In-Depth Study of Low-Complexity Domains: From Structural Diversity to Disease Mechanisms
Abstract
1. Introduction
2. Structural Characteristics and Classification of LCDs
2.1. Sequence Composition and Structural Diversity

2.2. Structural Characteristics and Conformational Dynamics
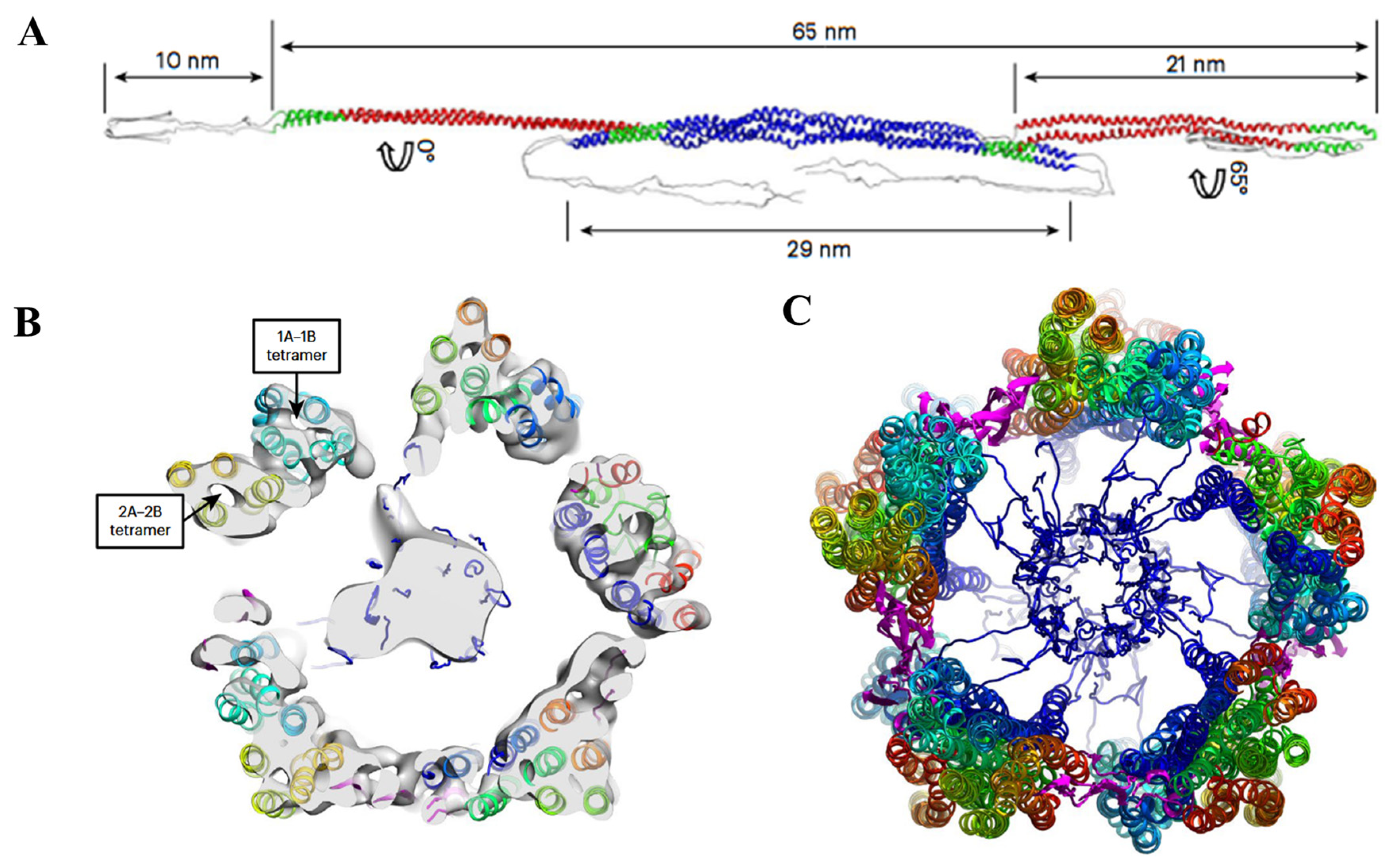
2.3. LCDs and Intermediate Silk Proteins
2.4. Liquid Crystal Characteristics and Phase Separation Ability
3. Biological Functions of LCDs
3.1. Protein Interactions and Formation of Multi Protein Complexes
3.2. LLPS and Formation of Biomolecule Aggregates
3.3. Signal Transmission and Environmental Response
3.4. Transcription Regulation and Gene Expression
3.5. Cytoskeleton Organization and Cellular Structure Maintenance
3.6. Nuclear Pore Transport
4. LCDs and Diseases
4.1. LCD Dysfunction in Neurodegenerative Diseases
4.2. LCD Dysfunction in Cancer
4.3. LCD Dysfunction in Other Diseases
| Protein | LCDs Type | Function | Related Diseases | Mutations/Modifications | Reference |
|---|---|---|---|---|---|
| FUS | G/Y-rich | transcriptional regulation | ALS | P525L, R495X | [51,67] |
| TDP-43 | G/N-rich | RNA binding and stress granules | ALS and FTD | P320L, M337V | [50,68] |
| Tau | P/S-rich | Microtubule stability | AD and CTE | Excessive phosphorylation | [54] |
| EWSR1 | Q/Y-rich | Stable transcriptional activation and LLPS | Ewing sarcoma | EWS: FLI1 fusion | [61] |
| hnRNPA2 | R/D-rich | RNA splicing and transport | MSP and ALS | D290V | [52,53] |
| hnRNPH1 | R/G/G-rich | RNA splicing and transcription | acute lymphoblastic leukemia | Y408S | [62] |
| KMT2D | Q-rich | Chromatin modification | Pancreatic cancer | LCDs loss mutation | [17] |
4.4. Therapeutic Targeting of LCDs
5. Research Methods and Technological Advances in LCDs
5.1. Biochemical and Biophysical Methods
5.2. Structural Biology Methods
5.3. Cell Biology Methods
5.4. Computational Biology and Bioinformatics Methods
5.5. Emerging Technologies and Future Development Directions
| Method Category | Specific Technique | Objective/Goal | Example Application |
|---|---|---|---|
| Biochemical & Biophysical | Circular Dichroism (CD) | Characterize secondary structure composition (α-helix, β-sheet, random coil) | Characterizing structural transitions in various LCD types [2] |
| Nuclear Magnetic Resonance (NMR) | Residue-level insights into dynamics, local environment, and interactions | Analyzing the role of tyrosine in EWS LCD phase separation [26] | |
| Analytical Ultracentrifugation (AUC) | Study self-association, oligomeric state, and aggregation | Characterizing self-association of the EWS LCD [26] | |
| Dynamic/Static Light Scattering (DLS/SLS) | Characterize phase separation kinetics, droplet size, and thermodynamics | Studying condensation initiation of hnRNPA1 LCD [77] | |
| Fluorescence Recovery After Photobleaching (FRAP) | Assess fluidity and dynamics of molecules within condensates | Demonstrating liquid-like properties of SWI/SNF complex LCDs [13] | |
| Structural Biology | Cryo-Electron Microscopy (Cryo-EM) | Determine high-resolution structures of aggregates and filaments | Solving structures of Tau fibrils from Alzheimer’s brain [83,84] |
| Cryo-Electron Tomography (Cryo-ET) | In situ structural analysis of complexes within cells | Revealing the role of vimentin head/tail LCDs in filament assembly [22] | |
| Hydrogen/Deuterium Exchange MS (HDX-MS) | Probe structural dynamics and solvent accessibility | Studying conformational changes and interactions of LCDs [86] | |
| Cell Biology | Fluorescence Microscopy | Visualize subcellular localization and phase separation in live cells | Observing nucleocytoplasmic distribution and condensation of FUS LCD [88] |
| Fluorescence Resonance Energy Transfer (FRET) | Detect direct protein–protein/protein–RNA interactions | US LCD: Validate binding to RNA during phase separation [91,92] | |
