Cellular and Molecular Effects of Targeting the CBP/β-Catenin Interaction with PRI-724 in Melanoma Cells, Drug-Naïve and Resistant to Inhibitors of BRAFV600 and MEK1/2
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds
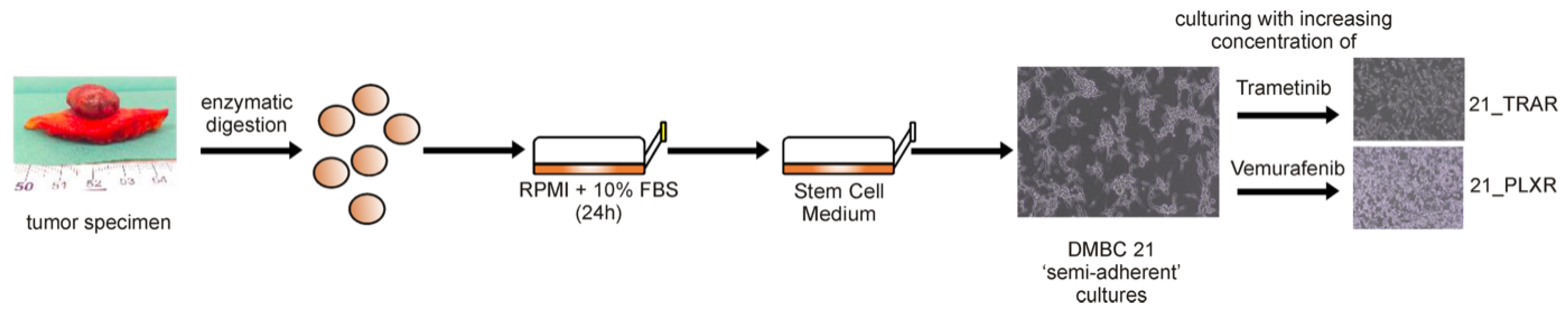
2.2. Generation and Culture of Drug-Naïve and Trametinib- and Vemurafenib-Resistant Melanoma Cells
2.3. Assessment of Acid Phosphatase Activity (APA)
2.4. Time-Lapse Microscopy (IncuCyte® ZOOM)
2.5. Caspase-3/7 Activation Assessed by Time-Lapse Fluorescence Microscopy
2.6. Flow Cytometry
2.7. Western Blotting
2.8. Invasion Assays
2.9. RNA Sequencing Analysis
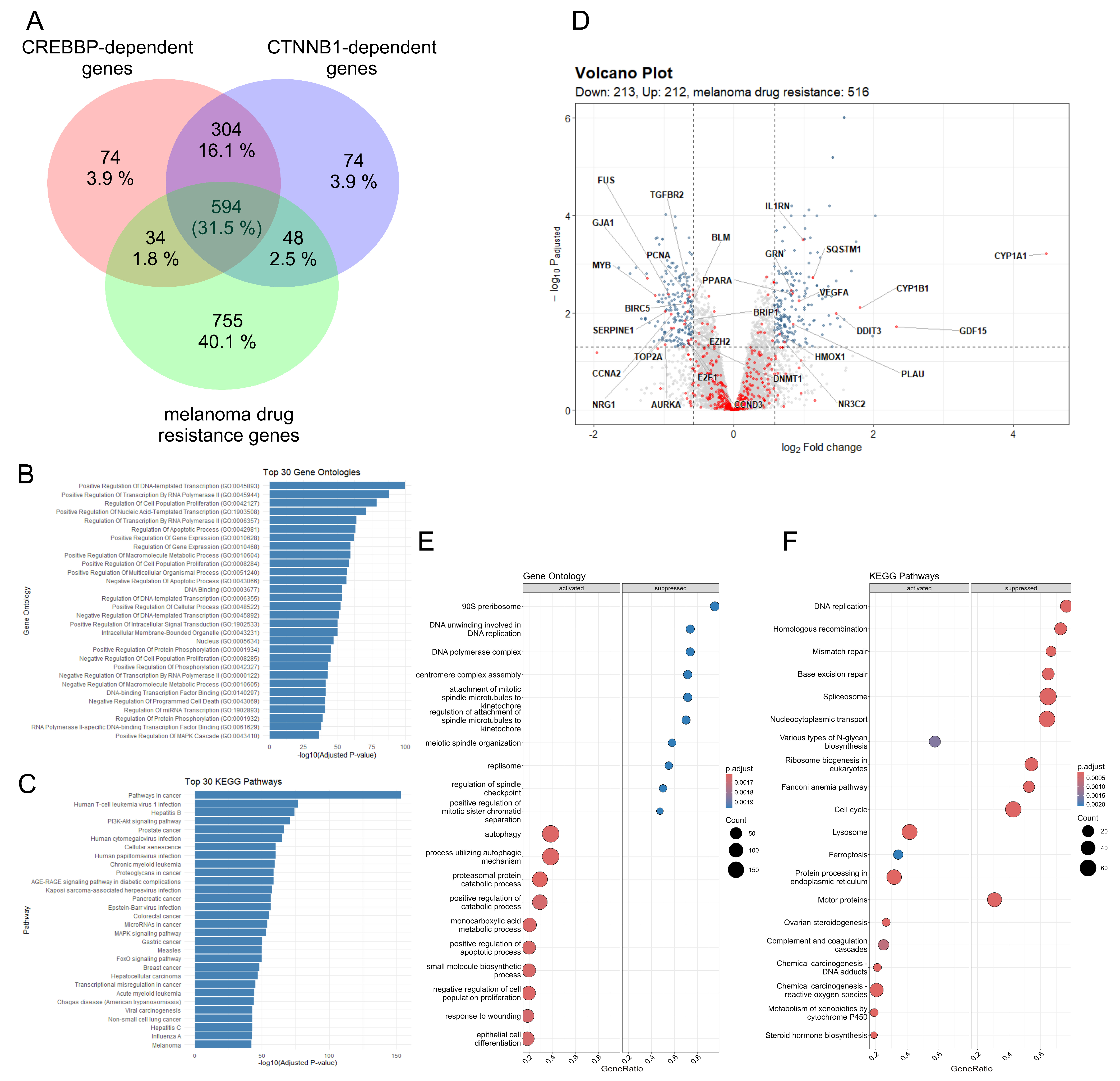
2.10. Bioinformatics Analysis
2.11. Statistical Analysis
3. Results
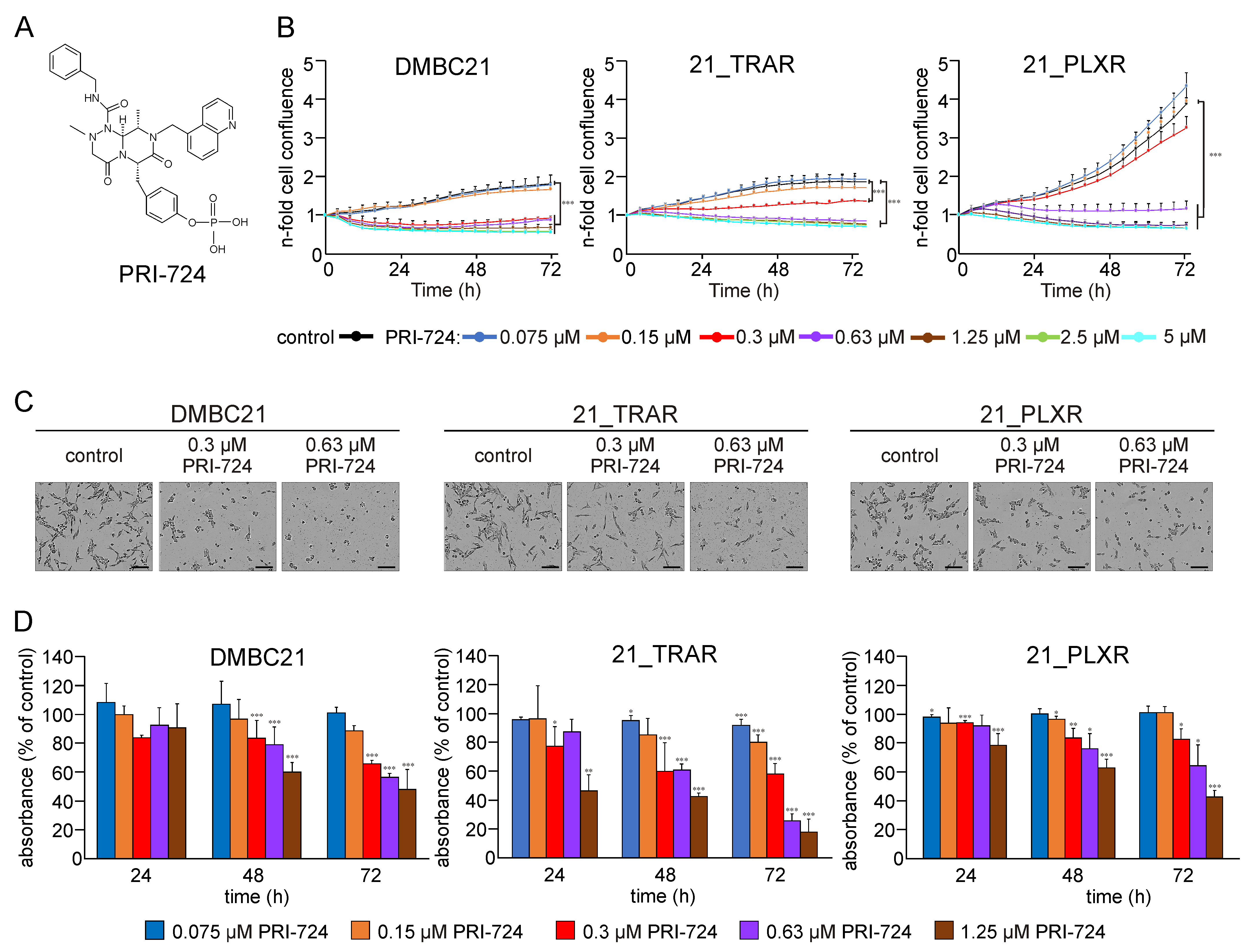
3.1. PRI-724 Reduces Viability of Drug-Naïve and Trametinib- and Vemurafenib-Resistant Melanoma Cells
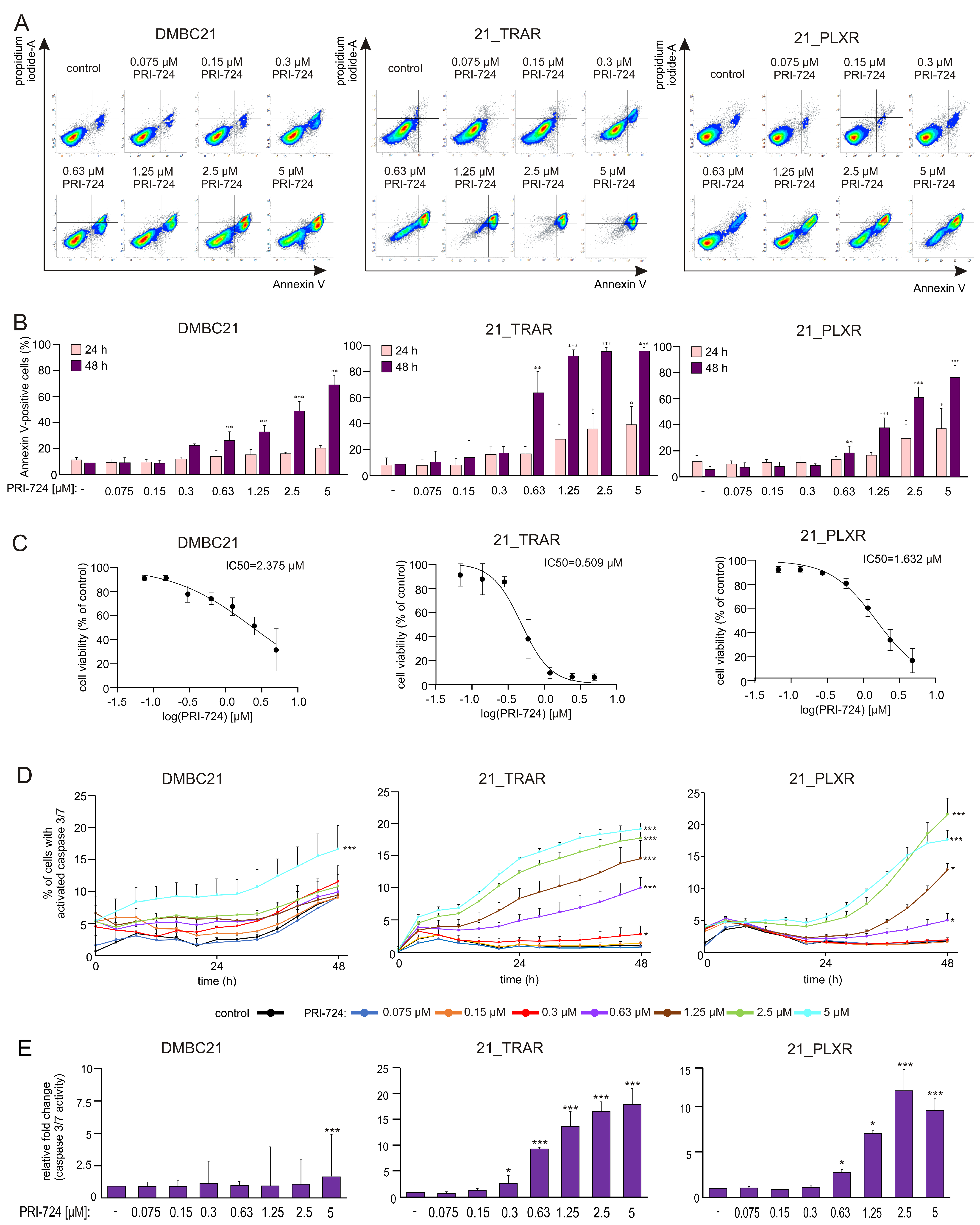
3.2. PRI-724 Effectively Induces Apoptosis in a Concentration-Dependent Manner in Drug-Naïve and Trametinib- and Vemurafenib-Resistant Melanoma Cells
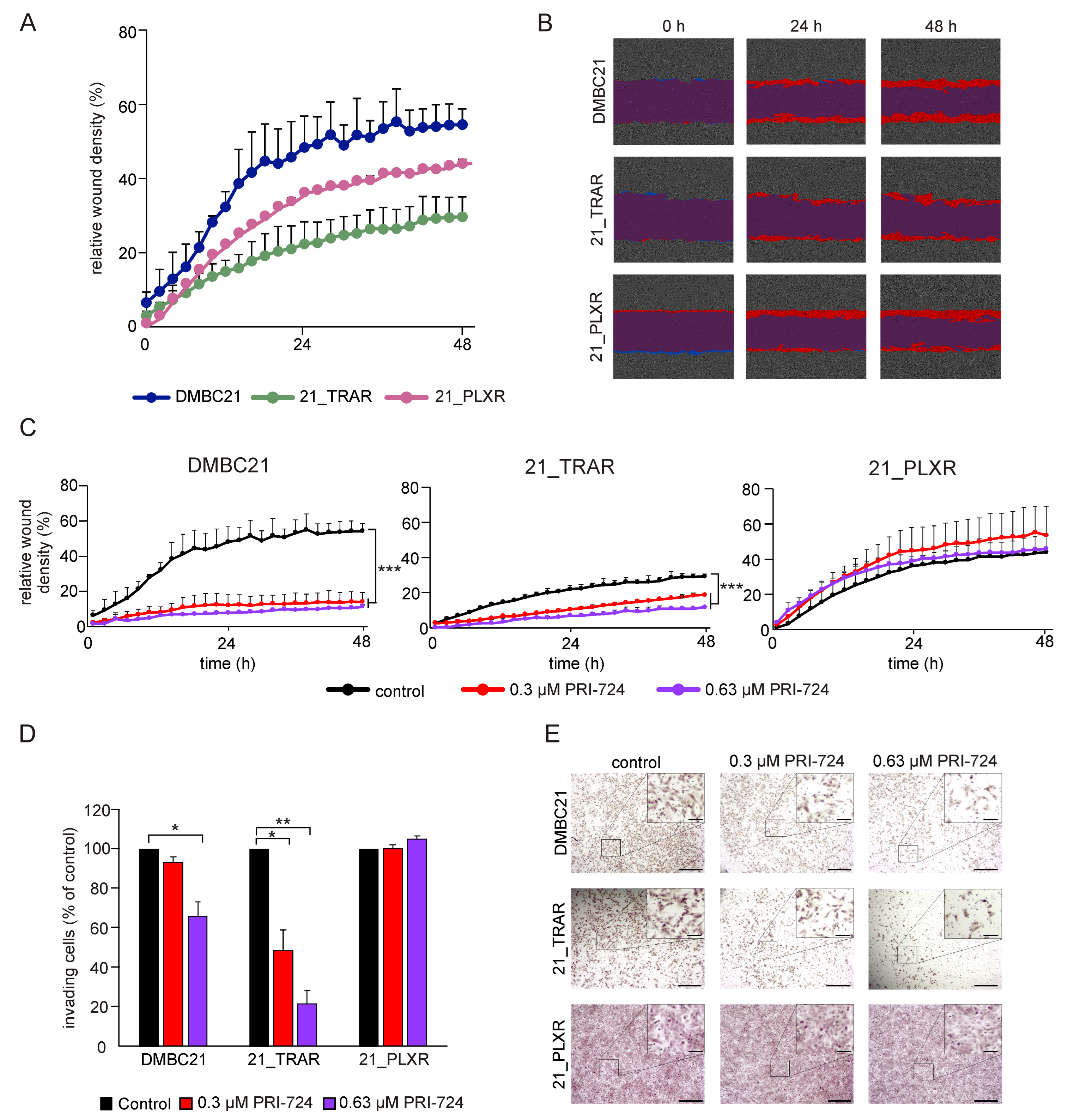
3.3. Treatment with PRI-724 Attenuates the Invasive Potential of Drug-Naïve and Trametinib-and Vemurafenib-Resistant Melanoma Cells
3.4. The CBP/β-Catenin Signaling Pathway May Play a Significant Role in Development and Maintenance of Drug Resistance in Melanoma
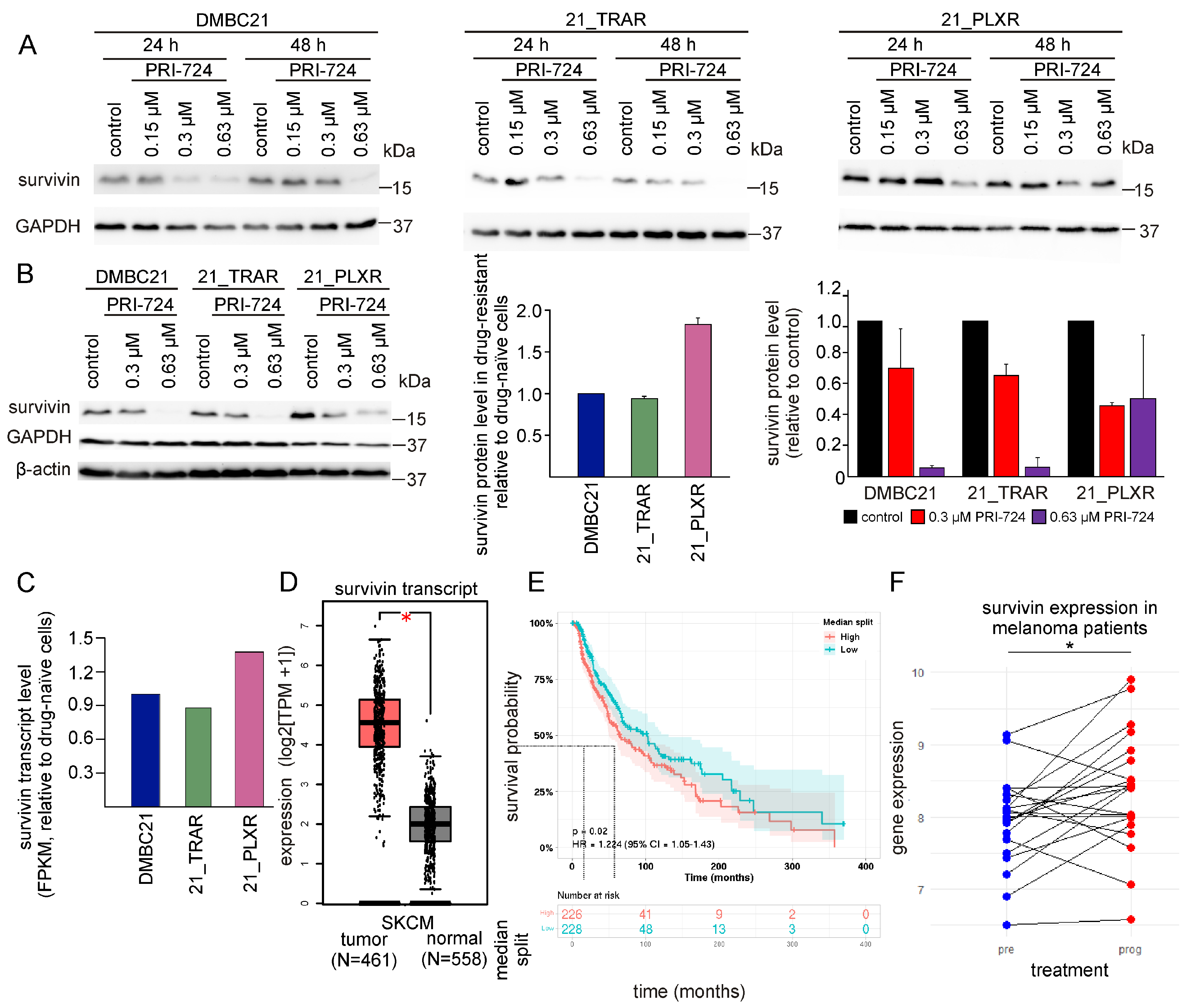
3.5. PRI-724 Downregulates Survivin (BIRC5)
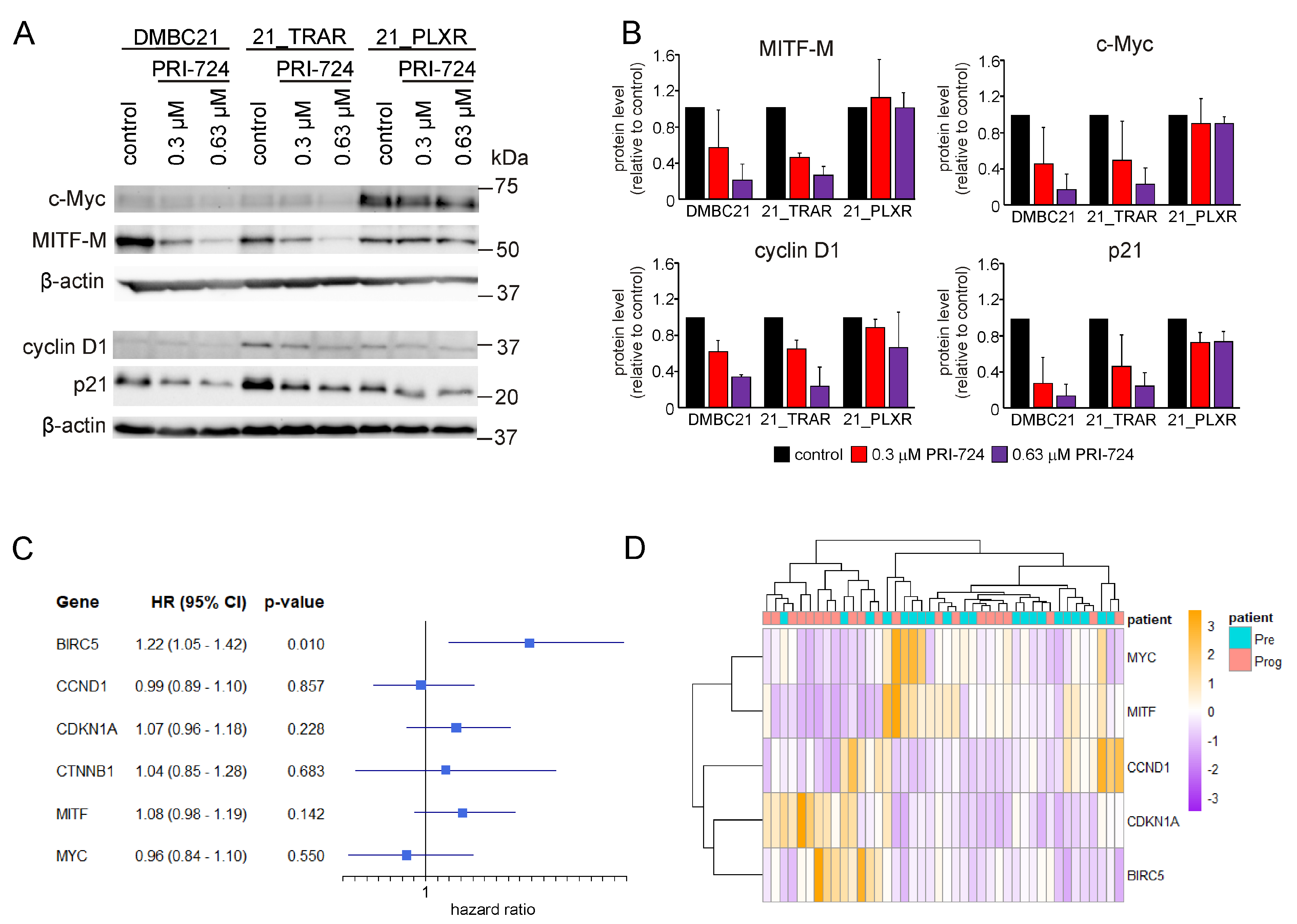
3.6. PRI-724 Downregulates the Level of CBP/β-Catenin Dependent Proteins in Drug-Naïve and Trametinib- and Vemurafenib-Resistant Melanoma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| bFGF | Basic fibroblast growth factor |
| BIRC5 | Baculoviral inhibitor of apoptosis repeat-containing 5 |
| BP | Biological process |
| BRAF | B-Raf murine sarcoma viral oncogene homolog B |
| CBP | CREB-binding protein |
| CC | Cellular component |
| CI | Confidence interval |
| DMBC | Department of Molecular Biology of Cancer |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| GCTs | Testicular germ cell tumors |
| GEO | Gene Expression Omnibus |
| GEPIA2 | Gene Expression Profiling Interactive Analysis 2 |
| GO | Gene ontology |
| GTEx | Genotype-Tissue Expression |
| HBSS | Hanks’ balanced salt solution |
| HR | Hazard ratio |
| HRP | Horseradish peroxidase |
| HSP-90 | Heat shock protein-90 |
| IAP | Inhibitor of apoptosis |
| ICIs | Immune checkpoint inhibitors |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MF | Molecular function |
| MI | Mitotic index |
| MMC | Mitomycin C |
| NES | Normalized enrichment score |
| ORA | Over-representation analysis |
