Glymphatic Dysfunction in Neuro-Pulmonary Complications Following Subarachnoid Hemorrhage: A New Perspective on Brain–Lung Axis Disruption
Abstract
1. Introduction
2. Current Evidence and Pathophysiology
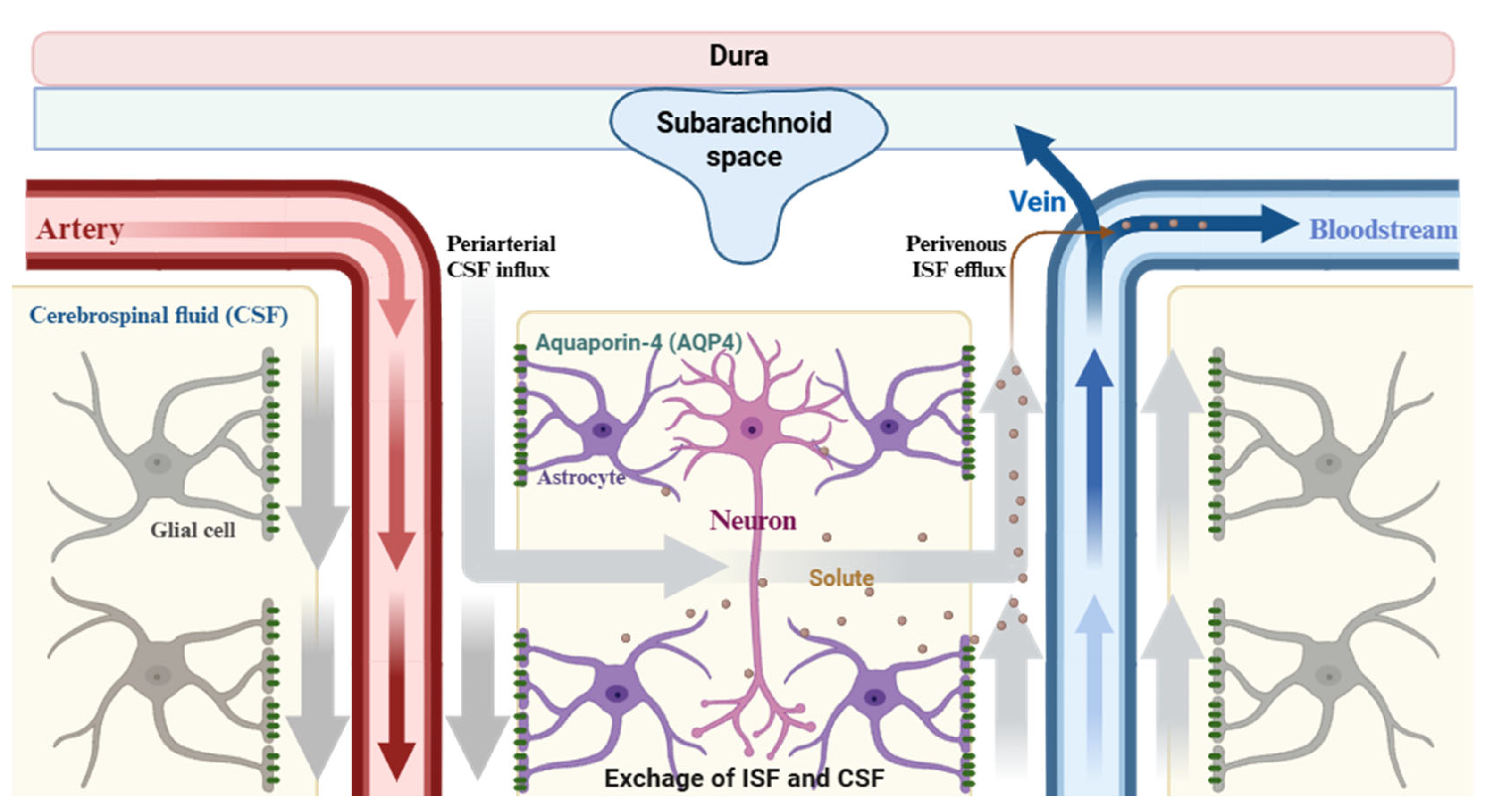
2.1. An Evolving Understanding of Glymphatic Biology
- 1.
- Elevated intracranial pressure (ICP) reduces the pressure gradient driving CSF influx into periarterial spaces [47];
- 2.
- Depolarization of AQP4 channels due to reactive astrogliosis disrupts the directional water flow necessary for convective exchange [42];
- 3.
- Obstruction of perivascular spaces by blood components and cellular debris hinders CSF movement and promotes local inflammation [48].