| Protein Complementation Assay (PCA) | Detect weak, transient protein–protein interactions | Mapping the dynamic interaction network of LCDs [93] | |
| Super-Resolution Microscopy (STED/STORM) | Visualize subcellular localization and fine structure of LCD condensates. | Resolve spatial organization [97] | |
| Computational Biology | Molecular Dynamics (MD) Simulations | Model conformational changes and interaction dynamics at atomic resolution | Revealing how tyrosine residues mediate π–π stacking in EWS LCD [26,95] |
| Bioinformatics (e.g., fLPS, LCD-Compass) | Identify LCDs and predict properties from sequence data | Proteome-wide discovery and classification of LCDs [3] |
6. Conclusions and Prospect
6.1. Main Findings of LCDs
6.2. Challenges and Limitations of LCD Research
6.3. Future Research Directions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LCDs | Low-complexity domains |
| LLPS | Liquid–liquid phase separation |
| ALS | Amyotrophic lateral sclerosis |
| FTD | Frontotemporal dementia |
| IFs | Intermediate filaments |
| cryo-EM | Cryo-electron microscopy |
| cryo-ET | Cryo-electron tomography |
| CD | Circular dichroism |
| NMR | Nuclear magnetic resonance |
| AUC | Analytical ultracentrifugation |
| DLS | Dynamic light scattering |
| SLS | Static light scattering |
| FCS | Fluorescence correlation spectroscopy |
| FRAP | Fluorescence recovery after photobleaching |
| FRET | Fluorescence resonance energy transfer |
| BRET | Bioluminescence resonance energy transfer |
| MD | Molecular dynamics |
| STED | Stimulated emission depletion microscopy |
| STORM | Stochastic optical reconstruction microscopy |
| SIM | Structured illumination microscopy |
| fLPS | functional Liquid–Liquid Phase Separation predictor |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| PHFs | Paired helical filaments |
| SFs | Straight filaments |
| CTE | Chronic traumatic encephalopathy |
| MSP | Multisystem proteinopathy |
| HDX-MS | Hydrogen/deuterium exchange mass spectrometry |
| PrP | Prion protein |
| KMT2D | Lysine methyltransferase 2D |
| FET | FUS/EWS/TAF15 |
| SWI/SNF | Switch/sucrose non-fermentable |
References
- Kato, M.; Han, T.W.; Xie, S.; Shi, K.; Du, X.; Wu, L.C.; Mirzaei, H.; Goldsmith, E.J.; Longgood, J.; Pei, J.; et al. Cell-free formation of RNA granules: Low complexity sequence domains form dynamic fibers within hydrogels. Cell 2012, 149, 753–767. [Google Scholar] [CrossRef]
- Cascarina, S.M.; Elder, M.R.; Ross, E.D. Atypical structural tendencies among low-complexity domains in the Protein Data Bank proteome. PLoS Comput. Biol. 2020, 16, e1007487. [Google Scholar] [CrossRef]
- Cascarina, S.M.; Ross, E.D. Identification of Low-Complexity Domains by Compositional Signatures Reveals Class-Specific Frequencies and Functions Across the Domains of Life. PLoS Comput. Biol. 2024, 20, e1011372. [Google Scholar] [CrossRef]
- Kang, H.; Luan, B.; Zhou, R. Glassy dynamics in mutant huntingtin proteins. J. Chem. Phys. 2018, 149, 072333. [Google Scholar] [CrossRef]
- Lee, J.; Cho, H.; Kwon, I. Phase separation of low-complexity domains in cellular function and disease. Exp. Mol. Med. 2022, 54, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Hyman, A.A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 196–213. [Google Scholar] [CrossRef]
- Haider, R.; Shipley, B.; Surewicz, K.; Hinczewski, M.; Surewicz, W.K. Pathological C-terminal phosphomimetic substitutions alter the mechanism of liquid-liquid phase separation of TDP-43 low complexity domain. Protein Sci. 2024, 33, e5179. [Google Scholar] [CrossRef] [PubMed]
- Cascarina, S.M.; King, D.C.; Osborne Nishimura, E.; Ross, E.D. LCD-Composer: An intuitive, composition-centric method enabling the identification and detailed functional mapping of low-complexity domains. NAR Genom. Bioinform. 2021, 3, lqab048. [Google Scholar] [CrossRef]
