The Molecular Mechanism of PDE1 Regulation
Highlights
- The molecular mechanism of PDE1 calcium regulation is identified.
- PDE1 autoinhibition operates via a mechanism similar to that of PDE4.
- The activation mode of PDE1 is distinct and unique.
- A second mechanistic class of phosphodiesterase regulation is established.
- The findings may open new opportunities for pharmacological interventions targeting PDE1.
Abstract
1. Introduction
2. Materials and Methods
2.1. Constructs and Alignments
2.2. Phosphodiesterase Expression
2.3. Phosphodiesterase Assays
2.4. Structure Predictions
2.5. Statistics and Data Analysis
3. Results
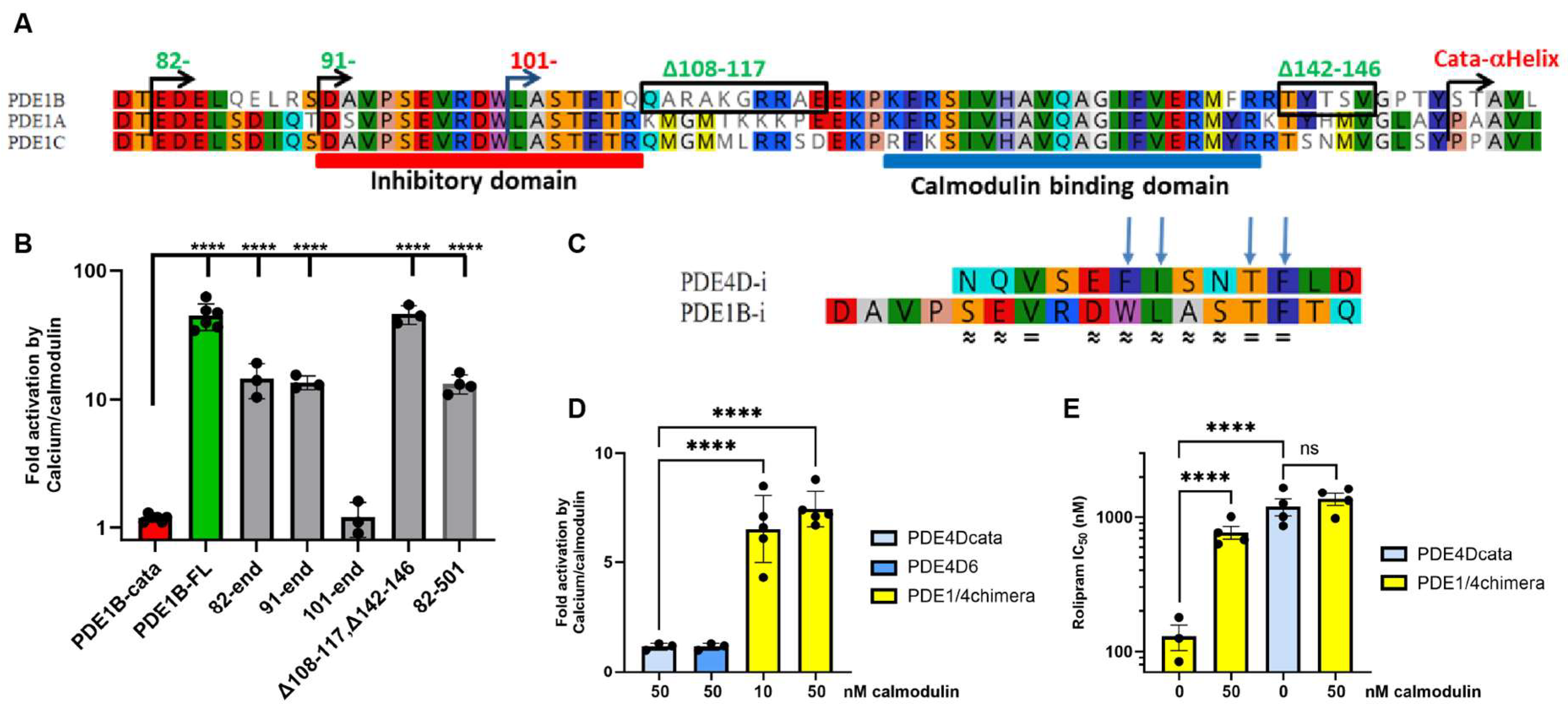
3.1. PDE1B Activation and Regulatory Domain Boundaries
3.2. The PDE1B Inhibitory Domain Has Similarities to PDE4 and Can Regulate PDE4 Activity in a PDE1/4 Chimera