| OSCC | Oral squamous cell carcinoma |
| PBS | Phosphate-buffered saline |
| PET | Polyethylene terephthalate |
| PI | Propidium iodide |
| PI3K/Akt | Phosphatidylinositol 3-kinase/protein kinase B |
| PreCOG | Prediction of Clinical Outcomes from Genomic Profiles |
| SCM | Stem cell medium |
| RWD | Relative wound density |
| SKCM | Skin cutaneous melanoma |
| STAT3 | Signal transducer and activator of transcription 3 |
| TCGA | The Cancer Genome Atlas |
| TKI | Tyrosine kinase inhibitor |
| YAP | Yes-associated protein |
References
- Ernst, M.; Giubellino, A. The Current State of Treatment and Future Directions in Cutaneous Malignant Melanoma. Biomedicines 2022, 10, 822. [Google Scholar] [CrossRef]
- Hossain, S.M. Phenotype Switching and the Melanoma Microenvironment, Impact on Immunotherapy and Drug Resistance. Int. J. Mol. Sci. 2023, 24, 1601. [Google Scholar] [CrossRef]
- Boutros, A.; Croce, E.; Ferrari, M.; Gili, R.; Massaro, G.; Marconcini, R.; Arecco, L.; Tanda, E.T.; Spagnolo, F. The treatment of advanced melanoma: Current approaches and new challenges. Crit. Rev. Oncol. Hematol. 2024, 196, 104276. [Google Scholar] [CrossRef]
- Wagstaff, W.; Mwamba, R.N.; Grullon, K.; Armstrong, M.; Zhao, P.; Hendren-Santiago, B.; Qin, K.H.; Li, A.J.; Hu, D.A.; Youssef, A.; et al. Melanoma: Molecular genetics, metastasis, targeted therapies, immunotherapies, and therapeutic resistance. Genes Dis. 2022, 9, 1608–1623. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, H.; Li, C. Signal pathways of melanoma and targeted therapy. Sig. Transduct. Target Ther. 2021, 6, 424. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Czyz, M. Anti-apoptotic proteins on guard of melanoma cell survival. Cancer Lett. 2013, 331, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Peery, R.C.; Liu, J.Y.; Zhang, J.T. Targeting survivin for therapeutic discovery: Past, present, and future promises. Drug Discov. Today 2017, 22, 1466–1477. [Google Scholar] [CrossRef]
- Albadari, N.; Li, W. Survivin small molecules inhibitors: Recent advances and challenges. Molecules 2023, 28, 1376. [Google Scholar] [CrossRef]
- Martínez-Sifuentes, M.A.; Bassol-Mayagoitia, S.; Nava-Hernández, M.P.; Ruiz-Flores, P.; Ramos-Treviño, J.; Haro-Santa Cruz, J.; Hernández-Ibarra, J.A. Survivin in breast cancer: A review. Genet. Test Mol. Biomark. 2022, 26, 411–421. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Jia, Y.; Zhang, Q.; Zhu, P.; Ma, X. α5-nAChR and survivin: Two potential biological targets in lung adenocarcinoma. J. Cell Physiol. 2021, 236, 1787–1797. [Google Scholar] [CrossRef]