2.2. Glymphatic Dysfunction Following SAH
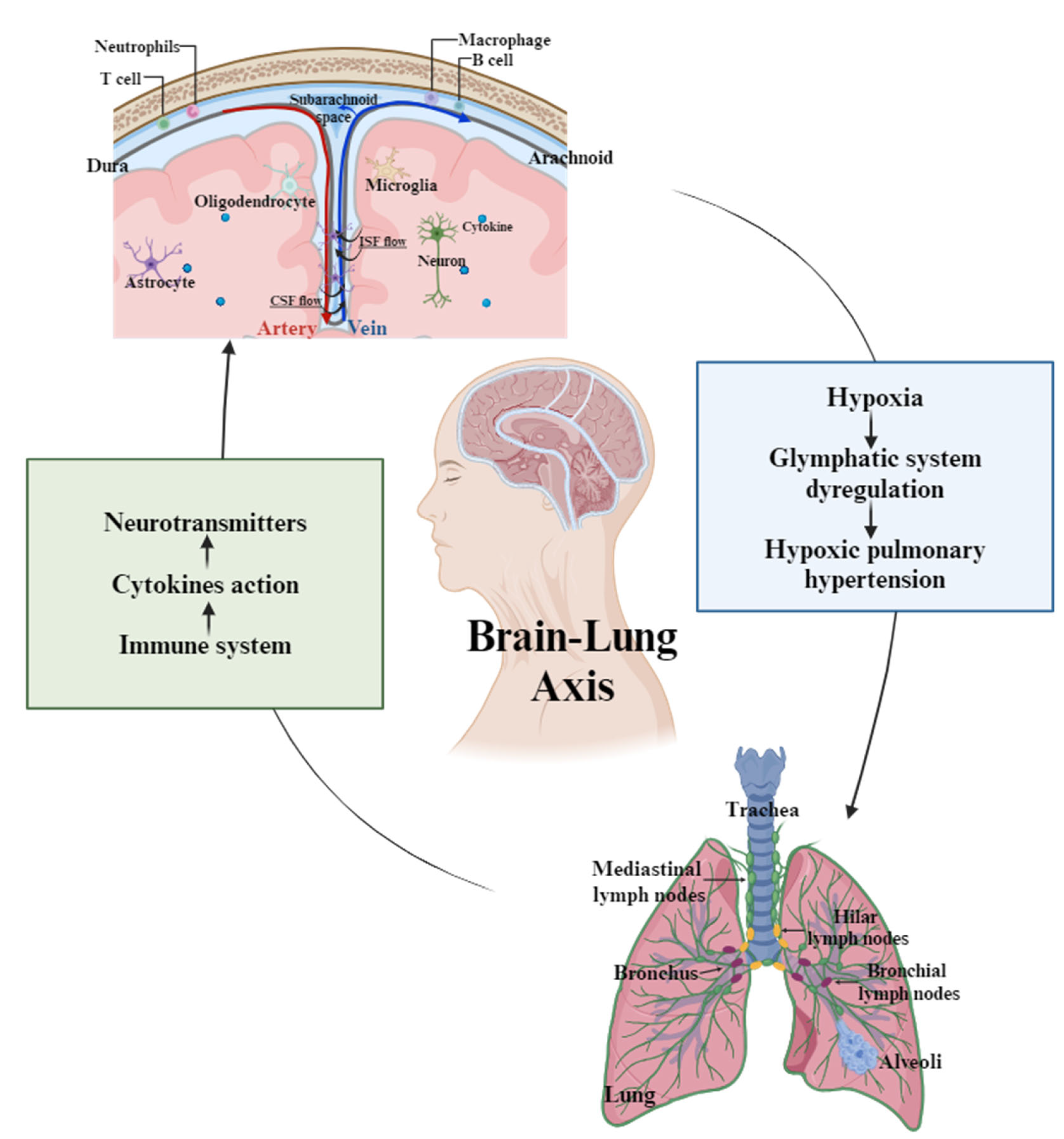
2.3. Pulmonary Complications Linked to Glymphatic Impairment
2.4. Therapeutic Perspectives: Targeting Glymphatic and Lymphatic Pathways
2.4.1. Modulating Aquaporin-4 Function
- -
- Case Study and Research Findings:
- -
- Clinical Implications and Challenges:
2.4.2. Enhancing CSF Dynamics
- -
- Innovative Approaches to CSF Dynamics:
- -
- Sleep Enhancement and Glymphatic Activity:
2.4.3. Targeting Meningeal Lymphatic Drainage
- -
- Restoring Meningeal Lymphatic Function:
- -
- Clinical Challenges and Potential:
2.4.4. Anti-Inflammatory and Neuroimmune Modulation
- -
- Cytokine Inhibition in SAH Models:
- -
- Emerging Anti-inflammatory Agents:
2.4.5. Emerging Technologies and Biomarker Development
- -
- Biomarkers for Glymphatic Dysfunction:
3. Discussion
- -
- Pathophysiological Mechanisms of Glymphatic Dysfunction and Systemic Inflammation:
- -
- Impact on Pulmonary Function and the Brain–Lung Axis:
- -
- Therapeutic Implications and Future Directions:
- -
- Limitations and Knowledge Gaps:
- -
- Concluding Remarks: Toward Brain–Body Communication in Neurocritical Care:
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Macdonald, R.L.; Schweizer, T.A. Spontaneous subarachnoid haemorrhage. Lancet 2017, 389, 655–666. [Google Scholar] [CrossRef]
- Etminan, N.; Macdonald, R.L. Management of aneurysmal subarachnoid hemorrhage. Handb. Clin. Neurol. 2017, 140, 195–228. [Google Scholar] [CrossRef]
- Abraham, M.K.; Chang, W.W. Subarachnoid Hemorrhage. Emerg. Med. Clin. N. Am. 2016, 34, 901–916. [Google Scholar] [CrossRef]
- Cabral, N.L.; Goncalves, A.R.; Longo, A.L.; Moro, C.H.; Costa, G.; Amaral, C.H.; Fonseca, L.A.; Eluf-Neto, J. Incidence of stroke subtypes, prognosis and prevalence of risk factors in Joinville, Brazil: A 2 year community based study. J. Neurol. Neurosurg. Psychiatry 2009, 80, 755–761. [Google Scholar] [CrossRef]
- Rowland, M.J.; Garry, P.; Ezra, M.; Corkill, R.; Baker, I.; Jezzard, P.; Westbrook, J.; Douaud, G.; Pattinson, K.T.S. Early brain injury and cognitive impairment after aneurysmal subarachnoid haemorrhage. Sci. Rep. 2021, 11, 23245. [Google Scholar] [CrossRef]
- Suarez, J.I.; Tarr, R.W.; Selman, W.R. Aneurysmal subarachnoid hemorrhage. N. Engl. J. Med. 2006, 354, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Ramagopalan, S.V.; Pakpoor, J.; Seminog, O.; Goldacre, R.; Graham, L.; Goldacre, M.J. Risk of subarachnoid haemorrhage in people admitted to hospital with selected immune-mediated diseases: Record-linkage studies. BMC Neurol. 2013, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Busl, K.M.; Claassen, J.; Diringer, M.N.; Helbok, R.; Park, S.; Rabinstein, A.; Treggiari, M.; Vergouwen, M.D.I.; Citerio, G. Contemporary management of aneurysmal subarachnoid haemorrhage. An update for the intensivist. Intensive Care Med. 2024, 50, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Kim, J.E.; Park, S.Q.; Ko, J.K.; Kim, D.W.; Park, J.C.; Yeon, J.Y.; Chung, S.Y.; Chung, J.; Joo, S.P.; et al. Korean Clinical Practice Guidelines for Aneurysmal Subarachnoid Hemorrhage. J. Korean Neurosurg. Soc. 2018, 61, 127–166. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Uchikawa, H.; Kajiwara, S.; Morioka, M. Central sympathetic nerve activation in subarachnoid hemorrhage. J. Neurochem. 2022, 160, 34–50. [Google Scholar] [CrossRef]
- Solar, P.; Zamani, A.; Lakatosova, K.; Joukal, M. The blood-brain barrier and the neurovascular unit in subarachnoid hemorrhage: Molecular events and potential treatments. Fluids Barriers CNS 2022, 19, 29. [Google Scholar] [CrossRef]
- Cossu, G.; Messerer, M.; Oddo, M.; Daniel, R.T. To look beyond vasospasm in aneurysmal subarachnoid haemorrhage. Biomed. Res. Int. 2014, 2014, 628597. [Google Scholar] [CrossRef] [PubMed]
- Francoeur, C.L.; Mayer, S.A. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit. Care 2016, 20, 277. [Google Scholar] [CrossRef] [PubMed]
- Kassell, N.F.; Sasaki, T.; Colohan, A.R.; Nazar, G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke 1985, 16, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, C.; Jaimes, F. Organ Dysfunction in Sepsis: An Ominous Trajectory From Infection To Death. Yale J. Biol. Med. 2019, 92, 629–640. [Google Scholar]
- Mazeraud, A.; Robba, C.; Rebora, P.; Iaquaniello, C.; Vargiolu, A.; Rass, V.; Bogossian, E.G.; Helbok, R.; Taccone, F.S.; Citerio, G. Acute Distress Respiratory Syndrome After Subarachnoid Hemorrhage: Incidence and Impact on the Outcome in a Large Multicenter, Retrospective Cohort. Neurocrit. Care 2021, 34, 1000–1008. [Google Scholar] [CrossRef]
- Wu, J.; Gao, W.; Zhang, H. Development of acute lung injury or acute respiratory distress syndrome after subarachnoid hemorrhage, predictive factors, and impact on prognosis. Acta Neurol. Belg. 2023, 123, 1331–1337. [Google Scholar] [CrossRef]
