Ladarixin Potential over the Effects of IL-8 and of Serum from Patients with Abdominal Aortic Aneurysm on Human Aortic Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood for Cell Conditioning
2.2. Cell Culture
2.3. Immunofluorescence
2.4. Gel Zymography
2.5. NADH/βNADPH Diaphorase Histochemistry
2.6. ATP Assays
2.7. RNA Isolation and Quantitative Real-Time PCR
2.8. Statistics
3. Results
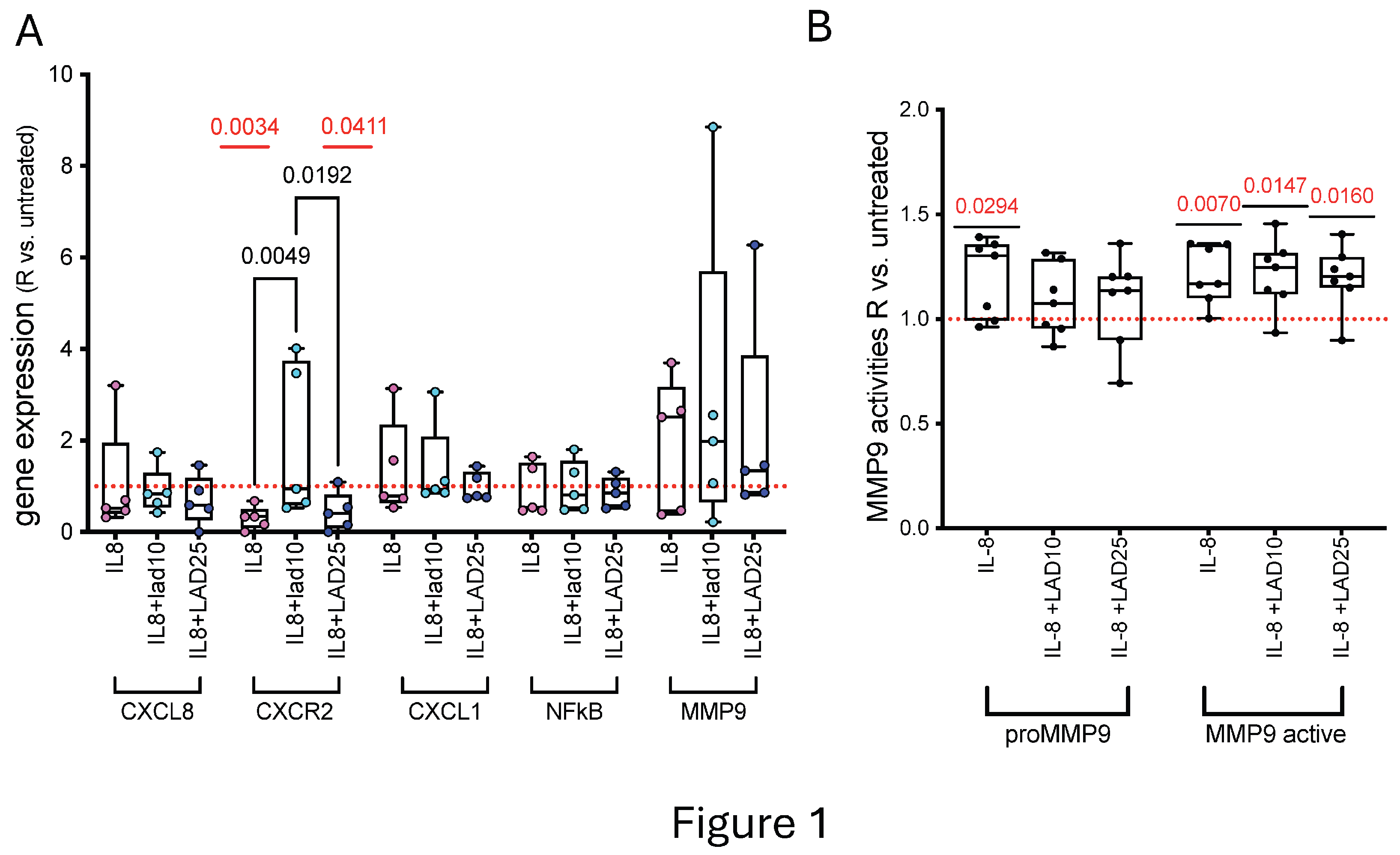
3.1. Primary HAOEC Conditioned with IL-8 and Treated with Lad: Gene Expression and MMP9 Activities
3.2. Primary HAOEC Conditioned with IL-8 and Treated with Lad: Energetics and Cytoskeleton
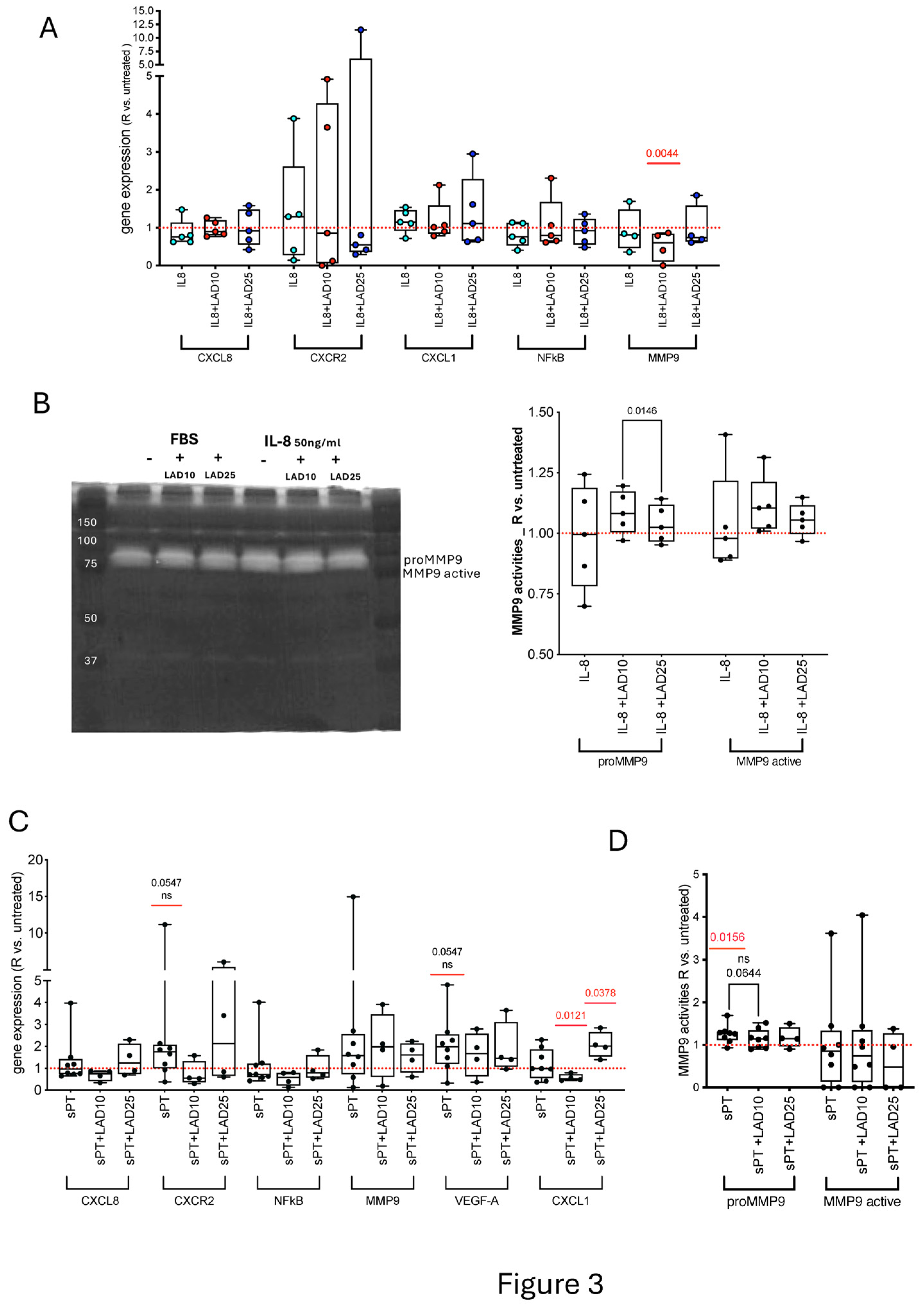
3.3. Primary HAOEC Conditioned with Human Serum and Treated with Ladarixin: Gene Expression and MMP9 Activities
3.4. Primary HAOEC Conditioned with Human Serum and Treated with Lad: Energetics and Cytoskeleton
3.5. Primary HAOSMC: Conditioned with IL-8 and Treated with Lad
3.6. Primary HAOSMC Conditioned with Human Serum and Treated with Ladarixin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golledge, J.; Thanigaimani, S.; Powell, J.T.; Tsao, P.S. Pathogenesis and management of abdominal aortic aneurysm. Eur. Heart J. 2023, 44, 2682–2697. [Google Scholar] [CrossRef]
- Michalska, M.; Grochowiecki, T.; Jakimowicz, T.; Nazarewski, S. A Review of the Impact of Neutrophils and Neutrophil Extracellular Traps (NETs) on the Development of Aortic Aneurysms in Animal and Human Studies. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e935134. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Deng, H.; Zhou, Z.; Xiong, X.; Gao, L. Endothelium as a Potential Target for Treatment of Abdominal Aortic Aneurysm. Oxidative Med. Cell. Longev. 2018, 2018, 6306542. [Google Scholar] [CrossRef]
- Franck, G.; Dai, J.; Fifre, A.; Ngo, S.; Justine, C.; Michineau, S.; Allaire, E.; Gervais, M. Reestablishment of the endothelial lining by endothelial cell therapy stabilizes experimental abdominal aortic aneurysms. Circulation 2013, 127, 1877–1887. [Google Scholar] [CrossRef]
- Rateri, D.L.; Moorleghen, J.J.; Balakrishnan, A.; Owens, A.P., 3rd; Howatt, D.A.; Subramanian, V.; Poduri, A.; Charnigo, R.; Cassis, L.A.; Daugherty, A. Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor−/− mice. Circ. Res. 2011, 108, 574–581. [Google Scholar] [CrossRef]
- Kokje, V.B.C.; Gäbel, G.; Dalman, R.L.; Koole, D.; Northoff, B.H.; Holdt, L.M.; Hamming, J.F.; Lindeman, J.H.N. CXCL8 hyper-signaling in the aortic abdominal aneurysm. Cytokine 2018, 108, 96–104. [Google Scholar] [CrossRef]