- Cascarina, S.M.; Ross, E.D. Expansion and functional analysis of the SR-related protein family across the domains of life. RNA 2022, 28, 1298–1314. [Google Scholar] [CrossRef]
- Cascarina, S.M.; Ross, E.D. Proteome-scale relationships between local amino acid composition and protein fates and functions. PLoS Comput. Biol. 2018, 14, e1006256. [Google Scholar] [CrossRef]
- McKnight, S.L. Protein domains of low sequence complexity-dark matter of the proteome. Genes. Dev. 2024, 38, 205–212. [Google Scholar] [CrossRef]
- Davis, R.B.; Supakar, A.; Ranganath, A.K.; Moosa, M.M.; Banerjee, P.R. Heterotypic interactions can drive selective co-condensation of prion-like low-complexity domains of FET proteins and mammalian SWI/SNF complex. Nat. Commun. 2024, 15, 1168. [Google Scholar] [CrossRef]
- Chong, S.; Dugast-Darzacq, C.; Liu, Z.; Dong, P.; Dailey, G.M.; Cattoglio, C.; Heckert, A.; Banala, S.; Lavis, L.; Darzacq, X.; et al. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science 2018, 361, eaar2555. [Google Scholar] [CrossRef] [PubMed]
- Vernon, R.M.; Forman-Kay, J.D. First-generation predictors of biological protein phase separation. Curr. Opin. Struct. Biol. 2019, 58, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Tu, B.P.; McKnight, S.L. Redox-mediated regulation of low complexity domain self-association. Curr. Opin. Genet. Dev. 2021, 67, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, L.; Jia, H.; Lin, Z.; Zhong, R.; Li, Y.; Jiang, C.; Liu, S.; Zhou, X.; Zhang, E. The low-complexity domains of the KMT2D protein regulate histone monomethylation transcription to facilitate pancreatic cancer progression. Cell Mol. Biol. Lett. 2021, 26, 45. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, N.; Shi, Y.; Ma, S.; Wang, Y.; Zheng, W.; Sun, R.; Song, Y.; Chen, M.; Qu, L.; et al. BRD4L cooperates with MYC to block local tumor invasion via suppression of S100A10. Cell Signal 2024, 119, 111173. [Google Scholar] [CrossRef]
- Patel, S.S.; Belmont, B.J.; Sante, J.M.; Rexach, M.F. Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell 2007, 129, 83–96. [Google Scholar] [CrossRef]
- Vodnala, M.; Choi, E.B.; Fong, Y.W. Low complexity domains, condensates, and stem cell pluripotency. World J. Stem Cells 2021, 13, 416–438. [Google Scholar] [CrossRef]
- Eldirany, S.A.; Lomakin, I.B.; Ho, M.; Bunick, C.G. Recent insight into intermediate filament structure. Curr. Opin. Cell Biol. 2021, 68, 132–143. [Google Scholar] [CrossRef]
- Eibauer, M.; Weber, M.S.; Kronenberg-Tenga, R.; Beales, C.T.; Boujemaa-Paterski, R.; Turgay, Y.; Sivagurunathan, S.; Kraxner, J.; Koster, S.; Goldman, R.D.; et al. Vimentin filaments integrate low-complexity domains in a complex helical structure. Nat. Struct. Mol. Biol. 2024, 31, 939–949. [Google Scholar] [CrossRef]
- Kobler, J. On the structure of low sets [complexity classes]. In Proceedings of the Structure in Complexity Theory. Tenth Annual IEEE Conference, Minneapolis, MN, USA, 19–22 June 1995; pp. 246–261. [Google Scholar] [CrossRef]
- Pakravan, D.; Michiels, E.; Bratek-Skicki, A.; De Decker, M.; Van Lindt, J.; Alsteens, D.; Derclaye, S.; Van Damme, P.; Schymkowitz, J.; Rousseau, F.; et al. Liquid-Liquid Phase Separation Enhances TDP-43 LCD Aggregation but Delays Seeded Aggregation. Biomolecules 2021, 11, 548. [Google Scholar] [CrossRef]
- Reines, D. Recent advances in understanding RNA polymerase II structure and function. Fac. Rev. 2020, 9, 11. [Google Scholar] [CrossRef]
- Johnson, C.N.; Sojitra, K.A.; Sohn, E.J.; Moreno-Romero, A.K.; Baudin, A.; Xu, X.; Mittal, J.; Libich, D.S. Insights into Molecular Diversity within the FUS/EWS/TAF15 Protein Family: Unraveling Phase Separation of the N-Terminal Low-Complexity Domain from RNA-Binding Protein EWS. J. Am. Chem. Soc. 2024, 146, 8071–8085. [Google Scholar] [CrossRef]
