Biological Mechanisms Involved in Muscle Dysfunction in COPD: An Integrative Damage–Regeneration–Remodeling Framework
Abstract
1. Introduction
2. Damage (Injury and Molecular Derailment)
2.1. Mechanical Load-Related Injury
2.2. Oxidative and Nitrosative Injury
2.3. Proteostasis Failure-I: Mismatching Between Protein Synthesis and Proteolysis—Ubiquitin–Proteasome System-, Autophagy-, and Ca2+-Dependent Pathways
2.4. Mitochondrial and Quality Control
2.5. Proteostasis Failure-II: Endoplasmic Reticulum Stress and Unfolded Protein Response
2.6. Microvascular and Neuromuscular Junction Contributors
2.7. Lipotoxicity and Myosteatosis
3. Regeneration and Their Bottlenecks
3.1. Satellite Cells and Myogenesis
3.2. Inflammatory Orchestration of Muscle Regeneration
3.3. Growth Signaling and Anabolic Resistance
4. Remodeling (Adaptive vs. Maladaptive Trajectories)
4.1. Respiratory Muscles: Predominant Adaptive Oxidative Remodeling Under Chronic Load
4.2. Limb Muscles: Glycolytic Shift and “Quality Loss”
4.3. Extracellular Matrix and Fibrosis. Neuromuscular Junction Remodeling
4.4. Time Course and Partial Reversibility
5. Translational Implications Mapped to Damage–Regeneration–Remodeling
5.1. Reduce Damage in Respiratory Muscles
5.2. Reduce Damage in Limb Muscles
5.3. Enable Regeneration
5.4. Steer Remodeling
6. Summarizing: What Differs Between Respiratory and Limb Muscles, and Why Does It Matter? (Figure 1)
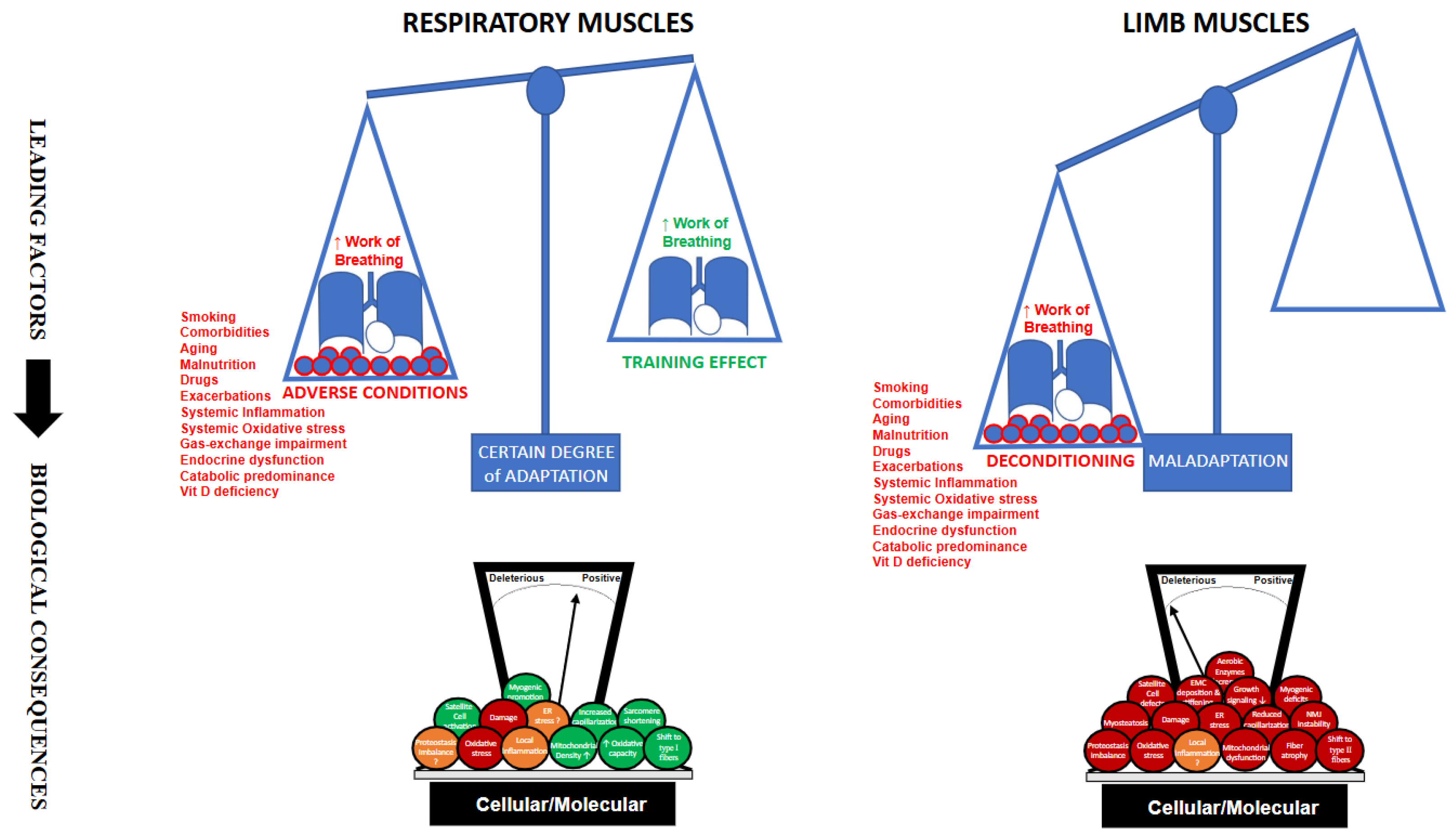
7. Conclusions
Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Global Initiative for Chronic Obstructive Lung Disease; Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease: 2025 Report. Available online: https://www.goldcopd.org/2025-gold-report/ (accessed on 27 October 2025).
