Calcium Dynamics in Astrocyte-Neuron Communication from Intracellular to Extracellular Signaling
Abstract
1. Introduction
2. Intracellular Calcium Signaling in Astrocytes: Established Mechanisms
2.1. Sources of Astrocytic Ca2+ Elevations
2.2. Spatial Organization of Ca2+ Signals
2.3. Temporal Dynamics
2.4. Functional Consequences of Intracellular Ca2+
3. The Transition: From Intracellular to Extracellular Calcium Dynamics
3.1. Mechanisms Linking Intracellular Ca2+ to Extracellular Changes
3.2. Extracellular Ca2+ Buffering Systems
3.3. Spatial Constraints and Microenvironments
4. Extracellular Calcium as a Rapid Signaling Mediator
4.1. Evidence for Rapid [Ca2+]o Changes
4.2. Mechanisms of Neuronal Sensitivity to [Ca2+]o
4.3. Regional and Cell-Type Specificity
4.4. Functional Implications
5. Ephaptic Coupling: A New Paradigm for Astrocyte–Neuron Communication
5.1. Theoretical Framework
5.2. Astrocyte-Mediated Ephaptic Mechanisms
5.3. Functional Advantages
6. Pathological Implications and Disease Relevance
6.1. Disrupted Ca2+ Homeostasis in Neurological Disorders
6.2. Developmental Disorders
6.3. Therapeutic Implications
7. Technical Considerations and Methodological Advances
7.1. Current Techniques for Studying [Ca2+]o
7.2. Challenges and Limitations
7.3. Future Technological Needs
| Method | Spatial Resolution | Temporal Resolution | Advantages | Limitations | Typical Applications | Refs. |
|---|---|---|---|---|---|---|
| Calcium-selective microelectrodes | Limited (micrometer scale, relatively large tip size) | High (submillisecond range) | Direct quantitative measurements, high temporal resolution, minimal buffering effects, real-time monitoring | Invasive, sensitivity to drift and interference, poor spatial precision for microdomains, limited to point measurements | Detecting rapid [Ca2+]o fluctuations during synaptic activity and network events | [133,134,135,142] |
| Small-molecule fluorescent dyes | Moderate (micrometer scale) | Moderate (millisecond to second range, dependent on dye kinetics) | Improved spatial resolution over electrodes, visualization of spatial patterns, variety of affinity ranges available | Limited extracellular compartment specificity, potential dye diffusion and loading issues, phototoxicity during prolonged imaging, buffering effects at high concentrations; photobleaching | Mapping [Ca2+]o spatial distributions in tissue preparations and acute slices | [124,125,136,139] |
| Confocal microscopy | High (subcellular, ~200–500 nm lateral) | Moderate to high (milliseconds to seconds, depending on scanning mode) | Good optical sectioning; reduced out-of-focus fluorescence, compatible with multiple fluorophores | Limited penetration depth (<100 μm typically), phototoxicity and photobleaching; temporal resolution limited by scanning speed | Detailed spatial mapping of [Ca2+]o in superficial layers, co-localization studies | [49,124,139] |
