Kv7 Channels as an Important Contributor to Alcohol-Induced Modulation of Neuronal Excitability in Neonatal Rat Superior Cervical Ganglion
Highlights
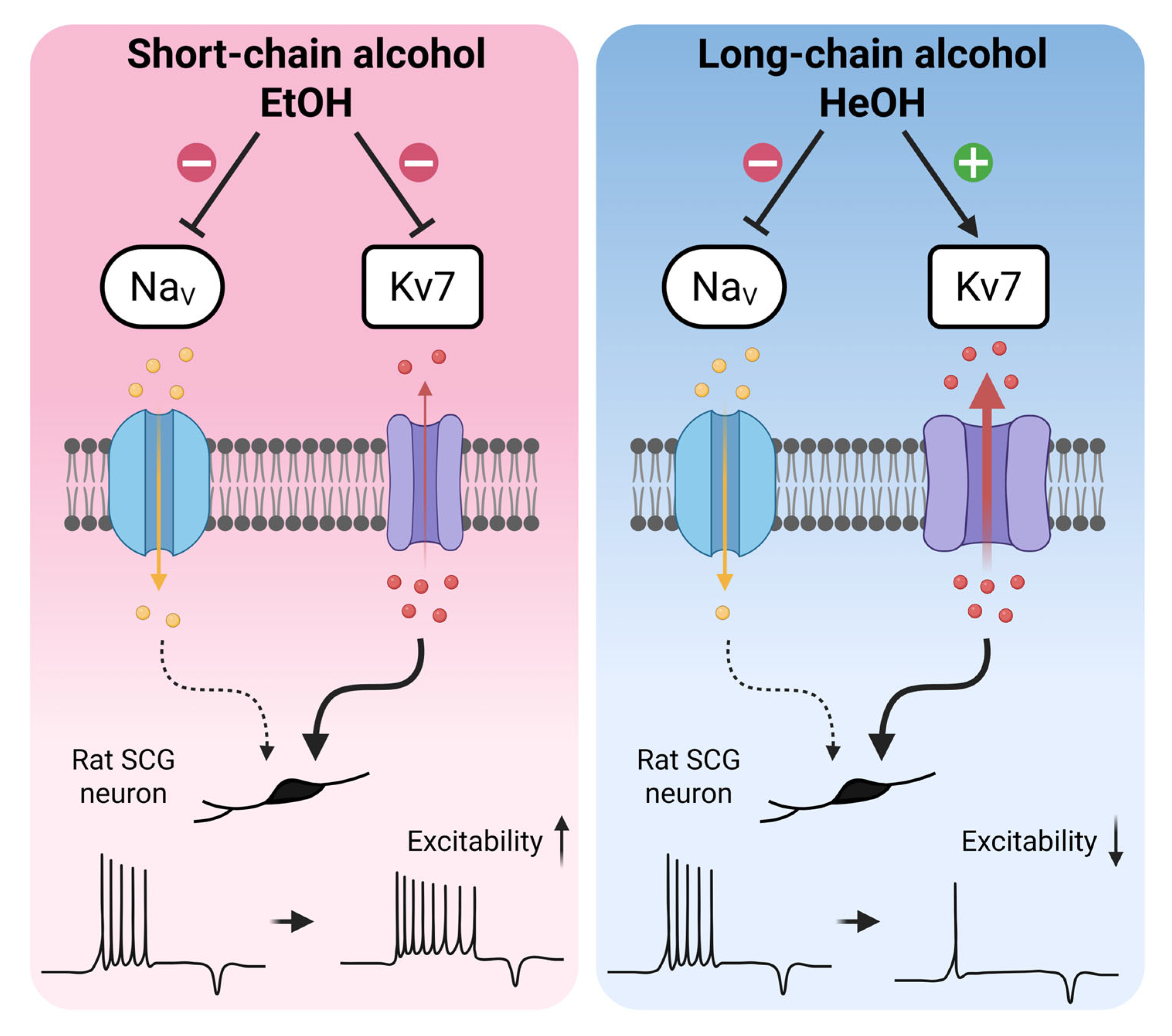
- Neuronal excitability changes induced by alcohols are determined by Kv7 modulation rather than sodium inhibition in rat neonatal SCG neurons.
- Short-chain alcohols enhance excitability through Kv7 inhibition, while long-chain alcohols reduce excitability via Kv7 activation.
- Kv7 channels represent an important target for n-alcohols in the regulation of neuronal excitability.
- The chain length-dependent modulation of Kv7 channels by alcohols provides mechanistic insights and guidance for Kv7 channel-targeted drug development.
