Calmodulin D133H Disrupts Cav1.2 and Kv7.1 Regulation to Prolong Cardiac Action Potentials in Long QT Syndrome
Highlights
- The calmodulin variant D133H disrupts Ca2+-dependent inactivation of Cav1.2 and reduces activation of Kv7.1.
- D133H reduces Ca2+ affinity and alters interactions with Cav1.2 and Kv7.1 binding domains.
- The dual impact on Cav1.2 and Kv7.1 reveals cross-channel regulatory coupling as a key determinant of ventricular repolarisation.
- These mechanistic insights broaden understanding of how specific CaM variants remodel cardiac electrical signalling in Long QT syndrome.
Abstract
1. Introduction
2. Materials and Methods
- Cav1.2-IQ1665−1685: KFYATFLIQEYFRKFKKRKEQ;
- Cav1.2-NSCaTE51−67: SWQAAIDAARQAKLMGS;
- Kv7.1-HB507−536: REHHRATIKVIRRMQYFVAKKKFQQARKPY.
3. Results
4. Discussion
4.1. Structural and Biophysical Impact on CaM
4.2. Functional Consequences for Cav1.2 and Kv7.1
4.3. Electrophysiological Mechanisms
4.4. Broader Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartz, P.J.; Stramba-Badiale, M.; Crotti, L.; Pedrazzini, M.; Besana, A.; Bosi, G.; Gabbarini, F.; Goulene, K.; Insolia, R.; Mannarino, S.; et al. Prevalence of the congenital long-QT syndrome. Circulation 2009, 120, 1761–1767. [Google Scholar] [CrossRef]
- Amin, A.S.; Pinto, Y.M.; Wilde, A.A. Long QT syndrome: Beyond the causal mutation. J. Physiol. 2013, 591, 4125–4139. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.; Howard, L.; Liu, M.; O’Brien, T.; Ward, D.; Shen, S.; Prendiville, T. Long QT Syndrome: Genetics and Future Perspective. Pediatr. Cardiol. 2019, 40, 1419–1430. [Google Scholar] [CrossRef]
- Roden, D.M.; Lazzara, R.; Rosen, M.; Schwartz, P.J.; Towbin, J.; Vincent, G.M. Multiple mechanisms in the long-QT syndrome. Current knowledge, gaps, and future directions. The SADS Foundation Task Force on LQTS. Circulation 1996, 94, 1996–2012. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Crotti, L.; Insolia, R. Long-QT syndrome: From genetics to management. Circ. Arrhythm. Electrophysiol. 2012, 5, 868–877. [Google Scholar] [CrossRef]
- Wilde, A.A.M.; Amin, A.S.; Postema, P.G. Diagnosis, management and therapeutic strategies for congenital long QT syndrome. Heart 2022, 108, 332–338. [Google Scholar] [CrossRef]
- Adler, A.; Novelli, V.; Amin, A.S.; Abiusi, E.; Care, M.; Nannenberg, E.A.; Feilotter, H.; Amenta, S.; Mazza, D.; Bikker, H.; et al. An International, Multicentered, Evidence-Based Reappraisal of Genes Reported to Cause Congenital Long QT Syndrome. Circulation 2020, 141, 418–428. [Google Scholar] [CrossRef]
- Plumereau, Q.; Theriault, O.; Pouliot, V.; Moreau, A.; Morel, E.; Fressart, V.; Denjoy, I.; Deliniere, A.; Bessiere, F.; Chevalier, P.; et al. Novel G1481V and Q1491H SCN5A Mutations Linked to Long QT Syndrome Destabilize the Nav1.5 Inactivation State. CJC Open 2021, 3, 256–266. [Google Scholar] [CrossRef]
- Landstrom, A.P.; Boczek, N.J.; Ye, D.; Miyake, C.Y.; De la Uz, C.M.; Allen, H.D.; Ackerman, M.J.; Kim, J.J. Novel long QT syndrome-associated missense mutation, L762F, in CACNA1C-encoded L-type calcium channel imparts a slower inactivation tau and increased sustained and window current. Int. J. Cardiol. 2016, 220, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Boczek, N.J.; Best, J.M.; Tester, D.J.; Giudicessi, J.R.; Middha, S.; Evans, J.M.; Kamp, T.J.; Ackerman, M.J. Exome sequencing and systems biology converge to identify novel mutations in the L-type calcium channel, CACNA1C, linked to autosomal dominant long QT syndrome. Circ. Cardiovasc. Genet. 2013, 6, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, M.; Wang, Q.; Kato, K.; Ohno, S.; Ding, W.G.; Toyoda, F.; Itoh, H.; Kimura, H.; Makiyama, T.; Ito, M.; et al. Long QT syndrome type 8: Novel CACNA1C mutations causing QT prolongation and variant phenotypes. Europace 2014, 16, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.J.M.; Crozier, I.G.; Binfield, A.L.; Love, D.R.; Lehnert, K.; Gibson, K.; Lintott, C.J.; Snell, R.G.; Jacobsen, J.C.; Jones, P.P.; et al. Penetrance and expressivity of the R858H CACNA1C variant in a five-generation pedigree segregating an arrhythmogenic channelopathy. Mol. Genet. Genomic Med. 2019, 7, e00476. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Koller, M.; Flura, M.; Mathews, S.; Strehler-Page, M.A.; Krebs, J.; Penniston, J.T.; Carafoli, E.; Strehler, E.E. Multiple divergent mRNAs code for a single human calmodulin. J. Biol. Chem. 1988, 263, 17055–17062. [Google Scholar] [CrossRef]
- Bogdanov, V.; Mariangelo, J.I.E.; Soltisz, A.M.; Sakuta, G.; Pokrass, A.; Beard, C.; Orengo, B.H.; Kalinin, R.; Ulker, A.; Yunker, B.; et al. Distinct intracellular spatiotemporal expression of Calmodulin genes underlies functional diversity of Calmodulin-dependent signalling in cardiac myocytes. Cardiovasc. Res. 2025, 121, 1052–1065. [Google Scholar] [CrossRef]