| Two-photon microscopy | High (subcellular, ~300–700 nm lateral) | Variable (milliseconds to seconds, depending on scanning configuration and indicator) | Deep tissue penetration (up to ~1 mm), reduced phototoxicity and photobleaching, compatible with in vivo imaging in awake animals | May underestimate amplitude of rapid transient Ca2+ changes, slower scanning can miss fast events, expensive instrumentation, still subject to phototoxicity during chronic imaging | [Ca2+]o visualization in intact neural circuits, deep tissue and in vivo studies | [46,49,69,138,143] |
| GECIs for extracellular monitoring | Cellular to subcellular resolution | Developing (currently limited by indicator kinetics) | Long-term monitoring; cell-type and compartment specificity, genetic targeting, integration with optogenetics | Technology still under development, need to optimize affinity and kinetics for extracellular environment, potential buffering effects, phototoxicity during chronic imaging, expression level variability | Targeted monitoring of extracellular Ca2+ dynamics in specific cell populations or microdomains | [126,127,128,136,138] |
| Advanced scanning techniques (resonant scanners, acousto-optic deflectors, spinning disk) | High (subcellular) | Very high (submillisecond to millisecond range) | Enhanced temporal resolution, reduced motion artifacts, improved ability to capture fast Ca2+ transients | Hardware-dependent performance, may sacrifice signal-to-noise ratio for speed, specialized and expensive equipment | Capturing rapid [Ca2+]o dynamics during high-frequency neuronal activity | [143] |
| Implantable biosensors | Under development (potentially cellular scale) | Under development (potentially millisecond range) | Potential for chronic in vivo monitoring; minimal invasiveness; real-time physiological measurements | Emerging technology, biocompatibility concerns, calibration challenges, long-term stability issues | Linking [Ca2+]o dynamics to physiological states and behavior in freely moving animals | [126,128,136] |
| Computational modeling | Theoretical (nanometer to tissue scale, parameter-dependent) | Theoretical (microsecond to second range, timestep-dependent) | Predict microdomain dynamics inaccessible to current techniques, disentangle overlapping processes; identify causal mechanisms, test hypotheses in silico | Requires empirical validation, model assumptions and simplifications may limit accuracy, dependent on quality of input parameters | Biophysical simulations of synaptic activity, astrocytic buffering effects, and [Ca2+]o dynamics in restricted spaces | [8,41,74,135,141] |
8. Future Directions and Unresolved Questions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Qi, Y.; Chen, W.; Zhou, T.; Zang, Y.; Li, J. Astrocyte Metabolism and Signaling Pathways in the CNS. Front. Neurosci. 2023, 17, 1217451. [Google Scholar] [CrossRef]
- Murphy-Royal, C.; Ching, S.; Papouin, T. A Conceptual Framework for Astrocyte Function. Nat. Neurosci. 2023, 26, 1848. [Google Scholar] [CrossRef]
- Castro, M.A.D.; Volterra, A. Astrocyte Control of the Entorhinal Cortex—Dentate Gyrus Circuit: Relevance to Cognitive Processing and Impairment in Pathology. Glia 2021, 70, 1536. [Google Scholar] [CrossRef] [PubMed]