Abstract
1. Introduction
2. Materials and Methods
2.1. SCG Neuronal Preparations
2.2. Animals
2.3. Cell Culture and Transfection
2.4. Electrophysiological Recording
2.5. Solution and Materials
2.6. Reverse Transcription-PCR
2.7. Single-Cell RNA Sequencing Data Analysis
2.8. Data Analysis and Statistical Analysis
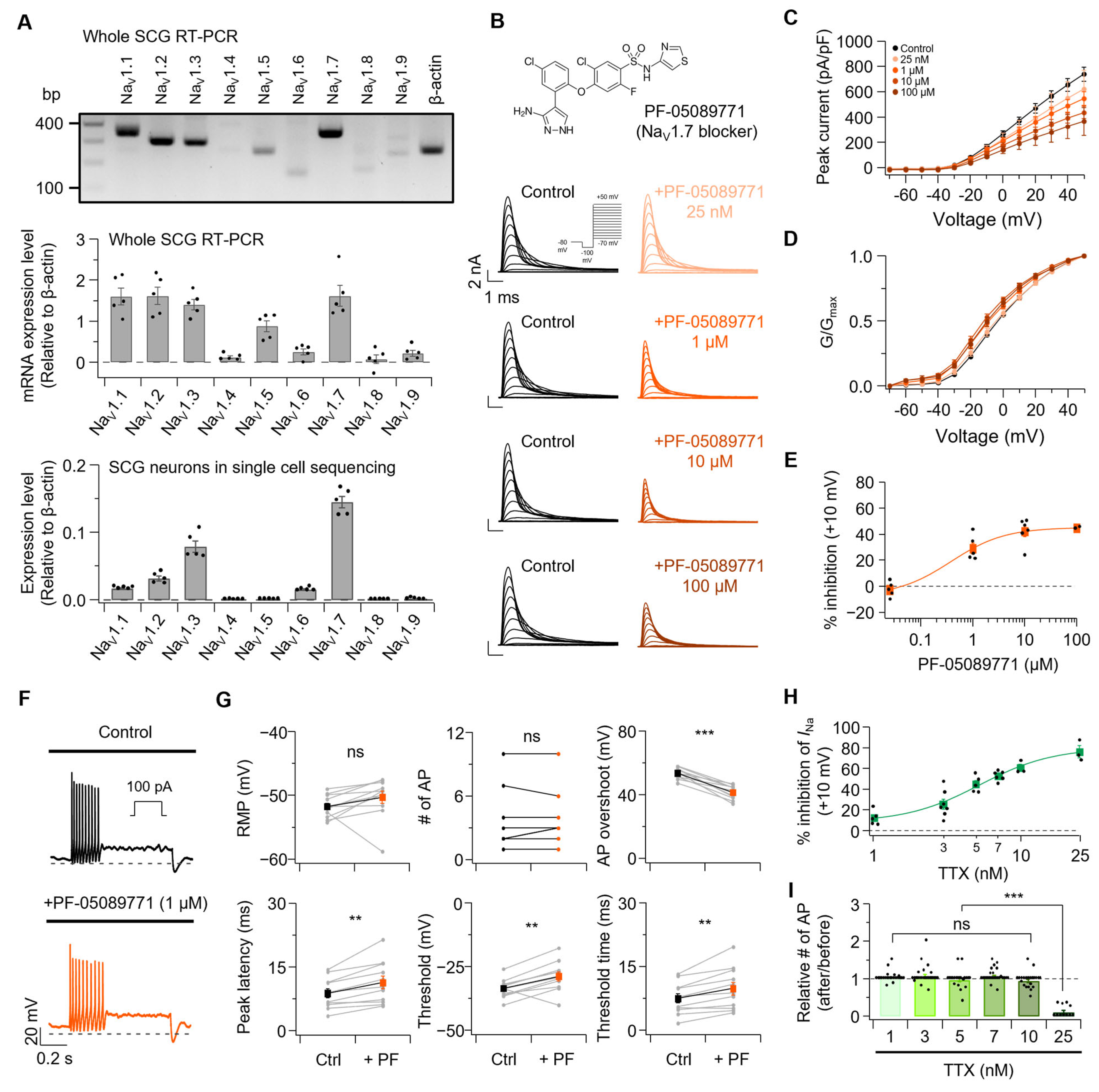
3. Results
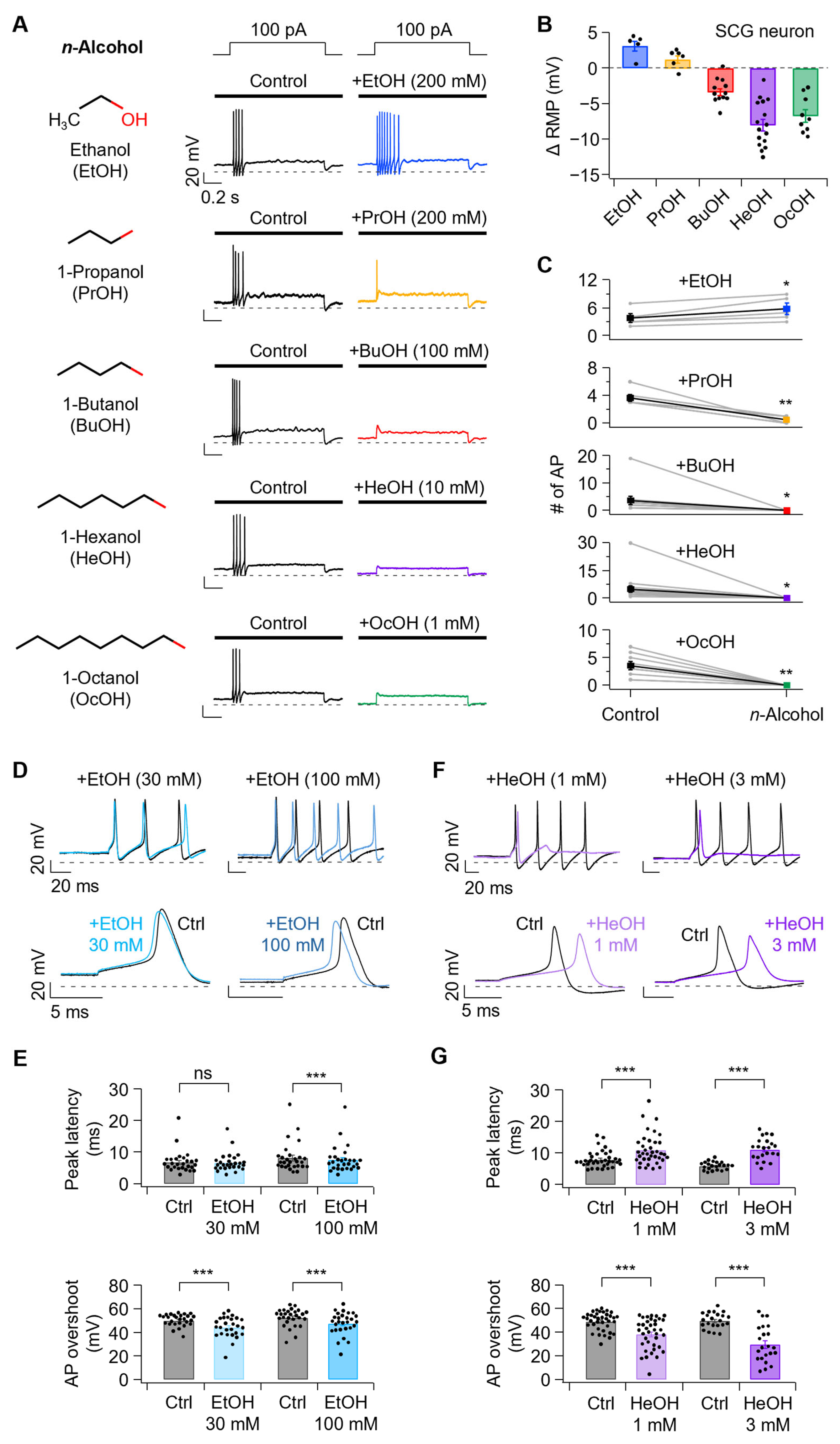
3.1. n-Alcohols Modulate Neuronal Excitability in SCG Neurons
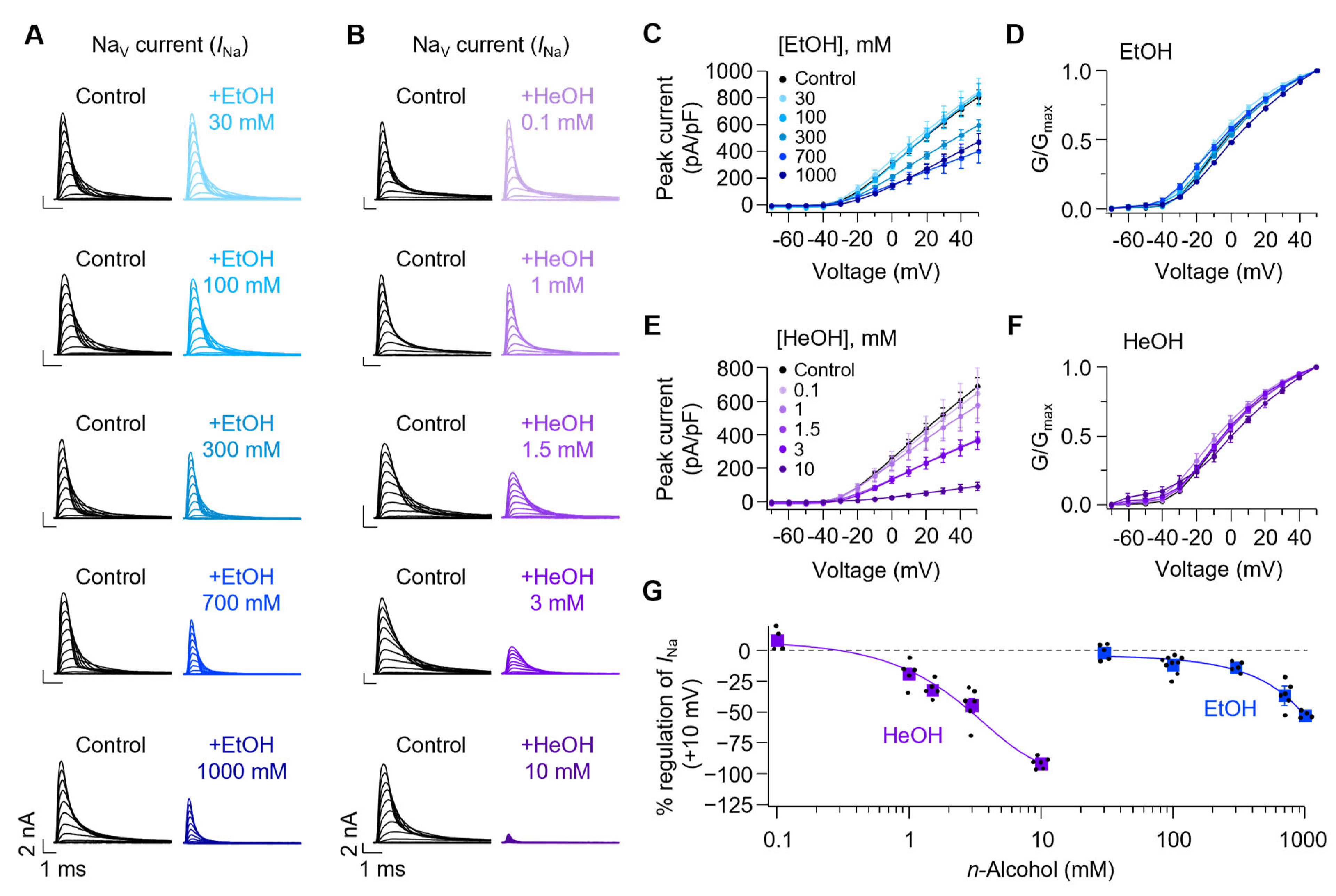
3.2. Both EtOH and HeOH Suppress Sodium Currents, Confirming Previous Findings
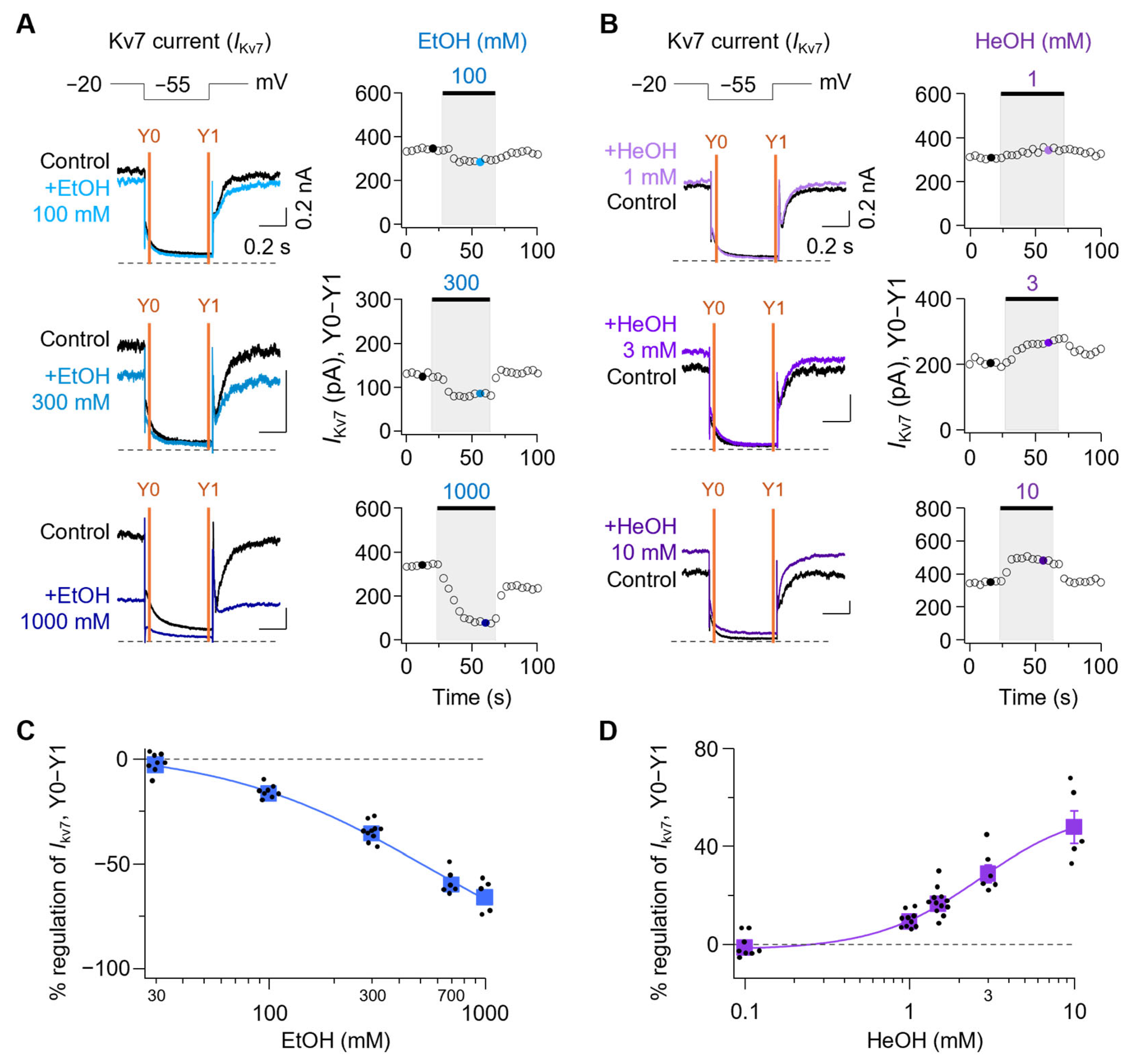
3.3. Kv7 Currents Are Oppositely Regulated by EtOH and HeOH
3.4. Comparison of EtOH and HeOH Effects with Selective NaV and Kv7 Channel Modulation
3.5. A Combination of NaV and Kv7 Modulators Could Mimic EtOH and HeOH
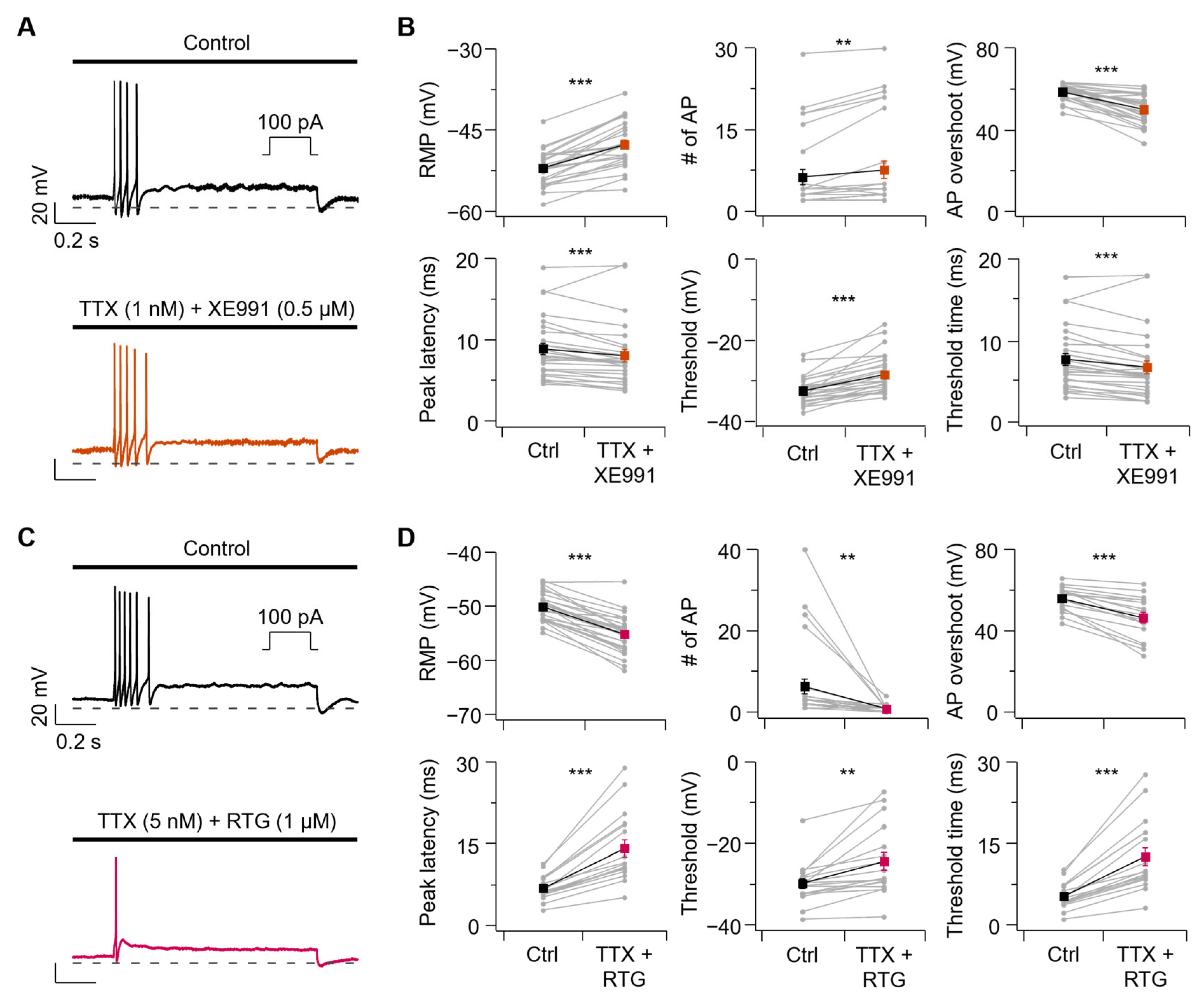
3.6. Sodium Channel Inhibition Alone Has a Limited Impact on AP Firing in SCG Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Action potential |
| BuOH | Butanol |
| EtOH | Ethanol |
| HeOH | Hexanol |
| Kv7 | Voltage-gated potassium channel subfamily 7 (KCNQ channels) |
| NaV | Voltage-gated sodium channel |
| OcOH | Octanol |
| PI(4,5)P2 | Phosphatidylinositol 4,5-bisphosphate |
| PIPKIγ | Phosphatidylinositol-4-phosphate 5-kinase type Iγ |
| PrOH | Propanol |
| RMP | Resting membrane potential |
| RTG | Retigabine |
| SCG | Superior cervical ganglion |
References
- Alifimoff, J.K.; Firestone, L.L.; Miller, K.W. Anaesthetic potencies of primary alkanols: Implications for the molecular dimensions of the anaesthetic site. Br. J. Pharmacol. 1989, 96, 9. [Google Scholar] [CrossRef]
- Mascia, M.P.; Machu, T.K.; Harris, R.A. Enhancement of homomeric glycine receptor function by long chain alcohols and anaesthetics. Br. J. Pharmacol. 1996, 119, 1331–1336. [Google Scholar] [CrossRef]
- Wick, M.J.; Mihic, S.J.; Ueno, S.; Mascia, M.P.; Trudell, J.R.; Brozowski, S.J.; Ye, Q.; Harrison, N.L.; Harris, R.A. Mutations of γ-aminobutyric acid and glycine receptors change alcohol cutoff: Evidence for an alcohol receptor? Proc. Natl. Acad. Sci. USA 1998, 95, 6504–6509. [Google Scholar] [CrossRef]
- Krasowski, M.D.; Harrison, N.L. The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations. Br. J. Pharmacol. 2000, 129, 731–743. [Google Scholar] [CrossRef]