- Warrier, N.M.; Krishnan, R.K.; Prabhu, V.; Hariharapura, R.C.; Agarwal, P.; Kumar, P. Survivin inhibition by piperine sensitizes glioblastoma cancer stem cells and leads to better drug response. Int. J. Mol. Sci. 2022, 23, 7604. [Google Scholar] [CrossRef]
- Wang, H.; Jin, S.; Lu, H.; Mi, S.; Shao, W.; Zuo, X.; Yin, H.; Zeng, S.; Shimamoto, F.; Qi, G. Expression of survivin, MUC2 and MUC5 in colorectal cancer and their association with clinicopathological characteristics. Oncol. Lett. 2017, 14, 1011–1016. [Google Scholar] [CrossRef]
- Branco, P.C.; Pontes, C.A.; Rezende-Teixeira, P.; Amengual-Rigo, P.; Alves-Fernandes, D.K.; Maria-Engler, S.S.; da Silva, A.B.; Pessoa, O.D.L.; Jimenez, P.C.; Mollasalehi, N.; et al. Survivin modulation in the antimelanoma activity of prodiginines. Eur. J. Pharmacol. 2020, 888, 173465. [Google Scholar] [CrossRef] [PubMed]
- Kondapuram, S.K.; Ramachandran, H.K.; Arya, H.; Coumar, M.S. Targeting survivin for cancer therapy: Strategies, small molecule inhibitors and vaccine based therapeutics in development. Life Sci. 2023, 335, 122260. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.; Adida, C.; Altieri, D.C. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 1997, 3, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Huang, C.; Liu, J.Y.; Zhang, J.T. Small Molecule Inhibitors Targeting the “Undruggable” Survivin: The Past, Present, and Future from a Medicinal Chemist’s Perspective. J. Med. Chem. 2023, 66, 16515–16545. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Liu, H.; Xu, H.; Zheng, H.; Qian, F.; Li, W.; Zhao, C.; Wang, Z.; Wang, X. Notch-1 signaling facilitates survivin expression in human non-small cell lung cancer cells. Cancer Biol. Ther. 2011, 11, 14–21. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Lyu, Z.; Chen, Y.; Ji, X.; Cao, H.; Jin, M.; Zhu, J.; Yang, J.; Ling, R.; et al. Positive feedback loop between mitochondrial fission and Notch signaling promotes survivin-mediated survival of TNBC cells. Cell Death Dis. 2018, 9, 1050. [Google Scholar] [CrossRef]
- Muramatsu, T.; Imoto, I.; Matsui, T.; Kozaki, K.; Haruki, S.; Sudol, M.; Shimada, Y.; Tsuda, H.; Kawano, T.; Inazawa, J. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis 2011, 32, 389–398. [Google Scholar] [CrossRef]
- Vlčková, K.; Ondrušová, L.; Vachtenheim, J.; Réda, J.; Dundr, P.; Zadinová, M.; Žáková, P.; Poučková, P. Survivin, a novel target of the Hedgehog/GLI signaling pathway in human tumor cells. Cell Death Dis. 2016, 7, e2048. [Google Scholar] [CrossRef]
- Asanuma, H.; Torigoe, T.; Kamiguchi, K.; Hirohashi, Y.; Ohmura, T.; Hirata, K.; Sato, M.; Sato, N. Survivin expression is regulated by coexpression of human epidermal growth factor receptor 2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cells. Cancer Res. 2005, 65, 11018–11025. [Google Scholar] [CrossRef]