- Tsai, W.C.; Guo, S.; Olaopa, M.A.; Field, L.J.; Yang, J.; Shen, C.; Chang, C.P.; Chen, P.S.; Rubart, M. Complex Arrhythmia Syndrome in a Knock-In Mouse Model Carrier of the N98S Calm1 Mutation. Circulation 2020, 142, 1937–1955. [Google Scholar] [CrossRef]
- Crotti, L.; Johnson, C.N.; Graf, E.; De Ferrari, G.M.; Cuneo, B.F.; Ovadia, M.; Papagiannis, J.; Feldkamp, M.D.; Rathi, S.G.; Kunic, J.D.; et al. Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation 2013, 127, 1009–1017. [Google Scholar] [CrossRef]
- Negi, S. Effect of Calcium Ion Removal, Ionic Strength, and Temperature on the Conformation Change in Calmodulin Protein at Physiological pH. J. Biophys. 2014, 2014, 329703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tanaka, T.; Ikura, M. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat. Struct. Biol. 1995, 2, 758–767. [Google Scholar] [CrossRef]
- Chattopadhyaya, R.; Meador, W.E.; Means, A.R.; Quiocho, F.A. Calmodulin structure refined at 1.7 A resolution. J. Mol. Biol. 1992, 228, 1177–1192. [Google Scholar] [CrossRef]
- Zhang, M.; Abrams, C.; Wang, L.; Gizzi, A.; He, L.; Lin, R.; Chen, Y.; Loll, P.J.; Pascal, J.M.; Zhang, J.F. Structural basis for calmodulin as a dynamic calcium sensor. Structure 2012, 20, 911–923. [Google Scholar] [CrossRef]
- Sorensen, A.B.; Sondergaard, M.T.; Overgaard, M.T. Calmodulin in a heartbeat. FEBS J. 2013, 280, 5511–5532. [Google Scholar] [CrossRef]
- Sorensen, B.R.; Shea, M.A. Interactions between domains of apo calmodulin alter calcium binding and stability. Biochemistry 1998, 37, 4244–4253. [Google Scholar] [CrossRef] [PubMed]
- Halling, D.B.; Liebeskind, B.J.; Hall, A.W.; Aldrich, R.W. Conserved properties of individual Ca2+-binding sites in calmodulin. Proc. Natl. Acad. Sci. USA 2016, 113, E1216–E1225. [Google Scholar] [CrossRef] [PubMed]
- Biekofsky, R.R.; Martin, S.R.; Browne, J.P.; Bayley, P.M.; Feeney, J. Ca2+ coordination to backbone carbonyl oxygen atoms in calmodulin and other EF-hand proteins: 15N chemical shifts as probes for monitoring individual-site Ca2+ coordination. Biochemistry 1998, 37, 7617–7629. [Google Scholar] [CrossRef]
- Kameyama, M.; Minobe, E.; Shao, D.; Xu, J.; Gao, Q.; Hao, L. Regulation of Cardiac CaV1.2 Channels by Calmodulin. Int. J. Mol. Sci. 2023, 24, 6409. [Google Scholar] [CrossRef] [PubMed]
- Chin, D.; Means, A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef]
- Yan, H.; Wang, C.; Marx, S.O.; Pitt, G.S. Calmodulin limits pathogenic Na+ channel persistent current. J. Gen. Physiol. 2017, 149, 277–293. [Google Scholar] [CrossRef]
- Kang, P.W.; Chakouri, N.; Diaz, J.; Tomaselli, G.F.; Yue, D.T.; Ben-Johny, M. Elementary mechanisms of calmodulin regulation of NaV1.5 producing divergent arrhythmogenic phenotypes. Proc. Natl. Acad. Sci. USA 2021, 118, e2025085118. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, T.; Oda, T.; Chakraborty, A.; Chen, L.; Uchinoumi, H.; Knowlton, A.A.; Fruen, B.R.; Cornea, R.L.; Meissner, G.; et al. Cardiac myocyte Z-line calmodulin is mainly RyR2-bound, and reduction is arrhythmogenic and occurs in heart failure. Circ. Res. 2014, 114, 295–306. [Google Scholar] [CrossRef]
- Chang, A.; Abderemane-Ali, F.; Hura, G.L.; Rossen, N.D.; Gate, R.E.; Minor, D.L., Jr. A Calmodulin C-Lobe Ca2+-Dependent Switch Governs Kv7 Channel Function. Neuron 2018, 97, 836–852.E6. [Google Scholar] [CrossRef]
- Saljic, A.; Muthukumarasamy, K.M.; la Cour, J.M.; Boddum, K.; Grunnet, M.; Berchtold, M.W.; Jespersen, T. Impact of arrhythmogenic calmodulin variants on small conductance Ca2+-activated K+ (SK3) channels. Physiol. Rep. 2019, 7, e14210. [Google Scholar] [CrossRef]
- Maier, L.S.; Bers, D.M. Calcium, calmodulin, and calcium-calmodulin kinase II: Heartbeat to heartbeat and beyond. J. Mol. Cell Cardiol. 2002, 34, 919–939. [Google Scholar] [CrossRef]
- Pitt, G.S. Calmodulin and CaMKII as molecular switches for cardiac ion channels. Cardiovasc. Res. 2007, 73, 641–647. [Google Scholar] [CrossRef]
- Bers, D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Keating, M.T.; Sanguinetti, M.C. Molecular and cellular mechanisms of cardiac arrhythmias. Cell 2001, 104, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Snutch, T.P.; Tomlinson, W.J.; Leonard, J.P.; Gilbert, M.M. Distinct calcium channels are generated by alternative splicing and are differentially expressed in the mammalian CNS. Neuron 1991, 7, 45–57. [Google Scholar] [CrossRef]
- Hofmann, F.; Flockerzi, V.; Kahl, S.; Wegener, J.W. L-type CaV1.2 calcium channels: From in vitro findings to in vivo function. Physiol. Rev. 2014, 94, 303–326. [Google Scholar] [CrossRef]