- Agusti, A.; Bel, E.; Thomas, M.; Vogelmeier, C.; Brusselle, G.; Holgate, S.; Humbert, M.; Jones, P.; Gibson, P.G.; Vestbo, J.; et al. Treatable traits: Toward precision medicine of chronic airway diseases. Eur. Respir. J. 2016, 47, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Mariniello, D.F.; d’Agnano, V.; Cennamo, D.; Conte, S.; Quarcio, G.; Notizia, L.; Pagliaro, R.; Schiattarella, A.; Salvi, R.; Bianco, A.; et al. Comorbidities in COPD: Current and future treatment challenges. J. Clin. Med. 2024, 13, 743. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; Gea, J. Molecular and biological pathways of skeletal muscle dysfunction in chronic obstructive pulmonary disease. Chronic Respir. Dis. 2016, 13, 297–311. [Google Scholar] [CrossRef]
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigaré, R.; Dekhuijzen, P.N.R.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. An official American Thoracic Society/European Respiratory Society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 189, e15–e62. [Google Scholar] [CrossRef]
- Laveneziana, P.; Albuquerque, A.; Aliverti, A.; Babb, T.; Barreiro, E.; Dres, M.; Bruno-Pierre, D.; Fauroux, B.; Gea, J.; Guenette, J.A.; et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur. Respir. J. 2019, 53, 1801214. [Google Scholar] [CrossRef]
- O’Donnell, D.E.; Laveneziana, P.; Webb, K.A.; Neder, J.A. Chronic obstructive pulmonary disease: Clinical integrative physiology. Clin. Chest Med. 2014, 35, 51–69. [Google Scholar] [CrossRef]
- Loring, S.H.; Mead, J. Action of the diaphragm on the rib cage inferred from a force-balance analysis. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982, 53, 756–760. [Google Scholar] [CrossRef]
- Similowski, T.; Yan, S.; Gauthier, A.P.; Macklem, P.T.; Bellemare, F. Contractile properties of the human diaphragm during chronic hyperinflation. N. Engl. J. Med. 1991, 325, 917–923. [Google Scholar] [CrossRef]
- Marini, J.J. Dynamic hyperinflation and auto-positive end-expiratory pressure: Lessons learned over 30 years. Am. J. Respir. Crit. Care Med. 2011, 184, 756–762. [Google Scholar] [CrossRef]
- De Troyer, A.; Kirkwood, P.A.; Wilson, T.A. Respiratory action of the intercostal muscles. Physiol. Rev. 2005, 85, 717–756. [Google Scholar] [CrossRef]
- Ehsan, M.; Khan, R.U.; Wakefield, D.; Qureshi, R.; Murray, L.; Zuwallack, R.; Leidy, N.K. A longitudinal study evaluating the effects of exacerbations on physical activity in patients with chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2013, 10, 559–564. [Google Scholar] [CrossRef]
- Pitta, F.; Troosters, T.; Spruit, M.A.; Probst, V.S.; Decramer, M.; Gosselink, R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 171, 972–977. [Google Scholar] [CrossRef]
- Henrot, P.; Dupin, I.; Schilfarth, P.; Esteves, P.; Blervaque, L.; Zysman, M.; Gouzi, F.; Hayot, M.; Pomiés, P.; Berger, P. Main pathogenic mechanisms and recent advances in COPD peripheral skeletal muscle wasting. Int. J. Mol. Sci. 2023, 24, 6454. [Google Scholar] [CrossRef] [PubMed]
- Jaitovich, A.; Barreiro, E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. What we know and can do for our patients. Am. J. Respir. Crit. Care Med. 2018, 198, 175–186. [Google Scholar] [CrossRef]
- Gea, J.; Agustí, A.; Roca, J. Pathophysiology of muscle dysfunction in COPD. J. Appl. Physiol. 2013, 114, 1222–1234. [Google Scholar] [CrossRef]
- Drummond, S.E.; Burns, D.P.; El Maghrani, S.; Ziegler, O.; Healy, V.; O’Halloran, K.D. Chronic intermittent hypoxia-induced diaphragm muscle weakness is NADPH oxidase-2 dependent. Cells 2023, 12, 1834. [Google Scholar] [CrossRef]
- Henríquez-Olguín, C.; Boronat, S.; Cabello-Verrugio, C.; Jaimovich, E.; Hidalgo, E.; Jensen, T.J. The emerging roles of nicotinamide adenine dinucleotide phosphate oxidase 2 in skeletal muscle redox signaling and metabolism. Antioxid. Redox Signal. 2019, 31, 1371–1410. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kurebayashi, N.; Murayama, T. The ryanodine receptor as a sensor for intracellular environments in muscles. Int. J. Mol. Sci. 2021, 22, 10795. [Google Scholar] [CrossRef]
- Xu, H.; Van Remmen, H. The sarcoendoplasmic reticulum calcium ATPase (SERCA) pump: A potential target for intervention in aging and skeletal muscle pathologies. Skelet. Muscle 2021, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; de la Puente, B.; Minguella, J.; Corominas, J.M.; Serrano, S.; Hussain, S.N.A.; Gea, J. Oxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 171, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Pascual, S.; Casadevall, C.; Orozco-Levi, M.; Barreiro, E. Muscle dysfunction in chronic obstructive pulmonary disease. J. Thorac. Dis. 2015, 7, E418–E438. [Google Scholar] [CrossRef]
- Barreiro, E.; Gea, J.; Corominas, J.M.; Hussain, S.N.A. Nitric oxide synthases and protein oxidation in the quadriceps femoris of patients with chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2003, 29, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Mofarrahi, M.; Hussain, S.N.A. Skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease. Int. J. Chronic Obs. Pulm. Dis. 2008, 3, 637–658. [Google Scholar] [CrossRef]
- Doucet, M.; Russell, A.P.; Léger, B.; Debigaré, R.; Joanisse, D.R.; Caron, M.A.; LeBlanc, P.; Maltais, F. Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007, 176, 261–269. [Google Scholar] [CrossRef]
- Plant, P.J.; Brooks, D.; Faughnan, M.; Bayley, T.; Bain, J.; Singer, L.; Correa, J.; Pearce, D.; Binnie, M.; Batt, J. Cellular markers of muscle atrophy in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2010, 42, 461–471. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th ed.). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Goll, D.E.; Neti, G.; Mares, S.W.; Thompson, V.F. Myofibrillar protein turnover: The proteasome and the calpains. J. Anim. Sci. 2008, 86 (Suppl. S14), E19–E35. [Google Scholar] [CrossRef]
- Du, J.; Wang, X.; Miereles, C.; Bailey, J.L.; Debigare, R.; Zheng, B.; Price, S.R.; Mitch, W.E. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J. Clin. Investig. 2004, 113, 115–123. [Google Scholar] [CrossRef]