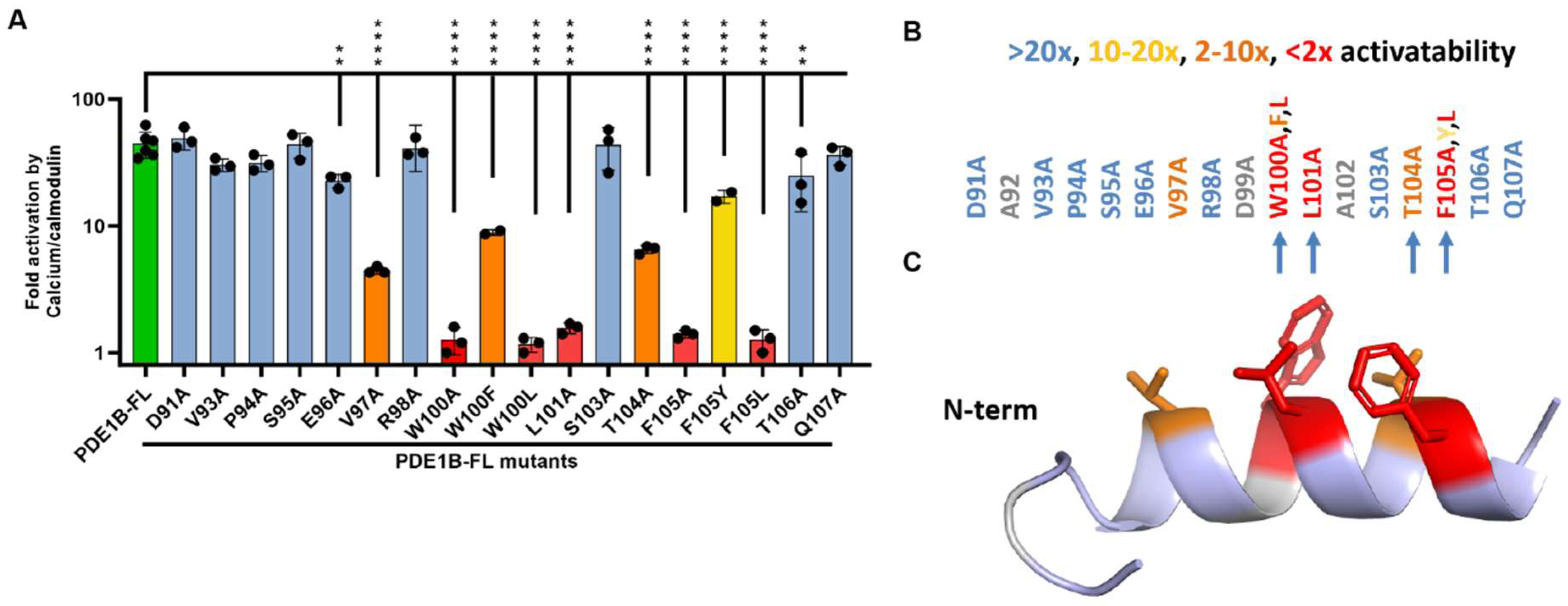
3.3. Alanine Scan of the PDE1B Inhibitory Domain
3.4. Inhibitory Domain Peptides Can Inhibit PDE1B in Trans
3.5. Calmodulin EC50 Inversely Correlates with Maximal Activation of Inhibitory Domain Mutants
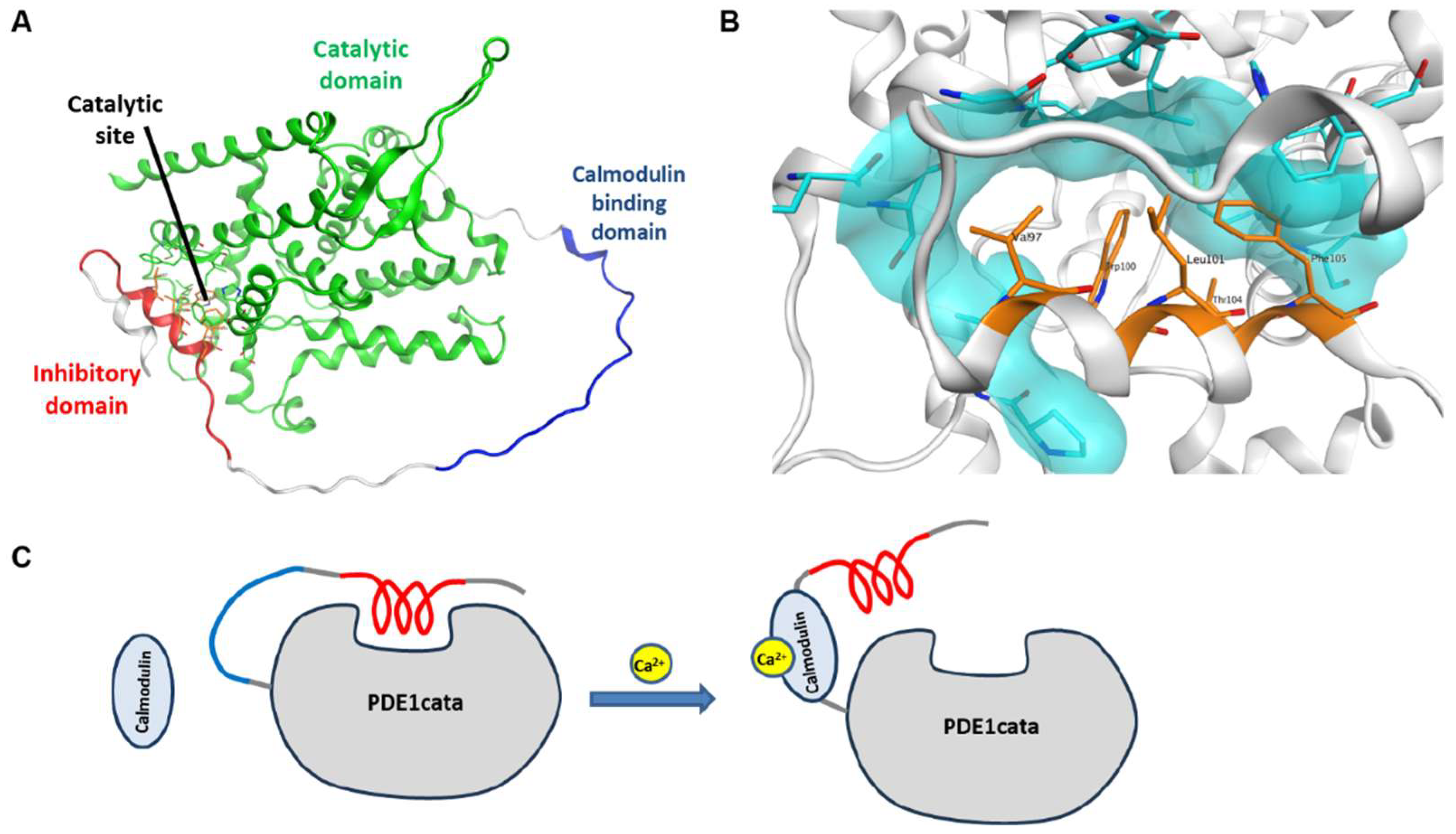
3.6. Structure and Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, F.; Murata, T.; Shimizu, K.; Degerman, E.; Maurice, D.; Manganiello, V. Cyclic Nucleotide Phosphodiesterases: Important Signaling Modulators and Therapeutic Targets. Oral Dis. 2015, 21, e25–e50. [Google Scholar] [CrossRef]
- Betolngar, D.B.; Mota, E.; Fabritius, A.; Nielsen, J.; Hougaard, C.; Christoffersen, C.T.; Yang, J.; Kehler, J.; Griesbeck, O.; Castro, L.R.V.; et al. Phosphodiesterase 1 Bridges Glutamate Inputs with No- and Dopamine-Induced Cyclic Nucleotide Signals in the Striatum. Cereb. Cortex 2019, 29, 5022–5036. [Google Scholar] [CrossRef]