- Oxford, G.S.; Swenson, R.P. n-Alkanols potentiate sodium channel inactivation in squid giant axons. Biophys. J. 1979, 26, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Mullin, M.J.; Hunt, W.A. Effects of ethanol on the functional properties of sodium channels in brain synaptosomes. In Recent Developments in Alcoholism: Memory Deficits Sociology of Treatment Ion Channels Early Problem Drinking; Springer: New York, NY, USA, 1987; pp. 303–311. [Google Scholar] [CrossRef]
- Klein, G.; Gardiwal, A.; Schaefer, A.; Panning, B.; Breitmeier, D. Effect of ethanol on cardiac single sodium channel gating. Forensic Sci. Int. 2007, 171, 131–135. [Google Scholar] [CrossRef]
- Horishita, T.; Harris, R.A. n-Alcohols inhibit voltage-gated Na+ channels expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 2008, 326, 270–277. [Google Scholar] [CrossRef]
- Wirkner, K.; Eberts, C.; Poelchen, W.; Allgaier, C.; Illes, P. Mechanism of inhibition by ethanol of NMDA and AMPA receptor channel functions in cultured rat cortical neurons. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 362, 568–576. [Google Scholar] [CrossRef]
- Hicklin, T.R.; Wu, P.H.; Radcliffe, R.A.; Freund, R.K.; Goebel-Goody, S.M.; Correa, P.R.; Proctor, W.R.; Lombroso, P.J.; Browning, M.D. Alcohol inhibition of the NMDA receptor function, long-term potentiation, and fear learning requires striatal-enriched protein tyrosine phosphatase. Proc. Natl. Acad. Sci. USA 2011, 108, 6650–6655. [Google Scholar] [CrossRef]
- Mihic, S.J.; Harris, R.A. Inhibition of rho1 receptor GABAergic currents by alcohols and volatile anesthetics. J. Pharmacol. Exp. Ther. 1996, 277, 411–416. [Google Scholar] [CrossRef]
- Davies, D.L.; Alkana, R.L. Direct Evidence for a Cause-Effect Link Between Ethanol Potentiation of GABAA Receptor Function and Intoxication from Hyperbaric Studies in C57, LS, and SS Mice. Alcohol. Clin. Exp. Res. 2001, 25, 1098–1106. [Google Scholar] [CrossRef]
- Molander, A.; Söderpalm, B. Accumbal strychnine-sensitive glycine receptors: An access point for ethanol to the brain reward system. Alcohol. Clin. Exp. Res. 2005, 29, 27–37. [Google Scholar] [CrossRef]
- Perkins, D.I.; Trudell, J.R.; Crawford, D.K.; Alkana, R.L.; Davies, D.L. Targets for ethanol action and antagonism in loop 2 of the extracellular domain of glycine receptors. J. Neurochem. 2008, 106, 1337–1349. [Google Scholar] [CrossRef]
