.; Han, A.X.; Mavodza, G.; Newell, J.; Strauss, J.F.; McAllister, J.M. The PCOS GWAS Candidate Gene ZNF217 Influences Theca Cell Expression of DENND1A.V2, CYP17A1, and Androgen Production. J. Endocr. Soc. 2022, 6, bvac078. [Google Scholar] [CrossRef]
- Teves, M.E.; Modi, B.P.; Kulkarni, R.; Han, A.X.; Marks, J.S.; Subler, M.A.; Windle, J.; Newall, J.M.; McAllister, J.M.; Strauss, J.F., III. Human DENND1A.V2 Drives Cyp17a1 Expression and Androgen Production in Mouse Ovaries and Adrenals. Int. J. Mol. Sci. 2020, 21, 2545. [Google Scholar] [CrossRef]
- Joseph, S.; Ubba, V.; Wang, Z.; Feng, M.; Dsilva, M.K.; Suero, S.; Waheed, D.; Snyder, N.W.; Yang, X.; Wang, H.; et al. Ovarian-Specific Cyp17A1 Overexpression in Female Mice: A Novel Model of Endogenous Testosterone Excess. Endocrinology 2025, 166, bqaf071. [Google Scholar] [CrossRef] [PubMed]
- Udhane, S.S.; Flück, C.E. Regulation of human (adrenal) androgen biosynthesis—New insights from novel throughput technology studies. Biochem. Pharmacol. 2016, 102, 20–33. [Google Scholar] [CrossRef]
- Lee, J.; Yamazaki, T.; Dong, H.; Jefcoate, C. A single cell level measurement of StAR expression and activity in adrenal cells. Mol. Cell. Endocrinol. 2017, 441, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wickenheisser, J.K.; Biegler, J.M.; Nelson-DeGrave, V.L.; Legro, R.S.; Strauss, J.F.; McAllister, J.M. Cholesterol Side-Chain Cleavage Gene Expression in Theca Cells: Augmented Transcriptional Regulation and mRNA Stability in Polycystic Ovary Syndrome. PLoS ONE 2012, 7, e48963. [Google Scholar] [CrossRef]
- Owens, L.A.; Kristensen, S.G.; Lerner, A.; Christopoulos, G.; Lavery, S.; Hanyaloglu, A.C.; Hardy, K.; Andersen, C.Y.; Franks, S. Gene Expression in Granulosa Cells From Small Antral Follicles From Women With or Without Polycystic Ovaries. J. Clin. Endocrinol. Metab. 2019, 104, 6182–6192. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Richards, J.S. Ontogeny of the ovary in polycystic ovary syndrome. Fertil. Steril. 2013, 100, 23–38. [Google Scholar] [CrossRef]
- Kong, F.-S.; Feng, J.; Yao, J.-P.; Lu, Y.; Guo, T.; Sun, M.; Ren, C.-Y.; Jin, Y.-Y.; Ma, Y.; Chen, J.-H. Dysregulated RNA editing of EIF2AK2 in polycystic ovary syndrome: Clinical relevance and functional implications. BMC Med. 2024, 22, 229. [Google Scholar] [CrossRef]
- Kong, F.-S.; Lu, Z.; Zhou, Y.; Lu, Y.; Ren, C.-Y.; Jia, R.; Zeng, B.; Huang, P.; Wang, J.; Ma, Y.; et al. Transcriptome analysis identification of A-to-I RNA editing in granulosa cells associated with PCOS. Front. Endocrinol. 2023, 14, 1170957. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Fei, Y.; Page, M. Biological Significance of RNA Editing in Cells. Mol. Biotechnol. 2012, 52, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Barbarroja, N.; Rodriguez-Cuenca, S.; Nygren, H.; Camargo, A.; Pirraco, A.; Relat, J.; Cuadrado, I.; Pellegrinelli, V.; Medina-Gomez, G.; Lopez-Pedrera, C.; et al. Increased Dihydroceramide/Ceramide Ratio Mediated by Defective Expression of degs1 Impairs Adipocyte Differentiation and Function. Diabetes 2014, 64, 1180–1192. [Google Scholar] [CrossRef]
- Chappell, N.R.; Zhou, B.; Hosseinzadeh, P.; Schutt, A.; Gibbons, W.E.; Blesson, C.S. Hyperandrogenemia alters mitochondrial structure and function in the oocytes of obese mouse with polycystic ovary syndrome. F&S Sci. 2020, 2, 101–112. [Google Scholar] [CrossRef]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef] [PubMed]
- Hesampour, F.; Jahromi, B.N.; Tahmasebi, F.; Gharesi-Fard, B. Association between Interleukin-32 and Interleukin-17A Single Nucleotide Polymorphisms and Serum Levels with Polycystic Ovary Syndrome. Iran. J. Allergy Asthma Immunol. 2019, 18, 91–99. [Google Scholar] [CrossRef]
- Gong, Y.; Luo, S.; Fan, P.; Zhu, H.; Li, Y.; Huang, W. Growth hormone activates PI3K/Akt signaling and inhibits ROS accumulation and apoptosis in granulosa cells of patients with polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2020, 18, 121. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Hu, M.; Zhang, Y.; Cui, P.; Li, X.; Li, J.; Vestin, E.; Brännström, M.; Shao, L.R.; et al. Differential Expression Patterns of Glycolytic Enzymes and Mitochondria-Dependent Apoptosis in PCOS Patients with Endometrial Hyperplasia, an Early Hallmark of Endometrial Cancer, In Vivo and the Impact of Metformin In Vitro. Int. J. Biol. Sci. 2019, 15, 714–725. [Google Scholar] [CrossRef]