- Goenaga, J.; Araque, A.; Kofuji, P.; Chao, D.H.M. Calcium Signaling in Astrocytes and Gliotransmitter Release. Front. Synaptic Neurosci. 2023, 15, 1138577. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Z.; Han, B.; Xiang, X.; Huang, W.; Yao, H. Revisiting Astrocytic Calcium Signaling in the Brain. Fundam. Res. 2024, 4, 1365. [Google Scholar] [CrossRef]
- Schulte, A.; Bieniussa, L.; Gupta, R.; Samtleben, S.; Bischler, T.; Doering, K.; Sodmann, A.; Rittner, H.L.; Blum, R. Homeostatic Calcium Fluxes, ER Calcium Release, SOCE, and Calcium Oscillations in Cultured Astrocytes Are Interlinked by a Small Calcium Toolkit. Cell Calcium 2022, 101, 102515. [Google Scholar] [CrossRef]
- Rose, C.R.; Ziemens, D.; Verkhratsky, A. On the Special Role of NCX in Astrocytes: Translating Na+-Transients into Intracellular Ca2+ Signals. Cell Calcium 2020, 86, 102154. [Google Scholar] [CrossRef]
- Pittà, M.D.; Volman, V.; Berry, H.; Parpura, V.; Volterra, A.; Ben-Jacob, E. Computational Quest for Understanding the Role of Astrocyte Signaling in Synaptic Transmission and Plasticity. Front. Comput. Neurosci. 2012, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Semyanov, A.; Henneberger, C.; Agarwal, A. Making Sense of Astrocytic Calcium Signals—From Acquisition to Interpretation. Nat. Rev. Neurosci. 2020, 21, 551. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Smith, N.; Xu, Q.; Fujita, T.; Baba, A.; Matsuda, T.; Takano, T.; Bekar, L.K.; Nedergaard, M. Astrocytes Modulate Neural Network Activity by Ca2+-Dependent Uptake of Extracellular K+. Sci. Signal. 2012, 5, ra26. [Google Scholar] [CrossRef]
- Martín, R.; Bajo-Grañeras, R.; Moratalla, R.; Moratalla, R.; Perea, G.; Araque, A. Circuit-Specific Signaling in Astrocyte-Neuron Networks in Basal Ganglia Pathways. Science 2015, 349, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Fellin, T.; Haydon, P.G. The Tripartite Synapse: Roles for Gliotransmission in Health and Disease. Trends Mol. Med. 2007, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Rodríguez, J.J.; Parpura, V. Calcium Signalling in Astroglia. Mol. Cell. Endocrinol. 2011, 353, 45. [Google Scholar] [CrossRef]
- Radin, D.P.; Cerne, R.; Witkin, J.M.; Lippa, A. High-Impact AMPAkines Elevate Calcium Levels in Cortical Astrocytes by Mobilizing Endoplasmic Reticular Calcium Stores. Neuroglia 2024, 5, 344–355. [Google Scholar] [CrossRef]
- Lines, J.; Baraibar, A.M.; Nanclares, C.; Martin, E.; Aguilar, J.; Kofuji, P.; Navarrete, M.; Araque, A. A Spatial Threshold for Astrocyte Calcium Surge. eLife 2024, 12, RP90046. [Google Scholar] [CrossRef]
- Imrie, G.; Farhy-Tselnicker, I. Astrocyte Regulation of Behavioral Outputs: The Versatile Roles of Calcium. Front. Cell. Neurosci. 2025, 19, 1606265. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.I.; Kavalali, E.T. Nano-Organization of Synaptic Calcium Signaling. Biochem. Soc. Trans. 2024, 52, 1459. [Google Scholar] [CrossRef]
- Wright, W.J.; Hedrick, N.G.; Komiyama, T. Distinct Synaptic Plasticity Rules Operate across Dendritic Compartments in Vivo during Learning. Science 2025, 388, 322. [Google Scholar] [CrossRef]