- Smuder, A.J.; Kavazis, A.N.; Hudson, M.B.; Nelson, W.B.; Powers, S.K. Oxidation enhances myofibrillar protein degradation via calpain and caspase-3. Free Radic. Biol. Med. 2010, 49, 1152–1160. [Google Scholar] [CrossRef]
- Pomiès, P.; Blaquière, M.; Maury, J.; Mercier, J.; Gouzi, F.; Hayot, M. Involvement of the FoxO1/MuRF1/Atrogin-1 signaling pathway in the oxidative stress-induced atrophy of cultured chronic obstructive pulmonary disease myotubes. PLoS ONE 2016, 11, e0160092. [Google Scholar] [CrossRef] [PubMed]
- Natanek, S.A.; Riddoch-Contreras, J.; Marsh, G.S.; Hopkinson, N.S.; Moxham, J.; Man, W.D.; Kemp, P.R.; Polkey, M.I. MuRF-1 and atrogin-1 protein expression and quadriceps fiber size and muscle mass in stable patients with COPD. COPD 2013, 10, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E. Muscle Atrophy in Chronic Obstructive Pulmonary Disease: Molecular basis and potential therapeutic targets. J. Thorac. Dis. 2018, 10 (Suppl. S12), S1415–S1424. [Google Scholar] [CrossRef] [PubMed]
- Fermoselle, C.; Rabinovich, R.; Ausin, P.; Puig-Vilanova, E.; Coronell, C.; Sanchez, F.; Roca, J.; Gea, J.; Barreiro, E. Does oxidative stress modulate limb muscle atrophy in severe COPD? Eur. Respir. J. 2012, 40, 851–862. [Google Scholar] [CrossRef]
- Martínez-Llorens, J.M.; Orozco-Levi, M.; Masdeu, M.J.; Coronell, C.; Ramírez-Sarmiento, A.; Sanjuas, C.; Broquetas, J.M.; Gea, J. Global muscle dysfunction and exacerbation of COPD: A cohort study. Med. Clin. 2004, 122, 521–527. [Google Scholar] [CrossRef]
- Maltais, F.; LeBlanc, P.; Whittom, F.; Simard, C.; Marquis, K.; Bélanger, M.; Breton, M.J.; Jobin, J. Oxidative enzyme activities of the vastus lateralis muscle and the functional status in patients with COPD. Thorax 2000, 55, 848–853. [Google Scholar] [CrossRef]
- Gosker, H.R.; Hesselink, M.K.; Duimel, H.; Ward, K.A.; Schols, A.M. Reduced mitochondrial density in the vastus lateralis muscle of patients with COPD. Eur. Respir. J. 2007, 30, 73–79. [Google Scholar] [CrossRef]
- Puente-Maestu, L.; Lázaro, A.; Humanes, B. Metabolic derangements in COPD muscle dysfunction. J. Appl. Physiol. 2013, 114, 1282–1290. [Google Scholar] [CrossRef]
- Leermakers, P.A.; Schols, A.M.W.J.; Kneppers, A.E.M.; Kelders, M.C.J.M.; de Theije, C.C.; Lainscak, M.; Gosker, H.R. Molecular signalling towards mitochondrial breakdown is enhanced in skeletal muscle of patients with chronic obstructive pulmonary disease (COPD). Sci. Rep. 2018, 8, 15007. [Google Scholar] [CrossRef]
- Guo, Y.; Gosker, H.R.; Schols, A.M.W.J.; Kapchinsky, S.; Bourbeau, J.; Sandri, M.; Jagoe, R.T.; Debigaré, R.; Maltais, F.; Taivassalo, T.; et al. Autophagy in locomotor muscles of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1313–1320. [Google Scholar] [CrossRef]
- Gosker, H.R.; van Mameren, H.; van Dijk, P.J.; Engelen, M.P.; van der Vusse, G.J.; Wouters, E.F.; Schols, A.M. Skeletal muscle fibre-type shifting and metabolic profile in patients with chronic obstructive pulmonary disease. Eur. Respir. J. 2002, 19, 617–625. [Google Scholar] [CrossRef]
- Remels, A.H.; Schrauwen, P.; Broekhuizen, R.; Willems, J.; Kersten, S.; Gosker, H.R.; Schols, A.M. Peroxisome proliferator-activated receptor expression is reduced in skeletal muscle in COPD. Eur. Respir. J. 2007, 30, 245–252. [Google Scholar] [CrossRef]
- Lei, Y.; Gan, M.; Qu, Y.; Chen, Q.; Wang, X.; Liao, T.; Zhao, M.; Chen, L.; Zhang, S.; Zhao, Y.; et al. The role of mitochondrial dynamics and mitophagy in skeletal muscle atrophy: From molecular mechanisms to therapeutic insights. Cell. Mol. Biol. Lett. 2024, 29, 59. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, M.; Shrikrishna, D.; Vitoriano, S.; Natanek, S.A.; Tanner, R.J.; Hart, N.; Kemp, P.R.; Moxham, J.; Polkey, M.I.; Hopkinson, N.S. Skeletal muscle adiposity is associated with physical activity, exercise capacity and fibre shift in COPD. Eur. Respir. J. 2014, 44, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.L.; Sioutas, A.; Kentson, M.; Jacobson, P.; Lundberg, P.; Leinhard, O.D.; Forsgren, M.F. Skeletal Myosteatosis is Associated with Systemic Inflammation and a Loss of Muscle Bioenergetics in Stable COPD. J. Inflamm. Res. 2022, 15, 4367–4384. [Google Scholar] [CrossRef]
- Correa de Araujo, R.; Addison, O.; Miljkovik, I.; Goodpaster, B.H.; Bergman, B.C.; Clark, R.V.; Elena, J.W.; Esser, K.A.; Ferrucci, L.; Harris-Love, M.O.; et al. Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging. Front. Physiol. 2020, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Pasto, M.; Carmona, M.A.; Orozco-Levi, M.; Palomeque, J.; Broquetas, J. Metabolic characteristics of the deltoid muscle in patients with chronic obstructive pulmonary disease. Eur. Respir. J. 2001, 17, 939–945. [Google Scholar] [CrossRef]
- Adami, A.; Corvino, R.B.; Calmelat, R.A.; Porszasz, J.; Casaburi, R.; Rossiter, H.B. Muscle oxidative capacity is reduced in both upper and lower limbs in COPD. Med. Sci. Sports Exerc. 2020, 52, 2061–2068. [Google Scholar] [CrossRef]
- Orozco-Levi, M.; Gea, J.; Lloreta, J.L.; Félez, M.; Minguella, J.; Serrano, S.; Broquetas, J.M. Subcellular adaptation of the human diaphragm in chronic obstructive pulmonary disease. Eur. Respir. J. 1999, 13, 371–378. [Google Scholar] [CrossRef]
- Ottenheijm, C.A.C.; Heunks, L.M.A.; Sieck, G.C.; Zhan, W.Z.; Jansen, S.M.; Degens, H.; de Boo, T.; Dekhuijzen, P.N.R. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 172, 200–205. [Google Scholar] [CrossRef]
- Marín-Corral, J.; Minguella, J.; Ramírez-Sarmiento, A.L.; Hussain, S.N.; Gea, J.; Barreiro, E. Oxidised proteins and superoxide anion production in the diaphragm of severe COPD patients. Eur. Respir. J. 2009, 33, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, P.; Li, J.; Liu, X.; Wu, W. Effect of oxidative stress on diaphragm dysfunction and exercise intervention in chronic obstructive pulmonary disease. Front. Physiol. 2021, 12, 684453. [Google Scholar] [CrossRef]
