Dual ROCK1/2–MYLK4 Kinase Inhibition Preserves Visual Function in a Rat Model of Neuromyelitis Optica Spectrum Disorder Optic Neuritis
Highlights
- Intravitreal administration of ITRI-E-(S)4046 (dual ROCK1/2–MYLK4 inhibitor) preserved visual-evoked potentials and retinal ganglion cell survival in an NMOSD rat optic neuritis model.
- TRI-ES suppressed optic nerve inflammation, demyelination, and glial activation while promoting anti-inflammatory (M2) macrophage polarization.
- Dual ROCK/MYLK4 inhibition represents a novel neuroprotective strategy that may complement immunotherapy in NMOSD-related optic neuritis.
- This study supports the translation of kinase-targeting therapeutics aimed at preserving visual function in autoimmune demyelinating diseases.
Abstract
1. Introduction
2. Materials and Methods
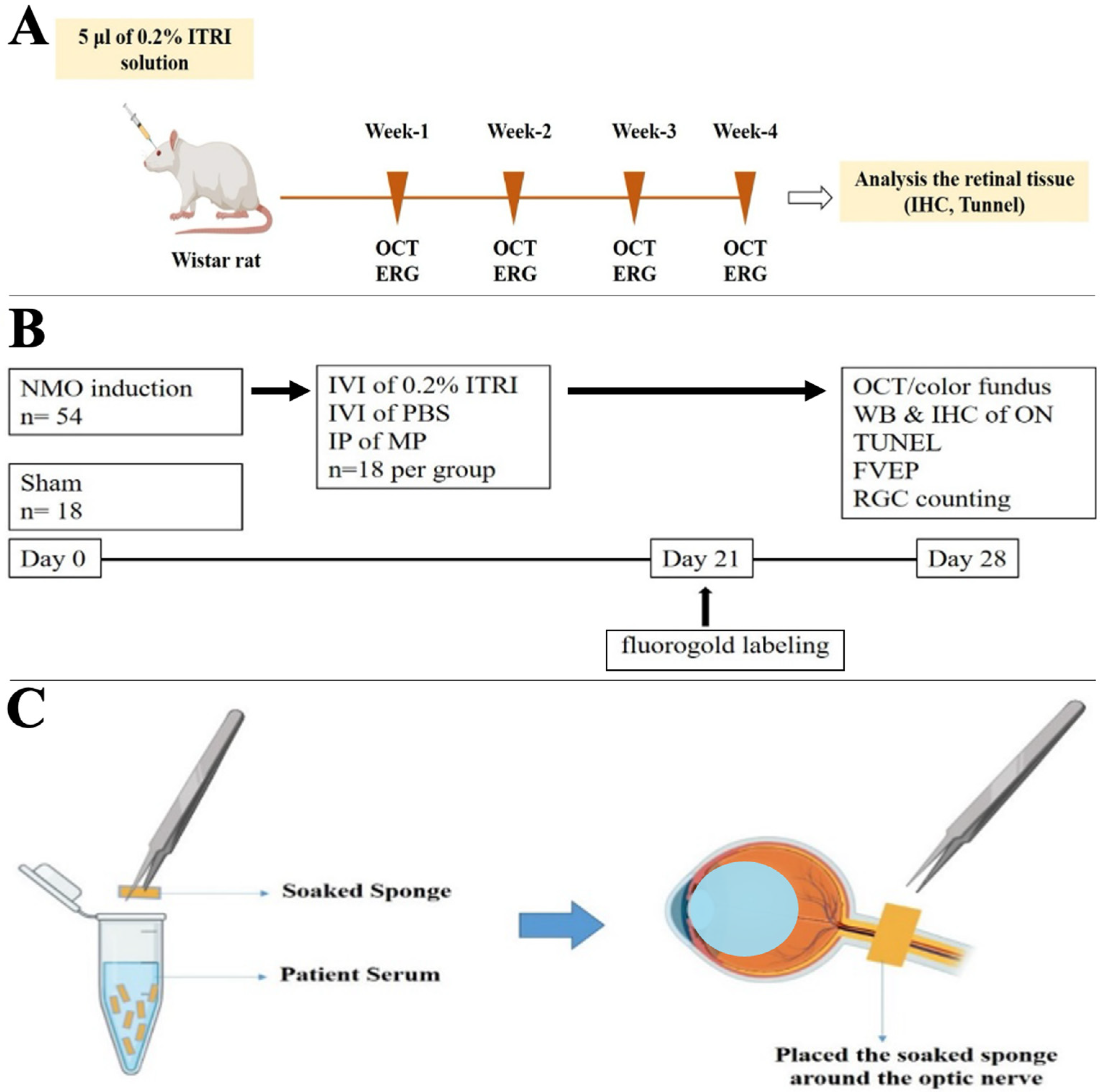
2.1. Animal Model and Study Design
2.2. NMOSD Induction Method
2.3. ITRI-ES Preparation and Administration
2.4. Outcome Measures
2.5. Statistical Analysis
2.6. Standard Protocol Approvals, Registrations, and Patient Consent
3. Results
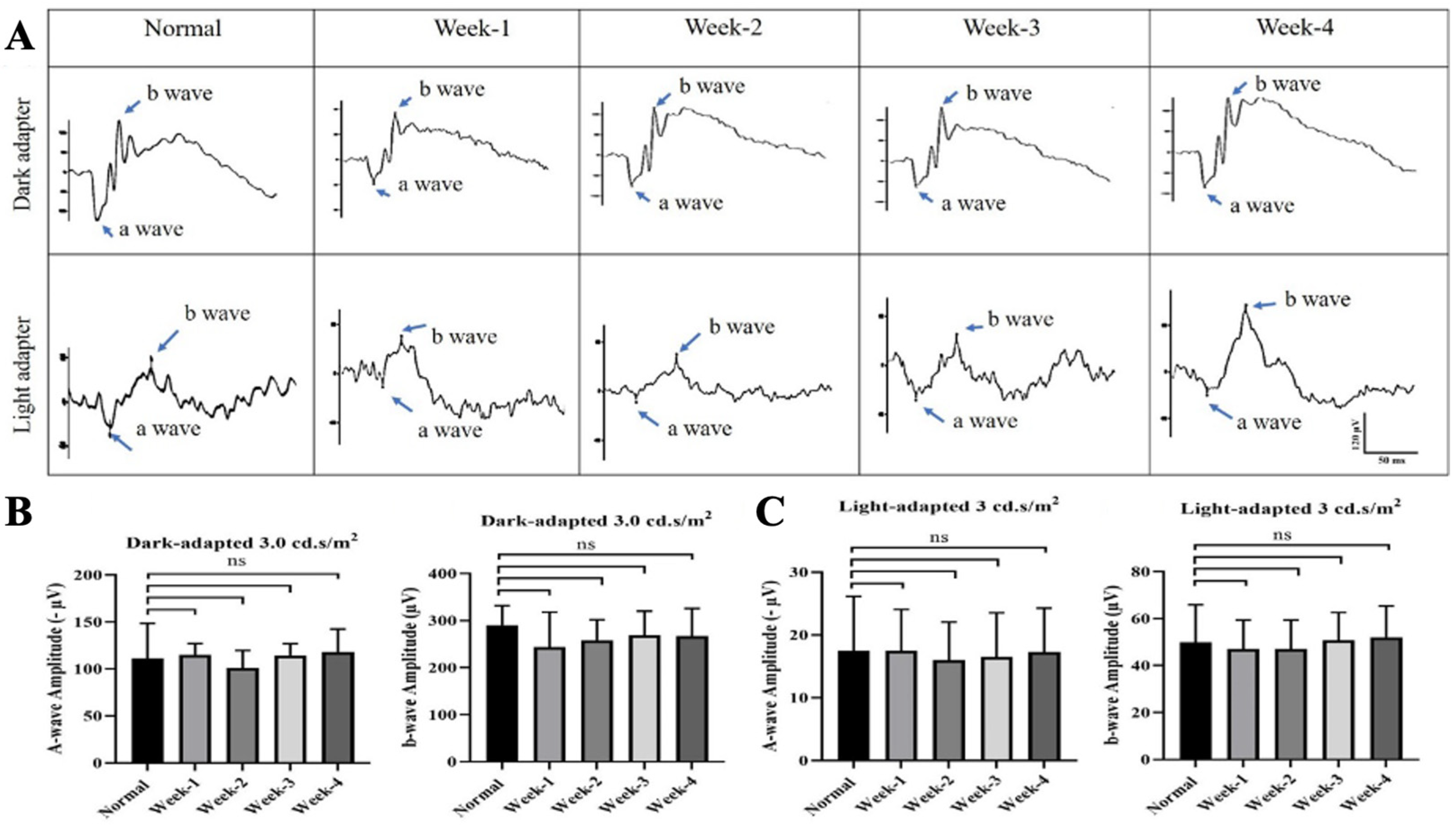
3.1. Safety of Intravitreal ITRI-ES
3.2. ITRI-ES Preserves Visual Function and RGCs in NMOSD Optic Neuritis
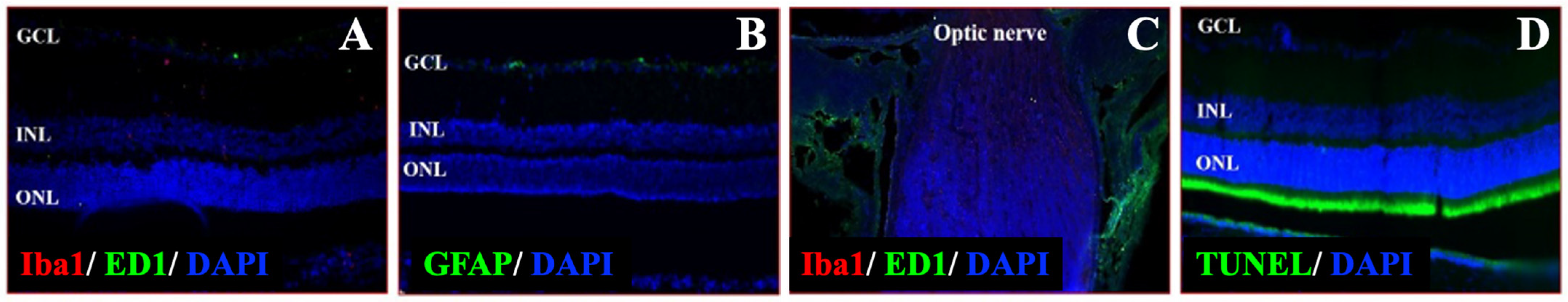
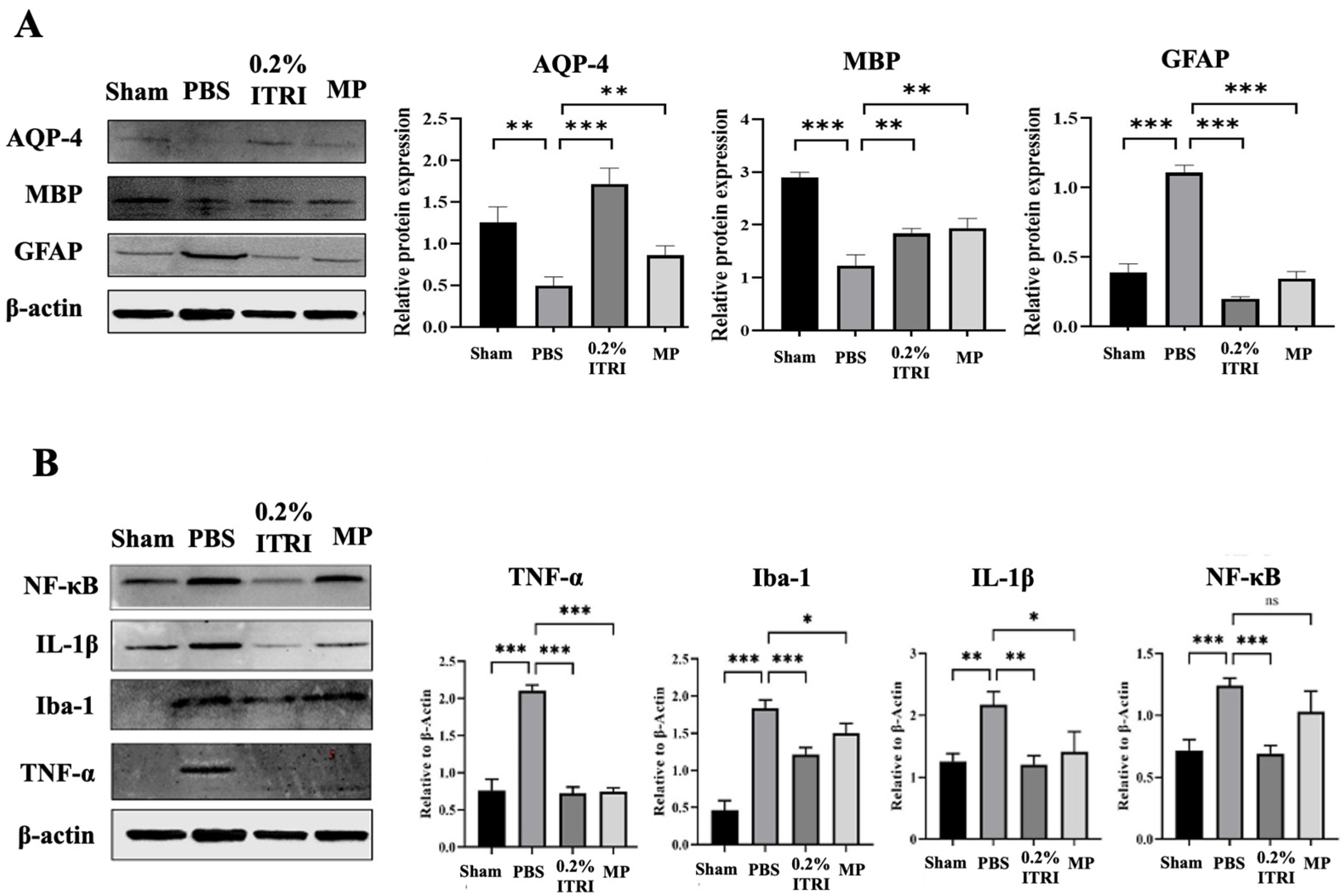
3.3. ITRI-ES Mitigates Optic Nerve Demyelination and Astrocyte Injury
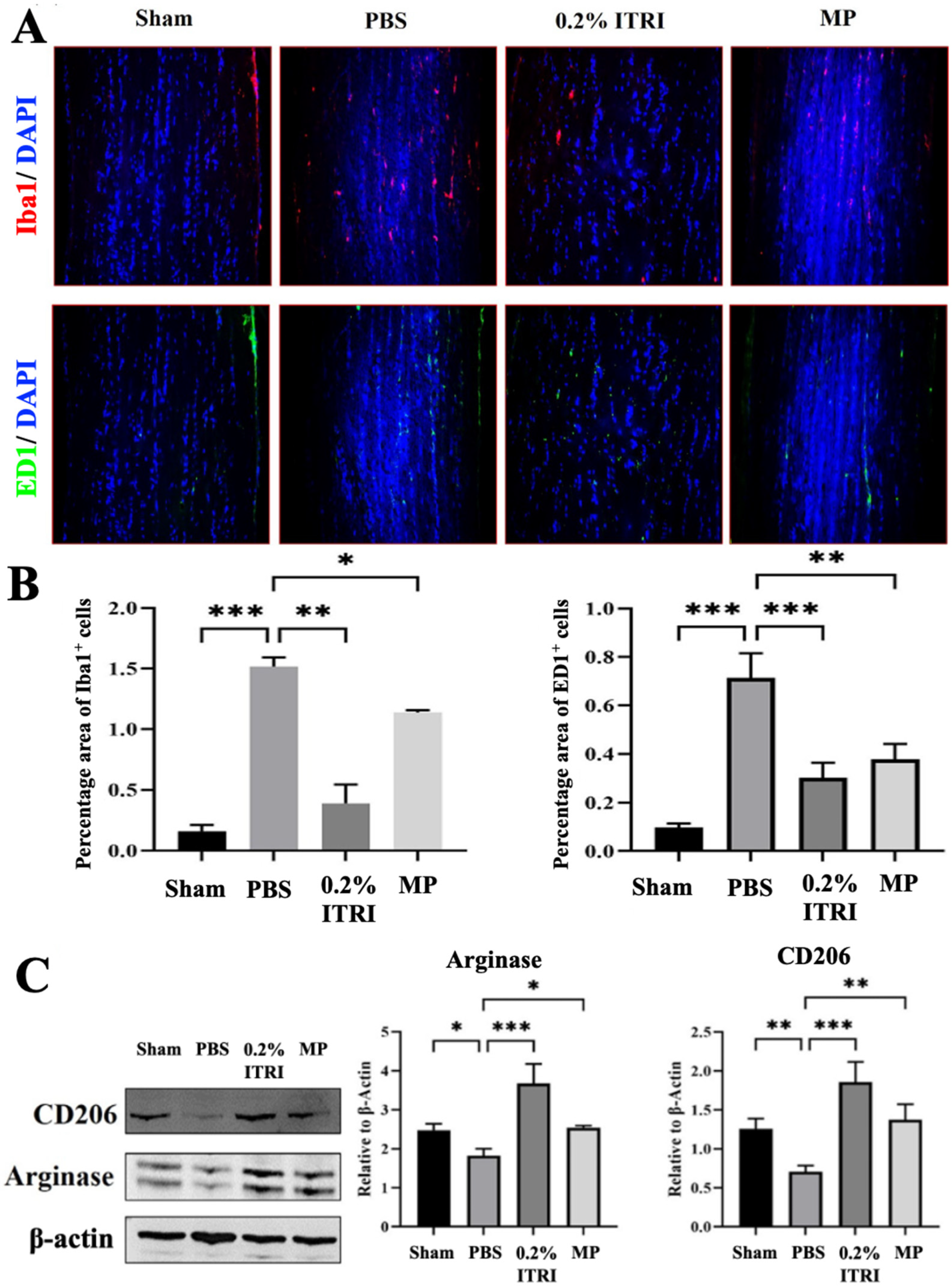
3.4. ITRI-ES Suppresses Neuroinflammation and Promotes an M2 Phenotype
3.5. Validation of NMOSD Pathological Features in the Animal Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AQP4 | Aquaporin-4 |
| Arg1 | Arginase 1 |
| CD206 | Cluster of Differentiation 206 |
| ERG | Electroretinography |
| GFAP | Glial Fibrillary Acidic Protein |
| HPF | High-Power Field |
| Iba1 | Ionized Calcium-Binding Adapter Molecule 1 |
| IL-1β | Interleukin-1 Beta |
| MBP | Myelin Basic Protein |
| MP | Methylprednisolone |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NMOSD | Neuromyelitis Optica |
| OCT | Optical Coherence Tomography |
| ON | Optic Neuritis |
| PBS | Phosphate-Buffered Saline |