- Chacon-Aponte, A.A.; Duran-Vargas, E.A.; Arevalo-Carrillo, J.A.; Lozada-Martinez, I.D.; Bolano-Romero, M.P.; Moscote-Salazar, L.R.; Grille, P.; Janjua, T. Brain-lung interaction: A vicious cycle in traumatic brain injury. Acute Crit. Care 2022, 37, 35–44. [Google Scholar] [CrossRef]
- Wang, R.H.; Lu, A.L.; Li, H.P.; Ma, Z.H.; Wu, S.B.; Lu, H.J.; Wen, W.X.; Huang, Y.; Wang, L.X.; Yuan, F. Prevalence, predictors, and outcomes of acute respiratory distress syndrome in severe stroke. Neurol. Sci. 2024, 45, 2719–2728. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Lv, T.; Zhao, B.; Hu, Q.; Zhang, X. The Glymphatic System: A Novel Therapeutic Target for Stroke Treatment. Front. Aging Neurosci. 2021, 13, 689098. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Silva, J.; Ferreira, R.; Trigo, D. Glymphatic system, AQP4, and their implications in Alzheimer’s disease. Neurol. Res. Pract. 2021, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Mestre, H.; Mori, Y.; Nedergaard, M. The Brain’s Glymphatic System: Current Controversies. Trends Neurosci. 2020, 43, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. The glymphatic hypothesis: The theory and the evidence. Fluids Barriers CNS 2022, 19, 9. [Google Scholar] [CrossRef]
- Voumvourakis, K.I.; Sideri, E.; Papadimitropoulos, G.N.; Tsantzali, I.; Hewlett, P.; Kitsos, D.; Stefanou, M.; Bonakis, A.; Giannopoulos, S.; Tsivgoulis, G.; et al. The Dynamic Relationship between the Glymphatic System, Aging, Memory, and Sleep. Biomedicines 2023, 11, 2092. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Li, B. Glymphatic System Dysfunction in Central Nervous System Diseases and Mood Disorders. Front. Aging Neurosci. 2022, 14, 873697. [Google Scholar] [CrossRef]
- Gu, J.C.; Wu, H.; Chen, X.Z.; Feng, J.F.; Gao, G.Y.; Jiang, J.Y.; Mao, Q. Intracranial Pressure during External Ventricular Drainage Weaning Is an Outcome Predictor of Traumatic Brain Injury. Biomed. Res. Int. 2020, 2020, 8379134. [Google Scholar] [CrossRef]
- Eide, P.K.; Hansson, H.A. A New Perspective on the Pathophysiology of Idiopathic Intracranial Hypertension: Role of the Glia-Neuro-Vascular Interface. Front. Mol. Neurosci. 2022, 15, 900057. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, L.; Wang, X.; Si, X.; Lenahan, C.; Shi, H.; Shao, A.; Tang, J.; Chen, S.; Zhang, J.; et al. A new perspective on cerebrospinal fluid dynamics after subarachnoid hemorrhage: From normal physiology to pathophysiological changes. J. Cereb. Blood Flow Metab. 2022, 42, 543–558. [Google Scholar] [CrossRef]
- Pu, T.; Zou, W.; Feng, W.; Zhang, Y.; Wang, L.; Wang, H.; Xiao, M. Persistent Malfunction of Glymphatic and Meningeal Lymphatic Drainage in a Mouse Model of Subarachnoid Hemorrhage. Exp. Neurobiol. 2019, 28, 104–118. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Y.; Chen, L.; Wang, J.; Zhang, J.; Zhang, H.; Tian, S.; Zhang, A.; Zhang, J.; Zhang, J.H.; et al. Cerebrospinal fluid markers of neuroinflammation and coagulation in severe cerebral edema and chronic hydrocephalus after subarachnoid hemorrhage: A prospective study. J. Neuron. 2024, 21, 237. [Google Scholar] [CrossRef]
- Gomolka, R.S.; Hablitz, L.M.; Mestre, H.; Giannetto, M.; Du, T.; Hauglund, N.L.; Xie, L.; Peng, W.; Martinez, P.M.; Nedergaard, M.; et al. Loss of aquaporin-4 results in glymphatic system dysfunction via brain-wide interstitial fluid stagnation. eLife 2023, 12, e82232. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.L.; Chen, J.J.; Hu, G.; Xu, J.; Xiao, M.; Li, S. Aquaporin 4 in Astrocytes is a Target for Therapy in Alzheimer’s Disease. Curr. Pharm. Des. 2017, 23, 4948–4957. [Google Scholar] [CrossRef] [PubMed]
- Hilzendeger, A.M.; Shenoy, V.; Raizada, M.K.; Katovich, M.J. Neuroinflammation in pulmonary hypertension: Concept, facts, and relevance. Curr. Hypertens. Rep. 2014, 16, 469. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liang, R.; Yang, B.; Zhou, Y.; Liu, M.; Fang, F.; Ding, J.; Fan, Y.; Hu, G. Aquaporin-4 mediates communication between astrocyte and microglia: Implications of neuroinflammation in experimental Parkinson’s disease. Neuroscience 2016, 317, 65–75. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Fu, Z.; Kipnis, J. The Meningeal Lymphatic System: A New Player in Neurophysiology. Neuron 2018, 100, 375–388. [Google Scholar] [CrossRef]
- Bohr, T.; Hjorth, P.G.; Holst, S.C.; Hrabetova, S.; Kiviniemi, V.; Lilius, T.; Lundgaard, I.; Mardal, K.A.; Martens, E.A.; Mori, Y.; et al. The glymphatic system: Current understanding and modeling. iScience 2022, 25, 104987. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Wang, X.; Xu, H.; Fang, C.; Yuan, L.; Wang, K.; Zheng, J.; Qi, Y.; Chen, S.; et al. Clinical Potential of Immunotherapies in Subarachnoid Hemorrhage Treatment: Mechanistic Dissection of Innate and Adaptive Immune Responses. Aging Dis. 2023, 14, 1533–1554. [Google Scholar] [CrossRef]