- Yu, Y.; Li, G.; He, X.; Lin, Y.; Chen, Z.; Lin, X.; Xu, H. MicroRNA-21 regulate the cell apoptosis and cell proliferation of polycystic ovary syndrome (PCOS) granulosa cells through target toll like receptor TLR8. Bioengineered 2021, 12, 5789–5796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wu, L.; Wang, Q.; Zhao, X.; Chen, T.; Yin, C.; Yan, L.; Yang, X. Insulin-like growth factor 2 mRNA-binding protein 2-regulated alternative splicing of nuclear factor 1 C-type causes excessive granulosa cell proliferation in polycystic ovary syndrome. Cell Prolif. 2022, 55, e13216. [Google Scholar] [CrossRef]
- Li, R.Z.; Hou, J.; Wei, Y.; Luo, X.; Ye, Y.; Zhang, Y. hnRNPDL extensively regulates transcription and alternative splicing. Gene 2019, 687, 125–134. [Google Scholar] [CrossRef]
- Berardo, A.; Lornage, X.; Johari, M.; Evangelista, T.; Cejas, C.; Barroso, F.; Dubrovsky, A.; Bui, M.T.; Brochier, G.; Saccoliti, M.; et al. HNRNPDL-related muscular dystrophy: Expanding the clinical, morphological and MRI phenotypes. J. Neurol. 2019, 266, 2524–2534. [Google Scholar] [CrossRef] [PubMed]
- Nishikura, K. Functions and Regulation of RNA Editing by ADAR Deaminases. Annu. Rev. Biochem. 2010, 79, 321–349. [Google Scholar] [CrossRef]
- Meyer, C.; Garzia, A.; Mazzola, M.; Gerstberger, S.; Molina, H.; Tuschl, T. The TIA1 RNA-Binding Protein Family Regulates EIF2AK2-Mediated Stress Response and Cell Cycle Progression. Mol. Cell 2018, 69, 622–635.e6. [Google Scholar] [CrossRef]
- Cesaro, T.; Hayashi, Y.; Borghese, F.; Vertommen, D.; Wavreil, F.; Michiels, T. PKR activity modulation by phosphomimetic mutations of serine residues located three aminoacids upstream of double-stranded RNA binding motifs. Sci. Rep. 2021, 11, 9188. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, H.; Guo, Y.; Wei, C.; Guan, L.; Ju, W.; Lian, F. Cangfu Daotan Wan alleviates polycystic ovary syndrome with phlegm-dampness syndrome via disruption of the PKP3/ERCC1/MAPK axis. J. Ovarian Res. 2023, 16, 134. [Google Scholar] [CrossRef]
- Das, S.; Maizels, E.T.; DeManno, D.; Clair, E.S.; A Adam, S.; Hunzicker-Dunn, M. A stimulatory role of cyclic adenosine 3′,5′-monophosphate in follicle-stimulating hormone-activated mitogen-activated protein kinase signaling pathway in rat ovarian granulosa cells. Endocrinology 1996, 137, 967–974. [Google Scholar] [CrossRef]
- Tajima, K.; Dantes, A.; Yao, Z.; Sorokina, K.; Kotsuji, F.; Seger, R.; Amsterdam, A. Down-Regulation of Steroidogenic Response to Gonadotropins in Human and Rat Preovulatory Granulosa Cells Involves Mitogen-Activated Protein Kinase Activation and Modulation of DAX-1 and Steroidogenic Factor-1. J. Clin. Endocrinol. Metab. 2003, 88, 2288–2299. [Google Scholar] [CrossRef]
- Gruijters, M.J.; Visser, J.A.; Durlinger, A.L.; Themmen, A.P. Anti-Müllerian hormone and its role in ovarian function. Mol. Cell. Endocrinol. 2003, 211, 85–90. [Google Scholar] [CrossRef]
- Ke, Y.; Tang, D.; Yang, Q.; Zhao, H.; Zheng, J.; Zhu, C. Anti-Müllerian hormone in PCOS: Molecular regulation and emerging therapeutic strategies. Biomol. Biomed. 2025, 26, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.F.; Bergeron, F.; Delaney, J.G.; Harvey, L.-M.; Viger, R.S. In Vivo Ablation of the Conserved GATA-Binding Motif in the Amh Promoter Impairs Amh Expression in the Male Mouse. Endocrinology 2019, 160, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Manuylov, N.; Zhou, B.; Ma, Q.; Fox, S.; Pu, W.; Tevosian, S. Conditional ablation of Gata4 and Fog2 genes in mice reveals their distinct roles in mammalian sexual differentiation. Dev. Biol. 2011, 353, 229–241. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, L.; Sun, T.; Liu, Y.; Bao, Y.; Gu, M.; Fu, S.; He, X.; Shi, C.; Wang, Y.; et al. Massively Parallel CRISPR-Cas9 Knockout Screening in Sheep Granulosa Cells for FSH Response Genes. Animals 2024, 14, 898. [Google Scholar] [CrossRef]
- Kumar, S.; Punetha, M.; Jose, B.; Bharati, J.; Khanna, S.; Sonwane, A.; Green, J.A.; Whitworth, K.; Sarkar, M. Modulation of granulosa cell function via CRISPR-Cas fuelled editing of BMPR-IB gene in goats (Capra hircus). Sci. Rep. 2020, 10, 20446. [Google Scholar] [CrossRef]
- Oktem, O.; Urman, B. Understanding follicle growth in vivo. Hum. Reprod. 2010, 25, 2944–2954. [Google Scholar] [CrossRef]
- Takov, E.C.V. Embryology, Ovarian Follicle Development. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2025. [Google Scholar]