- Khammy, M.M.; Dalsgaard, T.; Larsen, P.H.; Christoffersen, C.T.; Clausen, D.; Rasmussen, L.K.; Folkersen, L.; Grunnet, M.; Kehler, J.; Aalkjaer, C.; et al. Pde1a Inhibition Elicits Cgmp-Dependent Relaxation of Rat Mesenteric Arteries. Br. J. Pharmacol. 2017, 174, 4186–4198. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.; Beck, L.; Kehler, J.; Christoffersen, C.T.; Bundgaard, C.; Mogensen, S.; Mow, T.J.; Pinilla, E.; Knudsen, J.S.; Hedegaard, E.R.; et al. Novel Selective Pde Type 1 Inhibitors Cause Vasodilatation and Lower Blood Pressure in Rats. Br. J. Pharmacol. 2017, 174, 2563–2575. [Google Scholar] [CrossRef]
- Lukyanenko, Y.O.; Younes, A.; Lyashkov, A.E.; Tarasov, K.V.; Riordon, D.R.; Lee, J.; Sirenko, S.G.; Kobrinsky, E.; Ziman, B.; Tarasova, Y.S.; et al. Ca(2+)/Calmodulin-Activated Phosphodiesterase 1a Is Highly Expressed in Rabbit Cardiac Sinoatrial Nodal Cells and Regulates Pacemaker Function. J. Mol. Cell Cardiol. 2016, 98, 73–82. [Google Scholar] [CrossRef]
- Li, P.; Zheng, H.; Zhao, J.; Zhang, L.; Yao, W.; Zhu, H.; Beard, J.D.; Ida, K.; Lane, W.; Snell, G.; et al. Discovery of Potent and Selective Inhibitors of Phosphodiesterase 1 for the Treatment of Cognitive Impairment Associated with Neurodegenerative and Neuropsychiatric Diseases. J. Med. Chem. 2016, 59, 1149–1164. [Google Scholar] [CrossRef] [PubMed]
- A Randomized, Double-Blind, Placebo-Controlled Multicenter Study to Assess the Efficacy and Safety of Lenrispodun as Adjunctive Therapy in the Treatment of Patients with Motor Fluctuations Due to Parkinson’s Disease. 2023. Available online: https://clinicaltrials.gov/study/NCT05766813 (accessed on 1 October 2025).