- Pappu, R.; Farag, M.; Borcherds, W.; Bremer, A.; Mittag, T. Phase Separation in Mixtures of Prion-Like Low Complexity Domains is Driven by the Interplay of Homotypic and Heterotypic Interactions. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Farag, M.; Borcherds, W.M.; Bremer, A.; Mittag, T.; Pappu, R.V. Phase separation of protein mixtures is driven by the interplay of homotypic and heterotypic interactions. Nat. Commun. 2023, 14, 5527. [Google Scholar] [CrossRef]
- Mukherjee, S.; Schafer, L.V. Thermodynamic forces from protein and water govern condensate formation of an intrinsically disordered protein domain. Nat. Commun. 2023, 14, 5892. [Google Scholar] [CrossRef] [PubMed]
- Molliex, A.; Temirov, J.; Lee, J.; Coughlin, M.; Kanagaraj, A.P.; Kim, H.J.; Mittag, T.; Taylor, J.P. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 2015, 163, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Na, Z.; Slavoff, S.A. P-Bodies: Composition, Properties, and Functions. Biochemistry 2018, 57, 2424–2431. [Google Scholar] [CrossRef] [PubMed]
- Oroz, J.; Felix, S.S.; Cabrita, E.J.; Laurents, D.V. Structural transitions in Orb2 prion-like domain relevant for functional aggregation in memory consolidation. J. Biol. Chem. 2020, 295, 18122–18133. [Google Scholar] [CrossRef]
- Kato, M.; Yang, Y.S.; Sutter, B.M.; Wang, Y.; McKnight, S.L.; Tu, B.P. Redox State Controls Phase Separation of the Yeast Ataxin-2 Protein via Reversible Oxidation of Its Methionine-Rich Low-Complexity Domain. Cell 2019, 177, 711–721.e8. [Google Scholar] [CrossRef]
- Peng, L.; Li, E.M.; Xu, L.Y. From start to end: Phase separation and transcriptional regulation. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194641. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.I.; Onimaru, K. Biomolecular condensates in cancer biology. Cancer Sci. 2022, 113, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cooper, J.A.; Chong, Y.S.; Naveed, A.; Mayoh, C.; Jayatilleke, N.; Liu, T.; Amos, S.; Kobelke, S.; Marshall, A.C.; et al. NONO enhances mRNA processing of super-enhancer-associated GATA2 and HAND2 genes in neuroblastoma. EMBO Rep. 2023, 24, e54977. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, Y.; Kato, M.; Mori, E.; Liszczak, G.; Sutherland, L.; Sysoev, V.O.; Murray, D.T.; Tycko, R.; McKnight, S.L. Transiently structured head domains control intermediate filament assembly. Proc. Natl. Acad. Sci. USA 2021, 118, e2022121118. [Google Scholar] [CrossRef]
- Guo, M.; Wong, I.Y.; Moore, A.S.; Medalia, O.; Lippincott-Schwartz, J.; Weitz, D.A.; Goldman, R.D. Vimentin intermediate filaments as structural and mechanical coordinators of mesenchymal cells. Nat. Cell Biol. 2025, 27, 1210–1218. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr. Making sense of low-complexity domains: The 2025 Lasker Basic Science Award. Proc. Natl. Acad. Sci. USA 2025, 122, e2519778122. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.B.; Gorlich, D. Transport Selectivity of Nuclear Pores, Phase Separation, and Membraneless Organelles. Trends Biochem. Sci. 2016, 41, 46–61. [Google Scholar] [CrossRef]
- Hulsmann, B.B.; Labokha, A.A.; Gorlich, D. The permeability of reconstituted nuclear pores provides direct evidence for the selective phase model. Cell 2012, 150, 738–751. [Google Scholar] [CrossRef]
- Naim, B.; Zbaida, D.; Dagan, S.; Kapon, R.; Reich, Z. Cargo surface hydrophobicity is sufficient to overcome the nuclear pore complex selectivity barrier. EMBO J. 2009, 28, 2697–2705. [Google Scholar] [CrossRef]
- Raveh, B.; Karp, J.M.; Sparks, S.; Dutta, K.; Rout, M.P.; Sali, A.; Cowburn, D. Slide-and-exchange mechanism for rapid and selective transport through the nuclear pore complex. Proc. Natl. Acad. Sci. USA 2016, 113, E2489–E2497. [Google Scholar] [CrossRef]