- Brown, D.A.; Adams, P.R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature 1980, 283, 673–676. [Google Scholar] [CrossRef]
- Wang, H.S.; Pan, Z.; Shi, W.; Brown, B.S.; Wymore, R.S.; Cohen, I.S.; Dixon, J.E.; McKinnon, D. KCNQ2 and KCNQ3 potassium channel subunits: Molecular correlates of the M-channel. Science 1998, 282, 1890–1893. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Passmore, G.M. Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 2009, 156, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.L.; Hoshi, N. Modulation of Kv7 channels and excitability in the brain. Cell. Mol. Life Sci. 2017, 74, 495–508. [Google Scholar] [CrossRef]
- Schroeder, B.C.; Kubisch, C.; Stein, V.; Jentsch, T.J. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature 1998, 396, 687–690. [Google Scholar] [CrossRef]
- Wickenden, A.D.; McNaughton-Smith, G. Kv7 channels as targets for the treatment of pain. Curr. Pharm. Des. 2009, 15, 1773–1798. [Google Scholar] [CrossRef]
- French, J.A.; Abou-Khalil, B.W.; Leroy, R.F.; Yacubian, E.M.T.; Shin, P.; Hall, S.; Mansbach, H.; Nohria, V. Randomized, double-blind, placebo-controlled trial of ezogabine (retigabine) in partial epilepsy. Neurology 2011, 76, 1555–1563. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, K.; Lee, H.; Suh, B.C. Ethanol elevates excitability of superior cervical ganglion neurons by inhibiting Kv7 channels in a cell type-specific and PI(4,5)P2-dependent manner. Int. J. Mol. Sci. 2019, 20, 4419. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, S.; Gillespie, J.M.; Hodge, J.J. KCNQ channels show conserved ethanol block and function in ethanol behaviour. PLoS ONE 2012, 7, e50279. [Google Scholar] [CrossRef]
- Kim, K.W.; Suh, B.C. Ethanol inhibits Kv7.2/7.3 channel open probability by reducing the PI(4,5)P2 sensitivity of Kv7.2 subunit. BMB Rep. 2021, 54, 311. [Google Scholar] [CrossRef]
- Jeong, D.J.; Kim, K.W.; Suh, B.C. Dual regulation of Kv7.2/7.3 channels by long-chain n-alcohols. J. Gen. Physiol. 2022, 155, e202213191. [Google Scholar] [CrossRef]
- Zareen, N.; Greene, L.A. Protocol for culturing sympathetic neurons from rat superior cervical ganglia (SCG). J. Vis. Exp. 2009, 23, 988. [Google Scholar] [CrossRef]
- Osorio, N.; Alcaraz, G.; Padilla, F.; Couraud, F.; Delmas, P.; Crest, M. Differential targeting and functional specialization of sodium channels in cultured cerebellar granule cells. J. Physiol. 2005, 569, 801–816. [Google Scholar] [CrossRef] [PubMed]