- Sanz-Gálvez, R.; Falardeau, D.; Kolta, A.; Inglebert, Y. The Role of Astrocytes from Synaptic to Non-Synaptic Plasticity. Front. Cell. Neurosci. 2024, 18, 1477985. [Google Scholar] [CrossRef]
- Purushotham, S.S.; Buskila, Y. Astrocytic Modulation of Neuronal Signalling. Front. Netw. Physiol. 2023, 3, 1205544. [Google Scholar] [CrossRef]
- Garcia, D.W.; Jacquir, S. Astrocyte-Mediated Neuronal Irregularities and Dynamics: The Complexity of the Tripartite Synapse. Biol. Cybern. 2024, 118, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Ceglia, R.D.; Ledonne, A.; Litvin, D.; Lind, B.L.; Carriero, G.; Latagliata, E.C.; Bindocci, E.; Castro, M.A.D.; Savtchouk, I.; Vitali, I.; et al. Specialized Astrocytes Mediate Glutamatergic Gliotransmission in the CNS. Nature 2023, 622, 120. [Google Scholar] [CrossRef] [PubMed]
- Lalo, U.; Pankratov, Y. Astrocyte Ryanodine Receptors Facilitate Gliotransmission and Astroglial Modulation of Synaptic Plasticity. Front. Cell. Neurosci. 2024, 18, 1382010. [Google Scholar] [CrossRef]
- van Dijk, L.; Giladi, M.; Refaeli, B.; Hiller, R.; Cheng, M.H.; Bahar, İ.; Khananshvili, D. Key Residues Controlling Bidirectional Ion Movements in Na+/Ca2+ Exchanger. Cell Calcium 2018, 76, 10. [Google Scholar] [CrossRef]
- Ottolia, M.; John, S.; Hazan, A.; Goldhaber, J.I. The Cardiac Na+-Ca2+ Exchanger: From Structure to Function. Compr. Physiol. 2021, 12, 2681–2717. [Google Scholar] [CrossRef]
- Cobb-Lewis, D.E.; Sansalone, L.; Khaliq, Z.M. Contributions of the Sodium Leak Channel NALCN to Pacemaking of Medial Ventral Tegmental Area and Substantia Nigra Dopaminergic Neurons. J. Neurosci. 2023, 43, 6841. [Google Scholar] [CrossRef]
- Martin-de-Saavedra, M.D.; Santos, M.D.; Culotta, L.; Varea, O.; Spielman, B.; Parnell, E.; Forrest, M.P.; Gao, R.; Yoon, S.; McCoig, E.; et al. Shed CNTNAP2 Ectodomain Is Detectable in CSF and Regulates Ca2+ Homeostasis and Network Synchrony via PMCA2/ATP2B2. Neuron 2021, 110, 627. [Google Scholar] [CrossRef]
- Kowalski, A.; Betzer, C.; Larsen, S.T.; Gregersen, E.; Newcombe, E.A.; Bermejo, M.C.; Langkilde, A.E.; Kragelund, B.B.; Jensen, P.H.; Nissen, P. Monomeric α-Synuclein Activates the Plasma Membrane Calcium Pump. EMBO J. 2023, 1, 42. [Google Scholar] [CrossRef] [PubMed]
- Greger, I.H. Regulating Calcium Flux through AMPA Glutamate Receptors. Cell Calcium 2024, 123, 102934. [Google Scholar] [CrossRef]
- Caudal, L.C.; Gobbo, D.; Scheller, A.; Kirchhoff, F. The Paradox of Astroglial Ca2+ Signals at the Interface of Excitation and Inhibition. Front. Cell. Neurosci. 2020, 14, 609947. [Google Scholar] [CrossRef]
- Tewari, B.P.; Woo, A.M.; Prim, C.; Chaunsali, L.; Patel, D.C.; Kimbrough, I.F.; Engel, K.; Browning, J.L.; Campbell, S.L.; Sontheimer, H. Astrocytes Require Perineuronal Nets to Maintain Synaptic Homeostasis in Mice. Nat. Neurosci. 2024, 27, 1475. [Google Scholar] [CrossRef] [PubMed]