- Frey, S.; Gorlich, D. A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell 2007, 130, 512–523. [Google Scholar] [CrossRef]
- Huang, S.H.; Parandhaman, M.; Jyothi Ravi, M.; Janda, D.C.; Amemiya, S. Nanoscale Hydrophobicity of Transport Barriers in the Nuclear Pore Complex as Compared with the Liquid/Liquid Interface by Scanning Electrochemical Microscopy. Anal. Chem. 2025, 97, 2745–2753. [Google Scholar] [CrossRef]
- Nedelsky, N.B.; Taylor, J.P. Bridging biophysics and neurology: Aberrant phase transitions in neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 272–286. [Google Scholar] [CrossRef]
- Kelaini, S.; Chan, C.; Cornelius, V.A.; Margariti, A. RNA-Binding Proteins Hold Key Roles in Function, Dysfunction, and Disease. Biology 2021, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sumrow, L.; Tashiro, K.; Sutherland, L.; Liu, D.; Qin, T.; Kato, M.; Liszczak, G.; McKnight, S.L. Mutations linked to neurological disease enhance self-association of low-complexity protein sequences. Science 2022, 377, eabn5582. [Google Scholar] [CrossRef] [PubMed]
- Altemus, J.J.; Lay, M.A.; Thompson, V.F.; Schwartz, J.C. Purification of Low-Complexity Domain Proteins FUS, EWSR1, and Their Fusions. Curr. Protoc. 2025, 5, e70136. [Google Scholar] [CrossRef]
- Garg, D.K.; Bhat, R. Modulation of assembly of TDP-43 low-complexity domain by heparin: From droplets to amyloid fibrils. Biophys. J. 2022, 121, 2568–2582. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Mannen, T.; Cagatay, T.; Fujiwara, A.; Matsumura, H.; Niesman, A.B.; Brautigam, C.A.; Chook, Y.M.; Yoshizawa, T. Mechanism of karyopherin-beta2 binding and nuclear import of ALS variants FUS(P525L) and FUS(R495X). Sci. Rep. 2021, 11, 3754. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, Y.; Liu, X.; Tang, Y.; Lao, Z.; Wei, G. Dissecting how ALS-associated D290V mutation enhances pathogenic aggregation of hnRNPA2(286)(-)(291) peptides: Dynamics and conformational ensembles. Int. J. Biol. Macromol. 2023, 241, 124659. [Google Scholar] [CrossRef]
- Lu, J.; Ge, P.; Sawaya, M.R.; Hughes, M.P.; Boyer, D.R.; Cao, Q.; Abskharon, R.; Cascio, D.; Tayeb-Fligelman, E.; Eisenberg, D.S. Cryo-EM structures of the D290V mutant of the hnRNPA2 low-complexity domain suggests how D290V affects phase separation and aggregation. J. Biol. Chem. 2024, 300, 105531. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhang, Z.; Ye, K. Tau in neurodegenerative diseases: Molecular mechanisms, biomarkers, and therapeutic strategies. Transl. Neurodegener. 2024, 13, 40. [Google Scholar] [CrossRef]
- Wegmann, S. Liquid-Liquid Phase Separation of Tau Protein in Neurobiology and Pathology. Adv. Exp. Med. Biol. 2019, 1184, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, S.; Eftekharzadeh, B.; Tepper, K.; Zoltowska, K.M.; Bennett, R.E.; Dujardin, S.; Laskowski, P.R.; MacKenzie, D.; Kamath, T.; Commins, C.; et al. Tau protein liquid-liquid phase separation can initiate tau aggregation. EMBO J. 2018, 37, e98049. [Google Scholar] [CrossRef] [PubMed]
- Ainani, H.; Bouchmaa, N.; Ben Mrid, R.; El Fatimy, R. Liquid-liquid phase separation of protein tau: An emerging process in Alzheimer’s disease pathogenesis. Neurobiol. Dis. 2023, 178, 106011. [Google Scholar] [CrossRef]
- Qi, C.; Verheijen, B.M.; Kokubo, Y.; Shi, Y.; Tetter, S.; Murzin, A.G.; Nakahara, A.; Morimoto, S.; Vermulst, M.; Sasaki, R.; et al. Tau filaments from amyotrophic lateral sclerosis/parkinsonism-dementia complex adopt the CTE fold. Proc. Natl. Acad. Sci. USA 2023, 120, e2306767120. [Google Scholar] [CrossRef] [PubMed]
- Falcon, B.; Zivanov, J.; Zhang, W.; Murzin, A.G.; Garringer, H.J.; Vidal, R.; Crowther, R.A.; Newell, K.L.; Ghetti, B.; Goedert, M.; et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 2019, 568, 420–423. [Google Scholar] [CrossRef]
