Ayurvedic Phytochemicals in Oncology: ADP-Ribosylation as a Molecular Nexus
Abstract
1. Introduction
2. Selected Ayurvedic Phytochemicals in Cancer-Mechanistic Insights into Molecular Targets and ADP-Ribosylation
3. Phytochemical-Induced Poly-ADP-Ribosylation in Carcinogenesis: Insights from Ayurvedic Thymus Species
4. Phytochemicals in p53 Signalling and ADP-Ribosylation Pathways
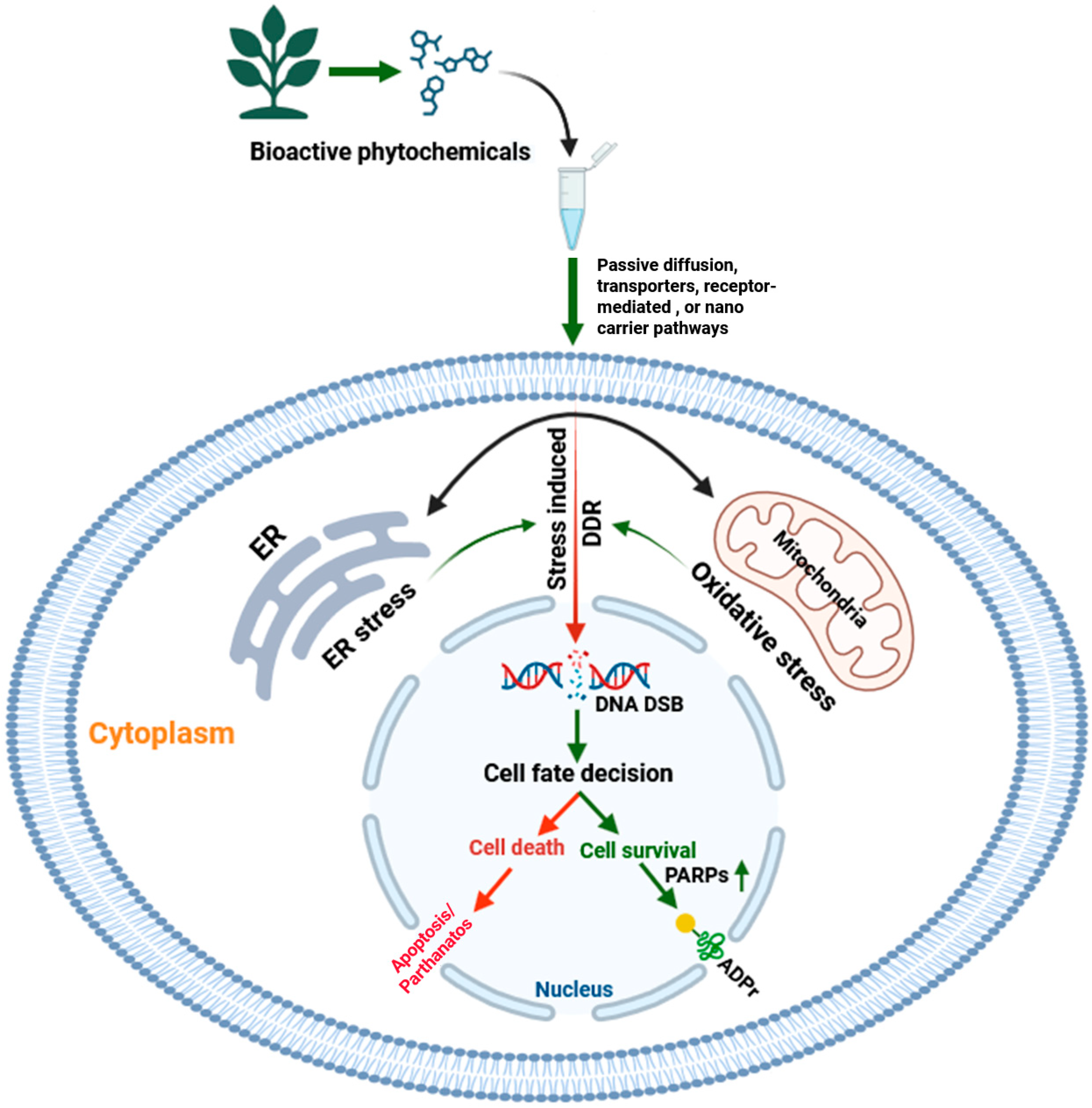
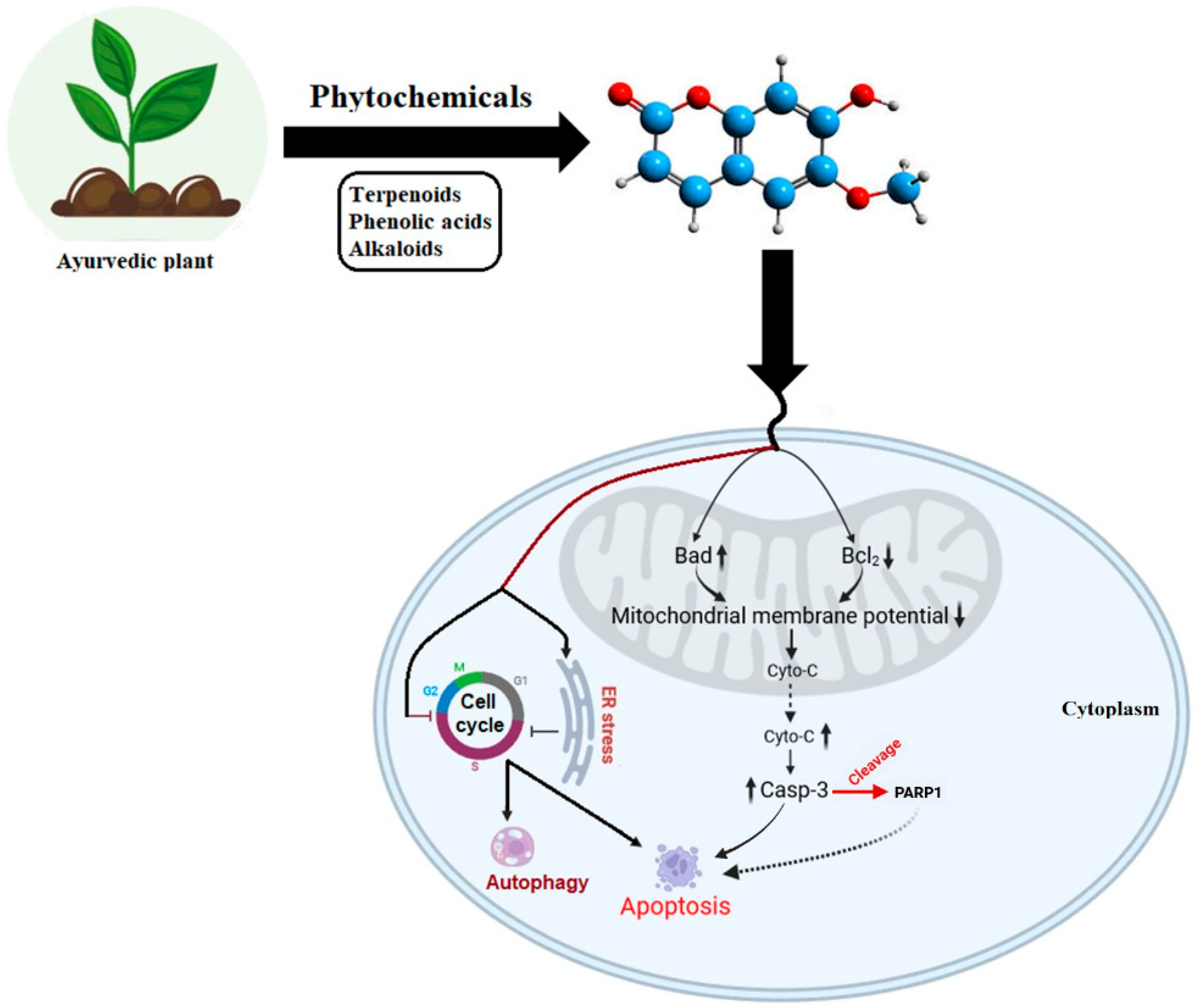
5. Phytochemical-Induced Coupling of Cellular Stress and the ADPr Axis in Apoptotic and Autophagic Cell Death
6. ADP-Ribosylation and Inflammatory Pathways: Insights from Ayurvedic Medicine
7. Integrative Cancer Therapies: ADP-Ribosylation in Ayurvedic and Allopathic Perspectives
8. Phytochemical–Nanoparticle Coupling and Targeted Delivery Strategies for Modulating ADP-Ribosylation Pathways in Cancer
9. Concluding Reflections
10. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TME | tumor microenvironment |
| CAM | complementary and alternative medicine |
| NSB | nano swarna bhasma |
| ADPr | ADP-ribosylation |
| ART | ADP-ribosyl transferases |
| NAD+ | β-nicotinamide adenine dinucleotide |
| PARP | poly (ADP-ribose) polymerase |
| 3D QSAR | three-dimensional quantitative structure–activity relationship |
| CDK1 | cyclin-dependent kinase 1 |
| Bax | Bcl-2-associated X protein |
| BRCA1 | breast cancer gene 1 |
| BRCA2 | breast cancer gene 2 |
| SIRT6 | sirtuin 6 |
| BAF170 | BRG1-Associated Factor 170 |
| IL | interleukin |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| RANKL | receptor activator of NF-kappa B ligand |
| MDR | multidrug resistance |
| MAPK | mitogen-activated protein kinase |
| ERK | extracellular signal-regulated kinase |
| PKA | protein kinase A |
| TNF-α | tumor necrosis factor-alpha |
| RAD51 | radiation sensitive 51. |
| Akt | protein kinase B |
| Bcl-2 | B-cell lymphoma 2 |
| COX-2 | cyclooxygenase-2 |
| MMP-9 | matrix metalloproteinase-9 |
| STAT3 | signal transducer and activator of transcription 3 |
| ROS | reactive oxygen species |
| EGCG | epigallocatechin-3-gallate |
| WFA | Withaferin A |
| BRAF | v-Raf murine sarcoma viral oncogene homolog B1 |
| Raf-1 | v-Raf-1 murine leukemia viral oncogene homolog 1 |
| HSP | heat shock protein |
| NHEJ | non-homologous end joining |
| CBP | CREB-binding protein |
| p300 | E1A-associated protein p300 |
| HATs | histone acetyltransferases |
| ATR | ataxia telangiectasia and Rad3-related |
| DR4 | death receptors 4 |
| DR5 | death receptors 5 |
| γH2AX | phosphorylated histone H2A variant X |
| p65/RelA | v-Rel avian reticuloendotheliosis viral oncogene homolog A |
| BEL-7402 | human hepatocellular carcinoma cell lines |
| LDH | lactate dehydrogenase |
| BAD | Bcl-2-associated death promoter |
| PEL | primary effusion lymphoma |
| ER | endoplasmic reticulum |
| JNK | c-jun N-terminal kinase |
| PI3K | phosphoinositide 3-kinase |
| mTOR | mammalian target of rapamycin |
| MCF-7 | Michigan Cancer Foundation-7 |
| Bcl-xL | B-cell lymphoma-extra large |
| CSCs | cancer stem cells |
| PEITC | phenethyl isothiocyanates |
| HER2 | human epidermal growth factor receptor 2 |
| EGFR | epidermal growth factor receptor |
| AP-1 | activator protein 1 |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| iNOS | inducible nitric oxide synthase |
| FDA | food and drug administration |
| TAMs | tumor-associated macrophages |
| RNS | reactive nitrogen species |
| AGEs | advanced glycation end products |
| RAGE | receptor for advanced glycation end-products |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| EGCG | epigallocatechin gallate |
| DDR | DNA damage response |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| Cas9 | CRISPR-associated protein 9 |
| ZFNs | zinc finger nucleases |
| TALENs | transcription activator-like effector nucleases |
| DSBs | double-strand breaks |
References
- Armitage, P.; Doll, R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br. J. Cancer 1954, 8, 1–12. [Google Scholar] [CrossRef]
- Sugimura, T. Multistep carcinogenesis: A 1992 perspective. Science 1992, 258, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Gorga, F. The Molecular Basis of Cancer. Bridgewater Rev. 1998, 17, 3–6. Available online: https://vc.bridgew.edu/br_rev/vol17/iss2/5 (accessed on 30 October 2025).
- Arnold, J.T. Integrating ayurvedic medicine into cancer research programs part 1: Ayurveda background and applications. J. Ayurveda Integr. Med. 2023, 14, 100676. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Tang, Y.Q.; Miao, H. Metabolism in tumor microenvironment: Implications for cancer immunotherapy. MedComm 2020, 1, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Vohra, R.; Singh, R.; Shrivastava, R. A scoping review on ‘Maharishi Amrit Kalash’, an ayurveda formulation for cancer prevention and management. J. Ayurveda Integr. Med. 2024, 15, 100866. [Google Scholar] [CrossRef]
- Tralongo, P.; Pescarenico, M.G.; Surbone, A.; Bordonaro, S.; Berretta, M.; DI Mari, A. Physical needs of long-term cancer patients. Anticancer Res. 2017, 37, 4733–4746. [Google Scholar] [CrossRef]
- Given, B.A.; Given, C.W.; Vachon, E.; Hershey, D. Do we have a clue: The treatment burden for the patient with cancer? Cancer Nurs. 2016, 39, 423–424. [Google Scholar] [CrossRef]
- Valdivieso, M.; Kujawa, A.M.; Jones, T.; Baker, L.H. Cancer survivors in the United States: A review of the literature and a call to action. Int. J. Med. Sci. 2012, 9, 63–73. [Google Scholar] [CrossRef]
- Mateo, J.; Steuten, L.; Aftimos, P.; André, F.; Davies, M.; Garralda, E.; Geissler, J.; Husereau, D.; Martinez-Lopez, I.; Normanno, N.; et al. Delivering precision oncology to patients with cancer. Nat. Med. 2022, 28, 658–665. [Google Scholar] [CrossRef]
- Samanta, K.; Reddy, G.S.V.S.R.; Sharma, N.K.; Kar, P. Deciphering the Role of Functional Ion Channels in Cancer Stem Cells (CSCs) and Their Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 7595. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dobos, G.J.; Rampp, T. The significance of Ayurvedic medicinal plants. J. Evid. Based Complement. Altern. Med. 2017, 22, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Tandon, N.; Yadav, S.S. Contributions of Indian council of medical research (ICMR) in the area of medicinal plants/traditional medicine. J. Ethnopharmacol. 2017, 197, 39–45. [Google Scholar] [CrossRef]
- Pandey, S.; Walpole, C.; Shaw, P.N.; Cabot, P.J.; Hewavitharana, A.K.; Batra, J. Bio-guided fractionation of papaya leaf juice for delineating the components responsible for the selective anti-proliferative effects on prostate cancer cells. Front. Pharmacol. 2018, 9, 1319. [Google Scholar] [CrossRef]