- Ryczko, D.; Hanini-Daoud, M.; Condamine, S.; Bréant, B.J.B.; Fougère, M.; Araya, R.; Kolta, A. S100β—Mediated Astroglial Control of Firing and Input Processing in Layer 5 Pyramidal Neurons of the Mouse Visual Cortex. J. Physiol. 2020, 599, 677. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.C.; Galland, F.; Guerra, M.C.; Rodrigues, L.; Taday, J.; Monteforte, P.T.; Hirata, H.; Gottfried, C.; Donato, R.; Smaili, S.S.; et al. Astroglial S100B Secretion Is Mediated by Ca2+ Mobilization from Endoplasmic Reticulum: A Study Using Forskolin and DMSO as Secretagogues. Int. J. Mol. Sci. 2023, 24, 16576. [Google Scholar] [CrossRef]
- Sumadewi, K.T.; de Liyis, B.G.; Linawati, N.M.; Widyadharma, I.P.E.; Astawa, I.N.M. Astrocyte Dysregulation as an Epileptogenic Factor: A Systematic Review. Egypt. J. Neurol. Psychiatry Neurosurg. 2024, 60, 69. [Google Scholar] [CrossRef]
- Hernández-Ortega, K.; Canul-Euan, A.A.; Solís-Paredes, J.M.; Borboa-Olivares, H.; Reyes-Muñoz, E.; Estrada-Gutiérrez, G.; Camacho-Arroyo, I. S100B Actions on Glial and Neuronal Cells in the Developing Brain: An Overview. Front. Neurosci. 2024, 18, 1425525. [Google Scholar] [CrossRef]
- Schwaller, B. Cytosolic Ca2+ Buffers Are Inherently Ca2+ Signal Modulators. Cold Spring Harb. Perspect. Biol. 2019, 12, a035543. [Google Scholar] [CrossRef]
- González, L.L.; Garrie, K.; Turner, M.D. Role of S100 Proteins in Health and Disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2020, 1867, 118677. [Google Scholar] [CrossRef]
- Hofer, A.M.; Brown, E.M. Extracellular Calcium Sensing and Signalling. Nat. Rev. Mol. Cell Biol. 2003, 4, 530. [Google Scholar] [CrossRef]
- Deemyad, T.; Lüthi, J.; Spruston, N. Astrocytes Integrate and Drive Action Potential Firing in Inhibitory Subnetworks. Nat. Commun. 2018, 9, 4336. [Google Scholar] [CrossRef]
- Toman, M.; Wade, J.; Verkhratsky, A.; Dallas, M.; Bithell, A.; Flanagan, B.; Harkin, J.; McDaid, L. The Influence of Astrocytic Leaflet Motility on Ionic Signalling and Homeostasis at Active Synapses. Sci. Rep. 2023, 13, 3050. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Araque, A. Astrocyte Regulation of Neural Circuit Activity and Network States. Glia 2022, 70, 1455. [Google Scholar] [CrossRef] [PubMed]
- Stedehouder, J.; Brizee, D.; Slotman, J.A.; Pascual-Garcia, M.; Leyrer, M.L.; Bouwen, B.L.J.; Dirven, C.M.; Gao, Z.; Berson, D.M.; Houtsmuller, A.B.; et al. Local Axonal Morphology Guides the Topography of Interneuron Myelination in Mouse and Human Neocortex. eLife 2019, 8, e48615. [Google Scholar] [CrossRef]
- Stedehouder, J.; Roberts, B.M.; Raina, S.; Bossi, S.; Liu, A.K.L.; Doig, N.M.; McGerty, K.; Magill, P.J.; Parkkinen, L.; Cragg, S.J. Rapid Modulation of Striatal Cholinergic Interneurons and Dopamine Release by Satellite Astrocytes. Nat. Commun. 2024, 15, 10017. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; McCarthy, K.D. Astrocyte Calcium Signaling: From Observations to Functions and the Challenges Therein. Cold Spring Harb. Perspect. Biol. 2015, 7, a020404. [Google Scholar] [CrossRef]
- Bindocci, E.; Savtchouk, I.; Liaudet, N.; Becker, D.; Carriero, G.; Volterra, A. Three-Dimensional Ca2+ Imaging Advances Understanding of Astrocyte Biology. Science 2017, 356, eaai8185. [Google Scholar] [CrossRef]