- Mapps, A.A.; Thomsen, M.B.; Boehm, E.; Zhao, H.; Hattar, S.; Kuruvilla, R. Diversity of satellite glia in sympathetic and sensory ganglia. Cell Rep. 2022, 38, 110328. [Google Scholar] [CrossRef]
- Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007, 8, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Platkiewicz, J.; Brette, R. A threshold equation for action potential initiation. PLoS Comput. Biol. 2010, 6, e1000850. [Google Scholar] [CrossRef]
- Ordemann, G.J.; Apgar, C.J.; Brager, D.H. D-type potassium channels normalize action potential firing between dorsal and ventral CA1 neurons of the mouse hippocampus. J. Neurophysiol. 2019, 121, 983–995. [Google Scholar] [CrossRef]
- Goethals, S.; Sierksma, M.C.; Nicol, X.; Réaux-Le Goazigo, A.; Brette, R. Electrical match between initial segment and somatodendritic compartment for action potential backpropagation in retinal ganglion cells. J. Neurophysiol. 2021, 126, 28–46. [Google Scholar] [CrossRef]
- Yu, Y.; Shu, Y.; McCormick, D.A. Cortical action potential backpropagation explains spike threshold variability and rapid-onset kinetics. J. Neurosci. 2008, 28, 7260–7272. [Google Scholar] [CrossRef]
- Fourcaud-Trocmé, N.; Zbili, M.; Duchamp-Viret, P.; Kuczewski, N. Afterhyperpolarization promotes the firing of mitral cells through a voltage-dependent modification of action potential threshold. Eneuro 2022, 9. [Google Scholar] [CrossRef]
- Koob, G.F.; Arends, M.A.; Le Moal, M. Drugs, Addiction, and the Brain; Academic Press: Waltham, MA, USA, 2014; pp. 173–219. [Google Scholar] [CrossRef]
- Asatryan, L.; Popova, M.; Perkins, D.; Trudell, J.R.; Alkana, R.L.; Davies, D.L. Ivermectin antagonizes ethanol inhibition in purinergic P2X4 receptors. J. Pharmacol. Exp. Ther. 2010, 334, 720–728. [Google Scholar] [CrossRef]
- Park, Y.Y.; Johnston, D.; Gray, R. Slowly inactivating component of Na+ current in peri-somatic region of hippocampal CA1 pyramidal neurons. J. Neurophysiol. 2013, 109, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Schrøder, R.L.; Jespersen, T.; Christophersen, P.; Strøbæk, D.; Jensen, B.S.; Olesen, S.P. KCNQ4 channel activation by BMS-204352 and retigabine. Neuropharmacology 2001, 40, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Tatulian, L.; Delmas, P.; Abogadie, F.C.; Brown, D.A. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J. Neurosci. 2001, 21, 5535–5545. [Google Scholar] [CrossRef] [PubMed]