- Lauzier, D.C.; Jayaraman, K.; Yuan, J.Y.; Diwan, D.; Vellimana, A.K.; Osbun, J.W.; Chatterjee, A.R.; Athiraman, U.; Dhar, R.; Zipfel, G.J. Early Brain Injury After Subarachnoid Hemorrhage: Incidence and Mechanisms. Stroke 2023, 54, 1426–1440. [Google Scholar] [CrossRef]
- Weiland, J.; Beez, A.; Westermaier, T.; Kunze, E.; Siren, A.L.; Lilla, N. Neuroprotective Strategies in Aneurysmal Subarachnoid Hemorrhage (aSAH). Int. J. Mol. Sci. 2021, 22, 5442. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. Fluid transport in the brain. Physiol. Rev. 2022, 102, 1025–1151. [Google Scholar] [CrossRef]
- Jiang, H.; Wei, H.; Zhou, Y.; Xiao, X.; Zhou, C.; Ji, X. Overview of the meningeal lymphatic vessels in aging and central nervous system disorders. Cell Biosci. 2022, 12, 202. [Google Scholar] [CrossRef]
- Wang, J.; Lv, T.; Jia, F.; Li, Y.; Ma, W.; Xiao, Z.P.; Yu, W.; Zhao, H.; Zhang, X.; Hu, Q. Subarachnoid hemorrhage distinctively disrupts the glymphatic and meningeal lymphatic systems in beagles. Theranostics 2024, 14, 6053–6070. [Google Scholar] [CrossRef]
- Reddy, O.C.; van der Werf, Y.D. The Sleeping Brain: Harnessing the Power of the Glymphatic System through Lifestyle Choices. Brain Sci. 2020, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Raghunandan, A.; Ladron-de-Guevara, A.; Tithof, J.; Mestre, H.; Du, T.; Nedergaard, M.; Thomas, J.H.; Kelley, D.H. Bulk flow of cerebrospinal fluid observed in periarterial spaces is not an artifact of injection. eLife 2021, 10, e65958. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, F.L.; Delle, C.; Nedergaard, M. The Glymphatic System (En)during Inflammation. Int. J. Mol. Sci. 2021, 22, 7491. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Zeppenfeld, D.M.; Venkataraman, A.; Plog, B.A.; Liao, Y.; Deane, R.; Nedergaard, M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 2013, 33, 18190–18199. [Google Scholar] [CrossRef]
- Klostranec, J.M.; Vucevic, D.; Bhatia, K.D.; Kortman, H.G.J.; Krings, T.; Murphy, K.P.; ter Brugge, K.G.; Mikulis, D.J. Current Concepts in Intracranial Interstitial Fluid Transport and the Glymphatic System: Part II-Imaging Techniques and Clinical Applications. Radiology 2021, 301, 516–532. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, M.; Chen, Y.; Yang, M.; Wang, Y. The Underlying Role of the Glymphatic System and Meningeal Lymphatic Vessels in Cerebral Small Vessel Disease. Biomolecules 2022, 12, 748. [Google Scholar] [CrossRef]
- Bolte, A.C.; Dutta, A.B.; Hurt, M.E.; Smirnov, I.; Kovacs, M.A.; McKee, C.A.; Ennerfelt, H.E.; Shapiro, D.; Nguyen, B.H.; Frost, E.L.; et al. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat. Commun. 2020, 11, 4524. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, N.M.; Wang, J.Z.; Pasarikovski, C.R.; Guha, D.; Al-Mufti, F.; Mamdani, M.; Saposnik, G.; Schweizer, T.A.; Macdonald, R.L. Management of raised intracranial pressure in aneurysmal subarachnoid hemorrhage: Time for a consensus? Neurosurg. Focus 2017, 43, E13. [Google Scholar] [CrossRef] [PubMed]
- Bilston, L.E.; Fletcher, D.F.; Brodbelt, A.R.; Stoodley, M.A. Arterial pulsation-driven cerebrospinal fluid flow in the perivascular space: A computational model. Comput. Methods Biomech. Biomed. Eng. 2003, 6, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, J.D.; Roumenina, L.T.; Perrella, G.; Rayes, J. Basic Mechanisms of Hemolysis-Associated Thrombo-Inflammation and Immune Dysregulation. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1349–1361. [Google Scholar] [CrossRef]
- Bozza, M.T.; Jeney, V. Pro-inflammatory Actions of Heme and Other Hemoglobin-Derived DAMPs. Front. Immunol. 2020, 11, 1323. [Google Scholar] [CrossRef]
- Iannucci, J.; Grammas, P. Thrombin, a Key Driver of Pathological Inflammation in the Brain. Cells 2023, 12, 1222. [Google Scholar] [CrossRef]
- Generoso, J.S.; Thorsdottir, S.; Collodel, A.; Dominguini, D.; Santo, R.R.E.; Petronilho, F.; Barichello, T.; Iovino, F. Dysfunctional Glymphatic System with Disrupted Aquaporin 4 Expression Pattern on Astrocytes Causes Bacterial Product Accumulation in the CSF during Pneumococcal Meningitis. mBio 2022, 13, e0188622. [Google Scholar] [CrossRef]
- Price, B.R.; Johnson, L.A.; Norris, C.M. Reactive astrocytes: The nexus of pathological and clinical hallmarks of Alzheimer’s disease. Ageing Res. Rev. 2021, 68, 101335. [Google Scholar] [CrossRef]