- Woodruff, T.K.; Shea, L.D. The Role of the Extracellular Matrix in Ovarian Follicle Development. Reprod. Sci. 2007, 14, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Franks, S.; Stark, J.; Hardy, K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum. Reprod. Updat. 2008, 14, 367–378. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y. Signaling pathways and targeted therapeutic strategies for polycystic ovary syndrome. Front. Endocrinol. 2023, 14, 1191759. [Google Scholar] [CrossRef]
- Ruan, Y.; Li, X.; Wang, X.; Zhai, G.; Lou, Q.; Jin, X.; He, J.; Mei, J.; Xiao, W.; Gui, J.; et al. New insights into the all-testis differentiation in zebrafish with compromised endogenous androgen and estrogen synthesis. PLoS Genet. 2024, 20, e1011170. [Google Scholar] [CrossRef]
- Lau, E.S.-W.; Zhang, Z.; Qin, M.; Ge, W. Knockout of Zebrafish Ovarian Aromatase Gene (cyp19a1a) by TALEN and CRISPR/Cas9 Leads to All-male Offspring Due to Failed Ovarian Differentiation. Sci. Rep. 2016, 6, 37357. [Google Scholar] [CrossRef]
- Carver, J.J.; Amato, C.M.; Yao, H.H.-C.; Zhu, Y. Adamts9 is required for the development of primary ovarian follicles and maintenance of female sex in zebrafish. Biol. Reprod. 2024, 111, 1107–1128. [Google Scholar] [CrossRef] [PubMed]
- Kushawaha, B.; Pelosi, E. Spotlight on Proteases: Roles in Ovarian Health and Disease. Cells 2025, 14, 921. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yue, Y.; Zhou, L.; Zhang, Z.; Shan, H.; He, H.; Ge, W. Disrupting Amh and androgen signaling reveals their distinct roles in zebrafish gonadal differentiation and gametogenesis. Commun. Biol. 2025, 8, 371. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, B.; Chen, W.; Ge, W. Anti-Müllerian hormone (Amh/amh) plays dual roles in maintaining gonadal homeostasis and gametogenesis in zebrafish. Mol. Cell. Endocrinol. 2020, 517, 110963. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; Xi, X.; Burn, M.; Karakaya, C.; Kallen, A.N. Aberrant H19 Expression Disrupts Ovarian Cyp17 and Testosterone Production and Is Associated with Polycystic Ovary Syndrome in Women. Reprod. Sci. 2021, 29, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, H.; Shi, X.; Warren, C.R.; Lotta, L.A.; Friesen, M.; Meissner, T.B.; Langenberg, C.; Wabitsch, M.; Wareham, N.; et al. Functional Screening of Candidate Causal Genes for Insulin Resistance in Human Preadipocytes and Adipocytes. Circ. Res. 2019, 126, 330–346. [Google Scholar] [CrossRef]
- Kahn, C.R. Knockout Mice Challenge our Concepts of Glucose Homeostasis and the Pathogenesis of Diabetes. J. Diabetes Res. 2003, 4, 169–182. [Google Scholar] [CrossRef]
- Garofalo, R.S.; Orena, S.J.; Rafidi, K.; Torchia, A.J.; Stock, J.L.; Hildebrandt, A.L.; Coskran, T.; Black, S.C.; Brees, D.J.; Wicks, J.R.; et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKBβ. J. Clin. Investig. 2003, 112, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Miki, H.; Yamauchi, T.; Suzuki, R.; Komeda, K.; Tsuchida, A.; Kubota, N.; Terauchi, Y.; Kamon, J.; Kaburagi, Y.; Matsui, J.; et al. Essential Role of Insulin Receptor Substrate 1 (IRS-1) and IRS-2 in Adipocyte Differentiation. Mol. Cell. Biol. 2001, 21, 2521–2532. [Google Scholar] [CrossRef]
- Kamble, P.G.; Hetty, S.; Vranic, M.; Almby, K.; Castillejo-López, C.; Abalo, X.M.; Pereira, M.J.; Eriksson, J.W. Proof-of-concept for CRISPR/Cas9 gene editing in human preadipocytes: Deletion of FKBP5 and PPARG and effects on adipocyte differentiation and metabolism. Sci. Rep. 2020, 10, 10565. [Google Scholar] [CrossRef]
- Claussnitzer, M.; Dankel, S.N.; Kim, K.-H.; Quon, G.; Meuleman, W.; Haugen, C.; Glunk, V.; Sousa, I.S.; Beaudry, J.L.; Puviindran, V.; et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. N. Engl. J. Med. 2015, 373, 895–907. [Google Scholar] [CrossRef]
- Roh, J.-I.; Lee, J.; Park, S.U.; Kang, Y.-S.; Lee, J.; Oh, A.-R.; Choi, D.J.; Cha, J.-Y.; Lee, H.-W. CRISPR-Cas9-mediated generation of obese and diabetic mouse models. Exp. Anim. 2018, 67, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Ma, Y.; Zhang, X.; Guan, F.; Chen, W.; Gao, K.; Qin, C.; Zhang, L. Preliminary Characterization of a Leptin Receptor Knockout Rat Created by CRISPR/Cas9 System. Sci. Rep. 2015, 5, 15942. [Google Scholar] [CrossRef]