- Hu, Z.; Liang, M.C.; Soong, T.W. Alternative Splicing of L-type CaV1.2 Calcium Channels: Implications in Cardiovascular Diseases. Genes 2017, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Dick, I.E.; Tadross, M.R.; Liang, H.; Tay, L.H.; Yang, W.; Yue, D.T. A modular switch for spatial Ca2+ selectivity in the calmodulin regulation of CaV channels. Nature 2008, 451, 830–834. [Google Scholar] [CrossRef]
- Erickson, M.G.; Liang, H.; Mori, M.X.; Yue, D.T. FRET two-hybrid mapping reveals function and location of L-type Ca2+ channel CaM preassociation. Neuron 2003, 39, 97–107. [Google Scholar] [CrossRef]
- Erickson, M.G.; Alseikhan, B.A.; Peterson, B.Z.; Yue, D.T. Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron 2001, 31, 973–985. [Google Scholar] [CrossRef]
- Bartels, P.; Salveson, I.; Coleman, A.M.; Anderson, D.E.; Jeng, G.; Estrada-Tobar, Z.M.; Man, K.N.M.; Yu, Q.; Kuzmenkina, E.; Nieves-Cintron, M.; et al. Half-calcified calmodulin promotes basal activity and inactivation of the L-type calcium channel CaV1.2. J. Biol. Chem. 2022, 298, 102701. [Google Scholar] [CrossRef] [PubMed]
- Fallon, J.L.; Halling, D.B.; Hamilton, S.L.; Quiocho, F.A. Structure of calmodulin bound to the hydrophobic IQ domain of the cardiac CaV1.2 calcium channel. Structure 2005, 13, 1881–1886. [Google Scholar] [CrossRef]
- Van Petegem, F.; Chatelain, F.C.; Minor, D.L., Jr. Insights into voltage-gated calcium channel regulation from the structure of the CaV1.2 IQ domain-Ca2+/calmodulin complex. Nat. Struct. Mol. Biol. 2005, 12, 1108–1115. [Google Scholar] [CrossRef]
- Kim, E.Y.; Rumpf, C.H.; Van Petegem, F.; Arant, R.J.; Findeisen, F.; Cooley, E.S.; Isacoff, E.Y.; Minor, D.L., Jr. Multiple C-terminal tail Ca2+/CaMs regulate CaV1.2 function but do not mediate channel dimerization. EMBO J. 2010, 29, 3924–3938. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Halling, D.B.; Black, D.J.; Pate, P.; Zhang, J.Z.; Pedersen, S.; Altschuld, R.A.; Hamilton, S.L. Apocalmodulin and Ca2+ calmodulin-binding sites on the CaV1.2 channel. Biophys. J. 2003, 85, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Benmocha, A.; Almagor, L.; Oz, S.; Hirsch, J.A.; Dascal, N. Characterization of the calmodulin-binding site in the N terminus of CaV1.2. Channels 2009, 3, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Taiakina, V.; Boone, A.N.; Fux, J.; Senatore, A.; Weber-Adrian, D.; Guillemette, J.G.; Spafford, J.D. The calmodulin-binding, short linear motif, NSCaTE is conserved in L-type channel ancestors of vertebrate CaV1.2 and CaV1.3 channels. PLoS ONE 2013, 8, e61765. [Google Scholar] [CrossRef]
- Asmara, H.; Minobe, E.; Saud, Z.A.; Kameyama, M. Interactions of calmodulin with the multiple binding sites of CaV1.2 Ca2+ channels. J. Pharmacol. Sci. 2010, 112, 397–404. [Google Scholar] [CrossRef]
- Liu, Z.; Vogel, H.J. Structural basis for the regulation of L-type voltage-gated calcium channels: Interactions between the N-terminal cytoplasmic domain and Ca2+-calmodulin. Front. Mol. Neurosci. 2012, 5, 38. [Google Scholar] [CrossRef]
- Zuhlke, R.D.; Pitt, G.S.; Deisseroth, K.; Tsien, R.W.; Reuter, H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature 1999, 399, 159–162. [Google Scholar] [CrossRef]
- Zuhlke, R.D.; Pitt, G.S.; Tsien, R.W.; Reuter, H. Ca2+-sensitive inactivation and facilitation of L-type Ca2+ channels both depend on specific amino acid residues in a consensus calmodulin-binding motif in the(alpha)1C subunit. J. Biol. Chem. 2000, 275, 21121–21129. [Google Scholar] [CrossRef] [PubMed]
- Zuhlke, R.D.; Reuter, H. Ca2+-sensitive inactivation of L-type Ca2+ channels depends on multiple cytoplasmic amino acid sequences of the alpha1C subunit. Proc. Natl. Acad. Sci. USA 1998, 95, 3287–3294. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.Z.; DeMaria, C.D.; Adelman, J.P.; Yue, D.T. Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Neuron 1999, 22, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Lundby, A.; Tseng, G.N.; Schmitt, N. Structural basis for K(V)7.1-KCNE(x) interactions in the I(Ks) channel complex. Heart Rhythm. 2010, 7, 708–713. [Google Scholar] [CrossRef]
- Tobelaim, W.S.; Dvir, M.; Lebel, G.; Cui, M.; Buki, T.; Peretz, A.; Marom, M.; Haitin, Y.; Logothetis, D.E.; Hirsch, J.A.; et al. Competition of calcified calmodulin N lobe and PIP2 to an LQT mutation site in Kv7.1 channel. Proc. Natl. Acad. Sci. USA 2017, 114, E869–E878. [Google Scholar] [CrossRef]
- Sun, J.; MacKinnon, R. Cryo-EM Structure of a KCNQ1/CaM Complex Reveals Insights into Congenital Long QT Syndrome. Cell 2017, 169, 1042–1050.e1049. [Google Scholar] [CrossRef]