- Miyazaki, K.; Ross, W.N. Fast Synaptically Activated Calcium and Sodium Kinetics in Hippocampal Pyramidal Neuron Dendritic Spines. eNeuro 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, K.; Nagao, M.; Konno, A.; Iwai, Y.; Vittani, M.; Kusk, P.; Mishima, T.; Hirai, H.; Nedergaard, M.; Hirase, H. Astrocytic GPCR-Induced Ca2+ Signaling Is Not Causally Related to Local Cerebral Blood Flow Changes. Int. J. Mol. Sci. 2023, 24, 13590. [Google Scholar] [CrossRef]
- Yoshida, E.; Terada, S.-I.; Tanaka, Y.; Kobayashi, K.; Ohkura, M.; Nakai, J.; Matsuzaki, M. In Vivo Wide-Field Calcium Imaging of Mouse Thalamocortical Synapses with an 8 K Ultra-High-Definition Camera. Sci. Rep. 2018, 8, 8324. [Google Scholar] [CrossRef]
- Walters, M.C.; Sonner, M.; Myers, J.H.; Ladle, D.R. Calcium Imaging of Parvalbumin Neurons in the Dorsal Root Ganglia. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Umpierre, A.D.; Bystrom, L.; Ying, Y.; Liu, Y.; Wu, L. Microglial Calcium Signaling Is Attuned to Neuronal Activity. bioRxiv 2019. [Google Scholar] [CrossRef]
- Durbin, R.J.; Heredia, D.J.; Gould, T.W.; Renden, R. Postsynaptic Calcium Extrusion at the Mouse Neuromuscular Junction Alkalinizes the Synaptic Cleft. J. Neurosci. 2023, 43, 5741. [Google Scholar] [CrossRef]
- Bazargani, N.; Attwell, D. Astrocyte Calcium Signaling: The Third Wave. Nat. Neurosci. 2016, 19, 182. [Google Scholar] [CrossRef]
- Gerasimov, E.; Erofeev, A.; Borodinova, A.; Bolshakova, A.; Balaban, P.; Bezprozvanny, I.; Vlasova, O.L. Optogenetic Activation of Astrocytes—Effects on Neuronal Network Function. Int. J. Mol. Sci. 2021, 22, 9613. [Google Scholar] [CrossRef]
- Wu, A.; Zhang, J.; Lun, W.-Z.; Geng, Z.; Yang, Y.; Wu, J.; Chen, G. Dynamic Changes of Media Prefrontal Cortex Astrocytic Activity in Response to Negative Stimuli in Male Mice. Neurobiol. Stress 2024, 33, 100676. [Google Scholar] [CrossRef]
- Lefton, K.B.; Wu, Y.; Dai, Y.; Okuda, T.; Zhang, Y.; Yen, A.; Rurak, G.M.; Walsh, S.; Manno, R.; Myagmar, B.; et al. Norepinephrine Signals through Astrocytes to Modulate Synapses. Science 2025, 388, 776. [Google Scholar] [CrossRef] [PubMed]
- Giudice, M.L.; Mihalik, B.; Dinnyés, A.; Kobolák, J. The Nervous System Relevance of the Calcium Sensing Receptor in Health and Disease. Molecules 2019, 24, 2546. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.; Mun, H.; Delbridge, L.; Kuchel, P.W.; Conigrave, A.D. Temperature Sensing by the Calcium-Sensing Receptor. Front. Physiol. 2023, 14, 1117352. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y. High Calcium Concentrations Reduce Cellular Excitability of Mouse MNTB Neurons. Brain Res. 2023, 1820, 148568. [Google Scholar] [CrossRef] [PubMed]
- Sundararaman, S.S.; van der Vorst, E.P.C. Calcium-Sensing Receptor (CaSR), Its Impact on Inflammation and the Consequences on Cardiovascular Health. Int. J. Mol. Sci. 2021, 22, 2478. [Google Scholar] [CrossRef]