- Snyder, G.L.; Prickaerts, J.; Wadenberg, M.L.; Zhang, L.; Zheng, H.; Yao, W.; Akkerman, S.; Zhu, H.; Hendrick, J.P.; Vanover, K.E.; et al. Preclinical Profile of Iti-214, an Inhibitor of Phosphodiesterase 1, for Enhancement of Memory Performance in Rats. Psychopharmacology 2016, 233, 3113–3124. [Google Scholar] [CrossRef] [PubMed]
- Rybalkin, S.D.; Rybalkina, I.G.; Shimizu-Albergine, M.; Tang, X.B.; Beavo, J.A. Pde5 Is Converted to an Activated State Upon Cgmp Binding to the Gaf a Domain. EMBO J. 2003, 22, 469–478. [Google Scholar] [CrossRef]
- Martinez, S.E.; Wu, A.Y.; Glavas, N.A.; Tang, X.B.; Turley, S.; Hol, W.G.; Beavo, J.A. The Two Gaf Domains in Phosphodiesterase 2a Have Distinct Roles in Dimerization and in Cgmp Binding. Proc. Natl. Acad. Sci. USA 2002, 99, 13260–13265. [Google Scholar] [CrossRef]
- Gupta, R.; Liu, Y.; Wang, H.; Nordyke, C.T.; Puterbaugh, R.Z.; Cui, W.; Varga, K.; Chu, F.; Ke, H.; Vashisth, H.; et al. Structural Analysis of the Regulatory Gaf Domains of Cgmp Phosphodiesterase Elucidates the Allosteric Communication Pathway. J. Mol. Biol. 2020, 432, 5765–5783. [Google Scholar] [CrossRef]
- Mou, H.; Cote, R.H. The Catalytic and Gaf Domains of the Rod Cgmp Phosphodiesterase (Pde6) Heterodimer Are Regulated by Distinct Regions of Its Inhibitory Gamma Subunit. J. Biol. Chem. 2001, 276, 27527–27534. [Google Scholar] [CrossRef]
- Matthiesen, K.; Nielsen, J. Binding of Cyclic Nucleotides to Phosphodiesterase 10a and 11a Gaf Domains Does Not Stimulate Catalytic Activity. Biochem. J. 2009, 423, 401–409. [Google Scholar] [CrossRef][Green Version]
- Jager, R.; Russwurm, C.; Schwede, F.; Genieser, H.G.; Koesling, D.; Russwurm, M. Activation of Pde10 and Pde11 Phosphodiesterases. J. Biol. Chem. 2012, 287, 1210–1219. [Google Scholar] [CrossRef]
- Pandit, J.; Forman, M.D.; Fennell, K.F.; Dillman, K.S.; Menniti, F.S. Mechanism for the Allosteric Regulation of Phosphodiesterase 2a Deduced from the X-Ray Structure of a near Full-Length Construct. Proc. Natl. Acad. Sci. USA 2009, 106, 18225–18230. [Google Scholar] [CrossRef]
- Sette, C.; Conti, M. Phosphorylation and Activation of a Camp-Specific Phosphodiesterase by the Camp-Dependent Protein Kinase. Involvement of Serine 54 in the Enzyme Activation. J. Biol. Chem. 1996, 271, 16526–16534. [Google Scholar] [CrossRef]
- MacKenzie, S.J.; Baillie, G.S.; McPhee, I.; MacKenzie, C.; Seamons, R.; McSorley, T.; Millen, J.; Beard, M.B.; van Heeke, G.; Houslay, M.D. Long Pde4 Camp Specific Phosphodiesterases Are Activated by Protein Kinase a-Mediated Phosphorylation of a Single Serine Residue in Upstream Conserved Region 1 (Ucr1). Br. J. Pharmacol. 2002, 136, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Burgin, A.B.; Magnusson, O.T.; Singh, J.; Witte, P.; Staker, B.L.; Bjornsson, J.M.; Thorsteinsdottir, M.; Hrafnsdottir, S.; Hagen, T.; Kiselyov, A.S.; et al. Design of Phosphodiesterase 4d (Pde4d) Allosteric Modulators for Enhancing Cognition with Improved Safety. Nat. Biotechnol. 2010, 28, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, P.; Aulabaugh, A.; Geoghegan, K.F.; McLellan, T.J.; Pandit, J. Engineered Stabilization and Structural Analysis of the Autoinhibited Conformation of Pde4. Proc. Natl. Acad. Sci. USA 2015, 112, E1414–E1422. [Google Scholar] [CrossRef]