- Sachyani, D.; Dvir, M.; Strulovich, R.; Tria, G.; Tobelaim, W.; Peretz, A.; Pongs, O.; Svergun, D.; Attali, B.; Hirsch, J.A. Structural basis of a Kv7.1 potassium channel gating module: Studies of the intracellular c-terminal domain in complex with calmodulin. Structure 2014, 22, 1582–1594. [Google Scholar] [CrossRef]
- Ghosh, S.; Nunziato, D.A.; Pitt, G.S. KCNQ1 assembly and function is blocked by long-QT syndrome mutations that disrupt interaction with calmodulin. Circ. Res. 2006, 98, 1048–1054. [Google Scholar] [CrossRef]
- Dvir, M.; Peretz, A.; Haitin, Y.; Attali, B. Recent molecular insights from mutated IKS channels in cardiac arrhythmia. Curr. Opin. Pharmacol. 2014, 15, 74–82. [Google Scholar] [CrossRef]
- Gao, J.; Makiyama, T.; Yamamoto, Y.; Kobayashi, T.; Aoki, H.; Maurissen, T.L.; Wuriyanghai, Y.; Kashiwa, A.; Imamura, T.; Aizawa, T.; et al. Novel Calmodulin Variant p.E46K Associated With Severe Catecholaminergic Polymorphic Ventricular Tachycardia Produces Robust Arrhythmogenicity in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ. Arrhythm. Electrophysiol. 2023, 16, e011387. [Google Scholar] [CrossRef]
- Jimenez-Jaimez, J.; Palomino Doza, J.; Ortega, A.; Macias-Ruiz, R.; Perin, F.; Rodriguez-Vazquez del Rey, M.M.; Ortiz-Genga, M.; Monserrat, L.; Barriales-Villa, R.; Blanca, E.; et al. Calmodulin 2 Mutation N98S Is Associated with Unexplained Cardiac Arrest in Infants Due to Low Clinical Penetrance Electrical Disorders. PLoS ONE 2016, 11, e0153851. [Google Scholar] [CrossRef]
- Gomez-Hurtado, N.; Boczek, N.J.; Kryshtal, D.O.; Johnson, C.N.; Sun, J.; Nitu, F.R.; Cornea, R.L.; Chazin, W.J.; Calvert, M.L.; Tester, D.J.; et al. Novel CPVT-Associated Calmodulin Mutation in CALM3 (CALM3-A103V) Activates Arrhythmogenic Ca Waves and Sparks. Circ. Arrhythm. Electrophysiol. 2016, 9, 8. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishikawa, T.; Makita, N.; Takefuta, K.; Nabeshima, T.; Nakayashiro, M. A novel de novo calmodulin mutation in a 6-year-old boy who experienced an aborted cardiac arrest. HeartRhythm Case Rep. 2017, 3, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Brohus, M.; Arsov, T.; Wallace, D.A.; Jensen, H.H.; Nyegaard, M.; Crotti, L.; Adamski, M.; Zhang, Y.; Field, M.A.; Athanasopoulos, V.; et al. Infanticide vs. inherited cardiac arrhythmias. Europace 2021, 23, 441–450. [Google Scholar] [CrossRef]
- Reed, G.J.; Boczek, N.J.; Etheridge, S.P.; Ackerman, M.J. CALM3 mutation associated with long QT syndrome. Heart Rhythm. 2015, 12, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Wren, L.M.; Jimenez-Jaimez, J.; Al-Ghamdi, S.; Al-Aama, J.Y.; Bdeir, A.; Al-Hassnan, Z.N.; Kuan, J.L.; Foo, R.Y.; Potet, F.; Johnson, C.N.; et al. Genetic Mosaicism in Calmodulinopathy. Circ. Genom. Precis. Med. 2019, 12, 375–385. [Google Scholar] [CrossRef]
- Zahavich, L.; Tarnopolsky, M.; Yao, R.; Mital, S. Novel Association of a De Novo CALM2 Mutation With Long QT Syndrome and Hypertrophic Cardiomyopathy. Circ. Genom. Precis. Med. 2018, 11, e002255. [Google Scholar] [CrossRef]
- Pipilas, D.C.; Johnson, C.N.; Webster, G.; Schlaepfer, J.; Fellmann, F.; Sekarski, N.; Wren, L.M.; Ogorodnik, K.V.; Chazin, D.M.; Chazin, W.J.; et al. Novel calmodulin mutations associated with congenital long QT syndrome affect calcium current in human cardiomyocytes. Heart Rhythm. 2016, 13, 2012–2019. [Google Scholar] [CrossRef]
- Kato, K.; Isbell, H.M.; Fressart, V.; Denjoy, I.; Debbiche, A.; Itoh, H.; Poinsot, J.; George, A.L., Jr.; Coulombe, A.; Shea, M.A.; et al. Novel CALM3 Variant Causing Calmodulinopathy With Variable Expressivity in a 4-Generation Family. Circ. Arrhythm. Electrophysiol. 2022, 15, e010572. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.R.; de la Rosa, A.L.; Do, D.; Shah, S. A rare case report of catecholaminergic polymorphic ventricular tachycardia with an uncommon CALM2 mutation. Eur. Heart J. Case Rep. 2024, 8, ytae340. [Google Scholar] [CrossRef]
- Nyegaard, M.; Overgaard, M.T.; Sondergaard, M.T.; Vranas, M.; Behr, E.R.; Hildebrandt, L.L.; Lund, J.; Hedley, P.L.; Camm, A.J.; Wettrell, G.; et al. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am. J. Hum. Genet. 2012, 91, 703–712. [Google Scholar] [CrossRef]
- Crotti, L.; Spazzolini, C.; Tester, D.J.; Ghidoni, A.; Baruteau, A.E.; Beckmann, B.M.; Behr, E.R.; Bennett, J.S.; Bezzina, C.R.; Bhuiyan, Z.A.; et al. Calmodulin mutations and life-threatening cardiac arrhythmias: Insights from the International Calmodulinopathy Registry. Eur. Heart J. 2019, 40, 2964–2975. [Google Scholar] [CrossRef]
- Marsman, R.F.; Barc, J.; Beekman, L.; Alders, M.; Dooijes, D.; van den Wijngaard, A.; Ratbi, I.; Sefiani, A.; Bhuiyan, Z.A.; Wilde, A.A.; et al. A mutation in CALM1 encoding calmodulin in familial idiopathic ventricular fibrillation in childhood and adolescence. J. Am. Coll. Cardiol. 2014, 63, 259–266. [Google Scholar] [CrossRef]