- Iliff, J.J.; Chen, M.J.; Plog, B.A.; Zeppenfeld, D.M.; Soltero, M.; Yang, L.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014, 34, 16180–16193. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Ni, W.; Gu, Y. Effects of deferoxamine on blood-brain barrier disruption after subarachnoid hemorrhage. PLoS ONE 2017, 12, e0172784. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Y.; Si, Y.; Lu, C.; Wang, J.; Wang, S.; Li, L.; Xie, W.; Yue, Z.; Yong, J.; et al. Shank3 ameliorates neuronal injury after cerebral ischemia/reperfusion via inhibiting oxidative stress and inflammation. Redox Biol. 2024, 69, 102983. [Google Scholar] [CrossRef]
- Mestre, H.; Tithof, J.; Du, T.; Song, W.; Peng, W.; Sweeney, A.M.; Olveda, G.; Thomas, J.H.; Nedergaard, M.; Kelley, D.H. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 2018, 9, 4878. [Google Scholar] [CrossRef]
- Huat, T.J.; Camats-Perna, J.; Newcombe, E.A.; Valmas, N.; Kitazawa, M.; Medeiros, R. Metal Toxicity Links to Alzheimer’s Disease and Neuroinflammation. J. Mol. Biol. 2019, 431, 1843–1868. [Google Scholar] [CrossRef] [PubMed]
- Amartumur, S.; Nguyen, H.; Huynh, T.; Kim, T.S.; Woo, R.S.; Oh, E.; Kim, K.K.; Lee, L.P.; Heo, C. Neuropathogenesis-on-chips for neurodegenerative diseases. Nat. Commun. 2024, 15, 2219. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Liu, J.; Liang, C.; Yang, L.; Wang, G. Aquaporin-4 in glymphatic system, and its implication for central nervous system disorders. Neurobiol. Dis. 2023, 179, 106035. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Ganesh, S.; Vetrivel, M. Neurogenic Pulmonary Edema in Traumatic Brain Injury. Indian. J. Crit. Care Med. 2017, 21, 329–331. [Google Scholar] [CrossRef]
- Stevens, R.D.; Nyquist, P.A. The systemic implications of aneurysmal subarachnoid hemorrhage. J. Neurol. Sci. 2007, 261, 143–156. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Karayannis, G.; Giamouzis, G.; Skoularigis, J.; Louridas, G.; Butler, J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J. Am. Coll. Cardiol. 2009, 54, 1747–1762. [Google Scholar] [CrossRef]
- Sun, Y.; Koyama, Y.; Shimada, S. Inflammation From Peripheral Organs to the Brain: How Does Systemic Inflammation Cause Neuroinflammation? Front. Aging Neurosci. 2022, 14, 903455. [Google Scholar] [CrossRef]
- Matthay, M.A.; Thompson, B.T.; Ware, L.B. The Berlin definition of acute respiratory distress syndrome: Should patients receiving high-flow nasal oxygen be included? Lancet Respir. Med. 2021, 9, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Golanov, E.V.; Bovshik, E.I.; Wong, K.K.; Pautler, R.G.; Foster, C.H.; Federley, R.G.; Zhang, J.Y.; Mancuso, J.; Wong, S.T.; Britz, G.W. Subarachnoid hemorrhage—Induced block of cerebrospinal fluid flow: Role of brain coagulation factor III (tissue factor). J. Cereb. Blood Flow Metab. 2018, 38, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.A.; Manolis, T.A.; Manolis, A.S. Neurohumoral Activation in Heart Failure. Int. J. Mol. Sci. 2023, 24, 15472. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Li, J.; Zhao, H.; Ma, Q. HMGB1: A New Target for Ischemic Stroke and Hemorrhagic Transformation. Transl. Stroke Res. 2025, 16, 990–1015. [Google Scholar] [CrossRef]
- Machhi, J.; Kevadiya, B.D.; Muhammad, I.K.; Herskovitz, J.; Olson, K.E.; Mosley, R.L.; Gendelman, H.E. Harnessing regulatory T cell neuroprotective activities for treatment of neurodegenerative disorders. Mol. Neurodegener. 2020, 15, 32. [Google Scholar] [CrossRef]
- Simon, M.J.; Iliff, J.J. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim. Biophys. Acta 2016, 1862, 442–451. [Google Scholar] [CrossRef]
- Manoj, H.; Gomes, S.M.; Thimmappa, P.Y.; Nagareddy, P.R.; Jamora, C.; Joshi, M.B. Cytokine signalling in formation of neutrophil extracellular traps: Implications for health and diseases. Cytokine Growth Factor Rev. 2025, 81, 27–39. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Qu, M.; Li, W.; Wu, D.; Cata, J.P.; Miao, C. Neutrophil, neutrophil extracellular traps and endothelial cell dysfunction in sepsis. Clin. Transl. Med. 2023, 13, e1170. [Google Scholar] [CrossRef]
- Sahu, S.K.; Ozanturk, A.N.; Kulkarni, D.H.; Ma, L.; Barve, R.A.; Dannull, L.; Lu, A.; Starick, M.; McPhatter, J.; Garnica, L.; et al. Lung epithelial cell-derived C3 protects against pneumonia-induced lung injury. Sci. Immunol. 2023, 8, eabp9547. [Google Scholar] [CrossRef]
- Li, G.; Cao, Y.; Tang, X.; Huang, J.; Cai, L.; Zhou, L. The meningeal lymphatic vessels and the glymphatic system: Potential therapeutic targets in neurological disorders. J. Cereb. Blood Flow Metab. 2022, 42, 1364–1382. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef] [PubMed]