- Lange, W.; Geißendörfer, J.; Schenzer, A.; Grötzinger, J.; Seebohm, G.; Friedrich, T.; Schwake, M. Refinement of the binding site and mode of action of the anticonvulsant Retigabine on KCNQ K+ channels. Mol. Pharmacol. 2009, 75, 272–280. [Google Scholar] [CrossRef]
- Liu, P.; Jo, S.; Bean, B.P. Modulation of neuronal sodium channels by the sea anemone peptide BDS-I. J. Neurophysiol. 2012, 107, 3155–3167. [Google Scholar] [CrossRef]
- Rush, A.M.; Dib-Hajj, S.D.; Liu, S.; Cummins, T.R.; Black, J.A.; Waxman, S.G. A single sodium channel mutation produces hyper-or hypoexcitability in different types of neurons. Proc. Natl. Acad. Sci. USA 2006, 103, 8245–8250. [Google Scholar] [CrossRef]
- Scholz, A.; Kuboyama, N.; Hempelmann, G.; Vogel, W. Complex blockade of TTX-resistant Na+ currents by lidocaine and bupivacaine reduce firing frequency in DRG neurons. J. Neurophysiol. 1998, 79, 1746–1754. [Google Scholar] [CrossRef]
- Scholz, A.; Vogel, W. Tetrodotoxin-resistant action potentials in dorsal root ganglion neurons are blocked by local anesthetics. Pain 2000, 89, 47–52. [Google Scholar] [CrossRef]
- Wu, J.V.; Kendig, J.J. Differential sensitivities of TTX-resistant and TTX-sensitive sodium channels to anesthetic concentrations of ethanol in rat sensory neurons. J. Neurosci. Res. 1998, 54, 433–443. [Google Scholar] [CrossRef]
- Conley, E.C.; Brammar, W.J. Ion Channel Factsbook: Volume 4, Voltage-Gated Channels; Academic Press: Waltham, MA, USA, 1999; pp. 657–702. [Google Scholar] [CrossRef]
- Passmore, G.M.; Selyanko, A.A.; Mistry, M.; Al-Qatari, M.; Marsh, S.J.; Matthews, E.A.; Dickenson, A.H.; Brown, T.A.; Burbidge, S.A.; Main, M.; et al. KCNQ/M currents in sensory neurons: Significance for pain therapy. J. Neurosci. 2003, 23, 7227–7236. [Google Scholar] [CrossRef]
- Arakaki, X.; Foster, H.; Su, L.; Do, H.; Wain, A.J.; Fonteh, A.N.; Zhou, F.; Harrington, M.G. Extracellular sodium modulates the excitability of cultured hippocampal pyramidal cells. Brain Res. 2011, 1401, 85–94. [Google Scholar] [CrossRef]