- Nguyen, T.H.N.; Bui, C.B.; Nguyen, V.T.V.; Nguyen, M.C.; Vu, N.T.T.; Nguyen, M.H. Long QT syndrome: Identification of a novel de novo mutation of calmodulin in a newborn girl. Biomed. Res. Ther. 2022, 9, 4822–4831. [Google Scholar] [CrossRef]
- Chaix, M.A.; Koopmann, T.T.; Goyette, P.; Alikashani, A.; Latour, F.; Fatah, M.; Hamilton, R.M.; Rioux, J.D. Novel CALM3 mutations in pediatric long QT syndrome patients support a CALM3-specific calmodulinopathy. HeartRhythm Case Rep. 2016, 2, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Boczek, N.J.; Gomez-Hurtado, N.; Ye, D.; Calvert, M.L.; Tester, D.J.; Kryshtal, D.O.; Hwang, H.S.; Johnson, C.N.; Chazin, W.J.; Loporcaro, C.G.; et al. Spectrum and Prevalence of CALM1-, CALM2-, and CALM3-Encoded Calmodulin Variants in Long QT Syndrome and Functional Characterization of a Novel Long QT Syndrome-Associated Calmodulin Missense Variant, E141G. Circ. Cardiovasc. Genet. 2016, 9, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Makita, N.; Yagihara, N.; Crotti, L.; Johnson, C.N.; Beckmann, B.M.; Roh, M.S.; Shigemizu, D.; Lichtner, P.; Ishikawa, T.; Aiba, T.; et al. Novel calmodulin mutations associated with congenital arrhythmia susceptibility. Circ. Cardiovasc. Genet. 2014, 7, 466–474. [Google Scholar] [CrossRef]
- Fujita, S.; Nakagawa, R.; Futatani, T.; Igarashi, N.; Fuchigami, T.; Saito, S.; Ohno, S.; Horie, M.; Hatasaki, K. Long QT syndrome with a de novo CALM2 mutation in a 4-year-old boy. Pediatr. Int. 2019, 61, 852–858. [Google Scholar] [CrossRef]
- Sondergaard, M.T.; Liu, Y.; Brohus, M.; Guo, W.; Nani, A.; Carvajal, C.; Fill, M.; Overgaard, M.T.; Chen, S.R.W. Diminished inhibition and facilitated activation of RyR2-mediated Ca2+ release is a common defect of arrhythmogenic calmodulin mutations. FEBS J. 2019, 286, 4554–4578. [Google Scholar] [CrossRef]
- Prakash, O.; Held, M.; McCormick, L.F.; Gupta, N.; Lian, L.Y.; Antonyuk, S.; Haynes, L.P.; Thomas, N.L.; Helassa, N. CPVT-associated calmodulin variants N53I and A102V dysregulate Ca2+ signalling via different mechanisms. J. Cell Sci. 2022, 135, jcs258796. [Google Scholar] [CrossRef]
- Da’as, S.I.; Thanassoulas, A.; Calver, B.L.; Beck, K.; Salem, R.; Saleh, A.; Kontogianni, I.; Al-Maraghi, A.; Nasrallah, G.K.; Safieh-Garabedian, B.; et al. Arrhythmogenic calmodulin E105A mutation alters cardiac RyR2 regulation leading to cardiac dysfunction in zebrafish. Ann. N. Y Acad. Sci. 2019, 1448, 19–29. [Google Scholar] [CrossRef]
- Sondergaard, M.T.; Tian, X.; Liu, Y.; Wang, R.; Chazin, W.J.; Chen, S.R.; Overgaard, M.T. Arrhythmogenic Calmodulin Mutations Affect the Activation and Termination of Cardiac Ryanodine Receptor-mediated Ca2+ Release. J. Biol. Chem. 2015, 290, 26151–26162. [Google Scholar] [CrossRef]
- Vassilakopoulou, V.; Calver, B.L.; Thanassoulas, A.; Beck, K.; Hu, H.; Buntwal, L.; Smith, A.; Theodoridou, M.; Kashir, J.; Blayney, L.; et al. Distinctive malfunctions of calmodulin mutations associated with heart RyR2-mediated arrhythmic disease. Biochim. Biophys. Acta 2015, 1850, 2168–2176. [Google Scholar] [CrossRef]
- Hwang, H.S.; Nitu, F.R.; Yang, Y.; Walweel, K.; Pereira, L.; Johnson, C.N.; Faggioni, M.; Chazin, W.J.; Laver, D.; George, A.L., Jr.; et al. Divergent regulation of ryanodine receptor 2 calcium release channels by arrhythmogenic human calmodulin missense mutants. Circ. Res. 2014, 114, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.; Hamborg, L.; Lau, K.; Brohus, M.; Sorensen, A.B.; Larsen, K.T.; Sommer, C.; Van Petegem, F.; Overgaard, M.T.; Wimmer, R. The arrhythmogenic N53I variant subtly changes the structure and dynamics in the calmodulin N-terminal domain, altering its interaction with the cardiac ryanodine receptor. J. Biol. Chem. 2020, 295, 7620–7634. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Gao, Q.; Yu, L.; Sun, X.; Feng, R.; Shao, D.; Yuan, Y.; Zhu, Z.; Sun, X.; Kameyama, M.; et al. The LQT-associated calmodulin mutant E141G induces disturbed Ca2+-dependent binding and a flickering gating mode of the CaV1.2 channel. Am. J. Physiol. Cell Physiol. 2020, 318, C991–C1004. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Gupta, N.; Milburn, A.; McCormick, L.; Deugi, V.; Fisch, P.; Wyles, J.; Thomas, N.L.; Antonyuk, S.; Dart, C.; et al. Calmodulin variant E140G associated with long QT syndrome impairs CaMKIIdelta autophosphorylation and L-type calcium channel inactivation. J. Biol. Chem. 2023, 299, 102777. [Google Scholar] [CrossRef]
- Gupta, N.; Richards, E.M.B.; Morris, V.S.; Morris, R.; Wadmore, K.; Held, M.; McCormick, L.; Prakash, O.; Dart, C.; Helassa, N. Arrhythmogenic calmodulin variants D131E and Q135P disrupt interaction with the L-type voltage-gated Ca2+ channel (CaV1.2) and reduce Ca2+-dependent inactivation. Acta Physiol. 2025, 241, e14276. [Google Scholar] [CrossRef]