- Khoobchandani, M.; Katti, K.K.; Karikachery, A.R.; Thipe, V.C.; Srisrimal, D.; Dhurvas Mohandoss, D.K.; Darshakumar, R.D.; Joshi, C.M.; Katti, K.V. New approaches in breast cancer therapy through green nanotechnology and nano-Ayurvedic medicine—Pre-clinical and pilot human clinical investigations. Int. J. Nanomed. 2020, 15, 181–197. [Google Scholar] [CrossRef]
- Huang, L.; Luo, S.; Tong, S.; Lv, Z.; Wu, J. The development of nanocarriers for natural products. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1967. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, J.R.; Yokomizo, K.; Fang, J. Nano-drug delivery system of natural products for disease prevention and treatment. Expert Opin. Drug Deliv. 2025, 22, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Katti, K.; Chanda, N.; Shukla, R.; Zambre, A.; Suibramanian, T.; Kulkarni, R.R.; Kannan, R.; Katti, K.V. Green nanotechnology from cumin phytochemicals: Generation of biocompatible gold nanoparticles. Int. J. Green Nanotechnol. Biomed. 2009, 1, B39–B52. [Google Scholar] [CrossRef]
- Yayintas, O.T.; Demir, N.; Canbolat, F.; Ayna, T.K.; Pehlivan, M. Characterization, biological activity, and anticancer effect of green-synthesized gold nanoparticles using Nasturtium officinale L. BMC Complement. Med. Ther. 2024, 24, 346. [Google Scholar] [CrossRef]
- Nune, S.K.; Chanda, N.; Shukla, R.; Katti, K.; Kulkarni, R.R.; Thilakavathi, S.; Mekapothula, S.; Kannan, R.; Katti, K.V. Green nanotechnology from tea: Phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J. Mater. Chem. 2009, 19, 2912–2920. [Google Scholar] [CrossRef]
- Khoobchandani, M.; Katti, K.; Maxwell, A.; Fay, W.P.; Katti, K.V. Laminin receptor-avid nanotherapeutic EGCg-AuNPs as a potential alternative therapeutic approach to prevent restenosis. Int. J. Mol. Sci. 2016, 17, 316. [Google Scholar] [CrossRef]
- Thipe, V.C.; Keyster, M.; Katti, K.V. Sustainable Nanotechnology: Mycotoxin Detection and Protection. In Nanobiotechnology Applications in Plant Protection; Abd-Elsalam, K., Prasad, R., Eds.; Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2018; pp. 323–349. [Google Scholar] [CrossRef]
- Yang, L.Y.; Lei, S.Z.; Xu, W.J.; Lai, Y.-X.; Zhang, Y.-Y.; Wang, Y.; Wang, Z.-L. Rising above: Exploring the therapeutic potential of natural product-based compounds in human cancer treatment. Tradit. Med. Res. 2025, 10, 18. [Google Scholar] [CrossRef]
- Menze, N.; Van der Watt, A.S.J.; Moxley, K.; Seedat, S. Profiles of traditional healers and their healing practices in the Eastern Cape province of South Africa. S. Afr. J. Psychiatry 2018, 24, a1305. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z. Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer. Biomedicines 2021, 9, 689. [Google Scholar] [CrossRef]
- Dutta, R.; Khalil, R.; Green, R.; Mohapatra, S.S.; Mohapatra, S. Withania somnifera (Ashwagandha) and Withaferin A: Potential in integrative oncology. Int. J. Mol. Sci. 2019, 20, 5310. [Google Scholar] [CrossRef] [PubMed]
- Ogiwara, H.; Ui, A.; Shiotani, B.; Zou, L.; Yasui, A.; Kohno, T. Curcumin suppresses multiple DNA damage response pathways and has potency as a sensitizer to PARP inhibitor. Carcinogenesis 2013, 34, 2486–2497. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Mishra, L.C.; Singh, B.B.; Dagenais, S. Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): A review. Altern. Med. Rev. 2000, 5, 334–346. [Google Scholar] [PubMed]
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008, 269, 199–225. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, N.; Gupta, A.; Valli, R.K.; Joshi, S.D.; Mills, J.T.; Hamel, E.; Khanna, P.; Jain, S.C.; Thakur, S.S.; Ravindranath, V. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc. Natl. Acad. Sci. USA 2012, 109, 3510–3515. [Google Scholar] [CrossRef]
- Patwardhan, B.; Gautam, M. Botanical immunodrugs: Scope and opportunities. Drug Discov. Today. 2005, 10, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Prasad, S.; Reuter, S.; Kannappan, R.; Yadev, V.R.; Park, B.; Kim, J.H.; Gupta, S.C.; Phromnoi, K.; Sundaram, C.; et al. Identification of novel anti-inflammatory agents from Ayurvedic medicine for prevention of chronic diseases: “reverse pharmacology” and “bedside to bench” approach. Curr. Drug Targets 2011, 12, 1595–1653. [Google Scholar] [CrossRef]
- Gupte, R.; Liu, Z.; Kraus, W.L. PARPs and ADP-ribosylation: Recent advances linking molecular functions to biological outcomes. Genes Dev. 2017, 31, 101–126. [Google Scholar] [CrossRef]
- Ray Chaudhuri, A.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef]
- Zrinej, J.; Elmchichi, L.; Alaqarbeh, M.; Tahar Lakhlifia, T.; Bouachrineac, M. Computational approach: 3D-QSAR, molecular docking, ADMET, molecular dynamics simulation investigations, and retrosynthesis of some curcumin analogues as PARP-1 inhibitors targeting colon cancer. New J. Chem. 2023, 47, 20987–21009. [Google Scholar] [CrossRef]
- El Hassab, M.A.; Eldehna, W.M.; Hassan, G.S.; Abou-Seri, S.M. Multi-stage structure-based virtual screening approach combining 3D pharmacophore, docking and molecular dynamic simulation towards the identification of potential selective PARP-1 inhibitors. BMC Chem. 2025, 19, 30. [Google Scholar] [CrossRef]
- Bono, A.; La Monica, G.; Alamia, F.; Mingoia, F.; Gentile, C.; Peri, D.; Lauria, A.; Martorana, A. In Silico Mixed Ligand/Structure-Based Design of New CDK-1/PARP-1 Dual Inhibitors as Anti-Breast Cancer Agents. Int. J. Mol. Sci. 2023, 24, 13769. [Google Scholar] [CrossRef]
- Zhu, Y.; Mehmoodc, A.; Li, D. Unraveling the inhibitory potential of Rosetta designed de novo cyclic peptides on PARP7 through molecular dynamics simulations. New J. Chem. 2024, 48, 7347–7355. [Google Scholar] [CrossRef]
- Wierbiłowicz, K.; Yang, C.S.; Almaghasilah, A.; Wesołowski, P.A.; Pracht, P.; Dworak, N.M.; Masur, J.; Wijngaarden, S.; Filippov, D.V.; Wales, D.J.; et al. Parp7 generates an ADP-ribosyl degron that controls negative feedback of androgen signaling. EMBO J. 2025, 44, 4720–4744. [Google Scholar] [CrossRef] [PubMed]
- Caba, K.; Tran-Nguyen, V.K.; Rahman, T.; Ballester, P.J. Comprehensive machine learning boosts structure-based virtual screening for PARP1 inhibitors. J. Cheminform. 2024, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and pharmacokinetic study of oral curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Kesarwani, K.; Gupta, R. Bioavailability enhancers of herbal origin: An overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Maeda, J.; Roybal, E.J.; Brents, C.A.; Uesaka, M.; Aizawa, Y.; Kato, T.A. Natural and glucosyl flavonoids inhibit poly(ADP-ribose) polymerase activity and induce synthetic lethality in BRCA mutant cells. Oncol. Rep. 2014, 31, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential mechanisms of quercetin in cancer prevention: Focus on cellular and molecular targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef]