- Lombardi, M.; Spartano, L.; Nicolo, S.; Ardita, V.; Chiesa, R.; Aramini, A.; Allegretti, M.; Baccellieri, D.; De Filippis, L.; Foglieni, C. Modulation of MMP9 and CXCR2/CXCL1/IL-8 axis in human abdominal aortic aneurysm tissues by ladarixin. Cardiovasc. Res. 2024, 120, 1832–1834. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Bosiacki, M.; Pilarczyk, M.; Kot, M.; Defort, P.; Walaszek, I.; Chlubek, D.; Baranowska-Bosiacka, I. The CXCL1-CXCR2 Axis as a Component of Therapy Resistance, a Source of Side Effects in Cancer Treatment, and a Therapeutic Target. Cancers 2025, 17, 1674. [Google Scholar] [CrossRef]
- Boccella, S.; Morace, A.M.; Giorgio, C.; Guida, F.; Perrone, M.; Manzo, I.; Belardo, C.; Jones, M.; Maione, S.; Aramini, A.; et al. Effects of CXCR1/2 Blockade with Ladarixin on Streptozotocin-Induced Type 1 Diabetes Mellitus and Peripheral Neuropathy and Retinopathy in Rat. Diabetes Metab. J. 2025, 49, 990–1005. [Google Scholar] [CrossRef]
- Napoli, M.; Sitaru, S.; Budke, A.; Kammerl, A.; Querner, G.; Immler, R.; Rohwedder, I.; Seifert, S.; Ferraro, B.; Weckbach, L.; et al. The dual non-competitive CXCR1/2 inhibitor ladarixin impairs neutrophil extravasation without altering intravascular adhesion. Haematologica 2025. [Google Scholar] [CrossRef] [PubMed]
- Aliev, G.; Smith, M.A.; Turmaine, M.; Neal, M.L.; Zimina, T.V.; Friedland, R.P.; Perry, G.; LaManna, J.C.; Burnstock, G. Atherosclerotic lesions are associated with increased immunoreactivity for inducible nitric oxide synthase and endothelin-1 in thoracic aortic intimal cells of hyperlipidemic Watanabe rabbits. Exp. Mol. Pathol. 2001, 71, 40–54. [Google Scholar] [CrossRef]
- Ejiri, J.; Inoue, N.; Tsukube, T.; Munezane, T.; Hino, Y.; Kobayashi, S.; Hirata, K.; Kawashima, S.; Imajoh-Ohmi, S.; Hayashi, Y.; et al. Oxidative stress in the pathogenesis of thoracic aortic aneurysm: Protective role of statin and angiotensin II type 1 receptor blocker. Cardiovasc. Res. 2003, 59, 988–996. [Google Scholar] [CrossRef]
- Morin, A.M.; Stanboli, A. Nitric oxide synthase in cultured endothelial cells of cerebrovascular origin: Cytochemistry. J. Neurosci. Res. 1993, 36, 272–279. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Zhang, S.; Xie, Z.; Ma, W.; Liu, S.; Fang, Y.; Zheng, S.; Huang, C.; Yan, G.; et al. Mitochondrial NAD(+) deficiency in vascular smooth muscle impairs collagen III turnover to trigger thoracic and abdominal aortic aneurysm. Nat. Cardiovasc. Res. 2025, 4, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed]