| RGC | Retinal Ganglion Cell |
| ROCK | Rho-kinase |
| TNF-α | Tumor Necrosis Factor Alpha |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
References
- Jacob, A.; McKeon, A.; Nakashima, I.; Sato, D.K.; Elsone, L.; Fujihara, K.; de Seze, J. Current concept of neuromyelitis optica (NMO) and NMO spectrum disorders. J. Neurol. Neurosurg. Psychiatry 2013, 84, 922–930. [Google Scholar] [CrossRef]
- Wen, Y.T.; Huang, C.W.; Liu, C.P.; Chen, C.H.; Tu, C.M.; Hwang, C.S.; Chen, Y.H.; Chen, W.R.; Lin, K.L.; Ho, Y.C.; et al. Inhibition of Retinal Ganglion Cell Loss By a Novel ROCK Inhibitor (E212) in Ischemic Optic Nerve Injury Via Antioxidative and Anti-Inflammatory Actions. Investig. Ophthalmol. Vis. Sci. 2021, 62, 21. [Google Scholar] [CrossRef]
- Jarius, S.; Wildemann, B. AQP4 antibodies in neuromyelitis optica: Diagnostic and pathogenetic relevance. Nat. Rev. Neurol. 2010, 6, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Asavapanumas, N.; Ratelade, J.; Papadopoulos, M.C.; Bennett, J.L.; Levin, M.H.; Verkman, A.S. Experimental mouse model of optic neuritis with inflammatory demyelination produced by passive transfer of neuromyelitis optica-immunoglobulin G. J. Neuroinflamm. 2014, 11, 16. [Google Scholar] [CrossRef]
- Misu, T.; Fujihara, K.; Kakita, A.; Konno, H.; Nakamura, M.; Watanabe, S.; Takahashi, T.; Nakashima, I.; Takahashi, H.; Itoyama, Y. Loss of aquaporin 4 in lesions of neuromyelitis optica: Distinction from multiple sclerosis. Brain 2007, 130 Pt 5, 1224–1234. [Google Scholar] [CrossRef]
- Kira, J. Autoimmunity in neuromyelitis optica and opticospinal multiple sclerosis: Astrocytopathy as a common denominator in demyelinating disorders. J. Neurol. Sci. 2011, 311, 69–77. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012, 11, 535–544. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, L.; Geng, J. Neuromyelitis optica spectrum disorder: Pathogenesis, treatment, and experimental models. Mult. Scler. Relat. Disord. 2019, 27, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.C.; Huang, T.L. What’s new in neuromyelitis optica spectrum disorder treatment? Taiwan J. Ophthalmol. 