- Chong, S.; Graham, T.G.W.; Dugast-Darzacq, C.; Dailey, G.M.; Darzacq, X.; Tjian, R. Tuning levels of low-complexity domain interactions to modulate endogenous oncogenic transcription. Mol. Cell 2022, 82, 2084–2097.e5. [Google Scholar] [CrossRef]
- Li, M.; Chen, C. Regulation of Metastasis in Ewing Sarcoma. Cancers 2022, 14, 4902. [Google Scholar] [CrossRef]
- Kim, G.H.; Kwon, I. Distinct roles of hnRNPH1 low-complexity domains in splicing and transcription. Proc. Natl. Acad. Sci. USA 2021, 118, e2109668118. [Google Scholar] [CrossRef]
- Thibault, P.A.; Ganesan, A.; Kalyaanamoorthy, S.; Clarke, J.W.E.; Salapa, H.E.; Levin, M.C. hnRNP A/B Proteins: An Encyclopedic Assessment of Their Roles in Homeostasis and Disease. Biology 2021, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, S.L. The roles of hnRNP A2/B1 in RNA biology and disease. Wiley Interdiscip. Rev. RNA 2021, 12, e1612. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Mohassel, P.; Donkervoort, S.; Guo, L.; O’Donovan, K.; Coughlin, M.; Lornage, X.; Foulds, N.; Hammans, S.R.; Foley, A.R.; et al. Heterozygous frameshift variants in HNRNPA2B1 cause early-onset oculopharyngeal muscular dystrophy. Nat. Commun. 2022, 13, 2306. [Google Scholar] [CrossRef] [PubMed]
- Prusiner, S.B. Shattuck lecture—Neurodegenerative diseases and prions. N. Engl. J. Med. 2001, 344, 1516–1526. [Google Scholar] [CrossRef]
- Kuang, L.; Kamelgarn, M.; Arenas, A.; Gal, J.; Taylor, D.; Gong, W.; Brown, M.; St Clair, D.; Kasarskis, E.J.; Zhu, H. Clinical and experimental studies of a novel P525R FUS mutation in amyotrophic lateral sclerosis. Neurol. Genet. 2017, 3, e172. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Babinchak, W.M.; Surewicz, W.K. Cryo-EM structure of amyloid fibrils formed by the entire low complexity domain of TDP-43. Nat. Commun. 2021, 12, 1620. [Google Scholar] [CrossRef]
- Usman, A.; Yusuf, A.; Isah, M.B.; Dang, M.; Zhang, X. Small Molecules as Regulators of Liquid-Liquid Phase Separation: Mechanisms and Strategies for New Drug Discovery. FASEB J. 2025, 39, e70773. [Google Scholar] [CrossRef]
- Kim, S.C.; Mitchell, S.J.; Qamar, S.; Whitcomb, D.J.; Ruepp, M.D.; St George-Hyslop, P.; Cho, K. Mimicking hypomethylation of FUS requires liquid-liquid phase separation to induce synaptic dysfunctions. Acta Neuropathol. Commun. 2023, 11, 199. [Google Scholar] [CrossRef]
- Selig, E.E.; Sohn, E.J.; Stoja, A.; Moreno-Romero, A.K.; Akula, S.; Xu, X.; Bishop, A.J.R.; Libich, D.S. Phase separation of the oncogenic fusion protein EWS::FLI1 is modulated by its DNA-binding domain. Proc. Natl. Acad. Sci. USA 2025, 122, e2221823122. [Google Scholar] [CrossRef]
- White, A.R.; Enever, P.; Tayebi, M.; Mushens, R.; Linehan, J.; Brandner, S.; Anstee, D.; Collinge, J.; Hawke, S. Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature 2003, 422, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Sprunger, M.L.; Jackrel, M.E. Prion-Like Proteins in Phase Separation and Their Link to Disease. Biomolecules 2021, 11, 1014. [Google Scholar] [CrossRef]
- Uliassi, E.; Nikolic, L.; Bolognesi, M.L.; Legname, G. Therapeutic strategies for identifying small molecules against prion diseases. Cell Tissue Res. 2023, 392, 337–347. [Google Scholar] [CrossRef]
- Howlett, G.J.; Minton, A.P.; Rivas, G. Analytical ultracentrifugation for the study of protein association and assembly. Curr. Opin. Chem. Biol. 2006, 10, 430–436. [Google Scholar] [CrossRef]
- Peran, I.; Martin, E.W.; Mittag, T. Walking Along a Protein Phase Diagram to Determine Coexistence Points by Static Light Scattering. Methods Mol. Biol. 2020, 2141, 715–730. [Google Scholar] [CrossRef]