- Zhao, P.; Meng, Q.; Liu, L.Z.; You, Y.P.; Liu, N.; Jiang, B.H. Regulation of survivin by PI3K/Akt/p70S6K1 pathway. Biochem. Biophys. Res. Commun. 2010, 395, 219–224. [Google Scholar] [CrossRef]
- Gu, Y.; Jin, S.; Wang, F.; Hua, Y.; Yang, L.; Shu, Y.; Zhang, Z.; Guo, R. Clinicopathological significance of PI3K, Akt and survivin expression in gastric cancer. Biomed. Pharmacother. 2014, 68, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Laka, K.; Makgoo, L.; Mbita, Z. Survivin Splice Variants in Arsenic Trioxide (As2O3)-Induced Deactivation of PI3K and MAPK Cell Signalling Pathways in MCF-7 Cells. Genes 2019, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.Z.; Mak, D.H.; Schober, W.D.; Cabreira-Hansen, M.; Beran, M.; McQueen, T.; Chen, W.; Andreeff, M. Regulation of survivin expression through Bcr-Abl/MAPK cascade: Targeting survivin overcomes imatinib resistance and increases imatinib sensitivity in imatinib-responsive CML cells. Blood 2006, 107, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Tracey, L.; Pérez-Rosado, A.; Artiga, M.J.; Camacho, F.I.; Rodríguez, A.; Martínez, N.; Ruiz-Ballesteros, E.; Mollejo, M.; Martinez, B.; Cuadros, M.; et al. Expression of the NF-kappaB targets BCL2 and BIRC5/Survivin characterizes small and aggressive B-cell lymphomas, respectively. J. Pathol. 2005, 206, 123–134. [Google Scholar] [CrossRef]
- Anandharaj, A.; Cinghu, S.; Park, W.Y. Rapamycin-mediated mTOR inhibition attenuates survivin and sensitizes glioblastoma cells to radiation therapy. Acta Biochim. Biophys. Sin. 2011, 43, 292–300. [Google Scholar] [CrossRef]
- Sehara, Y.; Sawicka, K.; Hwang, J.Y.; Latuszek-Barrantes, A.; Etgen, A.M.; Zukin, R.S. Survivin Is a transcriptional target of STAT3 critical to estradiol neuroprotection in global ischemia. J. Neurosci. 2013, 33, 12364–12374. [Google Scholar] [CrossRef]
- Senol, S.; Yildirim, A.; Ceyran, B.; Uruc, F.; Zemheri, E.; Ozkanli, S.; Akalin, I.; Ulus, I.; Caskurlu, T.; Aydin, A. Prognostic significance of survivin, β-catenin and p53 expression in urothelial carcinoma. Bosn. J. Basic Med. Sci. 2015, 15, 7–14. [Google Scholar] [CrossRef]
- Bu, H.; Liu, D.; Cui, J.; Cai, K.; Shen, F. Wnt/β-catenin signaling pathway is involved in induction of apoptosis by oridonin in colon cancer COLO205 cells. Transl. Cancer Res. 2019, 8, 1782–1794. [Google Scholar] [CrossRef]