- Mantione, M.E.; Lombardi, M.; Baccellieri, D.; Ferrara, D.; Castellano, R.; Chiesa, R.; Alfieri, O.; Foglieni, C. IL-1β/MMP9 activation in primary human vascular smooth muscle-like cells: Exploring the role of TNFα and P2X7. Int. J. Cardiol. 2019, 278, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Foglieni, C.; Cavarelli, M.; Piscopiello, M.; Fulgenzi, A.; Ferrero, M.E. Mn bioavailability by polarized Caco-2 cells: Comparison between Mn gluconate and Mn oxyprolinate. Nutr. J. 2011, 10, 77. [Google Scholar] [CrossRef]
- Lombardi, M.; Mantione, M.E.; Baccellieri, D.; Ferrara, D.; Castellano, R.; Chiesa, R.; Alfieri, O.; Foglieni, C. P2X7 receptor antagonism modulates IL-1β and MMP9 in human atherosclerotic vessels. Sci. Rep. 2017, 7, 4872. [Google Scholar] [CrossRef]
- Koch, A.E.; Kunkel, S.L.; Pearce, W.H.; Shah, M.R.; Parikh, D.; Evanoff, H.L.; Haines, G.K.; Burdick, M.D.; Strieter, R.M. Enhanced production of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneurysms. Am. J. Pathol. 1993, 142, 1423–1431. [Google Scholar]
- Levesque, M.; Bath, L.; Ducas, A.; Boyd, A. Increased Interleukin-8 Expression in Regions of High Intraluminal Thrombus Deposition in Human Abdominal Aortic Aneurysms. JVS-Vasc. Sci. 2020, 1, 256–257. [Google Scholar] [CrossRef]
- Middleton, R.K.; Bown, M.J.; Lloyd, G.M.; Jones, J.L.; London, N.J.; Sayers, R.D. Characterisation of Interleukin-8 and monocyte chemoattractant protein-1 expression within the abdominal aortic aneurysm and their association with mural inflammation. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2009, 37, 46–55. [Google Scholar] [CrossRef]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef]
- Yu, H.; Huang, X.; Ma, Y.; Gao, M.; Wang, O.; Gao, T.; Shen, Y.; Liu, X. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int. J. Biol. Sci. 2013, 9, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Hastings, N.E.; Feaver, R.E.; Lee, M.Y.; Wamhoff, B.R.; Blackman, B.R. Human IL-8 regulates smooth muscle cell VCAM-1 expression in response to endothelial cells exposed to atheroprone flow. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 725–731. [Google Scholar] [CrossRef]
- Yue, T.L.; Wang, X.; Sung, C.P.; Olson, B.; McKenna, P.J.; Gu, J.L.; Feuerstein, G.Z. Interleukin-8. A mitogen and chemoattractant for vascular smooth muscle cells. Circ. Res. 1994, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Mitchell, R.N.; Libby, P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 987–994. [Google Scholar] [CrossRef]