- Samidurai, A.; Xi, L.; Das, A.; Iness, A.N.; Vigneshwar, N.G.; Li, P.L.; Singla, D.K.; Muniyan, S.; Batra, S.K.; Kukreja, R.C. Role of Phosphodiesterase 1 in the Pathophysiology of Diseases and Potential Therapeutic Opportunities. Pharmacol. Ther. 2021, 226, 107858. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, R.L.; Stith-Coleman, I.E.; Vaughan, M. Proteolytic Activation of Calmodulin-Dependent Cyclic Nucleotide Phosphodiesterase. J. Biol. Chem. 1985, 260, 9009–9015. [Google Scholar] [CrossRef]
- Sonnenburg, W.K.; Seger, D.; Kwak, K.S.; Huang, J.; Charbonneau, H.; Beavo, J.A. Identification of Inhibitory and Calmodulin-Binding Domains of the Pde1a1 and Pde1a2 Calmodulin-Stimulated Cyclic Nucleotide Phosphodiesterases. J. Biol. Chem. 1995, 270, 30989–31000. [Google Scholar] [CrossRef]
- Liu, H.; Naismith, J.H. An Efficient One-Step Site-Directed Deletion, Insertion, Single and Multiple-Site Plasmid Mutagenesis Protoco. BMC Biotechnol. 2008, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Henikoff, S.; Henikoff, J.G. Amino Acid Substitution Matrices from Protein Blocks. Proc. Natl. Acad. Sci. USA 1992, 89, 10915–10919. [Google Scholar] [CrossRef]
- Lamiable, A.; Thevenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tuffery, P. Pep-Fold3: Faster De Novo Structure Prediction for Linear Peptides in Solution and in Complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with Alphafold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Evans, R.; O’Neill, M.; Pritzel, A.; Antropova, N.; Senior, A.; Green, T.; Žídek, A.; Bates, R.; Blackwell, S.; Yim, J.; et al. Protein Complex Prediction with Alphafold-Multimer. bioRxiv 2022. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. Alphafold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Card, G.L.; Suzuki, Y.; Artis, D.R.; Fong, D.; Gillette, S.; Hsieh, D.; Neiman, J.; West, B.L.; Zhang, C.; et al. A Glutamine Switch Mechanism for Nucleotide Selectivity by Phosphodiesterases. Mol. Cell 2004, 15, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Maupetit, J.; Derreumaux, P.; Tuffery, P. Improved Pep-Fold Approach for Peptide and Miniprotein Structure Prediction. J. Chem. Theory Comput. 2014, 10, 4745–4758. [Google Scholar] [CrossRef] [PubMed]
- Beard, M.B.; Olsen, A.E.; Jones, R.E.; Erdogan, S.; Houslay, M.D.; Bolger, G.B. Ucr1 and Ucr2 Domains Unique to the Camp-Specific Phosphodiesterase Family Form a Discrete Module Via Electrostatic Interactions. J. Biol. Chem. 2000, 275, 10349–10358. [Google Scholar] [CrossRef]
- Bolger, G.B.; Dunlop, A.J.; Meng, D.; Day, J.P.; Klussmann, E.; Baillie, G.S.; Adams, D.R.; Houslay, M.D. Dimerization of Camp Phosphodiesterase-4 (Pde4) in Living Cells Requires Interfaces Located in Both the Ucr1 and Catalytic Unit Domains. Cell Signal 2015, 27, 756–769. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, J.; Langgård, M.; Tengberg, J.F.; Kehler, J. The Molecular Mechanism of PDE1 Regulation. Cells 2025, 14, 1722. https://doi.org/10.3390/cells14211722
Nielsen J, Langgård M, Tengberg JF, Kehler J. The Molecular Mechanism of PDE1 Regulation. Cells. 2025; 14(21):1722. https://doi.org/10.3390/cells14211722
Chicago/Turabian StyleNielsen, Jacob, Morten Langgård, Josefine Fussing Tengberg, and Jan Kehler. 2025. "The Molecular Mechanism of PDE1 Regulation" Cells 14, no. 21: 1722. https://doi.org/10.3390/cells14211722
APA StyleNielsen, J., Langgård, M., Tengberg, J. F., & Kehler, J. (2025). The Molecular Mechanism of PDE1 Regulation. Cells, 14(21), 1722. https://doi.org/10.3390/cells14211722