- Chan, S.M.H.; Bernardo, I.; Mastronardo, C.; Mou, K.; De Luca, S.N.; Seow, H.J.; Dobric, A.; Brassington, K.; Selemidis, S.; Bozinovski, S.; et al. Apocynin prevents cigarette smoking-induced loss of skeletal muscle mass and function in mice by preserving proteostatic signalling. Br. J. Pharmacol. 2021, 178, 3049–3066. [Google Scholar] [CrossRef]
- Konokhova, Y.; Spendiff, S.; Jagoe, R.T.; Aare, S.; Kapchinsky, S.; MacMillan, N.J.; Rozakis, P.; Picard, M.; Aubertin-Leheudre, M.; Pion, C.H.; et al. Failed upregulation of TFAM protein and mitochondrial DNA in oxidatively defficient fibers of chronic obstructive pulmonary disease locomotor muscle. Skelet. Muscle 2016, 6, 10. [Google Scholar] [CrossRef]
- Barreiro, E.; Salazar-Degracia, A.; Sancho-Muñoz, A.; Aguiló, R.; Rodriguez-Fuster, A.; Gea, J. Endoplasmic reticulum stress and unfolded protein response in diaphragm muscle dyfunction of patients with stable chronic obstructive pulmonary disease. J. Appl. Physiol. 2019, 126, 1572–1586. [Google Scholar] [CrossRef] [PubMed]
- Jobin, J.; Maltais, F.; Doyon, J.F.; LeBlanc, P.; Simard, P.M.; Simard, A.A.; Simard, S. Chronic obstructive pulmonary disease: Capillarity and fiber-type characteristics of skeletal muscle. J. Cardiopulm. Rehabil. Prev. 1998, 18, 432–437. [Google Scholar] [CrossRef]
- Whittom, F.; Jobin, J.; Simard, P.M.; Leblanc, P.; Simard, C.; Bernard, S.; Belleau, R.; Maltais, F. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med. Sci. Sports Exerc. 1998, 30, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Vaes, A.W.; De Boever, P.; Franssen, F.M.E.; Uszko-Lencer, N.H.M.K.; Vanfleteren, L.E.G.W.; Spruit, M.A. Endothelial function in patients with COPD: Un updated systematic review of studies using flow-mediated dilatation. Expert Rev. Respir. Med. 2023, 17, 53–69. [Google Scholar] [CrossRef]
- Ives, S.J.; Layec, G.; Hart, C.R.; Trinity, J.D.; Gifford, J.R.; Garten, R.S.; Witman, M.A.H.; Sorensen, J.R.; Richardson, R.S. Pasive length movement in chronic obstructive pulmonary disease: Evidence of locomotor muscle vascular dysfunction. J. Appl. Physiol. 2020, 128, 1402–1411. [Google Scholar] [CrossRef]
- Gouzi, F.; Préfaut, C.; Abdellaoui, A.; Roudier, E.; de Rigal, P.; Molinari, N.; Laoudj-Chenivesse, D.; Mercier, J.; Birot, O.; Hayot, M. Blunted muscle angiogenic training-response in COPD patients versus sedentary controls. Eur. Respir. J. 2013, 41, 806–814. [Google Scholar] [CrossRef]
- Kapchinsky, S.; Vuda, M.; Miguez, K.; Elkrief, D.; de Souza, A.R.; Baglole, C.J.; Aare, S.; MacMillan, N.J.; Baril, J.; Rozakis, P.; et al. Smoke-induced neuromuscular junction degeneration precedes the fibre type shift and atrophy in chronic obstructive pulmonary disease. J. Physiol. 2018, 596, 2865–2881. [Google Scholar] [CrossRef]
- Taivassalo, T.; Hepple, R.T. Integrating mechanisms of exacerbated atrophy and other adverse skeletal muscle impact in, COPD. Front. Physiol. 2022, 13, 861617. [Google Scholar] [CrossRef]
- Karim, A.; Muhammad, T.; Qaisar, R. Prediction of sarcopenia using multiple biomarkers of neuromuscular junction degeneration in chronic obstructive pulmonary disease. J. Pers. Med. 2021, 11, 919. [Google Scholar] [CrossRef]
- Curtis, K.J.; Meyrick, V.M.; Mehta, B.; Haji, G.S.; Li, K.; Montgomery, H.; Mann, W.D.C.; Polkey, M.I.; Hopkinson, N.S. Angiotensin-Converting Enzyme Inhibition as an Adjunct to Pulmonary Rehabilitation in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2016, 194, 1349–1357. [Google Scholar] [CrossRef]
- Orozco-Levi, M.; Lloreta, J.; Gea, J. The “oil well analogy” as a comprehensive interpretation of factors leading to muscle injury and wasting. Ultrastruct. Pathol. 2006, 30, 247–252. [Google Scholar] [CrossRef]
- Orozco-Levi, M.; Gea, J.; Aguar, M.C.; Broquetas, J.M. Changes in the capillary content in the diaphragm of COPD patients: A sort of muscle remodelling? Am. J. Respir. Crit. Care Med. 1996, 153, A298 (abstract). [Google Scholar]
- Doucet, M.; Debigaré, R.; Joanisse, D.R.; Côté, C.; Leblanc, P.; Grégoire, J.; Deslauriers, J.; Vaillancourt, R.; Maltais, F. Adaptation of the diaphragm and the vastus lateralis in mild-to-moderate COPD. Eur. Respir. J. 2004, 24, 971–979. [Google Scholar] [CrossRef]
- Cao, Y.; Li, P.; Wang, Y.; Liu, X.; Wu, W. Diaphragm dysfunction and rehabilitation strategy in patients with chronic obstructive pulmonary disease. Front. Physiol. 2022, 13, 872277. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Fuentes, M.A.; Gea, J.; Aguar, M.C.; Minguella, J.; Lloreta, J.; Félez, M.; Broquetas, J. Capillary density and respiratory function in the external intercostal muscle. Arch. Bronconeumol. 1999, 35, 471–476. [Google Scholar] [CrossRef]
- Schrauwen, P.; Schrauwen-Hinderling, V.; Hoeks, J.; Hesselink, M.K. Mitochondrial dysfunction and lipotoxicity. Biochim. Biophys. Acta. 2010, 1801, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Kelley, D.E.; Thaete, F.L.; He, J.; Ross, R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J. Appl. Physiol. 2000, 89, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, J.; Esfandari, N.; Baracos, V.E.; Buteau, F.A.; Frenette, J.; Putman, C.T.; Mazurak, V.C. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014, 210, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Diallo, T.D.; Karrasch, S.; Jung, M.; Peters, A.; Lorbeer, R.; Schlett, C.L.; von Kruchtem, R.; Bamberg, F.; Rospleszcz, S.; Kiefer, L.S. Paraspinal myosteatosis is associated with COPD: A cross-sectional MRI analysis from the population-based KORA cohort. Respir. Res. 2025, 26, 217. [Google Scholar] [CrossRef]
- Robles, P.G.; Sussman, M.S.; Naraghi, A.; Brooks, D.; Goldstein, R.S.; White, L.M.; Mathur, S. Intramuscular fat infiltration contributes to impaired muscle function in COPD. Med. Sci. Sports Exerc. 2015, 47, 1334–1341. [Google Scholar] [CrossRef]
- Shields, G.S.; Coissi, G.S.; Jimenez-Royo, P.; Gambarota, G.; Dimber, R.; Hopkinson, N.S.; Matthews, P.M.; Brown, A.P.; Polkey, M.I. Bioenergetics and intermuscular fat in chronic obstructive pulmonary disease-associated quadriceps weakness. Muscle Nerve 2015, 51, 214–221. [Google Scholar] [CrossRef]