- Limpitikul, W.B.; Dick, I.E.; Joshi-Mukherjee, R.; Overgaard, M.T.; George, A.L., Jr.; Yue, D.T. Calmodulin mutations associated with long QT syndrome prevent inactivation of cardiac L-type Ca2+ currents and promote proarrhythmic behavior in ventricular myocytes. J. Mol. Cell Cardiol. 2014, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Makiyama, T.; Harita, T.; Sasaki, K.; Wuriyanghai, Y.; Hayano, M.; Nishiuchi, S.; Kohjitani, H.; Hirose, S.; Chen, J.; et al. Allele-specific ablation rescues electrophysiological abnormalities in a human iPS cell model of long-QT syndrome with a CALM2 mutation. Hum. Mol. Genet. 2017, 26, 1670–1677. [Google Scholar] [CrossRef]
- Wang, K.; Holt, C.; Lu, J.; Brohus, M.; Larsen, K.T.; Overgaard, M.T.; Wimmer, R.; Van Petegem, F. Arrhythmia mutations in calmodulin cause conformational changes that affect interactions with the cardiac voltage-gated calcium channel. Proc. Natl. Acad. Sci. USA 2018, 115, E10556–E10565. [Google Scholar] [CrossRef]
- Wang, K.; Brohus, M.; Holt, C.; Overgaard, M.T.; Wimmer, R.; Van Petegem, F. Arrhythmia mutations in calmodulin can disrupt cooperativity of Ca2+ binding and cause misfolding. J. Physiol. 2020, 598, 1169–1186. [Google Scholar] [CrossRef]
- Sondergaard, M.T.; Liu, Y.; Larsen, K.T.; Nani, A.; Tian, X.; Holt, C.; Wang, R.; Wimmer, R.; Van Petegem, F.; Fill, M.; et al. The Arrhythmogenic Calmodulin p.Phe142Leu Mutation Impairs C-domain Ca2+ Binding but Not Calmodulin-dependent Inhibition of the Cardiac Ryanodine Receptor. J. Biol. Chem. 2017, 292, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- McCormick, L.; Wadmore, K.; Milburn, A.; Gupta, N.; Morris, R.; Held, M.; Prakash, O.; Carr, J.; Barrett-Jolley, R.; Dart, C.; et al. Long QT syndrome-associated calmodulin variants disrupt the activity of the slowly activating delayed rectifier potassium channel. J. Physiol. 2023, 601, 3739–3764. [Google Scholar] [CrossRef] [PubMed]
- Da’as, S.I.; Thanassoulas, A.; Calver, B.L.; Saleh, A.; Abdelrahman, D.; Hasan, W.; Safieh-Garabedian, B.; Kontogianni, I.; Nasrallah, G.K.; Nounesis, G.; et al. Divergent Biochemical Properties and Disparate Impact of Arrhythmogenic Calmodulin Mutations on Zebrafish Cardiac Function. J. Cell Biochem. 2024, 125, e30619. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, S.P.; Niu, M.C. Calmodulinopathies: Throwing back the veil on the newest life-threatening genetic arrhythmia syndrome. Curr. Opin. Cardiol. 2021, 36, 61–66. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Crotti, L.; Nyegaard, M.; Overgaard, M.T. Role of Calmodulin in Cardiac Disease: Insights on Genotype and Phenotype. Circ. Genom. Precis. Med. 2024, 17, e004542. [Google Scholar] [CrossRef]
- Tsai, W.C.; Chen, P.S.; Rubart, M. Calmodulinopathy in inherited arrhythmia syndromes. Tzu Chi Med. J. 2021, 33, 339–344. [Google Scholar] [CrossRef]
- Chazin, W.J.; Johnson, C.N. Calmodulin Mutations Associated with Heart Arrhythmia: A Status Report. Int. J. Mol. Sci. 2020, 21, 1418. [Google Scholar] [CrossRef]
- Hussey, J.W.; Limpitikul, W.B.; Dick, I.E. Calmodulin Mutations in Human Disease. Channels 2023, 17, 2165278. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.W.; Woodbury, L.; Angsutararux, P.; Sambare, N.; Shi, J.; Marras, M.; Abella, C.; Bedi, A.; Zinn, D.; Cui, J.; et al. Arrhythmia-associated calmodulin variants interact with KCNQ1 to confer aberrant membrane trafficking and function. PNAS Nexus 2023, 2, pgad335. [Google Scholar] [CrossRef] [PubMed]
- Zissimopoulos, S.; Kirilenko, P.; Braza-Boils, A.; Zorio, E.; Wang, Y.; Gomez, A.M.; Cannell, M.B.; Latinkic, B.; Fowler, E.D. Compromised repolarization reserve in a murine model of catecholaminergic polymorphic ventricular tachycardia caused by RyR2-R420Q mutation. J. Mol. Cell Cardiol. 2025, 206, 127–140. [Google Scholar] [CrossRef]
- Sanguinetti, M.C.; Curran, M.E.; Zou, A.; Shen, J.; Spector, P.S.; Atkinson, D.L.; Keating, M.T. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 1996, 384, 80–83. [Google Scholar] [CrossRef]
- Wu, X.; Bers, D.M. Free and bound intracellular calmodulin measurements in cardiac myocytes. Cell Calcium 2007, 41, 353–364. [Google Scholar] [CrossRef]
- Kryshtal, D.O.; Gryshchenko, O.; Gomez-Hurtado, N.; Knollmann, B.C. Impaired calcium-calmodulin-dependent inactivation of CaV1.2 contributes to loss of sarcoplasmic reticulum calcium release refractoriness in mice lacking calsequestrin 2. J. Mol. Cell Cardiol. 2015, 82, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Findlay, I. Voltage- and cation-dependent inactivation of L-type Ca2+ channel currents in guinea-pig ventricular myocytes. J. Physiol. 2002, 541, 731–740. [Google Scholar] [CrossRef]