- Hao, H.; Lin, R.; Li, Z.; Shi, W.; Huang, T.; Niu, J.; Han, J.; Li, Q. MC4R deficiency in pigs results in hyperphagia and ultimately hepatic steatosis without high-fat diet. Biochem. Biophys. Res. Commun. 2019, 520, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhaut, N.H.; Jakob, S.; Anlag, K.; Eisenberger, T.; Sekido, R.; Kress, J.; Treier, A.-C.; Klugmann, C.; Klasen, C.; Holter, N.I.; et al. Somatic Sex Reprogramming of Adult Ovaries to Testes by FOXL2 Ablation. Cell 2009, 139, 1130–1142. [Google Scholar] [CrossRef]
- Wei, C.; Wang, F.; Liu, W.; Zhao, W.; Yang, Y.; Li, K.; Xiao, L.; Shen, J. CRISPR/Cas9 targeting of the androgen receptor suppresses the growth of LNCaP human prostate cancer cells. Mol. Med. Rep. 2017, 17, 2901–2906. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Xu, Y.; Wang, Q.; Namgoong, S.; Cui, X.; Kim, N. Effects of Growth Differentiation Factor 9 and Bone Morphogenetic Protein 15 on the in vitro Maturation of Porcine Oocytes. Reprod. Domest. Anim. 2013, 49, 219–227. [Google Scholar] [CrossRef]
- Fountas, S.; Petinaki, E.; Bolaris, S.; Kargakou, M.; Dafopoulos, S.; Zikopoulos, A.; Moustakli, E.; Sotiriou, S.; Dafopoulos, K. The Roles of GDF-9, BMP-15, BMP-4 and EMMPRIN in Folliculogenesis and In Vitro Fertilization. J. Clin. Med. 2024, 13, 3775. [Google Scholar] [CrossRef] [PubMed]
- McCarty, N.S.; Graham, A.E.; Studená, L.; Ledesma-Amaro, R. Multiplexed CRISPR technologies for gene editing and transcriptional regulation. Nat. Commun. 2020, 11, 1281. [Google Scholar] [CrossRef]
- Singh, S.; Pal, N.; Shubham, S.; Sarma, D.K.; Verma, V.; Marotta, F.; Kumar, M. Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J. Clin. Med. 2023, 12, 1454. [Google Scholar] [CrossRef]
- Shi, J.; Gao, Q.; Cao, Y.; Fu, J. Dennd1a, a susceptibility gene for polycystic ovary syndrome, is essential for mouse embryogenesis. Dev. Dyn. 2019, 248, 351–362. [Google Scholar] [CrossRef]
- Shaaban, Z.; Khoradmehr, A.; Amiri-Yekta, A.; Nowzari, F.; Shirazi, M.R.J.; Tamadon, A. Pathophysiologic Mechanisms of Insulin Secretion and Signaling-Related Genes in Etiology of Polycystic Ovary Syndrome. Genet. Res. 2021, 2021, 7781823. [Google Scholar] [CrossRef]
- Yan, Y.; Desvignes, T.; Bremiller, R.; Wilson, C.; Dillon, D.; High, S.; Draper, B.; Buck, C.L.; Postlethwait, J. Gonadal soma controls ovarian follicle proliferation through Gsdf in zebrafish. Dev. Dyn. 2017, 246, 925–945. [Google Scholar] [CrossRef]
- Xu, J.; Dun, J.; Yang, J.; Zhang, J.; Lin, Q.; Huang, M.; Ji, F.; Huang, L.; You, X.; Lin, Y. Letrozole Rat Model Mimics Human Polycystic Ovarian Syndrome and Changes in Insulin Signal Pathways. Med. Sci. Monit. 2020, 26, e923073. [Google Scholar] [CrossRef] [PubMed]
- Poojary, P.S.; Nayak, G.; Panchanan, G.; Rao, A.; Das Kundapur, S.; Kalthur, S.G.; Mutalik, S.; Adiga, S.K.; Zhao, Y.; Bakkum-Gamez, J.; et al. Distinctions in PCOS Induced by Letrozole Vs Dehydroepiandrosterone with High-fat Diet in Mouse Model. Endocrinology 2022, 163, bqac097. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, A.G. An Introduction to Genome-Wide Association Studies: GWAS for Dummies. Semin. Reprod. Med. 2016, 34, 196–204. [Google Scholar] [CrossRef]
- Tan, M.; Cheng, Y.; Zhong, X.; Yang, D.; Jiang, S.; Ye, Y.; Ding, M.; Guan, G.; Yang, D.; Zhao, X. LNK promotes granulosa cell apoptosis in PCOS via negatively regulating insulin-stimulated AKT-FOXO3 pathway. Aging 2021, 13, 4617–4633. [Google Scholar] [CrossRef]
- Han, S.; Zhang, Y.; Zheng, Y.; Liu, C.; Jiang, Y.; Zhao, S.; Zhao, H. Thada Is Dispensable for Female Fertility in Mice. Front. Endocrinol. 2022, 13, 787733. [Google Scholar] [CrossRef]
- Velmurugan, S.; Pauline, R.; Subbaraj, G.K. Association of candidate gene (INSR & THADA) polymorphism with polycystic ovary syndrome: Meta-analysis and statistical power analysis. J. Turk. Gynecol. Assoc. 2024, 25, 167–178. [Google Scholar] [CrossRef]
- Lit, K.K.; Zhirenova, Z.; Blocki, A. Insulin-like growth factor-binding protein 7 (IGFBP7): A microenvironment-dependent regulator of angiogenesis and vascular remodeling. Front. Cell Dev. Biol. 2024, 12, 1421438. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hui, L.; Shi, Y.; Wang, A.; Zhang, Y.; Yao, X.; Li, J. The role of IGFBP7 in the immune regulation of PCOS-like symptoms based on spleen transcriptome and TCR β CDR3 repertoire analysis. Mol. Cell. Endocrinol. 2025, 608, 112639. [Google Scholar] [CrossRef]