- Kahn, M. Taking the road less traveled—The therapeutic potential of CBP/β-catenin antagonists. Expert. Opin. Ther. Targets 2021, 25, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Schmidtova, S.; Kalavska, K.; Liskova, V.; Plava, J.; Miklikova, S.; Kucerova, L.; Matuskova, M.; Rojikova, L.; Cierna, Z.; Rogozea, A.; et al. Targeting of Deregulated Wnt/β-Catenin Signaling by PRI-724 and LGK974 Inhibitors in Germ Cell Tumor Cell Lines. Int. J. Mol. Sci. 2021, 22, 4263. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.F.; Spoettl, G.; Maurer, J.; Nölting, S.; Auernhammer, C.J. Inhibition of Wnt/β-Catenin Signaling in Neuroendocrine Tumors in vitro: Antitumoral Effects. Cancers 2020, 12, 345. [Google Scholar] [CrossRef]
- Kleszcz, R.; Szymanska, A.; Krajka-Kuzniak, V.; Baer-Dubowska, W.; Paluszczak, J. Inhibition of CBP/beta-catenin and porcupine attenuates Wnt signaling and induces apoptosis in head and neck carcinoma cells. Cell Oncol. 2019, 42, 505–520. [Google Scholar] [CrossRef]
- Fang, F.; Van Cleave, A.; Helmuth, R.; Torres, H.; Rickel, K.; Wollenzien, H.; Sun, H.; Zeng, E.; Zhao, J.; Tao, J. Targeting the Wnt/beta-catenin pathway in human osteosarcoma cells. Oncotarget 2018, 9, 36780–36792. [Google Scholar] [CrossRef]
- Gabata, R.; Harada, K.; Mizutani, Y.; Ouchi, H.; Yoshimura, K.; Sato, Y.; Kitao, A.; Kimura, K.; Kouji, H.; Miyashita, T.; et al. Anti-tumor activity of the small molecule inhibitor PRI-724 against beta-catenin-activated hepatocellular carcinoma. Anticancer Res. 2020, 40, 5211–5219. [Google Scholar] [CrossRef]
- He, Y.; Liu, D.; Ling, A.; Han, Z.; Cui, J.; Cheng, J.; Feng, Y.; Liu, W.; Gong, W.; Xia, Y.; et al. Inhibition of Wnt/β-catenin increases anti-tumor activity by synergizing with sorafenib in hepatocellular carcinoma. Cell Death Dis. 2025, 16, 466. [Google Scholar] [CrossRef]
- Martinez-Font, E.; Pérez-Capó, M.; Ramos, R.; Felipe, I.; Garcías, C.; Luna, P.; Terrasa, J.; Martín-Broto, J.; Vögler, O.; Alemany, R.; et al. Impact of Wnt/β-Catenin Inhibition on Cell Proliferation through CDC25A Downregulation in Soft Tissue Sarcomas. Cancers 2020, 12, 2556. [Google Scholar] [CrossRef]
- Kelch, M.A.; Vera-Guapi, A.; Beder, T.; Oswald, M.; Hiemisch, A.; Beil, N.; Wajda, P.; Ciesek, S.; Erfle, H.; Toptan, T.; et al. Machine learning on large scale perturbation screens for SARS-CoV-2 host factors identifies β-catenin/CBP inhibitor PRI-724 as a potent antiviral. Front. Microbiol. 2023, 14, 1193320. [Google Scholar] [CrossRef]
- Zhou, H.; Mak, P.Y.; Mu, H.; Mak, D.H.; Zeng, Z.; Cortes, J.; Liu, Q.; Andreeff, M.; Carter, B.Z. Combined inhibition of β-catenin and Bcr-Abl synergistically targets tyrosine kinase inhibitor-resistant blast crisis chronic myeloid leukemia blasts and progenitors in vitro and in vivo. Leukemia 2017, 31, 2065–2074. [Google Scholar] [CrossRef]