- Bers, D.M.; Patton, C.W.; Nuccitelli, R. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 2010, 99, 1–26. [Google Scholar] [CrossRef]
- Yus-Najera, E.; Santana-Castro, I.; Villarroel, A. The identification and characterization of a noncontinuous calmodulin-binding site in noninactivating voltage-dependent KCNQ potassium channels. J. Biol. Chem. 2002, 277, 28545–28553. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Vranken, W.F.; Boucher, W.; Stevens, T.J.; Fogh, R.H.; Pajon, A.; Llinas, M.; Ulrich, E.L.; Markley, J.L.; Ionides, J.; Laue, E.D. The CCPN data model for NMR spectroscopy: Development of a software pipeline. Proteins 2005, 59, 687–696. [Google Scholar] [CrossRef]
- Benoist, D.; Stones, R.; Benson, A.P.; Fowler, E.D.; Drinkhill, M.J.; Hardy, M.E.; Saint, D.A.; Cazorla, O.; Bernus, O.; White, E. Systems approach to the study of stretch and arrhythmias in right ventricular failure induced in rats by monocrotaline. Prog. Biophys. Mol. Biol. 2014, 115, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, N.; Goto, H.; Takasugi, M.; Verrier, R.L.; Kuwahara, T.; Kubota, T.; Toyoshi, H.; Nakashima, T.; Kawasaki, M.; Nishigaki, K.; et al. Prevalence of Microvolt T-Wave Alternans in Patients With Long QT Syndrome and Its Association With Torsade de Pointes. Circ. Arrhythm. Electrophysiol. 2016, 9, e003206. [Google Scholar] [CrossRef]
- Campiglio, M.; Coste de Bagneaux, P.; Ortner, N.J.; Tuluc, P.; Van Petegem, F.; Flucher, B.E. STAC proteins associate to the IQ domain of CaV1.2 and inhibit calcium-dependent inactivation. Proc. Natl. Acad. Sci. USA 2018, 115, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomstrom-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef]
- Abbas, R.; Abbas, A.; Khan, T.K.; Sharjeel, S.; Amanullah, K.; Irshad, Y. Sudden Cardiac Death in Young Individuals: A Current Review of Evaluation, Screening and Prevention. J. Clin. Med. Res. 2023, 15, 1–9. [Google Scholar] [CrossRef]
- Dal Cortivo, G.; Barracchia, C.G.; Marino, V.; D’Onofrio, M.; Dell’Orco, D. Alterations in calmodulin-cardiac ryanodine receptor molecular recognition in congenital arrhythmias. Cell Mol. Life Sci. 2022, 79, 127. [Google Scholar] [CrossRef]
- Dal Cortivo, G.; Marino, V.; Zamboni, D.; Dell’Orco, D. Impact of calmodulin missense variants associated with congenital arrhythmia on the thermal stability and the degree of unfolding. Hum. Genet. 2025, 144, 337–341. [Google Scholar] [CrossRef]
- Rocchetti, M.; Sala, L.; Dreizehnter, L.; Crotti, L.; Sinnecker, D.; Mura, M.; Pane, L.S.; Altomare, C.; Torre, E.; Mostacciuolo, G.; et al. Elucidating arrhythmogenic mechanisms of long-QT syndrome CALM1-F142L mutation in patient-specific induced pluripotent stem cell-derived cardiomyocytes. Cardiovasc. Res. 2017, 113, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Sung, R.J.; Wu, Y.H.; Lai, N.H.; Teng, C.H.; Luo, C.H.; Tien, H.C.; Lo, C.P.; Wu, S.N. Beta-adrenergic modulation of arrhythmogenesis and identification of targeted sites of antiarrhythmic therapy in Timothy (LQT8) syndrome: A theoretical study. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H33-44. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.P.; Kondo, C.S.; Sheldon, R.S.; Duff, H.J. Developmental changes in the delayed rectifier K+ channels in mouse heart. Circ. Res. 1996, 79, 79–85. [Google Scholar] [CrossRef]
- Fowler, E.D.; Kong, C.H.T.; Hancox, J.C.; Cannell, M.B. Late Ca2+ Sparks and Ripples During the Systolic Ca2+ Ca2+ Transient in Heart Muscle Cells. Circ. Res. 2018, 122, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Koenig, X.; Rubi, L.; Obermair, G.J.; Cervenka, R.; Dang, X.B.; Lukacs, P.; Kummer, S.; Bittner, R.E.; Kubista, H.; Todt, H.; et al. Enhanced currents through L-type calcium channels in cardiomyocytes disturb the electrophysiology of the dystrophic heart. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H564–H573. [Google Scholar] [CrossRef] [PubMed]
- Grantham, C.J.; Cannell, M.B. Ca2+ influx during the cardiac action potential in guinea pig ventricular myocytes. Circ. Res. 1996, 79, 194–200. [Google Scholar] [CrossRef]
- Brini, M.; Carafoli, E. The plasma membrane Ca2+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harb. Perspect. Biol. 2011, 3, a004168. [Google Scholar] [CrossRef]
- Chou, A.C.; Ju, Y.T.; Pan, C.Y. Calmodulin Interacts with the Sodium/Calcium Exchanger NCX1 to Regulate Activity. PLoS ONE 2015, 10, e0138856. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Salarian, M.; Chen, Y.; Veenstra, R.; Louis, C.F.; Yang, J.J. Gap junction regulation by calmodulin. FEBS Lett. 2014, 588, 1430–1438. [Google Scholar] [CrossRef]
- Peracchia, C. Gap Junction Channelopathies and Calmodulinopathies. Do Disease-Causing Calmodulin Mutants Affect Direct Cell-Cell Communication? Int. J. Mol. Sci. 2021, 22, 9169. [Google Scholar] [CrossRef]