- Tsoi, P.S.; Ferreon, J.C.; Ferreon, A.C.M. Initiation of hnRNPA1 Low-Complexity Domain Condensation Monitored by Dynamic Light Scattering. Int. J. Mol. Sci. 2024, 25, 6825. [Google Scholar] [CrossRef]
- Garcia-Saez, A.J.; Schwille, P. Fluorescence correlation spectroscopy for the study of membrane dynamics and protein/lipid interactions. Methods 2008, 46, 116–122. [Google Scholar] [CrossRef]
- Incicco, J.J.; Roy, D.; Stuchell-Brereton, M.D.; Soranno, A. Fluorescence Correlation Spectroscopy and Phase Separation. Methods Mol. Biol. 2023, 2563, 161–198. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Singh, N.; Patel, K.; Krishnamoorthy, G.; Maji, S.K. FRAP and FRET Investigation of alpha-Synuclein Fibrillization via Liquid-Liquid Phase Separation In Vitro and in HeLa Cells. Methods Mol. Biol. 2023, 2551, 395–423. [Google Scholar] [CrossRef]
- do Amaral, M.J.; Passos, A.R.; Mohapatra, S.; Freire, M.H.; Wegmann, S.; Cordeiro, Y. X-Ray Photon Correlation Spectroscopy, Microscopy, and Fluorescence Recovery After Photobleaching to Study Phase Separation and Liquid-to-Solid Transition of Prion Protein Condensates. Bio Protoc. 2025, 15, e5277. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.; Guo, L.; Gai, F.; Goulian, M. Fluorescence correlation spectroscopy measurements of the membrane protein TetA in Escherichia coli suggest rapid diffusion at short length scales. PLoS ONE 2012, 7, e48600. [Google Scholar] [CrossRef]
- Fitzpatrick, A.W.P.; Falcon, B.; He, S.; Murzin, A.G.; Murshudov, G.; Garringer, H.J.; Crowther, R.A.; Ghetti, B.; Goedert, M.; Scheres, S.H.W. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 2017, 547, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.; Tse, E.; Yang, H.; Shi, M.; Caro, C.D.; Wang, F.; Merz, G.E.; Prusiner, S.B.; Southworth, D.R.; Condello, C. Cryo-EM structures reveal tau filaments from Down syndrome adopt Alzheimer’s disease fold. Acta Neuropathol. Commun. 2024, 12, 94. [Google Scholar] [CrossRef]
- Schur, F.K. Toward high-resolution in situ structural biology with cryo-electron tomography and subtomogram averaging. Curr. Opin. Struct. Biol. 2019, 58, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sahin, C.; Leppert, A.; Landreh, M. Advances in mass spectrometry to unravel the structure and function of protein condensates. Nat. Protoc. 2023, 18, 3653–3661. [Google Scholar] [CrossRef]
- Kato, M.; McKnight, S.L. The low-complexity domain of the FUS RNA binding protein self-assembles via the mutually exclusive use of two distinct cross-beta cores. Proc. Natl. Acad. Sci. USA 2021, 118, e2114412118. [Google Scholar] [CrossRef] [PubMed]
- Ganser, L.R.; Niaki, A.G.; Yuan, X.; Huang, E.; Deng, D.; Djaja, N.A.; Ge, Y.; Craig, A.; Langlois, O.; Myong, S. The roles of FUS-RNA binding domain and low complexity domain in RNA-dependent phase separation. Structure 2024, 32, 177–187.e5. [Google Scholar] [CrossRef]
- Pfleger, K.D.; Eidne, K.A. Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET). Nat. Methods 2006, 3, 165–174. [Google Scholar] [CrossRef]
- Shi, J.; Tian, F.; Lyu, J.; Yang, M. Nanoparticle based fluorescence resonance energy transfer (FRET) for biosensing applications. J. Mater. Chem. B 2015, 3, 6989–7005. [Google Scholar] [CrossRef]
- Machleidt, T.; Woodroofe, C.C.; Schwinn, M.K.; Mendez, J.; Robers, M.B.; Zimmerman, K.; Otto, P.; Daniels, D.L.; Kirkland, T.A.; Wood, K.V. NanoBRET--A Novel BRET Platform for the Analysis of Protein-Protein Interactions. ACS Chem. Biol. 2015, 10, 1797–1804. [Google Scholar] [CrossRef]
- Joshi, A.; Walimbe, A.; Avni, A.; Rai, S.K.; Arora, L.; Sarkar, S.; Mukhopadhyay, S. Single-molecule FRET unmasks structural subpopulations and crucial molecular events during FUS low-complexity domain phase separation. Nat. Commun. 2023, 14, 7331. [Google Scholar] [CrossRef]