- Gorkhali, R.; Li, T.; Dong, B.; Bagchi, P.; Deng, X.; Pawar, S.; Duong, D.M.; Fang, N.; Seyfried, N.T.; Yang, J.J. Extracellular Calcium Alters Calcium-Sensing Receptor Network Integrating Intracellular Calcium-Signaling and Related Key Pathway. Sci. Rep. 2021, 11, 20576. [Google Scholar] [CrossRef]
- Elliott, E.R.; Cooper, R.L. The Effect of Calcium Ions on Resting Membrane Potential. Biology 2024, 13, 750. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wu, J.-X.; Chen, L. Structure of Voltage-Modulated Sodium-Selective NALCN-FAM155A Channel Complex. Nat. Commun. 2020, 11, 6199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wei, Y. Role of Sodium Leak Channel (NALCN) in Sensation and Pain: An Overview. Front. Pharmacol. 2024, 14, 1349438. [Google Scholar] [CrossRef]
- Octeau, J.C.; Gangwani, M.R.; Allam, S.L.; Tran, D.; Huang, S.; Hoang-Trong, T.M.; Golshani, P.; Rumbell, T.; Kozloski, J.; Khakh, B.S. Transient, Consequential Increases in Extracellular Potassium Ions Accompany Channelrhodopsin2 Excitation. Cell Rep. 2019, 27, 2249. [Google Scholar] [CrossRef]
- Čepkenović, B.; Friedland, F.; Noetzel, E.; Maybeck, V.; Offenhäusser, A. Single-Neuron Mechanical Perturbation Evokes Calcium Plateaus That Excite and Modulate the Network. Sci. Rep. 2023, 13, 20669. [Google Scholar] [CrossRef] [PubMed]
- Durkee, C.A.; Araque, A. Diversity and Specificity of Astrocyte–Neuron Communication. Neuroscience 2019, 396, 73. [Google Scholar] [CrossRef]
- Refaeli, R.; Doron, A.; Benmelech-Chovav, A.; Groysman, M.; Kreisel, T.; Loewenstein, Y.; Goshen, I. Features of Hippocampal Astrocytic Domains and Their Spatial Relation to Excitatory and Inhibitory Neurons. Glia 2021, 69, 2378. [Google Scholar] [CrossRef]
- Stobart, J.L.; Ferrari, K.D.; Barrett, M.; Glück, C.; Stobart, M.; Zuend, M.; Weber, B. Cortical Circuit Activity Evokes Rapid Astrocyte Calcium Signals on a Similar Timescale to Neurons. Neuron 2018, 98, 726. [Google Scholar] [CrossRef]
- Baravalle, R.; Canavier, C.C. Synchrony in Networks of Type 2 Interneurons Is More Robust to Noise with Hyperpolarizing Inhibition Compared to Shunting Inhibition in Both the Stochastic Population Oscillator and the Coupled Oscillator Regimes. eNeuro 2024, 11. [Google Scholar] [CrossRef]
- Navarrete, M.; Cuartero, M.I.; Palenzuela, R.; Draffin, J.E.; Konomi, A.; Serra, I.; Colié, S.; Castaño-Castaño, S.; Hasan, M.T.; Nebreda, Á.R.; et al. Astrocytic P38α MAPK Drives NMDA Receptor-Dependent Long-Term Depression and Modulates Long-Term Memory. Nat. Commun. 2019, 10, 2968. [Google Scholar] [CrossRef]
- Lalo, U.; Pankratov, Y. Role for Astrocytes in mGluR-Dependent LTD in the Neocortex and Hippocampus. Brain Sci. 2022, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
- Bohmbach, K.; Henneberger, C. Activity-Induced Reactivity of Astrocytes Impairs Cognition. PLoS Biol. 2024, 22, e3002712. [Google Scholar] [CrossRef]
- Ruffini, G.; Salvador, R.; Tadayon, E.; Sanchez-Todo, R.; Pascual-Leone, Á.; Santarnecchi, E. Realistic Modeling of Mesoscopic Ephaptic Coupling in the Human Brain. PLoS Comput. Biol. 2020, 16, e1007923. [Google Scholar] [CrossRef] [PubMed]