- Lawley, J.S.; Levine, B.D.; Williams, M.A.; Malm, J.; Eklund, A.; Polaner, D.M.; Subudhi, A.W.; Hackett, P.H.; Roach, R.C. Cerebral spinal fluid dynamics: Effect of hypoxia and implications for high-altitude illness. J. Appl. Physiol. 2016, 120, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Greve, H.J.; Dunbar, A.L.; Lombo, C.G.; Ahmed, C.; Thang, M.; Messenger, E.J.; Mumaw, C.L.; Johnson, J.A.; Kodavanti, U.P.; Oblak, A.L.; et al. The bidirectional lung brain-axis of amyloid-beta pathology: Ozone dysregulates the peri-plaque microenvironment. Brain 2023, 146, 991–1005. [Google Scholar] [CrossRef]
- Salvador, A.F.M.; Abduljawad, N.; Kipnis, J. Meningeal Lymphatics in Central Nervous System Diseases. Annu. Rev. Neurosci. 2024, 47, 323–344. [Google Scholar] [CrossRef]
- Li, R.; Zhao, M.; Yao, D.; Zhou, X.; Lenahan, C.; Wang, L.; Ou, Y.; He, Y. The role of the astrocyte in subarachnoid hemorrhage and its therapeutic implications. Front. Immunol. 2022, 13, 1008795. [Google Scholar] [CrossRef]
- Simon, D.W.; McGeachy, M.J.; Bayir, H.; Clark, R.S.; Loane, D.J.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 171–191. [Google Scholar] [CrossRef]
- Ding, Z.; Fan, X.; Zhang, Y.; Yao, M.; Wang, G.; Dong, Y.; Liu, J.; Song, W. The glymphatic system: A new perspective on brain diseases. Front. Aging Neurosci. 2023, 15, 1179988. [Google Scholar] [CrossRef]
- Rodriguez-Giraldo, M.; Gonzalez-Reyes, R.E.; Ramirez-Guerrero, S.; Bonilla-Trilleras, C.E.; Guardo-Maya, S.; Nava-Mesa, M.O. Astrocytes as a Therapeutic Target in Alzheimer’s Disease-Comprehensive Review and Recent Developments. Int. J. Mol. Sci. 2022, 23, 13630. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, K.; Zhu, J. Glymphatic system: An emerging therapeutic approach for neurological disorders. Front. Mol. Neurosci. 2023, 16, 1138769. [Google Scholar] [CrossRef]
- Mestre, H.; Hablitz, L.M.; Xavier, A.L.; Feng, W.; Zou, W.; Pu, T.; Monai, H.; Murlidharan, G.; Castellanos Rivera, R.M.; Simon, M.J.; et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife 2018, 7, e40070. [Google Scholar] [CrossRef]
- Arighi, A.; Arcaro, M.; Fumagalli, G.G.; Carandini, T.; Pietroboni, A.M.; Sacchi, L.; Fenoglio, C.; Serpente, M.; Sorrentino, F.; Isgro, G.; et al. Aquaporin-4 cerebrospinal fluid levels are higher in neurodegenerative dementia: Looking at glymphatic system dysregulation. Alzheimer’s Res. Ther. 2022, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Wang, M.X.; Ismail, O.; Braun, M.; Schindler, A.G.; Reemmer, J.; Wang, Z.; Haveliwala, M.A.; O’Boyle, R.P.; Han, W.Y.; et al. Loss of perivascular aquaporin-4 localization impairs glymphatic exchange and promotes amyloid beta plaque formation in mice. Alzheimer’s Res. Ther. 2022, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Li, J.; Wang, B.; Liu, Q.; Zhao, Y.; Zhang, H.; Wang, W.; Ren, W.; Cui, X.; Yang, X. Dynamic Evolution of the Glymphatic System at the Early Stages of Subarachnoid Hemorrhage. Front. Neurol. 2022, 13, 924080. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Jia, S.; Li, T.; Li, D.; Wang, X.; Liu, X.; Wang, Y.F. Alleviation of brain injury by applying TGN-020 in the supraoptic nucleus via inhibiting vasopressin neurons in rats of focal ischemic stroke. Life Sci. 2021, 264, 118683. [Google Scholar] [CrossRef]
- Li, X.; Xie, Z.; Zhou, Q.; Tan, X.; Meng, W.; Pang, Y.; Huang, L.; Ding, Z.; Hu, Y.; Li, R.; et al. TGN-020 Alleviate Inflammation and Apoptosis After Cerebral Ischemia-Reperfusion Injury in Mice Through Glymphatic and ERK1/2 Signaling Pathway. Mol. Neurobiol. 2024, 61, 1175–1186. [Google Scholar] [CrossRef]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef]
- Verkman, A.S.; Smith, A.J.; Phuan, P.W.; Tradtrantip, L.; Anderson, M.O. The aquaporin-4 water channel as a potential drug target in neurological disorders. Expert Opin. Ther. Targets 2017, 21, 1161–1170. [Google Scholar] [CrossRef]
- Palazzo, C.; Abbrescia, P.; Valente, O.; Nicchia, G.P.; Banitalebi, S.; Amiry-Moghaddam, M.; Trojano, M.; Frigeri, A. Tissue Distribution of the Readthrough Isoform of AQP4 Reveals a Dual Role of AQP4ex Limited to CNS. Int. J. Mol. Sci. 2020, 21, 1531. [Google Scholar] [CrossRef]
- Aoki-Yoshino, K.; Uchihara, T.; Duyckaerts, C.; Nakamura, A.; Hauw, J.J.; Wakayama, Y. Enhanced expression of aquaporin 4 in human brain with inflammatory diseases. Acta Neuropathol. 2005, 110, 281–288. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Pan, M.; Fiaz, M.; Hao, Y.; Yan, Y.; Sun, L.; Yan, F. Ultrasound-mediated blood-brain barrier opening: An effective drug delivery system for theranostics of brain diseases. Adv. Drug Deliv. Rev. 2022, 190, 114539. [Google Scholar] [CrossRef]
- Bothwell, S.W.; Omileke, D.; Patabendige, A.; Spratt, N.J. CSF Secretion Is Not Altered by NKCC1 Nor TRPV4 Antagonism in Healthy Rats. Brain Sci. 2021, 11, 1117. [Google Scholar] [CrossRef]