- Jiang, X.; Mak, P.Y.; Mu, H.; Tao, W.; Mak, D.H.; Kornblau, S.; Zhang, Q.; Ruvolo, P.; Burks, J.K.; Zhang, W.; et al. Disruption of Wnt/β-Catenin Exerts Antileukemia Activity and Synergizes with FLT3 Inhibition in FLT3-Mutant Acute Myeloid Leukemia. Clin. Cancer Res. 2018, 24, 2417–2429. [Google Scholar] [CrossRef]
- Hartman, M.L.; Sztiller-Sikorska, M.; Czyz, M. Whole-exome sequencing reveals novel genetic variants associated with diverse phenotypes of melanoma cells. Mol. Carcinog. 2019, 58, 588–602. [Google Scholar] [CrossRef]
- Czyz, M.; Sztiller-Sikorska, M.; Gajos-Michniewicz, A.; Osrodek, M.; Hartman, M.L. Plasticity of Drug-Naïve and Vemurafenib- or Trametinib-Resistant Melanoma Cells in Execution of Differentiation/Pigmentation Program. J. Oncol. 2019, 2019, 1697913. [Google Scholar] [CrossRef]
- Sztiller-Sikorska, M.; Hartman, M.; Talar, B.; Jakubowska, J.; Zalesna, I.; Czyz, M. Phenotypic diversity of patient-derived melanoma populations in stem cell medium. Lab. Investig. 2015, 95, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Kartha, V.K.; Abalkhail, K.A.; Sadykov, K.; Nguyen, B.C.; Laroche, F.; Feng, H.; Lee, J.; Pai, S.I.; Varelas, X.; Egloff, A.M.; et al. Functional and genomic analyses reveal therapeutic potential of targeting β-catenin/CBP activity in head and neck cancer. Genome Med. 2018, 10, 54. [Google Scholar] [CrossRef]
- Guan, J.; Gupta, R.; Filipp, F.V. Cancer systems biology of TCGA SKCM efficient detection of genomic drivers in melanoma. Sci. Rep. 2015, 5, 7857. [Google Scholar] [CrossRef] [PubMed]
- Rizos, H.; Menzies, A.M.; Pupo, G.M.; Carlino, M.S.; Fung, C.; Hyman, J.; Haydu, L.E.; Mijatov, B.; Becker, T.M.; Boyd, S.C.; et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: Spectrum and clinical impact. Clin. Cancer Res. 2014, 20, 1965–1977. [Google Scholar] [CrossRef]
- Osawa, Y.; Kojika, E.; Nishikawa, K.; Kimura, M.; Osakaya, S.; Miyauchi, H.; Kanto, T.; Kawakami, Y.; Kimura, K. Programmed cell death ligand 1 (PD-L1) blockade attenuates metastatic colon cancer growth in cAMP-response element-binding protein (CREB)-binding protein (CBP)/β-catenin inhibitor-treated livers. Oncotarget 2019, 10, 3013–3026. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Sztiller-Sikorska, M.; Gajos-Michniewicz, A.; Czyz, M. Dissecting mechanisms of melanoma resistance to BRAF and MEK inhibitors revealed genetic and non-genetic patient- and drug-specific alterations and remarkable phenotypic plasticity. Cells 2020, 9, 142. [Google Scholar] [CrossRef]
- Altarejos, J.Y.; Montminy, M. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 2011, 12, 141–151. [Google Scholar] [CrossRef]