- Mao, Z.; Hine, C.; Tian, X.; Van Meter, M.; Au, M.; Vaidya, A.; Seluanov, A.; Gorbunova, V. SIRT6 promotes DNA repair under stress by activating PARP1. Science 2011, 332, 1443–1446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Zhang, H.X. Effect of quercetin on the protein-substrate interactions in SIRT6: Insight from MD simulations. J. Mol. Graph. Model 2024, 130, 108778. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y. Potential terahertz therapeutic strategy for the prevention or mitigation of Alzheimer’s disease pathology. Light Sci. Appl. 2023, 12, 254. [Google Scholar] [CrossRef]
- Xavier, C.P.; Pereira-Wilson, C. Medicinal plants of the genuses Salvia and Hypericum are sources of anticolon cancer compounds: Effects on PI3K/Akt and MAP kinases pathways. PharmaNutrition 2016, 4, 112–122. [Google Scholar] [CrossRef]
- Rocha, J.; Eduardo-Figueira, M.; Barateiro, A.; Fernandes, A.; Brites, D.; Bronze, R.; Duarte, C.M.; Serra, A.T.; Pinto, R.; Freitas, M.; et al. Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin. Pharmacol. Toxicol. 2015, 116, 398–413. [Google Scholar] [CrossRef]
- Liu, W.Y.; Wang, H.; Xu, X.; Wang, X.; Han, K.K.; You, W.D.; Yang, Y.; Zhang, T. Natural compound rosmarinic acid displays anti-tumor activity in colorectal cancer cells by suppressing nuclear factor-kappa B signaling. World J. Clin. Oncol. 2025, 16, 105341. [Google Scholar] [CrossRef]
- Su, C.; Gius, J.P.; Van Steenberg, J.; Haskins, A.H.; Heishima, K.; Omata, C.; Iwayama, M.; Murakami, M.; Mori, T.; Maruo, K.; et al. Hypersensitivity of BRCA2 deficient cells to rosemary extract explained by weak PARP inhibitory activity. Sci. Rep. 2017, 7, 16704. [Google Scholar] [CrossRef]
- Subongkot, T.; Ngawhirunpat, T.; Opanasopit, P. Development of ultra deformable liposomes with fatty acids for enhanced dermal rosmarinic acid delivery. Pharmaceutics 2021, 13, 404. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ichikawa, H.; Takada, Y.; Sandur, S.K.; Shishodia, S.; Aggarwal, B.B. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol. Pharmacol. 2006, 69, 195–206. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, B.B. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.W.; Kuo, Y.H.; Lin, B.F. Anti-inflammatory activity of new compounds from Andrographis paniculata by NF-kappaB transactivation inhibition. J. Agric. Food Chem. 2010, 58, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.W.; Lin, B.F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Liu, Q.; You, G.; Ye, L.; Jin, Y.; Kong, L.; Guo, W.; Xu, Q.; Sun, Y. Advances in ameliorating inflammatory diseases and cancers by andrographolide: Pharmacokinetics, pharmacodynamics, and perspective. Med. Res. Rev. 2022, 42, 1147–1178. [Google Scholar] [CrossRef]
- Ichikawa, H.; Takada, Y.; Shishodia, S.; Jayaprakasam, B.; Nair, M.G.; Aggarwal, B.B. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol. Cancer Ther. 2006, 5, 1434–1445. [Google Scholar] [CrossRef]
- Mulabagal, V.; Subbaraju, G.V.; Rao, C.V.; Sivaramakrishna, C.; Dewitt, D.L.; Holmes, D.; Sung, B.; Aggarwal, B.B.; Tsay, H.S.; Nair, M.G. Withanolide sulfoxide from Aswagandha roots inhibits nuclear transcription factor-kappa-B, cyclooxygenase and tumor cell proliferation. Phytother. Res. 2009, 23, 987–992. [Google Scholar] [CrossRef]
- Widodo, N.; Kaur, K.; Shrestha, B.G.; Takagi, Y.; Ishii, T.; Wadhwa, R.; Kaul, S.C. Selective killing of cancer cells by leaf extract of Ashwagandha: Identification of a tumor-inhibitory factor and the first molecular insights to its effect. Clin. Cancer Res. 2007, 13, 2298–2306. [Google Scholar] [CrossRef]
- Grogan, P.T.; Sarkaria, J.N.; Timmermann, B.N.; Cohen, M.S. Oxidative cytotoxic agent withaferin A resensitizes temozolomide-resistant glioblastomas via MGMT depletion and induces apoptosis through Akt/mTOR pathway inhibitory modulation. Investig. New Drugs 2014, 32, 604–617. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, P.; Bajpai, G. Tinospora cordifolia (Giloy): An insight on the multifarious pharmacological paradigms of a most promising medicinal ayurvedic herb. Heliyon 2024, 10, e26125. [Google Scholar] [CrossRef]
- Patil, S.; Ashi, H.; Hosmani, J.; Almalki, A.Y.; Alhazmi, Y.A.; Mushtaq, S.; Parveen, S.; Baeshen, H.A.; Varadarajan, S.; Raj, A.T.; et al. Tinospora cordifolia (Thunb.) Miers (Giloy) inhibits oral cancer cells in a dose-dependent manner by inducing apoptosis and attenuating epithelial-mesenchymal transition. Saudi J. Biol. Sci. 2021, 28, 4553–4559. [Google Scholar] [CrossRef]
- Kumar, G.H.; Priyadarsini, R.V.; Vinothini, G.; Letchoumy, P.V.; Nagini, S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Investig. New Drugs. 2010, 28, 392–401. [Google Scholar] [CrossRef]
- Wu, Q.; Kohli, M.; Bergen, H.R., 3rd; Cheville, J.C.; Karnes, R.J.; Cao, H.; Young, C.Y.; Tindall, D.J.; McNiven, M.A.; Donkena, K.V. Preclinical evaluation of the supercritical extract of Azadirachta indica (neem) leaves in vitro and in vivo on inhibition of prostate cancer tumor growth. Mol. Cancer Ther. 2014, 13, 1067–1077. [Google Scholar] [CrossRef]
- Visavadiya, N.P.; Narasimhacharya, A.V. Asparagus root regulates cholesterol metabolism and improves antioxidant status in hypercholesteremic rats. Evid. Based Complement. Alternat. Med. 2009, 6, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.K.; Bhutani, M.; Nair, A.S.; Ahn, K.S.; Chakraborty, A.; Kadara, H.; Guha, S.; Sethi, G.; Aggarwal, B.B. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol. Cancer Res. 2007, 5, 943–955. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Biswas, K.; Mishra, A.K.; Patra, B.; Mishra, B.; Panda, J.; Avula, S.K.; Varma, R.S.; Panda, B.P.; Nayak, D. Amelioration of gold nanoparticles mediated through Ocimum oil extracts induces reactive oxygen species and mitochondrial instability against MCF-7 breast carcinoma. RSC Adv. 2024, 14, 27816–27830. [Google Scholar] [CrossRef]
- Jadhav, P.; Shaikh, A.Q.; Bachhav, R.S.; Rawal, D.; Lohar, S. A Review: Study of Tulsi as Immune Booster. Int. J. Res. Appl. Sci. Eng. Technol. 2025, 13, 770–775. [Google Scholar] [CrossRef]
- Hasan, M.R.; Alotaibi, B.S.; Althafar, Z.M.; Mujamammi, A.H.; Jameela, J. An update on the therapeutic anticancer potential of Ocimum sanctum L.: “Elixir of Life”. Molecules 2023, 28, 1193. [Google Scholar] [CrossRef]