2022, 12, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Hokari, M.; Yokoseki, A.; Arakawa, M.; Saji, E.; Yanagawa, K.; Yanagimura, F.; Toyoshima, Y.; Okamoto, K.; Ueki, S.; Hatase, T.; et al. Clinicopathological features in anterior visual pathway in neuromyelitis optica. Ann. Neurol. 2016, 79, 605–624. [Google Scholar] [CrossRef]
- Collongues, N.; de Seze, J. Current and future treatment approaches for neuromyelitis optica. Ther. Adv. Neurol. Disord. 2011, 4, 111–121. [Google Scholar] [CrossRef]
- Jarius, S.; Wildemann, B.; Paul, F. Neuromyelitis optica: Clinical features, immunopathogenesis and treatment. Clin. Exp. Immunol. 2014, 176, 149–164. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Bennett, J.L.; Verkman, A.S. Treatment of neuromyelitis optica: State-of-the-art and emerging therapies. Nat. Rev. Neurol. 2014, 10, 493–506. [Google Scholar] [CrossRef]
- Abboud, H.; Petrak, A.; Mealy, M.; Sasidharan, S.; Siddique, L.; Levy, M. Treatment of acute relapses in neuromyelitis optica: Steroids alone versus steroids plus plasma exchange. Mult. Scler. 2016, 22, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, P.; Abusamra, K.; Thandampallayam, M.; Kini, A. New advancements in the management of Neuromyelitis Optica spectrum disease: Literature review. Front. Ophthalmol. 2023, 3, 1130971. [Google Scholar] [CrossRef]
- Rowe, L.W.; Barry, Z.R.; Mackay, D.D.; Lai, K.E.; Ciulla, T.A. Autoimmune neuro-ophthalmic disorders: Pathophysiologic mechanisms and targeted biologic therapies. Expert Opin. Biol. Ther. 2025, 25, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, S.; Feng, J.; Qin, X. Advances in the treatment of neuromyelitis optic spectrum disorder. Ther. Adv. Neurol. Disord. 2025, 18, 17562864251328276. [Google Scholar] [CrossRef]
- Duan, T.; Verkman, A.S. Experimental animal models of aquaporin-4-IgG-seropositive neuromyelitis optica spectrum disorders: Progress and shortcomings. Brain Pathol. 2020, 30, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Kanamori, A.; Nakamura, M.; Takahashi, T.; Nakashima, I.; Negi, A. Sera from patients with seropositive neuromyelitis optica spectral disorders caused the degeneration of rodent optic nerve. Exp. Eye Res. 2014, 119, 61–69. [Google Scholar] [CrossRef]