- Qiao, X.; Hou, G.; Kang, J.; Wang, Q.Y.; Yin, Y. CT attenuation and cross-sectional area of the pectoralis are associated with clinical characteristics in chronic obstructive pulmonary disease patients. Front. Physiol. 2022, 13, 833796. [Google Scholar] [CrossRef]
- McDonald, M.L.N.; Diaz, A.A.; Rutten, E.; Lutz, S.M.; Harmouche, R.; San Jose Estepar, R.; Kinney, G.; Hokanson, J.E.; Gower, B.A.; Wouters, E.F.M.; et al. Chest computed tomography-derived low fat-free mass index and mortality in COPD. Eur. Respir. J. 2017, 50, 1701134. [Google Scholar] [CrossRef]
- Ezponda, A.; Casanova, C.; Cabrera, C.; Martin-Palmero, Á.; Marin-Oto, M.; Marín, J.M.; Pinto-Plata, V.; Divo, M.; Celli, B.R.; Zulueta, J.J. Psoas muscle density evaluated by chest CT and long-term mortality in COPD patients. Arch. Bronconeumol. 2021, 57, 533–539. [Google Scholar] [CrossRef]
- Emanuelsson, E.B.; Berry, D.B.; Reitzner, S.M.; Arif, M.; Mardinoglu, A.; Gustafsson, T.; Ward, S.R.; Sundberg, C.J.; Chapman, M.A. MRI characterization of skeletal muscle size and fatty infiltration in long-term trained and untrained individuals. Physiol. Rep. 2022, 10, e15398. [Google Scholar] [CrossRef]
- Sancho-Muñoz, A.; Guitart, M.; Rodríguez, D.A.; Gea, J.; Martínez-Llorens, J.; Barreiro, E. Deficient muscle regeneration potential in sarcopenic COPD patients: Role of satellite cells. J. Cell. Physiol. 2021, 236, 3083–3098. [Google Scholar] [CrossRef] [PubMed]
- Thériault, M.E.; Paré, M.-È.; Maltais, F.; Debigaré, R. Satellite cells senescence in limb muscle of severe patients with COPD. PLoS ONE 2012, 7, e39124. [Google Scholar] [CrossRef]
- Balnis, J.; Drake, L.A.; Singer, D.V.; Vincent, C.E.; Korponay, T.C.; D’Armiento, J.; Lee, C.G.; Elias, J.A.; Singer, H.A.; Jaitovich, A. Deaccelerated myogenesis and autophagy in genetically induced pulmonary emphysema. Am. J. Respir. Cell Mol. Biol. 2022, 66, 623–637. [Google Scholar] [CrossRef]
- Casadevall, C.; Sancho-Muñoz, A.; Vicente, I.; Pascual-Guardia, S.; Admetlló, M.; Gea, J. Influence of COPD systemic environment on the myogenic function of muscle precursor cells in vitro. Respir. Res. 2022, 23, 282. [Google Scholar] [CrossRef]
- Jaitovich, A. Impaired regenerative capacity contributes to skeletal muscle dysfunction in chronic obstructive pulmonary disease. Am. J. Physiol. Cell Physiol. 2022, 323, C974–C989. [Google Scholar] [CrossRef] [PubMed]
- Langen, R.C.J.; Der Velden, J.L.J.; Schols, A.M.W.; Kelders, M.C.J.M.; Wouters, E.F.M.; Janssen-Heininger, Y.M.W. Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J. 2004, 18, 227–237. [Google Scholar] [CrossRef]
- Szalay, K.; Razga, Z.; Duda, E. TNF inhibits myogenesis and downregulates the expression of myogenic regulatory factors myoD and myogenin. Eur. J. Cell Biol. 1997, 74, 391–398. [Google Scholar] [PubMed]
- Jones, S.E.; Barker, R.E.; Nolan, C.M.; Patel, S.; Maddocks, M.; Man, W.D.C. Pulmonary rehabilitation in patients with an acute exacerbation of chronic obstructive pulmonary disease. J. Thorac. Dis. 2018, 10 (Suppl. S12), S1390–S1399. [Google Scholar] [CrossRef] [PubMed]
- Heubel, A.D.; Kabbach, E.Z.; Leonardi, N.T.; Schafauser, N.S.; Kawakami, D.M.O.; Sentanin, A.C.; Di Lorenzo, V.A.P.; Silva, A.B.; Hurst, J.R.; Mendes, R.G. Respiratory and peripheral muscle strength influence recovery of exercise capacity after severe exacerbation of COPD? An observational prospective cohort study. Heart Lung 2023, 58, 91–97. [Google Scholar] [CrossRef]
- Gosker, H.R.; Langen, R.C.; Simons, S.O. Role of acute exacerbations in skeletal muscle impairment in COPD. Expert Rev. Respir. Med. 2021, 15, 103–115. [Google Scholar] [CrossRef]
- Simões, D.C.M.; Vogiatzis, I. Can muscle protein metabolism be specifically targeted by exercise training in COPD? J. Thorac. Dis. 2018, 10 (Suppl. S12), S1367–S1376. [Google Scholar] [CrossRef]
- Langen, R.C.J.; Schols, A.M.W.J. Inflammation: Friend or foe of muscle remodelling in COPD? Eur. Respir. J. 2007, 30, 605–607. [Google Scholar] [CrossRef]
- Casadevall, C.; Coronell, C.; Ramírez-Sarmiento, A.L.; Martínez-Llorens, J.M.; Barreiro, E.; Orozco-Levi, M.; Gea, J. Upregulation of pro-inflammatory cytokines in the intercostal muscles of COPD patients. Eur. Respir. J. 2007, 30, 701–707. [Google Scholar] [CrossRef]
- Casadevall, C.; Coronell, C.; Ausín, P.; Martínez-Llorens, J.M.; Orozco-Levi, M.; Barreiro, E.; Gea, J. Inflammatory cytokines and repair factors in the intercostal muscles of patients with severe COPD. Arch. Bronconeumol. 2009, 45, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; Schols, A.M.W.J.; Polkey, M.I.; Galdiz, J.B.; Gosker, H.R.; Swallow, E.B.; Coronell, C.; Gea, J. Cytokine profile in quadriceps muscles of patients with severe COPD. Thorax 2008, 63, 100–107. [Google Scholar] [CrossRef]
- Vogiatzis, I.; Stratakos, G.; Simoes, D.C.; Terzis, G.; Georgiadou, O.; Roussos, C.; Zakynthinos, S. Effects of rehabilitative exercise on peripheral muscle TNFalpha, IL-6, IGF-I and MyoD expresdion in patients with COPD. Thorax 2007, 62, 950–956. [Google Scholar] [CrossRef]
- Vogiatzis, I.; Simoes, D.C.; Stratakos, G.; Kourepini, E.; Terzis, G.; Manta, P.; Athanasopoulos, D.; Roussos, C.; Wagner, P.D.; Zakynthinos, S. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur. Respir. J. 2010, 36, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.; Kaiser, L.; Leferovich, J.; Tikunov, B. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N. Engl. J. Med. 1997, 337, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.; Nguyen, T.; Kaiser, L.R.; Rubinstein, N.A.; Maislin, G.; Gregory, C.; Rome, L.C.; Dudley, G.A.; Sieck, G.C.; Shrager, J.B. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: Clinical implications. Am. J. Respir. Crit. Care Med. 2003, 168, 706–713. [Google Scholar] [CrossRef]
- Newell, S.Z.; McKenzie, D.K.; Gandevia, S.C. Inspiratory and skeletal muscle strength and endurance and diaphragm activation in patients with chronic airflow limitation. Thorax 1989, 44, 903–912. [Google Scholar] [CrossRef]