- Nayak, P.; Thirunavoukkarasu, M. A review of the plant Boerhaavia diffusa: Its chemistry, pharmacology and therapeutical potential. J. Phytopharm. 2016, 5, 83–92. [Google Scholar] [CrossRef]
- Leslie Taylor, N.D. Boerhaavia diffusa. In The Healing Power of Rainforest Herbs; Square one Publishers, Inc.: New York, NY, USA, 2005; pp. 1–535. Available online: https://www.rain-tree.com/book2.htm (accessed on 30 October 2025).
- Manu, K.A.; Kuttan, G. Effect of Punarnavine, an alkaloid from Boerhaavia diffusa, on cell-mediated immune responses and TIMP-1 in B16F-10 metastatic melanoma-bearing mice. Immunopharmacol. Immunotoxicol. 2007, 29, 569–586. [Google Scholar] [CrossRef]
- Patel, N.; Mishra, R.; Rajput, D.; Gupta, A. A comprehensive review of the phytochemistry, pharmacology, pharmacokinetics, and green nanotechnological significance of Boerhavia diffusa Linn. Fitoterapia 2025, 184, 106599. [Google Scholar] [CrossRef]
- Aher, V.; Chattopadhyay, P.; Goyary, D.; Veer, V. Evaluation of the genotoxic and antigenotoxic potential of the alkaloid punarnavine from Boerhaavia diffusa. Planta Med. 2013, 79, 939–945. [Google Scholar] [CrossRef]
- Dey, T.; Dutta, P.; Manna, P.; Kalita, J.; Boruah, H.P.D.; Buragohain, A.K.; Unni, B. Anti-proliferative activities of Vasicinone on lung carcinoma cells mediated via activation of both mitochondria-dependent and independent pathways. Biomol. Ther. 2018, 26, 409–416. [Google Scholar] [CrossRef]
- Masutani, M.; Gunji, A.; Tsutsumi, M.; Ogawa, K.; Kamada, N.; Shirai, T.; Jishage, K.-I.; Nakagama, H.; Sugimura, T. Role of Poly-ADP-Ribosylation in Cancer Development. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6118/ (accessed on 30 October 2025).
- Bejan, D.S.; Sundalam, S.; Jin, H.; Morgan, R.K.; Kirby, I.T.; Siordia, I.R.; Tivon, B.; London, N.; Cohen, M.S. Structure-guided design and characterization of a clickable, covalent PARP16 inhibitor. Chem. Sci. 2022, 13, 13898–13906. [Google Scholar] [CrossRef]
- Masutani, M.; Nakagama, H.; Sugimura, T. Poly (ADP-ribose) and carcinogenesis. Genes Chromosomes Cancer 2003, 38, 339–348. [Google Scholar] [CrossRef]
- Prasad, S.C.; Thraves, P.J.; Bhatia, K.G.; Smulson, M.E.; Dritschilo, A. Enhanced poly (adenosine diphosphate ribose) polymerase activity and gene expression in Ewing’s sarcoma cells. Cancer Res. 1990, 50, 38–43. [Google Scholar] [PubMed]
- Bièche, I.; de Murcia, G.; Lidereau, R. Poly (ADP-ribose) polymerase gene expression status and genomic instability in human breast cancer. Clin. Cancer Res. 1996, 2, 1163–1167. [Google Scholar] [PubMed]
- Rajaee-Behbahani, N.; Schmezer, P.; Ramroth, H.; Bürkle, A.; Bartsch, H.; Dietz, A.; Becher, H. Reduced poly (ADP-ribosyl) ation in lymphocytes of laryngeal cancer patients: Results of a case-control study. Int. J. Cancer 2002, 98, 780–784. [Google Scholar] [CrossRef]
- Masutani, M.; Nozaki, T.; Sasaki, H.; Yamada, T.; Kohno, T.; Himizu, K.S.; Otoh, G.M.; Hiraishi, S.M.; Yokota, J.; Irohashi, H.S.; et al. Poly (ADP-ribose) polymerase-1 gene in human tumor cell lines: Its expression and structural alteration. Proc. Jpn. Acad. Ser. B 2004, 80, 114–118. [Google Scholar] [CrossRef]
- Augustin, A.; Spenlehauer, C.; Dumond, H.; Ménissier-De Murcia, J.; Piel, M.; Schmit, A.C.; Apiou, F.; Vonesch, J.L.; Kock, M.; Bornens, M.; et al. PARP-3 localizes preferentially to the daughter centriole and interferes with the G1/S cell cycle progression. J. Cell Sci. 2003, 116, 1551–1562. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Harsha, C.; Banik, K.; Gupta, S.C.; Aggarwal, B.B. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin. Sci. 2017, 131, 1781–1799. [Google Scholar] [CrossRef]
- Cohen, S.M.; Mukerji, R.; Timmermann, B.N.; Samadi, A.K.; Cohen, M.S. A novel combination of withaferin A and sorafenib shows synergistic efficacy against both papillary and anaplastic thyroid cancers. Am. J. Surg. 2012, 204, 895–901. [Google Scholar] [CrossRef]
- Adhvaryu, M.R.; Reddy, N.; Parabia, M.H. Anti-tumor activity of four Ayurvedic herbs in Dalton lymphoma ascites bearing mice and their short-term in vitro cytotoxicity on DLA-cell-line. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 409–418. [Google Scholar] [CrossRef]
- Alvarez-Gonzalez, R.; Zentgraf, H.; Frey, M.; Mendoza-Alvarez, H. Functional Interactions of PARP-1 with p53. In Poly(ADP-Ribosyl)ation. Molecular Biology Intelligence Unit; Springer: Boston, MA, USA, 2006. [Google Scholar] [CrossRef]
- Choi, Y.E.; Park, E. Curcumin enhances poly(ADP-ribose) polymerase inhibitor sensitivity to chemotherapy in breast cancer cells. J. Nutr. Biochem. 2015, 26, 1442–1447. [Google Scholar] [CrossRef]
- Fischbach, A.; Krüger, A.; Hampp, S.; Assmann, G.; Rank, L.; Hufnagel, M.; Stöckl, M.T.; Fischer, J.M.F.; Veith, S.; Rossatti, P.; et al. The C-terminal domain of p53 orchestrates the interplay between non-covalent and covalent poly(ADP-ribosyl)ation of p53 by PARP1. Nucleic Acids Res. 2018, 46, 804–822. [Google Scholar] [CrossRef]
- Koshkina, D.O.; Maluchenko, N.V.; Korovina, A.N.; Lobanova, A.A.; Feofanov, A.V.; Studitsky, V.M. Resveratrol Inhibits Nucleosome Binding and Catalytic Activity of PARP1. Biomolecules 2024, 14, 1398. [Google Scholar] [CrossRef]
- Sari, A.N.; Bhargava, P.; Dhanjal, J.K.; Putri, J.F.; Radhakrishnan, N.; Shefrin, S.; Ishida, Y.; Terao, K.; Sundar, D.; Kaul, S.C.; et al. Combination of Withaferin-A and CAPE Provides Superior Anticancer Potency: Bioinformatics and Experimental Evidence to Their Molecular Targets and Mechanism of Action. Cancers 2020, 12, 1160. [Google Scholar] [CrossRef]
- Watson, J.L.; Hill, R.; Yaffe, P.B.; Greenshields, A.; Walsh, M.; Lee, P.W.; Giacomantonio, C.A.; Hoskin, D.W. Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Lett. 2010, 297, 1–8. [Google Scholar] [CrossRef]
- Deng, Y.; Bi, R.; Guo, H.; Yang, J.; Du, Y.; Wang, C.; Wei, W. Andrographolide Enhances TRAIL-Induced Apoptosis via p53-Mediated Death Receptors Up-Regulation and Suppression of the NF-κB Pathway in Bladder Cancer Cells. Int. J. Biol. Sci. 2019, 15, 688–700. [Google Scholar] [CrossRef]
- Okuda, A.; Kurokawa, S.; Takehashi, M.; Maeda, A.; Fukuda, K.; Kubo, Y.; Nogusa, H.; Takatani-Nakase, T.; Okuda, S.; Ueda, K.; et al. Poly(ADP-ribose) polymerase inhibitors activate the p53 signaling pathway in neural stem/progenitor cells. BMC Neurosci. 2017, 18, 14. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hannan, M.A.; Dash, R.; Rahman, M.H.; Islam, R.; Uddin, M.J.; Sohag, A.A.M.; Rahman, M.H.; Rhim, H. Phytochemicals as a Complement to Cancer Chemotherapy: Pharmacological Modulation of the Autophagy-Apoptosis Pathway. Front. Pharmacol. 2021, 12, 639628. [Google Scholar] [CrossRef]