- Koch, J.C.; Tatenhorst, L.; Roser, A.E.; Saal, K.A.; Tönges, L.; Lingor, P. ROCK inhibition in models of neurodegeneration and its potential for clinical translation. Pharmacol. Ther. 2018, 189, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Nourinia, R.; Nakao, S.; Zandi, S.; Safi, S.; Hafezi-Moghadam, A.; Ahmadieh, H. ROCK inhibitors for the treatment of ocular diseases. Br. J. Ophthalmol. 2017; ahead of print. [Google Scholar] [CrossRef]
- Moura-Coelho, N.; Tavares Ferreira, J.; Bruxelas, C.P.; Dutra-Medeiros, M.; Cunha, J.P.; Pinto Proenca, R. Rho kinase inhibitors-a review on the physiology and clinical use in Ophthalmology. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.C.; Tonges, L.; Barski, E.; Michel, U.; Bahr, M.; Lingor, P. ROCK2 is a major regulator of axonal degeneration, neuronal death and axonal regeneration in the CNS. Cell Death Dis. 2014, 5, e1225. [Google Scholar] [CrossRef]
- Benarroch, E. What Is the Role of the Rho-ROCK Pathway in Neurologic Disorders? Neurology 2023, 101, 536–543. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Yu, J.; Feng, L.; Hou, S.; Liu, Y.; Guo, M.; Xie, Y.; Meng, J.; Zhang, H.; et al. Targeting the shift from M1 to M2 macrophages in experimental autoimmune encephalomyelitis mice treated with fasudil. PLoS ONE 2013, 8, e54841. [Google Scholar] [CrossRef]
- Chen, J.; Yin, W.; Tu, Y.; Wang, S.; Yang, X.; Chen, Q.; Zhang, X.; Han, Y.; Pi, R. L-F001, a novel multifunctional ROCK inhibitor, suppresses neuroinflammation in vitro and in vivo: Involvement of NF-kappaB inhibition and Nrf2 pathway activation. Eur. J. Pharmacol. 2017, 806, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Lin, F.P.; Tseng, H.C.; Ho, P.K.; Chen, Y.H.; Chen, Y.G.; Lu, D.W.; Chen, Y.H.; Chou, J.L.; Chen, H.C.; et al. Activation of the ROCK/MYLK Pathway Affects Complex Molecular and Morphological Changes of the Trabecular Meshwork Associated with Ocular Hypertension. Investig. Ophthalmol. Vis. Sci. 2024, 65, 17. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lin, W.Y.; Huang, Y.C.; Ho, W.Y.; Fu, C.W.; Tu, C.M.; Hwang, C.S.; Hung, C.L.; Lin, M.C.; Cheng, F.; et al. The Intraocular Pressure Lowering Effect of a Dual Kinase Inhibitor (ITRI-E-(S)4046) in Ocular Hypertensive Animal Models. Investig. Ophthalmol. Vis. Sci. 2021, 62, 12. [Google Scholar] [CrossRef]
- Garcia, J.G.; Liu, F.; Verin, A.D.; Birukova, A.; Dechert, M.A.; Gerthoffer, W.T.; Bamberg, J.R.; English, D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Investig. 2001, 108, 689–701. [Google Scholar] [CrossRef]
- Yu, J.Z.; Ding, J.; Ma, C.G.; Sun, C.H.; Sun, Y.F.; Lu, C.Z.; Xiao, B.G. Therapeutic potential of experimental autoimmune encephalomyelitis by Fasudil, a Rho kinase inhibitor. J. Neurosci. Res. 2010, 88, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.W.; Wu, P.Y.; Wen, Y.T.; Desai, T.D.; Huang, C.T.; Liu, P.K.; Tsai, R.K. Vitamin B3 Provides Neuroprotection via Antioxidative Stress in a Rat Model of Anterior Ischemic Optic Neuropathy. Antioxidants 2022, 11, 2422. [Google Scholar] [CrossRef]
- Lucchinetti, C.F.; Guo, Y.; Popescu, B.F.; Fujihara, K.; Itoyama, Y.; Misu, T. The pathology of an autoimmune astrocytopathy: Lessons learned from neuromyelitis optica. Brain Pathol. 2014, 24, 83–97. [Google Scholar] [CrossRef]
- Chen, T.; Lennon, V.A.; Liu, Y.U.; Bosco, D.B.; Li, Y.; Yi, M.H.; Zhu, J.; Wei, S.; Wu, L.J. Astrocyte-microglia interaction drives evolving neuromyelitis optica lesion. J. Clin. Investig. 2020, 130, 4025–4038. [Google Scholar] [CrossRef]
- Gajtko, A.; Bakk, E.; Hegedus, K.; Ducza, L.; Hollo, K. IL-1beta Induced Cytokine Expression by Spinal Astrocytes Can Play a Role in the Maintenance of Chronic Inflammatory Pain. Front. Physiol. 2020, 11, 543331. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.R.; Lin, Y.; Langan, T.J.; Iwakura, Y.; Chou, R.C. Innate Immune Functions of Astrocytes are Dependent Upon Tumor Necrosis Factor-Alpha. Sci. Rep. 2020, 10, 7047. [Google Scholar] [CrossRef]