- Kim, J.H.; Kushmerick, C.; Von Gersdorff, H. Presynaptic resurgent Na+ currents sculpt the action potential waveform and increase firing reliability at a CNS nerve terminal. J. Neurosci. 2010, 30, 15479–15490. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Stoler, O.; Scheller, A.; Khrapunsky, Y.; Goebbels, S.; Kirchhoff, F.; Gutnick, M.J.; Wolf, F.; Fleidervish, I.A. Role of sodium channel subtype in action potential generation by neocortical pyramidal neurons. Proc. Natl. Acad. Sci. USA 2018, 115, E7184–E7192. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.R.; Logothetis, D.E.; Hess, P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron 1989, 2, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Lechner, S.G.; Mayer, M.; Boehm, S. Activation of M1 muscarinic receptors triggers transmitter release from rat sympathetic neurons through an inhibition of M-type K+ channels. J. Physiol. 2003, 553, 789–802. [Google Scholar] [CrossRef]
- Roberts-Crowley, M.L.; Mitra-Ganguli, T.; Liu, L.; Rittenhouse, A.R. Regulation of voltage-gated Ca2+ channels by lipids. Cell Calcium 2009, 45, 589–601. [Google Scholar] [CrossRef]
- McFARLANE, S.; Cooper, E. Postnatal development of voltage-gated K currents on rat sympathetic neurons. J. Neurophysiol. 1992, 67, 1291–1300. [Google Scholar] [CrossRef]
- Vanterpool, C.K.; Vanterpool, E.A.; Pearce, W.J.; Buchholz, J.N. Advancing age alters the expression of the ryanodine receptor 3 isoform in adult rat superior cervical ganglia. J. Appl. Physiol. 2006, 101, 392–400. [Google Scholar] [CrossRef]
- Lopez, J.P.; Lücken, M.D.; Brivio, E.; Karamihalev, S.; Kos, A.; De Donno, C.; Benjamin, A.; Yang, H.; Dick, A.L.W.; Stoffel, R.; et al. Ketamine exerts its sustained antidepressant effects via cell-type-specific regulation of Kcnq2. Neuron 2022, 110, 2283–2298. [Google Scholar] [CrossRef]
- Covarrubias, M.; Rubin, E. Ethanol selectively blocks a noninactivating K+ current expressed in Xenopus oocytes. Proc. Natl. Acad. Sci. USA 1993, 90, 6957–6960. [Google Scholar] [CrossRef]
- Chiou, L.C.; Chuang, K.C.; Fan, S.H.; How, C.H.; Cheng, J.K. Does ethanol activate G-protein coupled inwardly rectifying K+ channels? Neuroreport 2002, 13, 163–165. [Google Scholar] [CrossRef]
- Handlechner, A.G.; Hermann, A.; Fuchs, R.; Weiger, T.M. Acetaldehyde-ethanol interactions on calcium-activated potassium (BK) channels in pituitary tumor (GH3) cells. Front. Behav. Neurosci. 2013, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Korkotian, E.; Bombela, T.; Odegova, T.; Zubov, P.; Segal, M. Ethanol affects network activity in cultured rat hippocampus: Mediation by potassium channels. PLoS ONE 2013, 8, e75988. [Google Scholar] [CrossRef]