- Masuelli, L.; Benvenuto, M.; Di Stefano, E.; Mattera, R.; Fantini, M.; De Feudis, G.; De Smaele, E.; Tresoldi, I.; Giganti, M.G.; Modesti, A.; et al. Curcumin blocks autophagy and activates apoptosis of malignant mesothelioma cell lines and increases the survival of mice intraperitoneally transplanted with a malignant mesothelioma cell line. Oncotarget 2017, 8, 34405. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordell, G.A.; Qiu, S.X. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 2005, 22, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Sha, T.; Guo, J.; Li, W.; Lu, J.; Chen, X. Cucurbitacin B induces DNA damage and autophagy mediated by reactive oxygen species (ROS) in MCF-7 breast cancer cells. J. Nat. Med. 2015, 69, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Marsden, V.S.; Ekert, P.G.; Van Delft, M.; Vaux, D.L.; Adams, J.M.; Strasser, A. Bcl-2-regulated apoptosis and cytochrome c release can occur independently of both caspase-2 and caspase-9. J. Cell Biol. 2004, 165, 775–780. [Google Scholar] [CrossRef]
- Kar, P.; Samanta, K.; Shaikh, S.; Chowdhury, A.; Chakraborti, T.; Chakraborti, S. Mitochondrial calpain system: An overview. Arch. Biochem. Biophys. 2010, 495, 1–7. [Google Scholar] [CrossRef]
- Zhang, J.F.; Liu, J.J.; Liu, P.Q.; Lin, D.J.; Li, X.D.; Chen, G.H. Oridonin inhibits cell growth by induction of apoptosis on human hepatocelluar carcinoma BEL-7402 cells. Hepatol. Res. 2006, 35, 104–110. [Google Scholar] [CrossRef]
- Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: Molecular mechanisms. Mol. Cancer 2013, 12, 171. [Google Scholar] [CrossRef]
- Gupta, P.; Srivastava, S.K. Antitumor activity of phenethyl isothiocyanate in HER2-positive breast cancer models. BMC Med. 2012, 10, 80. [Google Scholar] [CrossRef]
- Sun, Z.L.; Dong, J.L.; Wu, J. Juglanin induces apoptosis and autophagy in human breast cancer progression via ROS/JNK promotion. Biomed. Pharmacother. 2017, 85, 303–312. [Google Scholar] [CrossRef]
- Suskiewicz, M.J.; Prokhorova, E.; Rack, J.G.M.; Ahel, I. ADP-ribosylation from molecular mechanisms to therapeutic implications. Cell 2023, 186, 4475–4495. [Google Scholar] [CrossRef] [PubMed]
- Pleschke, J.M.; Kleczkowska, H.E.; Strohm, M.; Althaus, F.R. Poly (ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J. Biol. Chem. 2000, 275, 40974–40980. [Google Scholar] [CrossRef]
- Virag, L.; Marmer, D.J.; Szabó, C. Crucial role of apopain in the peroxynitrite-induced apoptotic DNA fragmentation. Free Radic. Biol. Med. 1998, 25, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Hassa, P.O.; Hottiger, M.O. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell Mol. Life Sci. 2002, 59, 1534–1553. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.A.; Kim, N.S.; Yu, S.W.; Wang, H.; Koh, D.W.; Sasaki, M.; Klaus, J.A.; Otsuka, T.; Zhang, Z.; Koehler, R.C.; et al. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc. Natl. Acad. Sci. USA 2006, 103, 18308–18313. [Google Scholar] [CrossRef]
- Kraus, W.L. PARPs and ADP-Ribosylation: 50 Years… and counting. Mol. Cell. 2015, 58, 902–910. [Google Scholar] [CrossRef]
- Bai, P.; Csóka, B. New route for the activation of poly(ADP-ribose) polymerase-1: A passage that links poly(ADP-ribose) polymerase-1 to lipotoxicity? Biochem. J. 2015, 469, e9–e11. [Google Scholar] [CrossRef] [PubMed]
- Sumantran, V.N.; Tillu, G. Cancer, inflammation, and insights from ayurveda. Evid. Based Complement. Altern. Med. 2012, 2012, 306346. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Karin, M. Nuclear factor-κB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef]
- Riehl, A.; Nemeth, J.; Angel, P.; Hess, J. The receptor RAGE: Bridging inflammation and cancer. Cell Commun. Signal. 2009, 7, 12–18. [Google Scholar] [CrossRef]
- Bierhaus, A.; Schiekofer, S.; Schwaninger, M.; Andrassy, M.; Humpert, P.M.; Chen, J.; Hong, M.; Luther, T.; Henle, T.; Klöting, I.; et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes 2001, 50, 2792–2808. [Google Scholar] [CrossRef]
- Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer. Oncology 2002, 16, 217–226. [Google Scholar]
- Ali, A.M.M.T.; Narayana, S.D.S.; Lulu, S.S.; Nag, S.; Sundararajan, V. Targeting NF-κB pathway for the anti-inflammatory potential of Bhadradarvadi kashayam on stimulated RAW 264.7 macrophages. Heliyon 2023, 19, e19270. [Google Scholar] [CrossRef]
- Sharma, A.; Arora, P. Anti-cancer activity of Cedrus deodara in 1,2-dimethly hydrazine (DMH) induced anti-cancer model in rats. Asian J. Pharm. Res. Dev. 2018, 6, 82–86. [Google Scholar] [CrossRef]
- Hwang, J.M.; Yu, J.Y.; Jang, Y.O.; Kim, B.T.; Hwang, K.J.; Jeon, Y.M.; Lee, J.C. A phenolic acid phenethyl urea compound inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines in cell culture. Int. Immunopharmacol. 2010, 10, 526–532. [Google Scholar] [CrossRef]
- Asif Amin, M.; Fox, D.A.; Ruth, J.H. Synovial cellular and molecular markers in rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 385–393. [Google Scholar] [CrossRef]
- Ritenbaugh, C.; Verhoef, M.; Fleishman, S.; Boon, H.; Leis, A. Whole systems research: A discipline for studying complementary and alternative medicine. Altern. Ther. Health Med. 2003, 9, 32–36. [Google Scholar]
- Bodeker, G. Integrative oncology meets immunotherapy: New prospects for combination therapy grounded in Eastern medical knowledge. Chin. J. Integr. Med. 2012, 18, 652–662. [Google Scholar] [CrossRef]
- Chopra, A.; Saluja, M.; Tillu, G.; Venugopalan, A.; Sarmukaddam, S.; Raut, A.K.; Bichile, L.; Narsimulu, G.; Handa, R.; Patwardhan, B. A Randomized Controlled Exploratory Evaluation of Standardized Ayurvedic Formulations in Symptomatic Osteoarthritis Knees: A Government of India NMITLI Project. Evid. Based Complement. Alternat. Med. 2011, 2011, 724291. [Google Scholar] [CrossRef]
- Mehra, R.; Makhija, R.; Vyas, N. A clinical study on the role of Ksara vasti and Triphala guggulu in Raktarsha (Bleeding piles). AYU 2011, 32, 192–195. [Google Scholar] [CrossRef]
- Braakhuis, B.J.; Tabor, M.P.; Kummer, J.A.; Leemans, C.R.; Brakenhoff, R.H. A genetic explanation of Slaughter’s concept of field cancerization: Evidence and clinical implications. Cancer Res. 2003, 63, 1727–1730. [Google Scholar]