- Martínez-Llorens, J.; Casadevall, C.; Lloreta, J.; Orozco-Levi, M.; Barreiro, E.; Broquetas, J.; Gea, J. Activation of satellite cells in the intercostal muscles of patients with chronic obstructive pulmonary disease. Arch. Bronconeumol. 2008, 44, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.; Brunet, A.; Medrano, G.; Debesse, B.; Derenne, J.P. Metabolic enzymatic activities in the intercostal and serratus muscles and in the latissimus dorsi of middle-aged normal men and patients with moderate obstructive pulmonary disease. Eur. Respir. J. 1988, 1, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Pastó, M.; Gea, J.; Blanco, M.; Orozco-Levi, M.; Pallás, O.; Masdeu, M.; Broquetas, J.M. Metabolic activity of the external intercostal muscle of patients with COPD. Arch. Bronconeumol. 2001, 37, 108–114. [Google Scholar] [CrossRef]
- Ribera, F.; N’Guessan, B.; Zoll, J.; Fortin, D.; Serrurier, B.; Mettauer, B.; Bigard, X.; Ventura-Clapier, R.; Lampert, E. Mitochondrial electron transport chain function is enhanced in inspiratory muscles of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003, 167, 873–879. [Google Scholar] [CrossRef]
- Gea, J. Myosin gene expression in the respiratory muscles. Eur. Respir. J. 1997, 10, 2404–2410. [Google Scholar] [CrossRef]
- Sanchez, J.; Derenne, J.P.; Debesse, B.; Riquet, M.; Monod, H. Typology of the respiratory muscles in normal men and in patients with moderate chronic respiratory diseases. Bull. Eur. Physiopathol. Respir. 1982, 18, 901–914. [Google Scholar] [PubMed]
- Levine, S.; Nguyen, T.; Friscia, M.; Zhu, J.; Szeto, W.; Kucharczuk, J.C.; Tikunov, B.A.; Rubinstein, N.A.; Kaiser, L.R.; Shrager, J.B. Parasternal intercostal muscle remodeling in severe chronic obstructive pulmonary disease. J. Appl. Physiol. 2006, 101, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Gosker, H.R.; Zeegers, M.P.; Wouters, E.F.; Schols, A.M. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: A systematic review and meta-analysis. Thorax 2007, 62, 944–949. [Google Scholar] [CrossRef]
- Allaire, J.; Maltais, F.; Doyon, J.-F.; Noël, M.; LeBlanc, P.; Carrier, G.; Simard, C.; Jobin, J. Peripheral muscle endurance and the oxidative profile of the quadriceps in patients with COPD. Thorax 2004, 59, 673–678. [Google Scholar] [CrossRef]
- Chiappa, G.R.; Borghi-Silva, A.; Ferreira, L.F.; Carrascosa, C.; Oliveira, C.C.; Maia, J.; Gimenes, A.C.; Queiroga, F.; Berton, D.; Ferreira, E.M.V.; et al. Kinetics of muscle deoxygenation are accelerated at the onset of heavy-intensity exercise in patients with COPD: Relaitonship to central cardiovascular dynamics. J. Appl. Physiol. 2008, 104, 1341–1350. [Google Scholar] [CrossRef]
- Orozco-Levi, M.; Coronell, C.; Ramírez-Sarmiento, A.; Lloreta, J.; Martínez-Llorens, J.; Galdiz, J.B.; Gea, J. Injury of peripheral muscles in smokers with chronic obstructive pulmonary disease. Ultrastruct. Pathol. 2012, 36, 228–238. [Google Scholar] [CrossRef]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef]
- Lieber, R.L.; Ward, S.R. Cellular mechanisms of tissue fibrosis. Structural and functional consequences of skeletal muscle fibrosis. Am. J. Physiol. Cell Physiol. 2013, 305, C241–C252. [Google Scholar] [CrossRef]
- Willis-Owen, S.A.G.; Thompson, A.; Kemp, P.R.; Polkey, M.I.; Cookson, W.O.C.M.; Moffatt, M.F.; Natanek, S.A. COPD is accompanied by co-ordinated transcriptional perturbation in the quadriceps affecting the mitochondria and extracellular matrix. Sci. Rep. 2018, 8, 12165. [Google Scholar] [CrossRef]
- Kritikaki, E.; Terzis, G.; Soundararajan, M.; Vogiatzis, I.; Simoes, D.C.M. Expression of intramuscular extracellular matrix proteins in vastus lateralis muscle fibres between atrophic and non-atrophic COPD. ERJ Open Res. 2024, 10, 00857–2023. [Google Scholar] [CrossRef]
- Deane, C.S.; Willis, C.R.G.; Phillips, B.E.; Atherton, P.J.; Harries, L.W.; Ames, R.M.; Szewczyk, N.J.; Etheridge, T. Transcriptomic meta-analysis of disuse muscle atrophy vs. resistance exercise-induced hypertrophy in young and older humans. J. Cachexia Sarcopenia Muscle 2021, 12, 629–645. [Google Scholar] [CrossRef]
- Niu, Y.; Yue, Y.; Zheng, Y.; Long, C.; Li, Q.; Chen, Y.; Chen, Z.; Ma, X. SWEmean of quadriceps, a potential index of complication evaluation to patients with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 1921–1928. [Google Scholar] [CrossRef]
- Güerri, R.; Gayete, A.; Balcells, E.; Ramirez-Sarmiento, A.; Vollmer, I.; Garcia-Aymerich, J.; Gea, J.; Orozco-Levi, M. Mass of intercostal muscles associates with risk of multiple exacerbations in COPD. Respir. Med. 2010, 104, 378–388. [Google Scholar] [CrossRef]
- Vilaró, J.; Ramirez-Sarmiento, A.; Martínez-Llorens, J.M.; Mendoza, T.; Alvarez, M.; Sánchez-Cayado, N.; Vega, A.; Gimeno, E.; Coronell, C.; Gea, J.; et al. Global muscle dysfunction as a risk factor of readmission to hospital due to COPD exacerbations. Respir. Med. 2010, 104, 1896–1902. [Google Scholar] [CrossRef]
- van de Bool, C.; Rutten, E.P.A.; van Helvoort, A.; Franssen, F.M.E.; Wouters, E.F.M.; Schols, A.M.W.J. A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J. Cachexia Sarcopenia Muscle 2017, 8, 748–758. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.C.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Cazzola, M.; Page, C.; Rogliani, P.; Calzetta, L.; Matera, M.G. Dual bronchodilation for the treatment of COPD: From bench to bedside. Br. J. Clin. Pharmacol. 2022, 88, 3657–3673. [Google Scholar] [CrossRef]
- Fishman, A.; Martinez, F.; Naunheim, K.; Piantadosi, S.; Wise, R.; Ries, A.; Weinmann, G.; Wood, D.E. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N. Engl. J. Med. 2003, 348, 2059–2073. [Google Scholar] [CrossRef]
- Criner, G.J.; Sue, R.; Wright, S.; Dransfield, M.T.; Rivas-Perez, H.; Wiese, T.; Sciurba, F.C.; Shah, P.L.; Wahidi, M.M.; Goulart de Oliveira, H.; et al. A multicenter randomized controlled trial of Zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE). Am. J. Respir. Crit. Care Med. 2018, 198, 1151–1164. [Google Scholar] [CrossRef]
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxic chronic obstructive lung disease: A clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann. Intern. Med. 1980, 93, 391–398. [Google Scholar] [CrossRef]