- Uldall, M.; Botfield, H.; Jansen-Olesen, I.; Sinclair, A.; Jensen, R. Acetazolamide lowers intracranial pressure and modulates the cerebrospinal fluid secretion pathway in healthy rats. Neurosci. Lett. 2017, 645, 33–39. [Google Scholar] [CrossRef]
- Plog, B.A.; Nedergaard, M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu. Rev. Pathol. 2018, 13, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Limanaqi, F.; Busceti, C.L.; Mastroiacovo, F.; Nicoletti, F.; Puglisi-Allegra, S.; Fornai, F. Glymphatic System as a Gateway to Connect Neurodegeneration From Periphery to CNS. Front. Neurosci. 2021, 15, 639140. [Google Scholar] [CrossRef] [PubMed]
- Astara, K.; Pournara, C.; de Natale, E.R.; Wilson, H.; Vavougios, G.D.; Lappas, A.S.; Politis, M.; Christodoulou, N.G. A novel conceptual framework for the functionality of the glymphatic system. J. Neurophysiol. 2023, 129, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Hady, K.K.; Okorie, C.U.A. Positive Airway Pressure Therapy for Pediatric Obstructive Sleep Apnea. Children 2021, 8, 979. [Google Scholar] [CrossRef]
- Hwang, D. Monitoring Progress and Adherence with Positive Airway Pressure Therapy for Obstructive Sleep Apnea: The Roles of Telemedicine and Mobile Health Applications. Sleep Med. Clin. 2016, 11, 161–171. [Google Scholar] [CrossRef]
- Hussain, R.; Tithof, J.; Wang, W.; Cheetham-West, A.; Song, W.; Peng, W.; Sigurdsson, B.; Kim, D.; Sun, Q.; Peng, S.; et al. Potentiating glymphatic drainage minimizes post-traumatic cerebral oedema. Nature 2023, 623, 992–1000. [Google Scholar] [CrossRef]
- Van den Bulcke, L.; Davidoff, H.; Heremans, E.; Potts, Y.; Vansteelandt, K.; De Vos, M.; Christiaens, D.; Emsell, L.; Jacobson, L.H.; Hoyer, D.; et al. Acoustic Stimulation to Improve Slow-Wave Sleep in Alzheimer’s Disease: A Multiple Night At-Home Intervention. Am. J. Geriatr. Psychiatry 2025, 33, 73–84. [Google Scholar] [CrossRef]
- Hein, Z.M.; Al-Zaghal, Z.A.S.; Muhammad Ghazali, M.; Jaffer, U.; Abdul Hamid, H.; Mehat, M.Z.; Che Ramli, M.D.; Che Mohd Nassir, C.M.N. Mechanistic insights into the sleep-glymphopathy-cerebral small vessel disease loop: Implications for epilepsy pathophysiology and therapy. Front. Neurosci. 2025, 19, 1546482. [Google Scholar] [CrossRef]
- Scott-Massey, A.; Boag, M.K.; Magnier, A.; Bispo, D.; Khoo, T.K.; Pountney, D.L. Glymphatic System Dysfunction and Sleep Disturbance May Contribute to the Pathogenesis and Progression of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 12928. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Sun, Y.R.; Pei, Y.H.; Ma, H.W.; Mu, Y.K.; Qin, L.H.; Yan, J.H. The lymphatic drainage systems in the brain: A novel target for ischemic stroke? Neural Regen. Res. 2023, 18, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Chang, Y.W.; Hong, C.C.; Yu, Y.H.; Su, J.L. The role of the VEGF-C/VEGFRs axis in tumor progression and therapy. Int. J. Mol. Sci. 2012, 14, 88–107. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Che, Y.; Murohara, T. Therapeutic Lymphangiogenesis Is a Promising Strategy for Secondary Lymphedema. Int. J. Mol. Sci. 2023, 24, 7774. [Google Scholar] [CrossRef]
- Dumitru, C.S.; Raica, M. Vascular Endothelial Growth Factor Family and Head and Neck Squamous Cell Carcinoma. Anticancer Res. 2023, 43, 4315–4326. [Google Scholar] [CrossRef]
- Juneja, P.; Ruhina Rahman, S.N.; Jakhar, D.; Mourya, A.K.; Tripathi, D.M.; Kaur, I.; Tiwari, V.; Rohilla, S.; Gupta, A.; Rawal, P.; et al. Recombinant VEGF-C (Cys156Ser) improves mesenteric lymphatic drainage and gut immune surveillance in experimental cirrhosis. JHEP Rep. 2023, 5, 100816. [Google Scholar] [CrossRef]
- Keuters, M.H.; Antila, S.; Immonen, R.; Plotnikova, L.; Wojciechowski, S.; Lehtonen, S.; Alitalo, K.; Koistinaho, J.; Dhungana, H. The Impact of VEGF-C-Induced Dural Lymphatic Vessel Growth on Ischemic Stroke Pathology. Transl. Stroke Res. 2025, 16, 781–799. [Google Scholar] [CrossRef]
- Lin, F.L.; Wang, P.Y.; Chuang, Y.F.; Wang, J.H.; Wong, V.H.Y.; Bui, B.V.; Liu, G.S. Gene Therapy Intervention in Neovascular Eye Disease: A Recent Update. Mol. Ther. 2020, 28, 2120–2138. [Google Scholar] [CrossRef]
- Guc, E.; Briquez, P.S.; Foretay, D.; Fankhauser, M.A.; Hubbell, J.A.; Kilarski, W.W.; Swartz, M.A. Local induction of lymphangiogenesis with engineered fibrin-binding VEGF-C promotes wound healing by increasing immune cell trafficking and matrix remodeling. Biomaterials 2017, 131, 160–175. [Google Scholar] [CrossRef]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef]
- Albuquerque, R.J.; Hayashi, T.; Cho, W.G.; Kleinman, M.E.; Dridi, S.; Takeda, A.; Baffi, J.Z.; Yamada, K.; Kaneko, H.; Green, M.G.; et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009, 15, 1023–1030. [Google Scholar] [CrossRef]