- Dhruva, A.; Hecht, F.M.; Miaskowski, C.; Kaptchuk, T.J.; Bodeker, G.; Abrams, D.; Lad, V.; Adler, S.R. Correlating traditional Ayurvedic and modern medical perspectives on cancer: Results of a qualitative study. J. Altern. Complement. Med. 2014, 20, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, P.; Yazdanpanah, O.; Benjamin, D.J.; Kalebasty, A.R. The Role of Ayurveda in Prostate Cancer Management. Integr. Cancer Ther. 2025, 24, 15347354251330906. [Google Scholar] [CrossRef] [PubMed]
- Bode, M. Revitalizing Ayurveda. Asian Med. 2025, 20, 1–31. [Google Scholar] [CrossRef]
- McTiernan, A.; Irwin, M.; Vongruenigen, V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. J. Clin. Oncol. 2010, 28, 4074–4080. [Google Scholar] [CrossRef]
- Bhatti, P.; Cushing-Haugen, K.L.; Wicklund, K.G.; Doherty, J.A.; Rossing, M.A. Nightshift work and risk of ovarian cancer. Occup. Environ. Med. 2013, 70, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, B.B.; Tergas, A.I.; Mateen, F.J.; Bhayani, N.H.; Oh, J. Nightshift work and risk of breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013, 138, 291–301. [Google Scholar] [CrossRef] [PubMed]
- El-Huneidi, W.; Anjum, S.; Mohammed, A.K.; Bin Eshaq, S.; Abdrabh, S.; Bustanji, Y.; Soares, N.C.; Semreen, M.H.; Alzoubi, K.H.; Abu-Gharbieh, E.; et al. Rosemarinic acid protects β-cell from STZ-induced cell damage via modulating NF-κβ pathway. Heliyon 2023, 9, e19234. [Google Scholar] [CrossRef] [PubMed]
- Gbr, A.A.; Abdel Baky, N.A.; Mohamed, E.A.; Zaky, H.S. Cardioprotective effect of pioglitazone and curcumin against diabetic cardiomyopathy in type 1 diabetes mellitus: Impact on CaMKII/NF-κB/TGF-β1 and PPAR-γ signaling pathway. Naunyn Schmiedeberg’s Arch. Pharmacol. 2021, 394, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, D.; Nazıroğlu, M. Curcumin diminishes cisplatin-induced apoptosis and mitochondrial oxidative stress through inhibition of TRPM2 channel signaling pathway in mouse optic nerve. J. Recept. Signal Transduct. Res. 2020, 40, 97–108. [Google Scholar] [CrossRef]
- Choi, S.J.; Kunwor, S.K.; Im, H.B.; Hwang, J.H.; Choi, D.; Han, D. Traditional and complementary medicine use among cancer patients in Nepal: A cross-sectional survey. BMC Complement. Med. Ther. 2022, 22, 70. [Google Scholar] [CrossRef]
- Sudhakar, A. History of cancer, ancient and modern treatment methods. J. Cancer Sci. Ther. 2009, 1, 1–4. [Google Scholar]
- Ottolino-Perry, K.; Diallo, J.S.; Lichty, B.D.; Bell, J.C.; McCart, J.A. Intelligent design: Combination therapy with oncolytic viruses. Mol. Ther. 2010, 18, 251–263. [Google Scholar] [CrossRef]
- Imran, A.; Qamar, H.Y.; Ali, Q.; Naeem, H.; Riaz, M.; Amin, S.; Kanwal, N.; Ali, F.; Sabar, M.F.; Nasir, I.A. Role of Molecular Biology in Cancer Treatment: A Review Article. Iran. J. Public Health 2017, 46, 1475–1485. [Google Scholar] [PubMed] [PubMed Central]
- Mahanta, P.; Nidagundi, P.S.; Sobagin, M.V. Concept & utility of Nanotechnology in the standardization of Rasadravya. J. Ayurveda Integr. Med. Sci. 2020, 4, 235–238. [Google Scholar] [CrossRef]
- Faraday, M. The Bakerian Lecture: Experimental Relations of Gold (and Other Metals) to Light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar] [CrossRef]
- Paul, S.; Chugh, A. Assessing the role of Ayurvedic ‘Bhasma’ as ethno-nanomedicine in the metal-based nanomedicine patent regime. J. Intellect. Prop. Rights 2011, 16, 509–515. [Google Scholar]
- Lv, Y.; Li, W.; Liao, W.; Jiang, H.; Liu, Y.; Cao, J.; Lu, W.; Feng, Y. Nano-Drug Delivery Systems Based on Natural Products. Int. J. Nanomed. 2024, 19, 541–569. [Google Scholar] [CrossRef]
- Aldea-Perona, A.M.; Beledo, J.F.; Frías Iniesta, J.; García, A.G.; Tamargo, J.; Zaragozá, F. Corrigendum to: An account on the history of pharmacology in Spain. Pharmacol. Res. 2024, 205, 107240. [Google Scholar] [CrossRef]
- Lüscher, B.; Ahel, I.; Altmeyer, M.; Ashworth, A.; Bai, P.; Chang, P.; Cohen, M.; Corda, D.; Dantzer, F.; Daugherty, M.D.; et al. ADP-ribosyltransferases, an update on function and nomenclature. FEBS J. 2022, 289, 7399–7410. [Google Scholar] [CrossRef]
- Zeng, Y.; Arisa, O.; Peer, C.J.; Fojo, A.; Figg, W.D. PARP inhibitors: A review of the pharmacology, pharmacokinetics, and pharmacogenetics. Semin. Oncol. 2024, 51, 19–24. [Google Scholar] [CrossRef]
- Rudolph, J.; Jung, K.; Luger, K. Inhibitors of PARP: Number crunching and structure gazing. Proc. Natl. Acad. Sci. USA 2022, 119, e2121979119. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Anasane, N.; Santos, C.A.D. Curcumin and curcumin-loaded nanoparticles: Antipathogenic and antiparasitic activities. Expert Rev. Anti Infect. Ther. 2020, 18, 367–379. [Google Scholar] [CrossRef]
- Pradhan, R.; Paul, S.; Das, B.; Sinha, S.; Dash, S.R.; Mandal, M.; Kundu, C.N. Resveratrol nanoparticle attenuates metastasis and angiogenesis by deregulating inflammatory cytokines through inhibition of CAFs in oral cancer by CXCL-12/IL-6-dependent pathway. J. Nutr. Biochem. 2023, 113, 109257. [Google Scholar] [CrossRef]
- Yilmaz, M.; Karanastasis, A.A.; Chatziathanasiadou, M.V.; Oguz, M.; Kougioumtzi, A.; Clemente, N.; Kellici, T.F.; Zafeiropoulos, N.E.; Avgeropoulos, A.; Mavromoustakos, T.; et al. Inclusion of Quercetin in Gold nanoparticles decorated with supramolecular hosts amplifies Its Tumor Targeting Properties. ACS Appl. Bio Mater. 2019, 2, 2715–2725. [Google Scholar] [CrossRef]
- Curtin, N.J.; Szabo, C. Poly(ADP-ribose) polymerase inhibition: Past, present and future. Nat. Rev. Drug Discov. 2020, 19, 711–736. [Google Scholar] [CrossRef] [PubMed]
- Virág, L.; Szabó, C. The therapeutic potential of poly (ADP-ribose) polymerase inhibitors. Pharmacol. Rev. 2002, 54, 375–429. [Google Scholar] [CrossRef]
- Wells, K.; Liu, T.; Zhu, L.; Yang, L. Immunomodulatory nanoparticles activate cytotoxic T cells for enhancement of the effect of cancer immunotherapy. Nanoscale 2024, 16, 17699–17722. [Google Scholar] [CrossRef]
- Qutub, M.; Hussain, U.M.; Tatode, A.; Premchandani, T.; Khan, R.; Umekar, M.; Taksande, J.; Singanwad, P. Nano-Engineered Epigallocatechin Gallate (EGCG) Delivery Systems: Overcoming Bioavailability Barriers to Unlock Clinical Potential in Cancer Therapy. AAPS PharmSciTech. 2025, 26, 137. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Afzal, O.; Yasmin, S.; Hussain, A.; Altamimi, M.A.; Webster, T.J.; Altamimi, A.S.A. Strategic nanocarriers to control neurodegenerative disorders: Concept, challenges, and future perspective. Int. J. Pharm. 2023, 633, 122614. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.; Jiang, Y.; Kim, J. Additively manufactured flexible piezoelectric lead zirconate titanate-nanocellulose films with outstanding mechanical strength, dielectric and piezoelectric properties. Mater. Today Sci. 2024, 21, 100478. [Google Scholar] [CrossRef]