- Chen, T.; Bosco, D.B.; Ying, Y.; Tian, D.S.; Wu, L.J. The Emerging Role of Microglia in Neuromyelitis Optica. Front. Immunol. 2021, 12, 616301. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Tan, W.; Zhou, Y. The role and mechanisms of Microglia in Neuromyelitis Optica Spectrum Disorders. Int. J. Med. Sci. 2021, 18, 3059–3065. [Google Scholar] [CrossRef]
- Saadoun, S.; Waters, P.; Bell, B.A.; Vincent, A.; Verkman, A.S.; Papadopoulos, M.C. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 2010, 133 Pt 2, 349–361. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, Y.; Qiu, W.; Peng, L.; Fang, L.; Xu, Y.; Yang, H. Structural and visual functional deficits in a rat model of neuromyelitis optica spectrum disorders related optic neuritis. Exp. Eye Res. 2018, 175, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; Yang, H.; Gardiner, S.K.; Cepurna, W.O.; Johnson, E.C.; Morrison, J.C.; Burgoyne, C.F. Rat optic nerve head anatomy within 3D histomorphometric reconstructions of normal control eyes. Exp. Eye Res. 2015, 139, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ratelade, J.; Zhang, H.; Saadoun, S.; Bennett, J.L.; Papadopoulos, M.C.; Verkman, A.S. Neuromyelitis optica IgG and natural killer cells produce NMO lesions in mice without myelin loss. Acta Neuropathol. 2012, 123, 861–872. [Google Scholar] [CrossRef]
- Howe, C.L.; Kaptzan, T.; Magana, S.M.; Ayers-Ringler, J.R.; LaFrance-Corey, R.G.; Lucchinetti, C.F. Neuromyelitis optica IgG stimulates an immunological response in rat astrocyte cultures. Glia 2014, 62, 692–708. [Google Scholar] [CrossRef]
- Hinson, S.R.; Clift, I.C.; Luo, N.; Kryzer, T.J.; Lennon, V.A. Autoantibody-induced internalization of CNS AQP4 water channel and EAAT2 glutamate transporter requires astrocytic Fc receptor. Proc. Natl. Acad. Sci. USA 2017, 114, 5491–5496. [Google Scholar] [CrossRef]
- Zhang, H.; Bennett, J.L.; Verkman, A.S. Ex vivo spinal cord slice model of neuromyelitis optica reveals novel immunopathogenic mechanisms. Ann. Neurol. 2011, 70, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Pan, J.; Mamtilahun, M.; Zhu, Y.; Wang, L.; Venkatesh, A.; Shi, R.; Tu, X.; Jin, K.; Wang, Y.; et al. Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics 2020, 10, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Kimbrough, D.J.; Fujihara, K.; Jacob, A.; Lana-Peixoto, M.A.; Leite, M.I.; Levy, M.; Marignier, R.; Nakashima, I.; Palace, J.; de Seze, J.; et al. Treatment of Neuromyelitis Optica: Review and Recommendations. Mult. Scler. Relat. Disord. 2012, 1, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Songthammawat, T.; Srisupa-Olan, T.; Siritho, S.; Kittisares, K.; Jitprapaikulsan, J.; Sathukitchai, C.; Prayoonwiwat, N. A pilot study comparing treatments for severe attacks of neuromyelitis optica spectrum disorders: Intravenous methylprednisolone (IVMP) with add-on plasma exchange (PLEX) versus simultaneous ivmp and PLEX. Mult. Scler. Relat. Disord. 2020, 38, 101506. [Google Scholar] [CrossRef]
- Levin, M.H.; Bennett, J.L.; Verkman, A.S. Optic neuritis in neuromyelitis optica. Prog. Retin. Eye Res. 2013, 36, 159–171. [Google Scholar] [CrossRef]
- Zeng, Q.; Dong, X.; Ruan, C.; Hu, B.; Luo, Y.; Luo, Z.; Xu, L.; Zhou, H.; Wang, R.; Yang, H. CD14(+)CD16(++) monocytes are increased in patients with NMO and are selectively suppressed by glucocorticoids therapy. J. Neuroimmunol. 2016, 300, 1–8. [Google Scholar] [CrossRef]
- Tan, H.B.; Zhong, Y.S.; Cheng, Y.; Shen, X. Rho/ROCK pathway and neural regeneration: A potential therapeutic target for central nervous system and optic nerve damage. Int. J. Ophthalmol. 2011, 4, 652–657. [Google Scholar] [CrossRef]
- Sanjari, N.; Pakravan, M.; Nourinia, R.; Esfandiari, H.; Hafezi-Moghadam, A.; Zandi, S.; Nakao, S.; Shah-Heidari, M.H.; Jamali, A.; Yaseri, M.; et al. Intravitreal Injection of a Rho-Kinase Inhibitor (Fasudil) for Recent-Onset Nonarteritic Anterior Ischemic Optic Neuropathy. J. Clin. Pharmacol. 2016, 56, 749–753. [Google Scholar] [CrossRef]
- Miao, H.; Crabb, A.W.; Hernandez, M.R.; Lukas, T.J. Modulation of factors affecting optic nerve head astrocyte migration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4096–4103. [Google Scholar] [CrossRef] [PubMed]