- Abiria, S.A.; Colbran, R.J. CaMKII associates with CaV1.2 L-type calcium channels via selected beta subunits to enhance regulatory phosphorylation. J. Neurochem. 2010, 112, 150–161. [Google Scholar] [CrossRef]
- Bers, D.M.; Grandi, E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J. Cardiovasc. Pharmacol. 2009, 54, 180–187. [Google Scholar] [CrossRef]
- Mustroph, J.; Maier, L.S.; Wagner, S. CaMKII regulation of cardiac K channels. Front. Pharmacol. 2014, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Ca2+-calmodulin-dependent protein kinase II regulation of cardiac excitation-transcription coupling. Heart Rhythm. 2011, 8, 1101–1104. [Google Scholar] [CrossRef]
- Thanassoulas, A.; Vassilakopoulou, V.; Calver, B.L.; Buntwal, L.; Smith, A.; Lai, C.; Kontogianni, I.; Livaniou, E.; Nounesis, G.; Lai, F.A.; et al. Life-threatening arrhythmogenic CaM mutations disrupt CaM binding to a distinct RyR2 CaM-binding pocket. Biochim. Biophys. Acta Gen. Subj. 2023, 1867, 130313. [Google Scholar] [CrossRef]
- Jensen, H.H.; Frantzen, M.T.; Wesseltoft, J.L.; Busuioc, A.O.; Moller, K.V.; Brohus, M.; Duun, P.R.; Nyegaard, M.; Overgaard, M.T.; Olsen, A. Human calmodulin mutations cause arrhythmia and affect neuronal function in C. elegans. Hum. Mol. Genet. 2023, 32, 2068–2083. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, S.K.; Kim, C.S.J.; Tester, D.J.; Gencarelli, M.; Tobert, K.E.; Gluscevic, M.; Ackerman, M.J. Single Construct Suppression and Replacement Gene Therapy for the Treatment of All CALM1-, CALM2-, and CALM3-Mediated Arrhythmia Disorders. Circ. Arrhythm. Electrophysiol. 2024, 17, e012036. [Google Scholar] [CrossRef]
- Kefalas, G.; Jouvet, N.; Baldwin, C.; Estall, J.L.; Larose, L. Peptide-based sequestration of the adaptor protein Nck1 in pancreatic beta cells enhances insulin biogenesis and protects against diabetogenic stresses. J. Biol. Chem. 2018, 293, 12516–12524. [Google Scholar] [CrossRef] [PubMed]



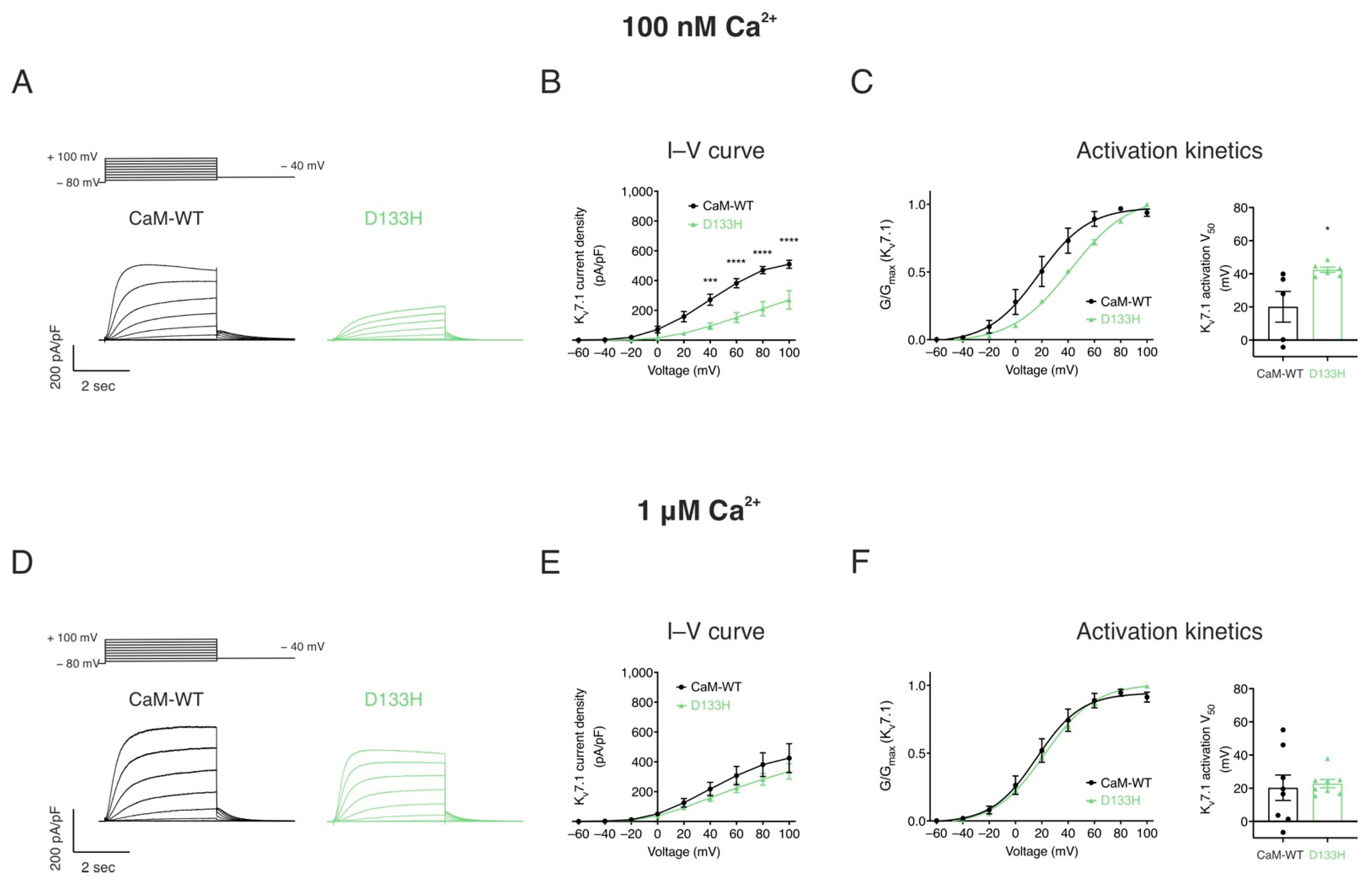
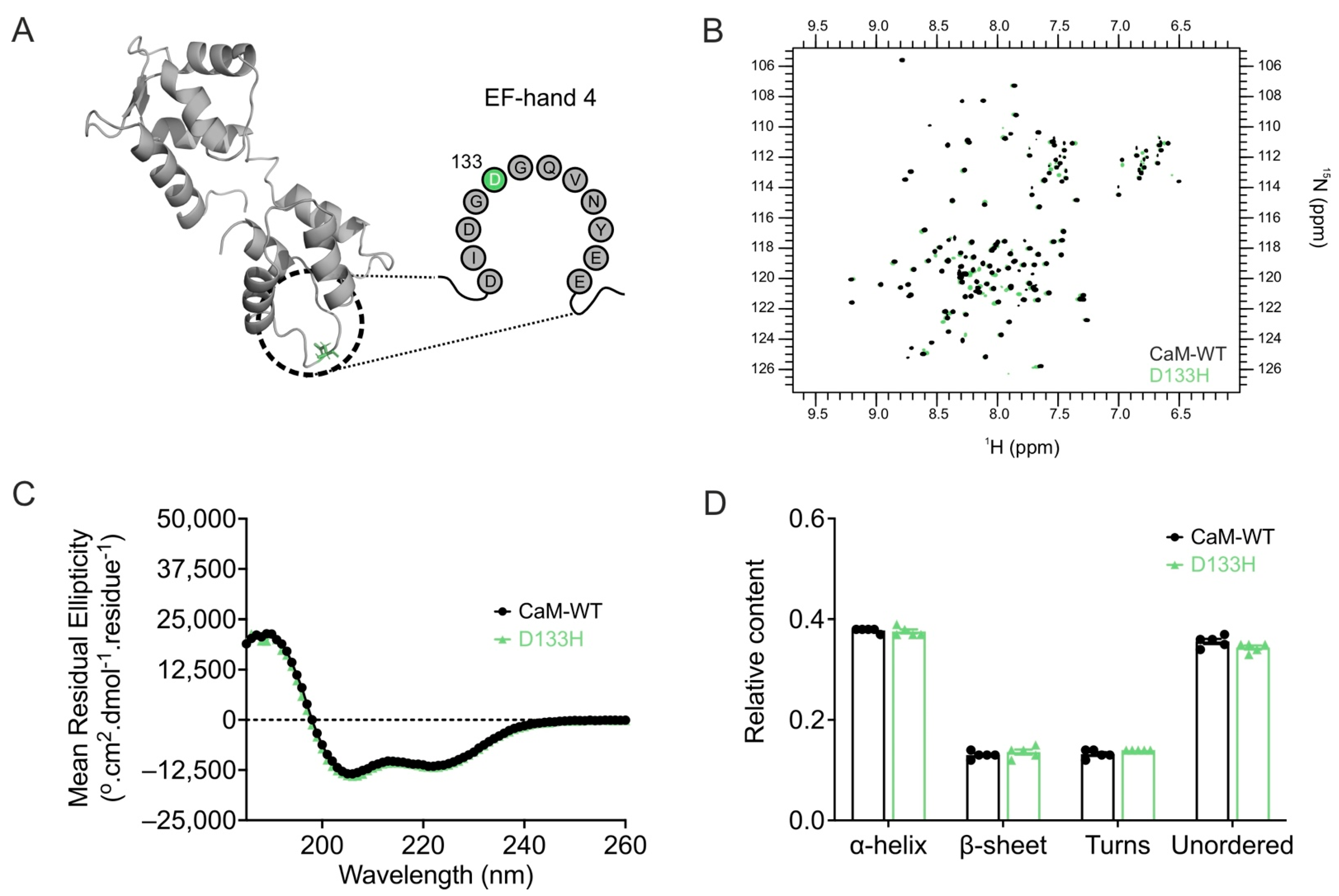

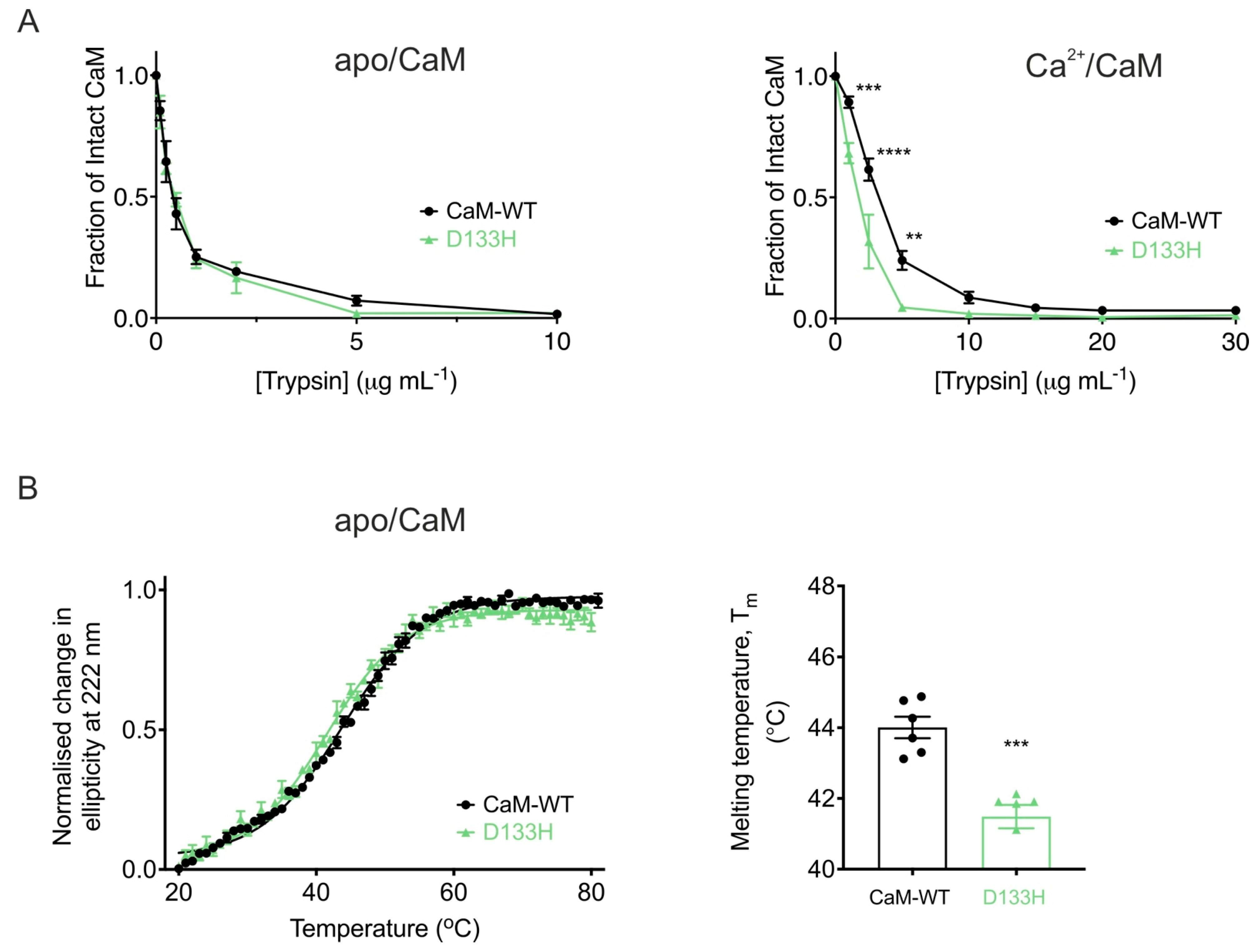

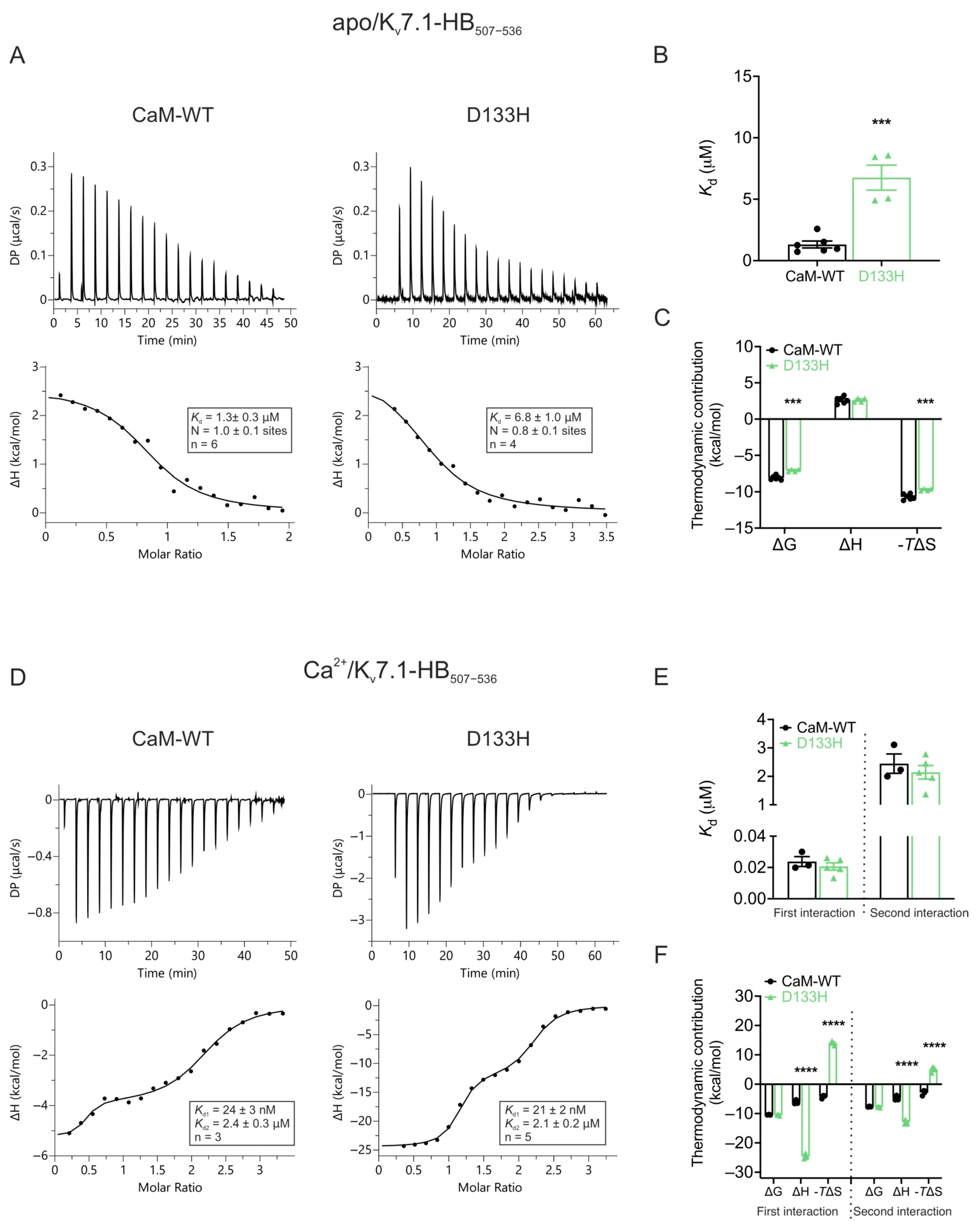
| Cav1.2 (Patch Clamp Electrophysiology) | |||||||
|---|---|---|---|---|---|---|---|
| Stable HEK293 | Mouse Cardiomyocyte | ||||||
| Peak Current Density (pA/pF) | Reversal Potential (mV) | V50 Activation (mV) | V50 Inactivation (mV) | CDI (f300) | APD90 at 1 Hz (ms) | CDI (f50) | |
| CaM-WT | −2.2 ± 0.4 | 58.7 ± 2.8 | 6.9 ± 3.8 | −17.0 ± 3.6 | 0.76 ± 0.04 | 170 ± 14 | 0.26 ± 0.04 |
| D133H | −3.4 ± 0.4 | 52.4 ± 1.6 | 6.9 ± 2.3 | −18.0 ± 1.8 | 0.21 ± 0.05 **** | 225 ± 18 * | 0.18 ± 0.08 |
| Kv7.1 (Patch Clamp Electrophysiology) | ||||
|---|---|---|---|---|
| 100 nM Ca2+ | 1 μM Ca2+ | |||
| Current Density at +100 mV (pA/pF) | V50 Activation (mV) | Current Density at +100 mV (pA/pF) | V50 Activation (mV) | |
| CaM-WT | 509.6 ± 27.0 | 20.1 ± 9.3 | 424.7 ± 97.0 | 20.3 ± 7.7 |
| D133H | 270.2 ± 62.2 **** | 42.4 ± 1.6 * | 321.2 ± 47.3 | 22.8 ± 2.5 |
| Ca2+ | Cav1.2 | Kv7.1 | |||
|---|---|---|---|---|---|
| IQ1665−1685 | NSCaTE51−67 | HB507−536 (apo) | HB507−536 (Ca2+) | ||
| CaM-WT | 0.9 ± 0.1 | 0.083 ± 0.005 | 1.6 ± 0.3 | 1.3 ± 0.3 | 0.024 ± 0.003 2.4 ± 0.3 |
| D133H | 22.2 ± 1.5 **** | 0.020 ± 0.005 **** | 9.3 ± 1.5 *** | 6.8 ± 1.0 *** | 0.021 ± 0.002 2.1 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, N.; McCormick, L.F.; Richards, E.M.B.; Wadmore, K.; Morris, R.; Morris, V.S.; Kirilenko, P.; Fowler, E.D.; Dart, C.; Helassa, N. Calmodulin D133H Disrupts Cav1.2 and Kv7.1 Regulation to Prolong Cardiac Action Potentials in Long QT Syndrome. Cells 2025, 14, 1763. https://doi.org/10.3390/cells14221763
Gupta N, McCormick LF, Richards EMB, Wadmore K, Morris R, Morris VS, Kirilenko P, Fowler ED, Dart C, Helassa N. Calmodulin D133H Disrupts Cav1.2 and Kv7.1 Regulation to Prolong Cardiac Action Potentials in Long QT Syndrome. Cells. 2025; 14(22):1763. https://doi.org/10.3390/cells14221763
Chicago/Turabian StyleGupta, Nitika, Liam F. McCormick, Ella M. B. Richards, Kirsty Wadmore, Rachael Morris, Vanessa S. Morris, Pavel Kirilenko, Ewan D. Fowler, Caroline Dart, and Nordine Helassa. 2025. "Calmodulin D133H Disrupts Cav1.2 and Kv7.1 Regulation to Prolong Cardiac Action Potentials in Long QT Syndrome" Cells 14, no. 22: 1763. https://doi.org/10.3390/cells14221763
APA StyleGupta, N., McCormick, L. F., Richards, E. M. B., Wadmore, K., Morris, R., Morris, V. S., Kirilenko, P., Fowler, E. D., Dart, C., & Helassa, N. (2025). Calmodulin D133H Disrupts Cav1.2 and Kv7.1 Regulation to Prolong Cardiac Action Potentials in Long QT Syndrome. Cells, 14(22), 1763. https://doi.org/10.3390/cells14221763