- Ayyadurai, P.; Ragavendran, C. Nano-bio-encapsulation of phyto-vaccines: A breakthrough in targeted cancer immunotherapy. Mol. Biol. Rep. 2024, 52, 58. [Google Scholar] [CrossRef]
- Aikins, M.E.; Xu, C.; Moon, J.J. Engineered Nanoparticles for Cancer Vaccination and Immunotherapy. Acc. Chem. Res. 2020, 53, 2094–2105. [Google Scholar] [CrossRef]
- Ranjbar, M.H.; Einafshar, E.; Javid, H.; Jafari, N.; Sajjadi, S.S.; Darban, R.A.; Hashemy, S.I. Enhancing the anticancer effects of rosmarinic acid in PC3 and LNCaP prostate cancer cells using titanium oxide and selenium-doped graphene oxide nanoparticles. Sci. Rep. 2025, 15, 11568. [Google Scholar] [CrossRef]
- Sinha, S.; Paul, S.; Acharya, S.S.; Das, C.; Dash, S.R.; Bhal, S.; Pradhan, R.; Das, B.; Kundu, C.N. Combination of Resveratrol and PARP inhibitor Olaparib efficiently deregulates homologous recombination repair pathway in breast cancer cells through inhibition of TIP60-mediated chromatin relaxation. Med. Oncol. 2024, 41, 49. [Google Scholar] [CrossRef]
- Li, H.; Qu, X.; Qian, W.; Song, Y.; Wang, C.; Liu, W. Andrographolide-loaded solid lipid nanoparticles enhance anti-cancer activity against head and neck cancer and precancerous cells. Oral Dis. 2022, 28, 142–149. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Maher, D.M.; Sundram, V.; Bell, M.C.; Jaggi, M.; Chauhan, S.C. Curcumin induces chemo/radio-sensitization in ovarian cancer cells and curcumin nanoparticles inhibit ovarian cancer cell growth. J. Ovarian Res. 2010, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Hatami, M.; Kouchak, M.; Kheirollah, A.; Khorsandi, L.; Rashidi, M. Quercetin-loaded solid lipid nanoparticles exhibit antitumor activity and suppress the proliferation of triple-negative MDA-MB 231 breast cancer cells: Implications for invasive breast cancer treatment. Mol. Biol. Rep. 2023, 50, 9417–9430. [Google Scholar] [CrossRef]
- Andreeva, T.V.; Maluchenko, N.V.; Efremenko, A.V.; Lyubitelev, A.V.; Korovina, A.N.; Afonin, D.A.; Kirpichnikov, M.P.; Studitsky, V.M.; Feofanov, A.V. Epigallocatechin gallate affects the structure of chromatosomes, nucleosomes and their complexes with PARP1. Int. J. Mol. Sci. 2023, 24, 14187. [Google Scholar] [CrossRef]
- Vodnik, V.V.; Mojić, M.; Stamenović, U.; Otoničar, M.; Ajdžanović, V.; Maksimović-Ivanić, D.; Mijatović, S.; Marković, M.M.; Barudžija, T.; Filipović, B.; et al. Development of genistein-loaded gold nanoparticles and their antitumor potential against prostate cancer cell lines. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112078. [Google Scholar] [CrossRef]
- Verdura, S.; Cuyàs, E.; Ruiz-Torres, V.; Micol, V.; Joven, J.; Bosch-Barrera, J.; Menendez, J.A. Lung Cancer Management with Silibinin: A Historical and Translational Perspective. Pharmaceuticals 2021, 14, 559. [Google Scholar] [CrossRef] [PubMed]
- Agarwalla, P.; Mukherjee, S.; Sreedhar, B.; Banerjee, R. Glucocorticoid receptor-mediated delivery of nano gold-withaferin conjugates for reversal of epithelial-to-mesenchymal transition and tumor regression. Nanomedicine 2016, 11, 2529–2546. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Spaccapelo, R.; Mancuso, A.; Nisticò, S.P.; Udongo, B.P.; Fresta, M.; Paolino, D. Sulforaphane-loaded ultradeformable vesicles as a potential natural nanomedicine for the treatment of skin cancer diseases. Pharmaceutics 2019, 12, 6. [Google Scholar] [CrossRef]
- Gupta, L.; Sharma, A.K.; Gothwal, A.; Khan, M.S.; Khinchi, M.P.; Qayum, A.; Singh, S.K.; Gupta, U. Dendrimer encapsulated and conjugated delivery of berberine: A novel approach mitigating toxicity and improving in vivo pharmacokinetics. Int. J. Pharm. 2017, 528, 88–99. [Google Scholar] [CrossRef]
- AbouAitah, K.; Stefanek, A.; Higazy, I.M.; Janczewska, M.; Swiderska-Sroda, A.; Chodara, A.; Wojnarowicz, J.; Szałaj, U.; Shahein, S.A.; Aboul-Enein, A.M.; et al. Effective Targeting of Colon Cancer Cells with Piperine Natural Anticancer Prodrug Using Functionalized Clusters of Hydroxyapatite Nanoparticles. Pharmaceutics 2020, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Nowroozzadeh, M.H.; Ghorat, F.; Iraji, A.; Hashempur, M.H. Piperine and its nanoformulations: A mechanistic review of their anti-cancer activities. Biomed. Pharmacother. 2025, 187, 118075. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, B.; Ma, H.; Li, Y.; Du, J.; Zhang, B.; Gao, Y.; Liu, Y.; Wu, C. Preparation of biomimetic selenium-baicalein nanoparticles and their targeted therapeutic application in nonsmall cell lung cancer. Mol. Pharm. 2024, 21, 4476–4489. [Google Scholar] [CrossRef]
- Kazmi, I.; Al-Abbasi, F.A.; Imam, S.S.; Afzal, M.; Nadeem, M.S.; Altayb, H.N.; Alshehri, S. Formulation and evaluation of Apigenin-loaded hybrid nanoparticles. Pharmaceutics 2022, 14, 783. [Google Scholar] [CrossRef]
- Wang, C.; Ren, X.; Han, Y.; Nan, D.; Zhang, Y.; Gao, Z. Development of a biomimetic nanoparticle platform for apigenin therapy in triple-negative breast cancer. Front. Oncol. 2025, 15, 1521529. [Google Scholar] [CrossRef]
- Adel, M.; Zahmatkeshan, M.; Akbarzadeh, A.; Rabiee, N.; Ahmadi, S.; Keyhanvar, P.; Rezayat, S.M.; Seifalian, A.M. Chemotherapeutic effects of Apigenin in breast cancer: Preclinical evidence and molecular mechanisms; enhanced bioavailability by nanoparticles. Biotechnol. Rep. 2022, 34, e00730. [Google Scholar] [CrossRef]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Cetin, B.; Wabl, C.A.; Gumusay, O. The DNA damaging revolution. Crit. Rev. Oncol. Hematol. 2020, 156, 103117. [Google Scholar] [CrossRef]
- Phillipps, J.; Zhou, A.Y.; Butt, O.H.; Ansstas, G. PARP inhibition and immunotherapy: A promising duo in fighting cancer. Transl. Cancer Res. 2023, 12, 2433–2437. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Chaturvedi, G.; Prakrithi, P.; Pathak, A.K.; Kutum, R.; Dakle, P.; Narang, A.; Manchanda, V.; Patil, R.; Aggarwal, D.; et al. Whole exome sequencing in healthy individuals of extreme constitution types Reveals differential disease Risk: A novel approach towards predictive medicine. J. Pers. Med. 2022, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Minas, T.Z.; Kiely, M.; Ajao, A.; Ambs, S. An overview of cancer health disparities: New approaches and insights and why they matter. Carcinogenesis 2021, 42, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.W.; Nagaraja, A.S.; Lutgendorf, S.K.; Green, P.A.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef]
- Gibbs, B.F. Differential modulation of IgE- dependent activation of human basophils by ambraxol and related secretolytic ana-logues. Int. J. Immunopathol. Pharmacol. 2009, 22, 919–927. [Google Scholar] [CrossRef]