- Murphy, P.B.; Rehal, S.; Arbane, G.; Bourke, S.; Calverley, P.M.A.; Crook, A.M.; Dowson, L.; Duffy, N.; Gibson, G.J.; Hughes, P.D.; et al. Effect of home noninvasive ventilation with oxygen vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: A randomized clinical trial. JAMA 2017, 317, 2177–2186. [Google Scholar] [CrossRef]
- Rochester, C.L.; Alison, J.A.; Carlin, B.; Jenkins, A.R.; Cox, N.S.; Bauldoff, G.; Bhatt, S.P.; Bourbeau, J.; Burtin, C.; Camp, P.G.; et al. Pulmonary rehabilitation for adults with chronic respiratory disease: An official American Thoracic Society clinical practice guideline. Am. J. Respir. Crit. Care Med. 2023, 208, e7–e26. [Google Scholar] [CrossRef]
- Nolan, C.M.; Rochester, C.L. Exercise training modalities for people with chronic obstructive pulmonary disease. COPD 2019, 16, 378–389. [Google Scholar] [CrossRef]
- Mador, M.J.; Bozkanat, E.; Aggarwal, A.; Shaffer, M.; Kufel, T.J. Endurance and strength training in Patients With COPD. Chest 2004, 125, 2036–2045. [Google Scholar] [CrossRef]
- Emtner, M.; Porszasz, J.; Burns, M.; Somfay, A.; Casaburi, R. Benefits of supplemental oxygen in exercise training in nonhypoxemic with chronic obstructive pulmonary disease patients. Am. J. Respir. Crit. Care Med. 2003, 168, 1034–1042. [Google Scholar] [CrossRef]
- Nonoyama, M.; Brooks, D.; Lacasse, Y.; Guyatt, G.H.; Goldstein, R.S. Oxygen therapy during exercise training in chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2007, 2007, CD005372. [Google Scholar] [CrossRef]
- Neunhäuserer, D.; Hudelmaier, M.; Niederseer, D.; Vecchiato, M.; Wirth, W.; Steide-Kloc, E.; Kaiser, B.; Lamprecht, B.; Studnicka, M.; Niebauer, J. The impact of exercise training and supplemental oxygen on peripheral muscles in Chronic Obstructive Pulmonary Disease. Med. Sci. Sports Exerc. 2023, 55, 2123–2131. [Google Scholar] [CrossRef]
- Labeix, P.; Court Fortune, I.; Muti, D.; Berger, M.; Chomette-Ballereau, S.; Barthelemy, J.C.; Féasson, L.; Costes, F. The effect of pressure ventilatory support on quadriceps endurance is maintained after exercise training in severe COPD patients. A longitudinal randomized, cross over study. Front. Physiol. 2022, 13, 1055023. [Google Scholar] [CrossRef]
- Lu, H.Y.; Chen, C.F.; Lee, D.L.; Tsai, Y.J.; Lin, P.C. Effects of early pulmonary rehabilitation on hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 881–893. [Google Scholar] [CrossRef]
- Du, Y.; Lin, J.; Wang, X.; Zhang, Y.; Ge, H.; Wang, Y.; Ma, Z.; Zhang, H.; Liu, J.; Wang, Z.; et al. Early pulmonary rehabilitation in acute exacerbation of chronic obstructive pulmonary disease: A meta-analysis of randomized controlled trials. COPD 2022, 19, 69–80. [Google Scholar] [CrossRef]
- Sillen, M.J.H.; Franssen, F.M.; Delbressine, J.M.; Vaes, A.W.; Wouters, E.F.; Spruit, M.A. Efficacy of lower-limb muscle training modalities in severely dyspnoeic individuals with COPD and quadriceps muscle weakness: Results from the DICES trial. Thorax 2014, 69, 525–531. [Google Scholar] [CrossRef]
- Wouters, E.F.; Posthuma, R.; Koopman, M.; Liu, W.Y.; Sillen, M.J.; Hajian, B.; Sastry, M.; Spruit, M.A.; Franssen, F.M. An update on pulmonary rehabilitation techniques for patients with chronic obstructive pulmonary disease. Expert Rev. Respir. Med. 2020, 14, 149–161. [Google Scholar] [CrossRef]
- Jin, S.; Huang, B.; Kong, Y.; Zhou, X.; Ma, J. Effect of neuromuscular electrical stimulation combined with respiratory rehabilitation training on pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. J. Cardiothorac. Surg. 2025, 20, 79. [Google Scholar] [CrossRef]
- Bustamante, V.; Casanova, J.; López de Santamaría, E.; Mas, S.; Sellarés, J.; Gea, J.; Gáldiz, J.B.; Barreiro, E.; ON BEHALF OF THE ENIGMA IN COPD PROJECT. Redox balance following magnetic stimulation training in the quadriceps of patients with severe COPD. Free Radic. Res. 2008, 42, 939–948. [Google Scholar] [CrossRef]
- Bustamante, V.; López de Santa María, E.; Gorostiza, A.; Jiménez, U.; Gáldiz, J.B. Muscle training with repetitive magnetic stimulation of the quadriceps in severe COPD patients. Respir. Med. 2010, 104, 237–245. [Google Scholar] [CrossRef]
- Casaburi, R.; Bhasin, S.; Cosentino, L.; Porszasz, J.; Somfay, A.; Lewis, M.I.; Fournier, M.; Storer, T.W. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2004, 170, 870–878. [Google Scholar] [CrossRef]
- Atlantis, E.; Fahey, P.; Cochrane, B.; Wittert, G.; Smith, S. Endogenous testosterone level and testosterone supplementation therapy in chronic obstructive pulmonary disease (COPD): A systematic review and meta-analysis. BMJ Open 2013, 3, e003127. [Google Scholar] [CrossRef]
- Hornikx, M.; Van Remoortel, H.; Lehouck, A.; Mathieu, C.; Maes, K.; Gayan-Ramirez, G.; Decramer, M.; Troosters, T.; Janssens, W. Vitamin D supplementation during rehabilitation in COPD: A seconday analysis of a randomized trial. Respir. Res. 2012, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Brauwers, B.; Machado, F.V.; Beijers, R.J.H.C.G.; Spruit, M.A. Combined exercise training and nutritional interventions or pharmacological treatments to improve exercise capacity and body composition in chronic obstructive pulmonary disease: A narrative review. Nutrients 2023, 15, 5136. [Google Scholar] [CrossRef]
- Jonker, R.; Deutz, N.E.P.; Schols, A.M.W.J.; Veley, E.A.; Harrykisson, R.; Zachria, A.J.; Engelen, M.P.K.J. Whole-body protein anabolism in COPD patients and healthy older adults is not enhanced by adding either carbohydrates or leucine to a serving of protein. Clin. Nutr. 2019, 38, 1684–1691. [Google Scholar] [CrossRef]
- de Bisschop, C.; Caron, F.; Ingrand, P.; Bretonneau, Q.; Dupuy, P.; Meurice, J.C. Does branched-chain amino acid supplementation improve pulmonary rehabilitation effect in COPD. Respir. Med. 2021, 189, 106642. [Google Scholar] [CrossRef]
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J.P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef]
- Surmachevska, N.; Tiwari, V. Corticosteroid Induced Myopathy. StatPearls [Internet]. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557731/ (accessed on 3 October 2025).