- Kosova, G.; Urbanek, M. Genetics of the polycystic ovary syndrome. Mol. Cell. Endocrinol. 2013, 373, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Li, Y.; Liao, N.; Liu, J.; Zhang, Q.; Luo, M.; Xiao, J.; Chen, Y.; Wang, M.; Chen, K.; et al. Identification of key genes associated with polycystic ovary syndrome (PCOS) and ovarian cancer using an integrated bioinformatics analysis. J. Ovarian Res. 2022, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Uddin, F.; Rudin, C.M.; Sen, T. CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future. Front. Oncol. 2020, 10, 1387. [Google Scholar] [CrossRef]
- Cao, C.; Ma, Q.; Mo, S.; Shu, G.; Liu, Q.; Ye, J.; Gui, Y. Single-Cell RNA Sequencing Defines the Regulation of Spermatogenesis by Sertoli-Cell Androgen Signaling. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Sun, Y.; Miao, N.; Sun, T. Detect accessible chromatin using ATAC-sequencing, from principle to applications. Hereditas 2019, 156, 29. [Google Scholar] [CrossRef]
- Sankaranarayanan, L.; Brewer, K.J.; Johnson, G.D.; Barrera, A.; Venukuttan, R.; Sisk, R.; Dunaif, A.; Reddy, T.E. Gene regulatory activity associated with PCOS revealed DENND1A-dependent testosterone production. bioRxiv 2024. bioRxiv:2024.2005.2023.595551. [Google Scholar] [CrossRef]
- Tee, M.K.; Speek, M.; Legeza, B.; Modi, B.; Teves, M.E.; McAllister, J.M.; Strauss, J.F.; Miller, W.L. Alternative splicing of DENND1A, a PCOS candidate gene, generates variant 2. Mol. Cell. Endocrinol. 2016, 434, 25–35. [Google Scholar] [CrossRef]
- McAllister, J.M.; Legro, R.S.; Modi, B.P.; Strauss, J.F. Functional genomics of PCOS: From GWAS to molecular mechanisms. Trends Endocrinol. Metab. 2015, 26, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Gainder, S.; Sachdeva, G.; Suri, V.; Sachdeva, N.; Chopra, S. Comparison of the different PCOS phenotypes based on clinical metabolic, and hormonal profile, and their response to clomiphene. Indian J. Endocrinol. Metab. 2019, 23, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Baba, T. Polycystic ovary syndrome: Criteria, phenotypes, race and ethnicity. Reprod. Med. Biol. 2025, 24, e12630. [Google Scholar] [CrossRef]
- Mózsik, L.; Hoekzema, M.; de Kok, N.A.W.; Bovenberg, R.A.L.; Nygård, Y.; Driessen, A.J.M. CRISPR-based transcriptional activation tool for silent genes in filamentous fungi. Sci. Rep. 2021, 11, 1118. [Google Scholar] [CrossRef]
- Makarova, K.S.; Grishin, N.V.; Shabalina, S.A.; Wolf, Y.I.; Koonin, E.V. A putative RNA-interference-based immune system in prokaryotes: Computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol. Direct 2006, 1, 7. [Google Scholar] [CrossRef]
- Kazemian, P.; Yu, S.-Y.; Thomson, S.B.; Birkenshaw, A.; Leavitt, B.R.; Ross, C.J.D. Lipid-Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Components. Mol. Pharm. 2022, 19, 1669–1686. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Tao, Y.; Liu, Y.; Li, J. Advances in delivery systems for CRISPR/Cas-mediated cancer treatment: A focus on viral vectors and extracellular vesicles. Front. Immunol. 2024, 15, 1444437. [Google Scholar] [CrossRef]
- Amarilla-Quintana, S.; Navarro, P.; Hernández, I.; Ramos, A.; Montero-Calle, A.; Cabezas-Sainz, P.; Barrero, M.J.; Megías, D.; Vilaplana-Martí, B.; Epifano, C.; et al. CRISPR targeting of FOXL2 c.402C>G mutation reduces malignant phenotype in granulosa tumor cells and identifies anti-tumoral compounds. Mol. Oncol. 2025, 19, 1092–1116. [Google Scholar] [CrossRef]
- Heinzelmann, E.; Piraino, F.; Costa, M.; Roch, A.; Norkin, M.; Garnier, V.; Homicsko, K.; Brandenberg, N. iPSC-derived and Patient-Derived Organoids: Applications and challenges in scalability and reproducibility as pre-clinical models. Curr. Res. Toxicol. 2024, 7, 100197. [Google Scholar] [CrossRef]
- Brooks, I.R.; Garrone, C.M.; Kerins, C.; Kiar, C.S.; Syntaka, S.; Xu, J.Z.; Spagnoli, F.M.; Watt, F.M. Functional genomics and the future of iPSCs in disease modeling. Stem Cell Rep. 2022, 17, 1033–1047. [Google Scholar] [CrossRef]
- Rubeis, G.; Steger, F. Risks and benefits of human germline genome editing: An ethical analysis. Asian Bioeth. Rev. 2018, 10, 133–141. [Google Scholar] [CrossRef]
- Chin, A.H.B.; Sun, N. Do not overlook the possibility of genome-edited somatic cells ending up in the human germline. J. Community Genet. 2024, 15, 749–752. [Google Scholar] [CrossRef]
- Kosicki, M.; Tomberg, K.; Bradley, A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018, 36, 765–771. [Google Scholar] [CrossRef]