- Ratelade, J.; Verkman, A.S. Inhibitor(s) of the classical complement pathway in mouse serum limit the utility of mice as experimental models of neuromyelitis optica. Mol. Immunol. 2014, 62, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Varrin-Doyer, M.; Spencer, C.M.; Schulze-Topphoff, U.; Nelson, P.A.; Stroud, R.M.; Cree, B.A.; Zamvil, S.S. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann. Neurol. 2012, 72, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Lucchinetti, C.F.; Mandler, R.N.; McGavern, D.; Bruck, W.; Gleich, G.; Ransohoff, R.M.; Trebst, C.; Weinshenker, B.; Wingerchuk, D.; Parisi, J.E.; et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain 2002, 125 Pt 7, 1450–1461. [Google Scholar] [CrossRef]
- Merle, H.; Olindo, S.; Jeannin, S.; Valentino, R.; Mehdaoui, H.; Cabot, F.; Donnio, A.; Hage, R.; Richer, R.; Smadja, D.; et al. Treatment of optic neuritis by plasma exchange (add-on) in neuromyelitis optica. Arch. Ophthalmol. 2012, 130, 858–862. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-T.; Hossen, M.; Chen, T.-W.; Fu, C.-W.; Chen, Y.-H.; Huang, T.-L.; Tsai, R.-K. Dual ROCK1/2–MYLK4 Kinase Inhibition Preserves Visual Function in a Rat Model of Neuromyelitis Optica Spectrum Disorder Optic Neuritis. Cells 2025, 14, 1712. https://doi.org/10.3390/cells14211712
Huang C-T, Hossen M, Chen T-W, Fu C-W, Chen Y-H, Huang T-L, Tsai R-K. Dual ROCK1/2–MYLK4 Kinase Inhibition Preserves Visual Function in a Rat Model of Neuromyelitis Optica Spectrum Disorder Optic Neuritis. Cells. 2025; 14(21):1712. https://doi.org/10.3390/cells14211712
Chicago/Turabian StyleHuang, Chin-Te, Monir Hossen, Tu-Wen Chen, Chih-Wei Fu, Yi-Hsun Chen, Tzu-Lun Huang, and Rong-Kung Tsai. 2025. "Dual ROCK1/2–MYLK4 Kinase Inhibition Preserves Visual Function in a Rat Model of Neuromyelitis Optica Spectrum Disorder Optic Neuritis" Cells 14, no. 21: 1712. https://doi.org/10.3390/cells14211712
APA StyleHuang, C.-T., Hossen, M., Chen, T.-W., Fu, C.-W., Chen, Y.-H., Huang, T.-L., & Tsai, R.-K. (2025). Dual ROCK1/2–MYLK4 Kinase Inhibition Preserves Visual Function in a Rat Model of Neuromyelitis Optica Spectrum Disorder Optic Neuritis. Cells, 14(21), 1712. https://doi.org/10.3390/cells14211712