- Shang, X.; Guo, X.; Li, B.; Pan, H.; Zhang, J.; Zhang, Y.; Miao, X. Microwave-assisted extraction of three bioactive alkaloids from Peganum harmala L. and their acaricidal activity against Psoroptes cuniculi in vitro. J. Ethnopharmacol. 2016, 192, 350–361. [Google Scholar] [CrossRef]
- Ahmad, R.; Srivastava, S.; Ghosh, S.; Khare, S.K. Phytochemical delivery through nanocarriers: A review. Colloids Surf. B Biointerfaces 2021, 197, 111389. [Google Scholar] [CrossRef]
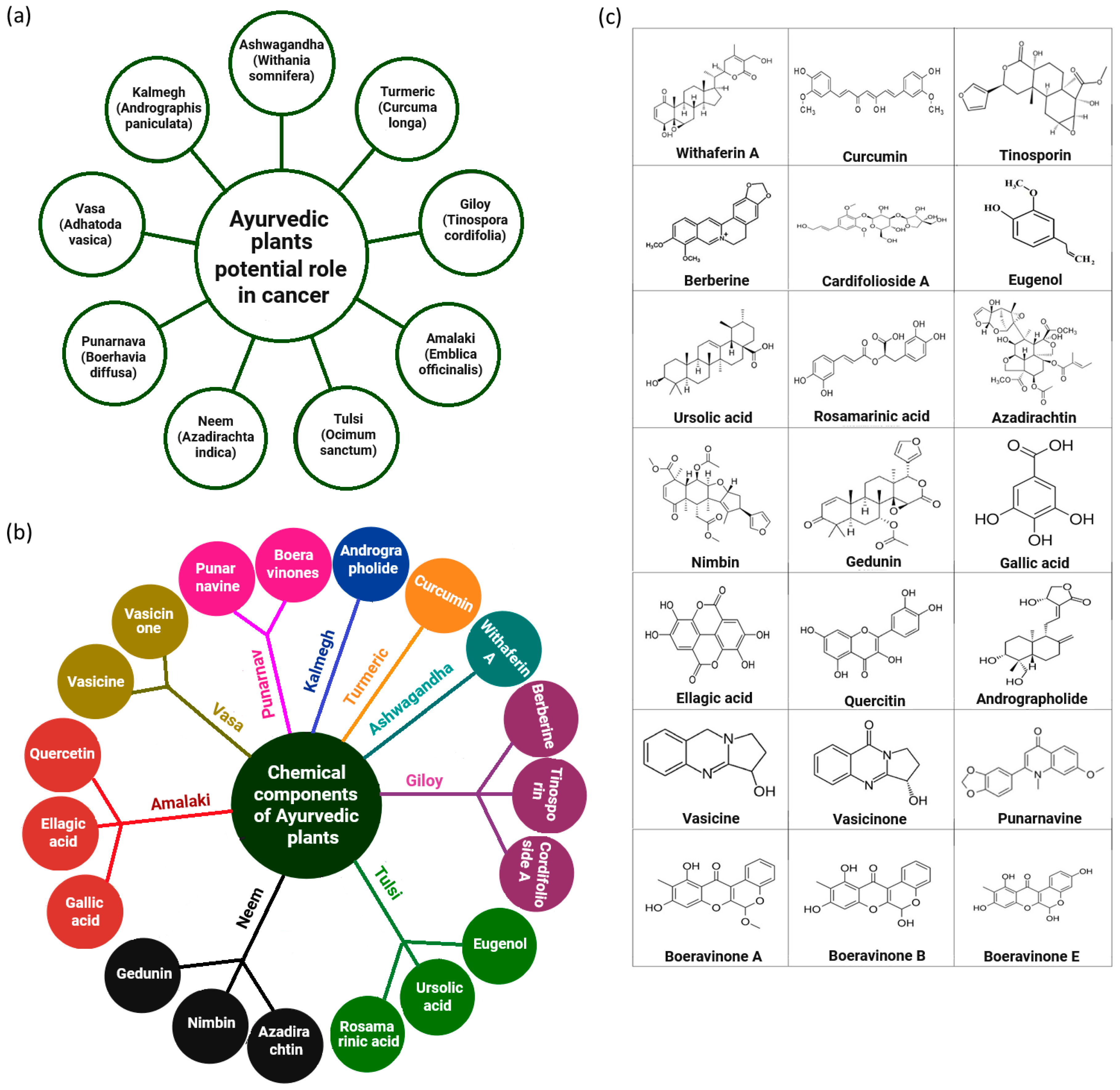
| Sl No. | Name of the Phytochemical | Deliverable Phytochemical–Nanoparticle Conjugate | Size of the Formulation (nm) | Zeta Potential Values (mV) | Mechanistic Insights: ADPr/PARP Pathway | Cancer Type(s) Studied | Clinical Status | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Rosamarinic acid | Rosmarinic acid titanium oxide and selenium-doped graphene oxide nanoparticles (rosamarinic acid@Se-TiO2-GO nanocomplex) | 344.8 ± 43.2 | −33.1 ± 2.64 | Cleaved PARP-1, via p53 upregulation induced by HDAC2 downregulation, promoted apoptosis. | Prostate cancer | Preclinical in vitro only | [163] |
| 2 | Resveratrol | Liposomal formulation | - | - | Synergizes with PARP inhibitors, modulates DNA repair pathways. | Breast, prostate | Preclinical | [164] |
| 3 | Andrographolide | Solid Lipid Nanoparticles (ADG-SLNs) | 286.1 ± 8.03 | −20.5 ± 0.3 | ADG-SLNs enhance apoptosis in HN models; PARP/ADPr involvement (cleaved PARP-1, PARylation) remains to be tested. | Head-and-neck cancer (HIOEC, Leuk-1, HN6, HN30 cells) | Preclinical in vitro only | [165] |
| 4 | Curcumin | Poly (lactic acid-co-glycolic acid) (PLGA) curcumin (Nano-CUR) | 70 ± 3.9 | - | Enhances PARP inhibition, increases DNA damage, induces apoptosis. | Breast, ovarian | Preclinical | [166] |
| 5 | Quercetin | Solid lipid nanoparticles | 154 | −27.7 | Inhibits PARP activity, enhances chemosensitivity. | Lung, colon cancers | Preclinical | [167] |
| 6 | Epigallocatechin gallate (EGCG) | Chitosan nanoparticles | - | - | Downregulates PARP expression, increases ROS-mediated DNA damage. | Prostate and breast cancers | Preclinical | [168] |
| 7 | Genistein | Genistein–gold nanoparticles conjugates (Gen@AuNPs) | 65 ± 1.7 | −35 ± 2.5 | Enhanced antiproliferative effect; PARP/ADPr mechanisms (e.g., cleaved PARP-1 or PARP arbitrated ADPr) yet to be investigated. | Prostate cancer | Preclinical (in vitro: PC3, DU145, and LNCaP cell lines) | [169] |
| 8 | Silibinin | No nano-conjugate reported | - | - | Prevents chemically induced lung tumors; overcomes drug resistance & metastatic traits; inhibits STAT3 in tumor and microenvironment-PARP/ADPr involvement needs further investigation. | Lung cancers | Preclinical | [170] |
| 9 | Withaferin A (WA) | Gold nanoparticles (AuNP) conjugated with dexamethasone (GR ligand) and withaferin A (Au-Dex-WA nanoconjugate). | - | - | Glucocorticoid receptor (GR)-dependent cytotoxicity, epithelial–mesenchymal transition (EMT) reversal, ATP-binding cassette sub-family G member 2 (ABCG2) downregulation; potential PARP/ADPr involvement (needs further investigation). | Mouse melanoma (EMT reversal, tumor regression); also studied in breast, lung (NSCLC), glioblastoma. | Au-Dex-WA nanoconjugate remains preclinical; WA tested in early-phase clinical studies. | [171] |
| 10 | Sulforaphane | Ultra deformable vesicles (ethosomes®, transfersomes®) | 102 ± 6 | −21 ± 2 | ROS-mediated DNA damage and apoptosis via DR5, AP-1, MAPKs, mitochondrial dysfunction, and NF-κB inhibition; PARP/ADPr involvement yet to be explored. | Skin cancer (melanoma, SK-MEL-28) | Preclinical in vitro (Melanoma cell lines) only | [172] |
| 11 | Berberine | Poly (amidoamine) (PAMAM) dendrimer encapsulated and conjugated formulation | - | - | Induced apoptosis via mitochondrial dysfunction, ROS generation, and modulation of Bcl-2 family proteins; possible PARP cleavage during apoptotic cascade (specific role of ADPr/PARP pathway not investigated—needs further exploration. | Cervical cancer | Preclinical (in vitro—HeLa cells; in vivo—mouse xenograft model). | [173] |
| 12 | Piperine | Piperine-loaded hydroxyapatite, polymeric, and lipid nanoparticles; also, curcumin–piperine nanoparticle combinations. | 63.73 ± 1.07 | −20.46 | Piperine triggers DNA damage and caspase-dependent PARP-1 cleavage in apoptosis; nanoparticle delivery enhances bioavailability and sustained release, though ADPr signaling needs further investigation. | Colon, prostate, and breast cancers | Preclinical models | [174,175] |
| 13 | Baicalein | Selenium–Baicalein nanoparticles (ACM-SSe-BE), coated with A549 cell membrane for homologous targeting | 135.2 ± 2.52 | −32.23 ± 1.19 | Enhances ROS generation, promotes apoptosis and proliferation inhibition; possible ADPr/PARP involvement remains to be further investigated | A549 (non-small-cell lung cancer) | Preclinical (in vitro and in vivo in animal models) | [176] |
| 14 | Apigenin | Polymer–lipid hybrid nanoparticles (PLHNPs), macrophage-membrane-coated PEG micellar system (m@PEG-AGN), nanocrystals, micelles, liposomes, poly (lactic-co-glycolic acid) (PLGA) | 125.73 ± 5.57 | −26.71 ± 1.93 | Causes cell cycle arrest, ROS-induced DNA damage, apoptosis; suppresses metastasis (MMP/Akt); PARP signaling through ADPr in physiological/pathophysiological context needs further investigation | Breast cancer (Triple-negative), colorectal carcinoma, and other cancer cell lines, often in vitro and/or in animal models | Apigenin remains at the preclinical stage. However, nanoformulations improve solubility, bioavailability & targeting, showing promise for clinical use | [177,178,179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, G.S.V.S.R.; Nandy, S.K.; Cherukuri, P.; Samanta, K.; Kar, P. Ayurvedic Phytochemicals in Oncology: ADP-Ribosylation as a Molecular Nexus. Cells 2025, 14, 1753. https://doi.org/10.3390/cells14221753
Reddy GSVSR, Nandy SK, Cherukuri P, Samanta K, Kar P. Ayurvedic Phytochemicals in Oncology: ADP-Ribosylation as a Molecular Nexus. Cells. 2025; 14(22):1753. https://doi.org/10.3390/cells14221753
Chicago/Turabian StyleReddy, Gali Sri Venkata Sai Rishma, Suman Kumar Nandy, Pitchaiah Cherukuri, Krishna Samanta, and Pulak Kar. 2025. "Ayurvedic Phytochemicals in Oncology: ADP-Ribosylation as a Molecular Nexus" Cells 14, no. 22: 1753. https://doi.org/10.3390/cells14221753
APA StyleReddy, G. S. V. S. R., Nandy, S. K., Cherukuri, P., Samanta, K., & Kar, P. (2025). Ayurvedic Phytochemicals in Oncology: ADP-Ribosylation as a Molecular Nexus. Cells, 14(22), 1753. https://doi.org/10.3390/cells14221753