- Aussel, C.; Cathomen, T.; Fuster-García, C. The hidden risks of CRISPR/Cas: Structural variations and genome integrity. Nat. Commun. 2025, 16, 7208. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Jiang, J.; Wu, M.; Lin, P. Applications and challenges of CRISPR-Cas gene-editing to disease treatment in clinics. Precis. Clin. Med. 2021, 4, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, X.; Gao, F.; Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 2023, 11, 1143157. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hwang, Y.; Lim, S.; Jang, H.-K.; Kim, H.-O. Advances in Nanoparticles as Non-Viral Vectors for Efficient Delivery of CRISPR/Cas9. Pharmaceutics 2024, 16, 1197. [Google Scholar] [CrossRef] [PubMed]
- Burdo, T.H.; Chen, C.; Kaminski, R.; Sariyer, I.K.; Mancuso, P.; Donadoni, M.; Smith, M.D.; Sariyer, R.; Caocci, M.; Liao, S.; et al. Preclinical safety and biodistribution of CRISPR targeting SIV in non-human primates. Gene Ther. 2023, 31, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Palacios, A.M.; Korus, P.; Wilkens, B.G.C.; Heshmatpour, N.; Patnaik, S.R. Revolutionizing in vivo therapy with CRISPR/Cas genome editing: Breakthroughs, opportunities and challenges. Front. Genome Ed. 2024, 6, 1342193. [Google Scholar] [CrossRef]
- Pandey, V.; Sharma, S.; Pokharel, Y.R. Exploring CRISPR-Cas: The transformative impact of gene editing in molecular biology. Mol. Ther.-Nucleic Acids 2025, 36, 102717. [Google Scholar] [CrossRef]
- Zhuo, C.; Zhang, J.; Lee, J.-H.; Jiao, J.; Cheng, D.; Liu, L.; Kim, H.-W.; Tao, Y.; Li, M. Spatiotemporal control of CRISPR/Cas9 gene editing. Signal Transduct. Target. Ther. 2021, 6, 238. [Google Scholar] [CrossRef] [PubMed]
- Brokowski, C.; Adli, M. CRISPR Ethics: Moral Considerations for Applications of a Powerful Tool. J. Mol. Biol. 2019, 431, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Ayanoğlu, F.B.; ELÇİN, A.E.; ELÇİN, Y.M. Bioethical issues in genome editing by CRISPR-Cas9 technology. Turk. J. Biol. 2020, 44, 110–120. [Google Scholar] [CrossRef]
| Gene | Role in PCOS | Potential CRISPR Application |
|---|---|---|
| DENND1A [57,58] | Overexpressed DENND1A isoform leads to excess androgen production | Perform knockout to decrease androgen biosynthesis |
| THADA [59] | Variants are linked to defects in energy metabolism | Model metabolic dysfunction or correct variants to improve insulin sensitivity |
| LHCGR [53,63] | Altered gene expression results in impaired folliculogenesis | Edit variants to restore normal receptor function |
| FSHR [53,62,63] | Reduced receptor sensitivity to FSH leads to defective follicle development and ovulatory dysfunction | CRISPRa to upregulate FSHR expression and increase responsiveness to FSH |
| INSR [60] | Polymorphisms disrupt insulin receptor signaling, which contributes to IR | Target and excise specific INSR polymorphisms to restore normal receptor function in metabolic tissues |
| YAP1 [97] | Increased activity in variants is associated with disruption in cellular processes and enlarged ovaries | CRISPRi to downregulate YAP1 overexpression in ovarian granulosa cells |
| RAB5B [98] | Upregulated expression is linked to IR | Correct or knockout RAB5B to restore normal insulin signaling |
| HMGA2 [54] | Altered expression is associated with hyperinsulinemia and IR | Model HMGA2 to further understand metabolic effects |
| KISS1 [64,65] | Polymorphisms exacerbate ovulatory dysfunction and contribute to abnormal BMI and LH levels | Target SNPs to restore normal GnRH feedback and ovulation |
| LEPR [69] | Variants disrupt leptin binding, impair insulin pathways, and increase IR | Edit variants to improve insulin sensitivity and normalize leptin signaling |
| SOD2 [72,73] | A16V variant could result in cellular damage and impair gonadotropin balance | Target and edit variant to limit cellular injury and normalize hormone levels |
| ERBB4 [77] | Dysfunction can lead to pronounced reproductive and metabolic abnormalities | Create gene knockout models to further investigate how it induces PCOS features |
| WWTR1 [79] | Altered expression can contribute to infertility, IR, and influences response to metformin | Inhibit gene to enhance metformin efficacy |
| CHEK2 [81,82] | Mutations in CHEK2 result in irregular oocyte apoptosis and follicular excess | Develop models to better understand how variants contribute to PCOS |
| ADIPOQ [84,86] | ADIPOQ polymorphisms are associated with reduced adiponectin levels | Model ADIPOQ SNPs to study adiponectin signaling and ethnic-specific effects |
| ADIPOR1 [85,89] | Variants impair adiponectin signaling and are linked to IR | Use mouse models to test the effect of treatment on clinical characteristics |