- Wang, T.; Yang, N.; Liang, C.; Xu, H.; An, Y.; Xiao, S.; Zheng, M.; Liu, L.; Wang, G.; Nie, L. Detecting Protein-Protein Interaction Based on Protein Fragment Complementation Assay. Curr. Protein Pept. Sci. 2020, 21, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.M. Optimizing strategy for the discovery of compositionally-biased or low-complexity regions in proteins. Sci. Rep. 2024, 14, 680. [Google Scholar] [CrossRef]
- Tejedor, A.R.; Collepardo-Guevara, R.; Ramirez, J.; Espinosa, J.R. Time-Dependent Material Properties of Aging Biomolecular Condensates from Different Viscoelasticity Measurements in Molecular Dynamics Simulations. J. Phys. Chem. B 2023, 127, 4441–4459. [Google Scholar] [CrossRef]
- Pedraza, P.E.; Hoyos, D.; Feito, A.; Gámez, F.; Sanchez-Burgo, I.; Collepardo-Guevara, R.; Tejedo, A.R.; Espinosa, J.R. Charged mutations in the FUS low-complexity domain modulate condensate ageing kinetics. bioRxiv 2025. [Google Scholar] [CrossRef]
- Wegel, E.; Gohler, A.; Lagerholm, B.C.; Wainman, A.; Uphoff, S.; Kaufmann, R.; Dobbie, I.M. Imaging cellular structures in super-resolution with SIM, STED and Localisation Microscopy: A practical comparison. Sci. Rep. 2016, 6, 27290. [Google Scholar] [CrossRef] [PubMed]
- Brucale, M.; Schuler, B.; Samori, B. Single-molecule studies of intrinsically disordered proteins. Chem. Rev. 2014, 114, 3281–3317. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, S.; Gao, Y. Single-molecule manipulation and detection. Acta Biochim. Biophys. Sin. 2018, 50, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Prakash, K.; Diederich, B.; Heintzmann, R.; Schermelleh, L. Super-resolution microscopy: A brief history and new avenues. Philos. Trans. A Math. Phys. Eng. Sci. 2022, 380, 20210110. [Google Scholar] [CrossRef]
- Lv, Q.; Zhou, F.; Liu, X.; Zhi, L. Artificial intelligence in small molecule drug discovery from 2018 to 2023: Does it really work? Bioorg Chem 2023, 141, 106894. [Google Scholar] [CrossRef]
- Lopez-Cabrejos, J.; Paixao, T.; Alvarez, A.B.; Luque, D.B. An Efficient and Low-Complexity Transformer-Based Deep Learning Framework for High-Dynamic-Range Image Reconstruction. Sensors 2025, 25, 1497. [Google Scholar] [CrossRef]
- Liu, X.; Peng, T.; Xu, M.; Lin, S.; Hu, B.; Chu, T.; Liu, B.; Xu, Y.; Ding, W.; Li, L.; et al. Spatial multi-omics: Deciphering technological landscape of integration of multi-omics and its applications. J. Hematol. Oncol. 2024, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.R.; Chong, S. Spatiotemporal characterization of endogenous transcription factor hubs by super-resolution microscopy. Biophys. J. 2025, 124, 245A. [Google Scholar] [CrossRef]
- Lionnet, T.; Wu, C. Single-molecule tracking of transcription protein dynamics in living cells: Seeing is believing, but what are we seeing? Curr. Opin. Genet. Dev. 2021, 67, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Pržulj, N.; Malod-Dognin, N. Simplicity within biological complexity. Bioinform. Adv. 2025, 5, vbae164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Zhou, K.; Xia, L.; Ren, K.; Xu, Y. In-Depth Study of Low-Complexity Domains: From Structural Diversity to Disease Mechanisms. Cells 2025, 14, 1752. https://doi.org/10.3390/cells14221752
Xu H, Zhou K, Xia L, Ren K, Xu Y. In-Depth Study of Low-Complexity Domains: From Structural Diversity to Disease Mechanisms. Cells. 2025; 14(22):1752. https://doi.org/10.3390/cells14221752
Chicago/Turabian StyleXu, Haixia, Kaili Zhou, Lianren Xia, Kejin Ren, and Yongjie Xu. 2025. "In-Depth Study of Low-Complexity Domains: From Structural Diversity to Disease Mechanisms" Cells 14, no. 22: 1752. https://doi.org/10.3390/cells14221752
APA StyleXu, H., Zhou, K., Xia, L., Ren, K., & Xu, Y. (2025). In-Depth Study of Low-Complexity Domains: From Structural Diversity to Disease Mechanisms. Cells, 14(22), 1752. https://doi.org/10.3390/cells14221752