- Ma, H.; Nguyen, C.; Lee, K.S.; Kahn, M. Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene 2005, 24, 3619–3631. [Google Scholar] [CrossRef]
- Gang, E.J.; Hsieh, Y.T.; Pham, J.; Zhao, Y.; Nguyen, C.; Huantes, S.; Park, E.; Naing, K.; Klemm, L.; Swaminathan, S.; et al. Small-molecule inhibition of CBP/catenin interactions eliminates drug-resistant clones in acute lymphoblastic leukemia. Oncogene 2014, 33, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Masiello, D.; McMillian, M.; Nguyen, C.; Wu, Y.; Melendez, E.; Smbatyan, G.; Kida, A.; He, Y.; Teo, J.L.; et al. CBP/catenin antagonist safely eliminates drug-resistant leukemia-initiating cells. Oncogene 2016, 35, 3705–3717. [Google Scholar] [CrossRef] [PubMed]
- Emami, K.H.; Nguyen, C.; Ma, H.; Kim, D.H.; Jeong, K.W.; Eguchi, M.; Moon, R.T.; Teo, J.L.; Oh, S.W.; Kim, H.Y.; et al. A small molecule inhibitor of β-catenin/CREB-binding protein transcription [corrected]. Proc. Natl. Acad. Sci. USA 2004, 101, 12682–12687. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Ma, H.; Oehler, V.G.; Gang, E.J.; Nguyen, C.; Masiello, D.; Liu, H.; Zhao, Y.; Radich, J.; Kahn, M. The gamma catenin/CBP complex maintains survivin transcription in β-catenin deficient/depleted cancer cells. Curr. Cancer Drug Targets 2011, 11, 213–225. [Google Scholar] [CrossRef]
- Manegold, P.; Lai, K.K.Y.; Wu, Y.; Teo, J.L.; Lenz, H.J.; Genyk, Y.S.; Pandol, S.J.; Wu, K.; Lin, D.P.; Chen, Y.; et al. Differentiation Therapy Targeting the β-Catenin/CBP Interaction in Pancreatic Cancer. Cancers 2018, 10, 95. [Google Scholar] [CrossRef]
- Henderson, W.R., Jr.; Chi, E.Y.; Ye, X.; Nguyen, C.; Tien, Y.T.; Zhou, B.; Borok, Z.; Knight, D.A.; Kahn, M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 2010, 107, 14309–14314. [Google Scholar] [CrossRef]
- Kovacs, D.; Migliano, E.; Muscardin, L.; Silipo, V.; Catricalà, C.; Picardo, M.; Bellei, B. The role of Wnt/β-catenin signaling pathway in melanoma epithelial-to-mesenchymal-like switching: Evidences from patients-derived cell lines. Oncotarget 2016, 7, 43295–43314. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; Martin, O.A.; Bonner, W.M. p21: A Two-Faced Genome Guardian. Trends. Mol. Med. 2017, 23, 310–319. [Google Scholar] [CrossRef]
- Xia, X.; Ma, Q.; Li, X.; Ji, T.; Chen, P.; Xu, H.; Li, K.; Fang, Y.; Weng, D.; Weng, Y.; et al. Cytoplasmic p21 is a potential predictor for cisplatin sensitivity in ovarian cancer. BMC Cancer 2011, 11, 399. [Google Scholar] [CrossRef]
- Vincent, A.J.; Ren, S.; Harris, L.G.; Devine, D.J.; Samant, R.S.; Fodstad, O.; Shevde, L.A. Cytoplasmic translocation of p21 mediates NUPR1-induced chemoresistance: NUPR1 and p21 in chemoresistance. FEBS Lett. 2012, 586, 3429–3434. [Google Scholar] [CrossRef]