- Brønstad, E.; Rognmo, Ø.; Tjonna, A.E.; Dedichen, H.H.B.; Kirkeby-Garstad, I.; Haberg, A.K.; Ingul, C.B.; Wisloff, U.; Steinshamn, S. High-intensity knee-extensor training restores skeletal muscle function in COPD patients. Eur. Respir. J. 2012, 40, 1130–1136. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Wang, Y.; Xia, J.; Liu, X. Effects of exercise intervention on peripheral skeletal muscle in stable patients with COPD. Front. Med. 2021, 8, 766841. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.F.; Yang, I.A.; Chang, Y.C.; Vaughan, A. Nutritional support in chronic obstructive pulmonary disease (COPD): An evidence update. J. Thorac. Dis. 2019, 11 (Suppl. S17), S2230–S2237. [Google Scholar] [CrossRef] [PubMed]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.; Sobotka, L.; et al. ESPEN practical guideline: Clinical nutrition and hydration in geriatrics. Clin. Nutr. 2022, 41, 958–989. [Google Scholar] [CrossRef]
- Loeckx, M.; Rodrigues, F.M.; Blondeel, A.; Everaerts, S.; Janssens, W.; Demeyer, H.; Troosters, T.Y. Sustaining training effects through physical activity coaching (STEP): A randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 121. [Google Scholar] [CrossRef]
- Demeyer, H.; Louvaris, Z.; Frei, A.; Rabinovich, R.A.; de Jong, C.; Gimeno-Santos, E.; Loeckx, M.; Buttery, S.C.; Rubio, N.; Van der Molen, T.; et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: A multicentre randomised controlled trial. Thorax 2017, 72, 415–423. [Google Scholar] [CrossRef]
- Ramirez-Sarmiento, A.; Orozco-Levi, M.; Guell, R.; Barreiro, E.; Hernandez, N.; Mota, S.; Sangenis, M.; Broquetas, J.M.; Casan, P.; Gea, J. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: Structural adaptation and physiologic outcomes. Am. J. Respir. Crit. Care Med. 2002, 166, 1491–1497. [Google Scholar] [CrossRef]
- Han, B.; Chen, Z.; Ruan, B.; Chen, Y.; Lv, Y.; Li, C.; Yu, L. Effects of inspiratory muscle training in people with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Life 2024, 14, 1470. [Google Scholar] [CrossRef]
- Xie, J.; Zhu, Y.; Wang, Y.; Mo, Y.; Shi, X.; Liang, W.M.; Ren, F.F.; Bai, Z.; Nie, F. Effects of pulmonary rehabilitation combined with inspiratory muscle training on lung function and exercise capacity in older patients with COPD: A systematic review and meta-analysis. Front. Med. 2025, 12, 1621375. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Forget, P.; Couturaud, F.; Reychler, G. Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis. Clin. Respir. J. 2018, 12, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Casadevall, C.; Pascual, S.; Orozco-Levi, M.; Barreiro, E. Clinical management of chronic obstructive pulmonary disease patients with muscle dysfunction. J. Thorac. Dis. 2016, 8, 3379–3400. [Google Scholar] [CrossRef] [PubMed]
| Domain | Mechanism (Examples) | Key Markers/Assays | Typical Pattern | Clinical Readouts |
|---|---|---|---|---|
| Damage RM | Mechanical load/Geometry (hyperinflation, PEEPi) Oxidative/nitrosative stress | MIP/SNIP, Pdi ↓ Diaphragm thickness ↓ (US) Length–tension disadvantage Protein carbonyls; 4-HNE; nitrotyrosine | Sarcolemmal damage Sarcomere damage Oxidative signatures | Dyspnea ↑; Strength ↓; Endurance ↓ Exercise limitation (6MWD and CPET ↓) |
| Damage LM | Oxidative/nitrosative stress Proteolysis Proteostasis failure Mitochondrial/ER stress Microvascular involvement NMJ involvement Lipotoxicity/myosteatosis | Protein carbonyls; 4-HNE; nitrotyrosine Atrogin-1, MuRF-1, LC3-II; ubiquitinated proteins; p62; calpain/caspase activity CS/SDH/COX ↓; mtDNA damage BiP/CHOP (UPR) Capillary-to-fiber ratio ↓ Changes in NMJ morphology Neurogenic grouping EMG abnormalities CT attenuation; MRI PDFF; Lipid intermediates | Oxidative signatures Predominant proteolysis Oxidative capacity ↓ Oxidative capacity ↓ NMJ instability Fat infiltration | Weakness Fatigability Muscle mass ↓ Poor muscle quality Strength ↓; Endurance ↓ Exercise limitation (6MWD and CPET ↓) Early acidosis Impaired O2 kinetics |
| Repair RM and LM | Satellite cells/myogenesis Inflammatory resolution Anabolic signaling | Pax7+ cells MyoD/myogenin Fusion indices M1→M2 polarization Cytokine panels IGF-1/AKT/mTOR Myostatin/activin | Both impaired in sarcopenia (diaphragm less studied) Impairs resolution in both Anabolic resistance in both Sex/Age effects | Injury-blunted Recovery after training Fibrosis-prone repair Persistent weakness Rehabilitation gains limited |
| Remodeling RM | Diaphragm conditioning | Shift to type I ↑ Mitochondrial content ↑ Capillarity ↑ | Aerobic metabolism ↑ | Fatigue resistance Strength not restored |
| Remodeling LM | Limb glycolytic shift Loss of muscle quality loss ECM/muscle fibrosis | Fiber atrophy Shift to type II Mitochondrial dysfunction Capillarity ↓ Intramuscular fat (CT/MRI) Collagen content Stiffness indices | Aerobic metabolism ↓ Myosteatosis ECM debris deposition | Weakness Strength ↓ Endurance ↓ Exercise limitation Reduced muscle quality Force transmission ↓ Post-exercise recovery ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gea, J.; Orozco-Levi, M.; Pascual-Guàrdia, S.; Casadevall, C.; Enríquez-Rodríguez, C.J.; Camps-Ubach, R.; Barreiro, E. Biological Mechanisms Involved in Muscle Dysfunction in COPD: An Integrative Damage–Regeneration–Remodeling Framework. Cells 2025, 14, 1731. https://doi.org/10.3390/cells14211731
Gea J, Orozco-Levi M, Pascual-Guàrdia S, Casadevall C, Enríquez-Rodríguez CJ, Camps-Ubach R, Barreiro E. Biological Mechanisms Involved in Muscle Dysfunction in COPD: An Integrative Damage–Regeneration–Remodeling Framework. Cells. 2025; 14(21):1731. https://doi.org/10.3390/cells14211731
Chicago/Turabian StyleGea, Joaquim, Mauricio Orozco-Levi, Sergi Pascual-Guàrdia, Carme Casadevall, César Jessé Enríquez-Rodríguez, Ramon Camps-Ubach, and Esther Barreiro. 2025. "Biological Mechanisms Involved in Muscle Dysfunction in COPD: An Integrative Damage–Regeneration–Remodeling Framework" Cells 14, no. 21: 1731. https://doi.org/10.3390/cells14211731
APA StyleGea, J., Orozco-Levi, M., Pascual-Guàrdia, S., Casadevall, C., Enríquez-Rodríguez, C. J., Camps-Ubach, R., & Barreiro, E. (2025). Biological Mechanisms Involved in Muscle Dysfunction in COPD: An Integrative Damage–Regeneration–Remodeling Framework. Cells, 14(21), 1731. https://doi.org/10.3390/cells14211731