- Kaewphaleuk, T.; Watanapa, W.B.; Panich, U. Ethanol enhances endothelial ionic currents and nitric oxide release via intermediate-conductance calcium-activated potassium channel. Life Sci. 2019, 228, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Tóth, M.E.; Vígh, L.; Sántha, M. Alcohol stress, membranes, and chaperones. Cell Stress Chaperones 2014, 19, 299–309. [Google Scholar] [CrossRef]
- Bae, M.K.; Jeong, D.K.; Park, N.S.; Lee, C.H.; Cho, B.H.; Jang, H.O.; Yun, I. The effect of ethanol on the physical properties of neuronal membranes. Mol. Cells 2005, 19, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Kostowski, W. Interactions of ethanol with ion channels: Possible implications for mechanisms of intoxication and dependence. In Ion Channels and Ion Pumps: Metabolic and Endocrine Relationships in Biology and Clinical Medicine; Springer: New York, NY, USA, 1994; pp. 436–454. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kaminow, B.; Yunusov, D.; Dobin, A. STARsolo: Accurate, fast and versatile mapping/quantification of single-cell and single-nucleus RNA-seq data. bioRxiv 2021. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, D.-J.; Woo, J.-N.; Yun, T.; Baek, M.; Suh, B.-C. Kv7 Channels as an Important Contributor to Alcohol-Induced Modulation of Neuronal Excitability in Neonatal Rat Superior Cervical Ganglion. Cells 2025, 14, 1723. https://doi.org/10.3390/cells14211723
Jeong D-J, Woo J-N, Yun T, Baek M, Suh B-C. Kv7 Channels as an Important Contributor to Alcohol-Induced Modulation of Neuronal Excitability in Neonatal Rat Superior Cervical Ganglion. Cells. 2025; 14(21):1723. https://doi.org/10.3390/cells14211723
Chicago/Turabian StyleJeong, Da-Jeong, Jin-Nyeong Woo, Tery Yun, Myungin Baek, and Byung-Chang Suh. 2025. "Kv7 Channels as an Important Contributor to Alcohol-Induced Modulation of Neuronal Excitability in Neonatal Rat Superior Cervical Ganglion" Cells 14, no. 21: 1723. https://doi.org/10.3390/cells14211723
APA StyleJeong, D.-J., Woo, J.-N., Yun, T., Baek, M., & Suh, B.-C. (2025). Kv7 Channels as an Important Contributor to Alcohol-Induced Modulation of Neuronal Excitability in Neonatal Rat Superior Cervical Ganglion. Cells, 14(21), 1723. https://doi.org/10.3390/cells14211723