- Lazarova, D.L.; Chiaro, C.; Wong, T.; Drago, E.; Rainey, A.; O’Malley, S.; Bordonaro, M. CBP Activity Mediates Effects of the Histone Deacetylase Inhibitor Butyrate on WNT Activity and Apoptosis in Colon Cancer Cells. J. Cancer 2013, 4, 481–490. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Ning, Y.; Yang, D.; Cole, S.; Kahn, M.; Zoghbi, M.; Berg, J.; Fujimori, M.; Inada, T.; Kouji, H.A. Phase I First-in-Human Study of PRI-724 in Patients (Pts) with Advanced Solid Tumors. J. Clin. Oncol. 2013, 31, 2501. [Google Scholar] [CrossRef]
- Ko, A.H.; Chiorean, E.G.; Kwak, E.L.; Lenz, H.J.; Nadler, P.I.; Wood, D.L.; Fujimori, M.; Inada, T.; Hiroyuki Kouji, H.; McWilliams, R.R. Final results of a phase Ib dose-escalation study of PRI-724, a CBP/beta-catenin modulator, plus gemcitabine (GEM) in patients with advanced pancreatic adenocarcinoma (APC) as second-line therapy after FOLFIRINOX or FOLFOX. J. Clin. Oncol. 2016, 34, e15721. [Google Scholar] [CrossRef]
- McWilliams, R.R.; Ko, A.H.; Chiorean, E.G.; Kwak, E.L.; Lenz, H.J.; Nadler, P.I.; Wood, D.L.; Fujimori, M.; Morita, K.; Inada, T. A Phase Ib Dose-Escalation Study of PRI-724, a CBP/Beta-Catenin Modulator, plus Gemcitabine (GEM) in Patients with Advanced Pancreatic Adenocarcinoma (APC) as Second-Line Therapy after FOLFIRINOX or FOLFOX. J. Clin. Oncol. 2015, 33, e15270. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajos-Michniewicz, A.; Wozniak, M.; Kluszczynska, K.A.; Czyz, M. Cellular and Molecular Effects of Targeting the CBP/β-Catenin Interaction with PRI-724 in Melanoma Cells, Drug-Naïve and Resistant to Inhibitors of BRAFV600 and MEK1/2. Cells 2025, 14, 1710. https://doi.org/10.3390/cells14211710
Gajos-Michniewicz A, Wozniak M, Kluszczynska KA, Czyz M. Cellular and Molecular Effects of Targeting the CBP/β-Catenin Interaction with PRI-724 in Melanoma Cells, Drug-Naïve and Resistant to Inhibitors of BRAFV600 and MEK1/2. Cells. 2025; 14(21):1710. https://doi.org/10.3390/cells14211710
Chicago/Turabian StyleGajos-Michniewicz, Anna, Michal Wozniak, Katarzyna Anna Kluszczynska, and Malgorzata Czyz. 2025. "Cellular and Molecular Effects of Targeting the CBP/β-Catenin Interaction with PRI-724 in Melanoma Cells, Drug-Naïve and Resistant to Inhibitors of BRAFV600 and MEK1/2" Cells 14, no. 21: 1710. https://doi.org/10.3390/cells14211710
APA StyleGajos-Michniewicz, A., Wozniak, M., Kluszczynska, K. A., & Czyz, M. (2025). Cellular and Molecular Effects of Targeting the CBP/β-Catenin Interaction with PRI-724 in Melanoma Cells, Drug-Naïve and Resistant to Inhibitors of BRAFV600 and MEK1/2. Cells, 14(21), 1710. https://doi.org/10.3390/cells14211710