| Target Gene | Model (Species/Cell) | Key Findings | PCOS Relevance | Model Advantages and Limitations |
|---|---|---|---|---|
| FSHR [108] | Human granulosa cells | dCas9 activation increased FSHR expression (strongest at 5 min with Gonal-f); estradiol levels also elevated | Demonstrates CRISPR modulation of gonadotropin signaling and steroidogenesis | Human-origin cell model; directly relevant to ovarian physiology. However, the study was conducted in a cell line because primary granulosa cells are difficult to target for epigenome editing. |
| IRS1 [162] | Human adipocytes | Loss of function led to insulin-stimulated AKT2 phosphorylation & glucose uptake; altered lipid metabolism | Links IRS1 loss to IR & metabolic features of PCOS | Abundant source of adipogenic cells and is suitable for gene editing due to their proliferation ability. The knockout of the effector genes cannot fully replicate the regulatory state. |
| PPARG [166] | Human preadipocytes | Knockout of PPARG prevents differentiation of preadipocytes into adipocytes | Shows how PPARG plays a role in regulating adipogenesis | Excluding selection markers enhanced knockout efficiency and allowed cells to preserve a high differentiation capacity, but inserting random indels may generate undesired epigenetic changes and result in false positives. |
| FTO (rs1421085) [167] | CRISPR repair of the risk allele restored ARID5B binding, repressed IRX3/IRX5, reactivated browning programs, and increased thermogenesis | Links obesity genetics to PCOS metabolic features | Shows direct manipulation of the FTO risk allele in primary human adipocytes, and highlights its ability to repress thermogenesis in adipocytes, independent of the central nervous system. | |
| DENND1A.V2 [57] | Human theca-like cells | Overexpression of DENND1A.V2 enhanced CYP17A1 expression and androgen output | Results mirror PCOS-associated hyperandrogenism | The model functionally reproduces the hyperandrogenic PCOS phenotype; however, the mechanism by which DENND1A.V2 regulates CYP17A1 expression remains unclear. However, only one variant was tested, limiting the scope. |
| CYP17A1 [119] | Mouse ovarian theca cells | Overexpression of CYP17A1 resulted in hyperandrogenism, prolonged estrous cycles, and cystic follicle morphology | Features mirror clinical and histologic findings in PCOS | Ovary-specific overexpression enables temporal control of androgen excess and closely reproduces hyperandrogenic PCOS features. However, systemic metabolic effects were mild, and interspecies differences may limit full translational applicability. |
| AMH [123,124] | Mouse testes and ovaries | CRISPR/Cas9 deletion of GATA-binding site in AMH promoter reduced AMH expression in fetal/neonate testes but not in adult ovaries | Insights into AMH transcriptional control can help explain dysregulated folliculogenesis. | The model demonstrates site-specific control, which facilitates the identification of transcriptional regulatory mechanisms rather than total gene loss. Since the deletion affected fetal/neonatal testes and not adult ovaries, the model cannot fully represent AMH dysregulation throughout life. |
| AKT2 [164] | Mice | Knockout mice exhibited growth deficiency, lipoatrophy, IR, hyperglycemia, hyperinsulinemia, and impaired muscle glucose uptake | Demonstrates AKT2’s role in insulin signaling, glucose metabolism, and adipose regulation | Enabled dissection of tissue- and sex-specific AKT2 functions, particularly in adipose tissue and pancreatic β-cells. The model also mimicked human type 2 diabetes progression, offering translational insight into β-cell compensation and failure. However, the phenotype was strain-dependent, reducing reproducibility across genetic backgrounds. |
| LEPR [168] | Loss of leptin receptor caused hyperphagia, obesity, hyperglycemia, IR, dyslipidemia, and glucose intolerance | Establishes leptin signaling as key to metabolic dysfunction seen in PCOS | The models successfully reproduce the hallmark metabolic disturbances seen in obesity and diabetes, and demonstrate high editing efficiency of CRISPR/Cas9, producing fully penetrant phenotypes without the need for multiple breeding generations. Yet, the line could not be maintained as the mice were severely obese and infertile. | |