- Breslin, J.W.; Gaudreault, N.; Watson, K.D.; Reynoso, R.; Yuan, S.Y.; Wu, M.H. Vascular endothelial growth factor-C stimulates the lymphatic pump by a VEGF receptor-3-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H709–H718. [Google Scholar] [CrossRef]
- Ahn, S.H.; Savarraj, J.P.J.; Parsha, K.; Hergenroeder, G.W.; Chang, T.R.; Kim, D.H.; Kitagawa, R.S.; Blackburn, S.L.; Choi, H.A. Inflammation in delayed ischemia and functional outcomes after subarachnoid hemorrhage. J. Neuron. 2019, 16, 213. [Google Scholar] [CrossRef]
- Liu, J.Q.; Zhao, X.T.; Qin, F.Y.; Zhou, J.W.; Ding, F.; Zhou, G.; Zhang, X.S.; Zhang, Z.H.; Li, Z.B. Isoliquiritigenin mitigates oxidative damage after subarachnoid hemorrhage in vivo and in vitro by regulating Nrf2-dependent Signaling Pathway via Targeting of SIRT1. Phytomedicine 2022, 105, 154262. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Zheng, Y.; Yu, Q.; Zeng, M.; Bai, L.; Yang, L.; Guo, M.; Jiang, X.; Gan, J. Inhibitors of the NLRP3 inflammasome pathway as promising therapeutic candidates for inflammatory diseases (Review). Int. J. Mol. Med. 2023, 51, 35. [Google Scholar] [CrossRef]
- Lee, D.S.; Suh, M.; Sarker, A.; Choi, Y. Brain Glymphatic/Lymphatic Imaging by MRI and PET. Nucl. Med. Mol. Imaging 2020, 54, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Harrison, I.F.; Ismail, O.; Machhada, A.; Colgan, N.; Ohene, Y.; Nahavandi, P.; Ahmed, Z.; Fisher, A.; Meftah, S.; Murray, T.K.; et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain 2020, 143, 2576–2593. [Google Scholar] [CrossRef] [PubMed]
- Sanicola, H.W.; Stewart, C.E.; Luther, P.; Yabut, K.; Guthikonda, B.; Jordan, J.D.; Alexander, J.S. Pathophysiology, Management, and Therapeutics in Subarachnoid Hemorrhage and Delayed Cerebral Ischemia: An Overview. Pathophysiology 2023, 30, 420–442. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Jitsuishi, T.; Hozumi, T.; Iwanami, J.; Kitajo, K.; Yamaguchi, H.; Mori, Y.; Mogi, M.; Sawai, S. Temporal expression profiling of DAMPs-related genes revealed the biphasic post-ischemic inflammation in the experimental stroke model. Mol. Brain 2020, 13, 57. [Google Scholar] [CrossRef]
- Inamasu, J.; Nakatsukasa, M.; Mayanagi, K.; Miyatake, S.; Sugimoto, K.; Hayashi, T.; Kato, Y.; Hirose, Y. Subarachnoid hemorrhage complicated with neurogenic pulmonary edema and takotsubo-like cardiomyopathy. Neurol. Med.-Chir. 2012, 52, 49–55. [Google Scholar] [CrossRef]
- Molnar, C.; Gal, J.; Szanto, D.; Fulop, L.; Szegedi, A.; Siro, P.; Nagy, E.V.; Lengyel, S.; Kappelmayer, J.; Fulesdi, B. Takotsubo cardiomyopathy in patients suffering from acute non-traumatic subarachnoid hemorrhage-A single center follow-up study. PLoS ONE 2022, 17, e0268525. [Google Scholar] [CrossRef]
- Ziaka, M.; Exadaktylos, A. Pathophysiology of acute lung injury in patients with acute brain injury: The triple-hit hypothesis. Crit. Care 2024, 28, 71. [Google Scholar] [CrossRef]
- Ziaka, M.; Exadaktylos, A. Brain-lung interactions and mechanical ventilation in patients with isolated brain injury. Crit. Care 2021, 25, 358. [Google Scholar] [CrossRef]
- Uchida, K. Waste Clearance in the Brain and Neuroinflammation: A Novel Perspective on Biomarker and Drug Target Discovery in Alzheimer’s Disease. Cells 2022, 11, 919. [Google Scholar] [CrossRef]
- Licastro, E.; Pignataro, G.; Iliff, J.J.; Xiang, Y.; Lo, E.H.; Hayakawa, K.; Esposito, E. Glymphatic and lymphatic communication with systemic responses during physiological and pathological conditions in the central nervous system. Commun. Biol. 2024, 7, 229. [Google Scholar] [CrossRef]
- Hou, C.; Liu, Q.; Zhang, H.; Wang, W.; Wang, B.; Cui, X.; Li, J.; Ren, W.; Yang, X. Nimodipine Attenuates Early Brain Injury by Protecting the Glymphatic System After Subarachnoid Hemorrhage in Mice. Neurochem. Res. 2022, 47, 701–712. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.C.; Oh, J.S. Glymphatic Dysfunction in Neuro-Pulmonary Complications Following Subarachnoid Hemorrhage: A New Perspective on Brain–Lung Axis Disruption. Cells 2025, 14, 1739. https://doi.org/10.3390/cells14211739
Lee EC, Oh JS. Glymphatic Dysfunction in Neuro-Pulmonary Complications Following Subarachnoid Hemorrhage: A New Perspective on Brain–Lung Axis Disruption. Cells. 2025; 14(21):1739. https://doi.org/10.3390/cells14211739
Chicago/Turabian StyleLee, Eun Chae, and Jae Sang Oh. 2025. "Glymphatic Dysfunction in Neuro-Pulmonary Complications Following Subarachnoid Hemorrhage: A New Perspective on Brain–Lung Axis Disruption" Cells 14, no. 21: 1739. https://doi.org/10.3390/cells14211739
APA StyleLee, E. C., & Oh, J. S. (2025). Glymphatic Dysfunction in Neuro-Pulmonary Complications Following Subarachnoid Hemorrhage: A New Perspective on Brain–Lung Axis Disruption. Cells, 14(21), 1739. https://doi.org/10.3390/cells14211739