- Reutershan, J.; Morris, M.A.; Burcin, T.L.; Smith, D.F.; Chang, D.; Saprito, M.S.; Ley, K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J. Clin. Investig. 2006, 116, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Galisteo, R.; Gutkind, J.S. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J. Biol. Chem. 2009, 284, 6038–6042. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Abouelasrar Salama, S.; Vandooren, J.; Mortier, A.; Janssens, R.; Vandendriessche, S.; Ganseman, E.; Martens, E.; Gouwy, M.; Neerinckx, B.; et al. From ELISA to Immunosorbent Tandem Mass Spectrometry Proteoform Analysis: The Example of CXCL8/Interleukin-8. Front. Immunol. 2021, 12, 644725. [Google Scholar] [CrossRef]
- Schönbeck, U.; Brandt, E.; Petersen, F.; Flad, H.D.; Loppnow, H. IL-8 specifically binds to endothelial but not to smooth muscle cells. J. Immunol. 1995, 154, 2375–2383. [Google Scholar] [CrossRef]
- Geiser, T.; Dewald, B.; Ehrengruber, M.U.; Clark-Lewis, I.; Baggiolini, M. The interleukin-8-related chemotactic cytokines GRO alpha, GRO beta, and GRO gamma activate human neutrophil and basophil leukocytes. J. Biol. Chem. 1993, 268, 15419–15424. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Zhou, C.; Zhang, Z.; Zhang, J.; Qin, X. Immune-dysregulated neutrophils characterized by upregulation of CXCL1 may be a potential factor in the pathogenesis of abdominal aortic aneurysm and systemic lupus erythematosus. Heliyon 2023, 9, e18037. [Google Scholar] [CrossRef]
- Zhao, J.; Nishimura, Y.; Kimura, A.; Ozawa, K.; Kondo, T.; Tanaka, T.; Yoshizumi, M. Chemokines protect vascular smooth muscle cells from cell death induced by cyclic mechanical stretch. Sci. Rep. 2017, 7, 16128. [Google Scholar] [CrossRef]
- Korbecki, J.; Maruszewska, A.; Bosiacki, M.; Chlubek, D.; Baranowska-Bosiacka, I. The Potential Importance of CXCL1 in the Physiological State and in Noncancer Diseases of the Cardiovascular System, Respiratory System and Skin. Int. J. Mol. Sci. 2022, 24, 205. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Sánchez, A.C.; Koltsova, E.K. Immune and inflammatory mechanisms of abdominal aortic aneurysm. Front. Immunol. 2022, 13, 989933. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, H.N.; Li, S.; Davila, A., Jr.; Chellappa, K.; Davis, J.G.; Guan, Y.; Frederick, D.W.; Chu, W.; Zhao, H.; et al. Rapamycin maintains NAD(+)/NADH redox homeostasis in muscle cells. Aging 2020, 12, 17786–17799. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, S.I.; Jinga, M.; Cochior, D.; Bontas, E.; Parepa, I.; Nita, D.; Gabriel, C.; Ţintoiu, I.C. Endothelial Dysfunction in Aortic Aneurysm. In New Approaches to Aortic Diseases from Valve to Abdominal Bifurcation; Academic Press: London, UK, 2018; pp. 25–39. [Google Scholar]
- Miyoshi, T.; Yamashita, K.; Arai, T.; Yamamoto, K.; Mizugishi, K.; Uchiyama, T. The role of endothelial interleukin-8/NADPH oxidase 1 axis in sepsis. Immunology 2010, 131, 331–339. [Google Scholar] [CrossRef]
- Seckler, J.M.; Shen, J.; Lewis, T.H.J.; Abdulameer, M.A.; Zaman, K.; Palmer, L.A.; Bates, J.N.; Jenkins, M.W.; Lewis, S.J. NADPH diaphorase detects S-nitrosylated proteins in aldehyde-treated biological tissues. Sci. Rep. 2020, 10, 21088. [Google Scholar] [CrossRef]
- Wilson, C.; Lee, M.D.; Buckley, C.; Zhang, X.; McCarron, J.G. Mitochondrial ATP Production is Required for Endothelial Cell Control of Vascular Tone. Function 2023, 4, zqac063. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spartano, L.; Lombardi, M.; Ardita, V.; Chiesa, R.; Aramini, A.; Allegretti, M.; Baccellieri, D.; De Filippis, L.; Foglieni, C. Ladarixin Potential over the Effects of IL-8 and of Serum from Patients with Abdominal Aortic Aneurysm on Human Aortic Cells. Cells 2025, 14, 1713. https://doi.org/10.3390/cells14211713
Spartano L, Lombardi M, Ardita V, Chiesa R, Aramini A, Allegretti M, Baccellieri D, De Filippis L, Foglieni C. Ladarixin Potential over the Effects of IL-8 and of Serum from Patients with Abdominal Aortic Aneurysm on Human Aortic Cells. Cells. 2025; 14(21):1713. https://doi.org/10.3390/cells14211713
Chicago/Turabian StyleSpartano, Lucia, Maria Lombardi, Vincenzo Ardita, Roberto Chiesa, Andrea Aramini, Marcello Allegretti, Domenico Baccellieri, Lidia De Filippis, and Chiara Foglieni. 2025. "Ladarixin Potential over the Effects of IL-8 and of Serum from Patients with Abdominal Aortic Aneurysm on Human Aortic Cells" Cells 14, no. 21: 1713. https://doi.org/10.3390/cells14211713
APA StyleSpartano, L., Lombardi, M., Ardita, V., Chiesa, R., Aramini, A., Allegretti, M., Baccellieri, D., De Filippis, L., & Foglieni, C. (2025). Ladarixin Potential over the Effects of IL-8 and of Serum from Patients with Abdominal Aortic Aneurysm on Human Aortic Cells. Cells, 14(21), 1713. https://doi.org/10.3390/cells14211713