| LNK (SH2B3) [183] | LNK knockout group exhibited a partially restored estrous cycle and an improved glucose metabolism | LNK may be a target for PCOS clinical treatment | The model combines genetic (LNK knockout) and hormonal (DHEA + high-fat diet) approaches, replicating both ovarian and metabolic abnormalities. Limitations include the unclear mechanism of FOXO3 (a transcription factor), and hyperandrogenism was underexplored. | |
| IGFBP7 [187] | IGFBP7 is linked to cystic follicular enlargement, reduced granulosa cell layers, immature and atretic follicles, and the disappearance of oocytes. Knockout of the IGFBP7 gene reversed the disruption caused by the administered DHEA | IGFBP7 plays a role in the development of PCOS | Combines genetic (IGFBP7 knockout) and hormonal (DHEA) models, and uses spleen transcriptomics to uncover immune-endocrine interplay. However, focus was on spleen immune cells, not ovarian tissue. | |
| CYP19A1A [155,156] | Zebrafish | Knockout of CYP19A1A abolished estrogen production, resulting in complete ovary-to-testis sex reversal and infertility | Highlights the role of estrogen in establishing and maintaining female reproductive architecture and follicle progression | Successful in vivo monitoring of gonadal differentiation. However, an off-targeting effect may occur due to the short recognition site of 18 nucleotides. |
| ADAMTS9 [157] | Knockout of ADAMTS9 led to underdeveloped ovaries composed entirely of early-stage oocytes that failed to ovulate | Demonstrates ADAMTS9’s role in follicle rupture and oocyte maturation | The ADAMTS9 knockout zebrafish can survive to adulthood, allowing for in vivo study. However, the zebrafish exhibited a strong sex bias towards males, leaving a small number of homozygous knockout females in the offspring of heterozygous crossings. | |
| GSDF [179] | Knockout female zebrafish exhibited infertility, oligo-ovulation, hyperandrogenism, IR, and obesity | Metabolic and reproductive phenotypes of PCOS were displayed | The model revealed a clear and reproducible phenotype, where all gsdf mutant fish developed as females, but it lacked rescue experiments to confirm specificity of the phenotype. | |
| BMPR1B [149] | Goat granulosa cells | Knockout of BMPR1B enhanced SMAD signaling, made cells more sensitive to gonadotropins, reduced cell growth/viability, and removed the usual BMP-4/7 suppression of progesterone production | Shows the gene’s importance in regulating granulosa cell growth and hormone production | The model successfully mimicked the biological phenotype seen in FecB-positive sheep, and used a primary granulosa cell culture, offering a controlled environment to directly assess Smad signaling and steroidogenesis. However, the model does not replicate systemic hormonal interactions, folliculogenesis, or ovulation seen in vivo. |
| MC4R [170] | Pigs | MC4R-deficient pigs developed marked hyperinsulinemia, IR, hyperorexia, hyperphagia, increased adiposity, and dysregulated lipid metabolism with substantial hepatic fat accumulation | Highlights melanocortin pathway’s role in obesity-related metabolic dysfunction relevant to PCOS | The model offers a better physiological and metabolic similarity to humans, but failed to assess long-term disease progression. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucheeri, S.; Alcibahy, Y.; Bucheeri, Y.; Bucheeri, S.; Alhermi, A.; Butler, A.E. CRISPR as a Tool to Uncover Gene Function in Polycystic Ovary Syndrome: A Literature Review of Experimental Models Targeting Ovarian and Metabolic Genes. Cells 2025, 14, 1769. https://doi.org/10.3390/cells14221769
Bucheeri S, Alcibahy Y, Bucheeri Y, Bucheeri S, Alhermi A, Butler AE. CRISPR as a Tool to Uncover Gene Function in Polycystic Ovary Syndrome: A Literature Review of Experimental Models Targeting Ovarian and Metabolic Genes. Cells. 2025; 14(22):1769. https://doi.org/10.3390/cells14221769
Chicago/Turabian StyleBucheeri, Shahd, Yasmine Alcibahy, Yara Bucheeri, Sarah Bucheeri, Abrar Alhermi, and Alexandra E. Butler. 2025. "CRISPR as a Tool to Uncover Gene Function in Polycystic Ovary Syndrome: A Literature Review of Experimental Models Targeting Ovarian and Metabolic Genes" Cells 14, no. 22: 1769. https://doi.org/10.3390/cells14221769
APA StyleBucheeri, S., Alcibahy, Y., Bucheeri, Y., Bucheeri, S., Alhermi, A., & Butler, A. E. (2025). CRISPR as a Tool to Uncover Gene Function in Polycystic Ovary Syndrome: A Literature Review of Experimental Models Targeting Ovarian and Metabolic Genes. Cells, 14(22), 1769. https://doi.org/10.3390/cells14221769