- Shivacharan, R.S.; Chiang, C.; Wei, X.; Subramanian, M.; Couturier, N.H.; Pakalapati, N.; Durand, D.M. Neural Recruitment by Ephaptic Coupling in Epilepsy. Epilepsia 2021, 62, 1505. [Google Scholar] [CrossRef]
- Cunha, G.M.; de Sousa, M.P.B.; Corso, G.; dos Santos Lima, G.Z. How Ephapticity Can Explain Brain Complexity? Res. Sq. 2023. [CrossRef]
- Cunha, G.M.; Corso, G.; de Sousa, M.P.B.; dos Santos Lima, G.Z. Can Ephapticity Contributes to the Brain Complexity? PLoS ONE 2024, 19, e0310640. [Google Scholar] [CrossRef]
- de Sousa, M.P.B.; Cunha, G.M.; Corso, G.; dos Santos Lima, G.Z. Thermal Effects and Ephaptic Entrainment in Hodgkin–Huxley Model. Sci. Rep. 2024, 14, 20075. [Google Scholar] [CrossRef]
- Anastassiou, C.A.; Koch, C. Ephaptic Coupling to Endogenous Electric Field Activity: Why Bother? Curr. Opin. Neurobiol. 2015, 31, 95. [Google Scholar] [CrossRef]
- Zhang, Y.; Tsang, T.K.; Bushong, E.A.; Chu, L.; Chiang, A.; Ellisman, M.H.; Reingruber, J.; Su, C. Asymmetric Ephaptic Inhibition between Compartmentalized Olfactory Receptor Neurons. Nat. Commun. 2019, 10, 1560. [Google Scholar] [CrossRef]
- Manninen, T.; Saudargienė, A.; Linne, M. Astrocyte-Mediated Spike-Timing-Dependent Long-Term Depression Modulates Synaptic Properties in the Developing Cortex. PLoS Comput. Biol. 2020, 16, e1008360. [Google Scholar] [CrossRef] [PubMed]
- Verhoog, Q.P.; Holtman, L.; Aronica, E.; van Vliet, E.A. Astrocytes as Guardians of Neuronal Excitability: Mechanisms Underlying Epileptogenesis. Front. Neurol. 2020, 11, 591690. [Google Scholar] [CrossRef]
- Hyung, S.; Park, J.; Jung, K. Application of Optogenetic Glial Cells to Neuron–Glial Communication. Front. Cell. Neurosci. 2023, 17, 1249043. [Google Scholar] [CrossRef]
- Eitelmann, S.; Everaerts, K.; Petersilie, L.; Rose, C.R.; Stephan, J. Ca2+-Dependent Rapid Uncoupling of Astrocytes upon Brief Metabolic Stress. Front. Cell. Neurosci. 2023, 17, 1151608. [Google Scholar] [CrossRef]
- Lines, J.; Corkrum, M.; Aguilar, J.; Araque, A. The Duality of Astrocyte Neuromodulation: Astrocytes Sense Neuromodulators and Are Neuromodulators. J. Neurochem. 2025, 169, e70054. [Google Scholar] [CrossRef]
- Robertson, J. The Astroglia Syncytial Theory of Consciousness. Int. J. Mol. Sci. 2025, 26, 5785. [Google Scholar] [CrossRef]
- Pinotsis, D.A.; Miller, E.K. In Vivo Ephaptic Coupling Allows Memory Network Formation. Cereb. Cortex 2023, 33, 9877. [Google Scholar] [CrossRef]
- Shah, D.; Gsell, W.; Wahis, J.; Luckett, E.S.; Jamoulle, T.; Vermaercke, B.; Preman, P.; Moechars, D.; Hendrickx, V.; Jaspers, T.; et al. Astrocyte Calcium Dysfunction Causes Early Network Hyperactivity in Alzheimer’s Disease. Cell Rep. 2022, 40, 111280. [Google Scholar] [CrossRef] [PubMed]
- Madeira, D.; Domingues, J.; Lopes, C.R.; Canas, P.M.; Cunha, R.A.; Agostinho, P. Modification of Astrocytic Cx43 Hemichannel Activity in Animal Models of AD: Modulation by Adenosine A2A Receptors. Cell. Mol. Life Sci. 2023, 80, 340. [Google Scholar] [CrossRef] [PubMed]
- Cheli, V.T.; González, D.A.S.; Smith, J.; Spreuer, V.; Murphy, G.G.; Paez, P.M. L-type Voltage—Operated Calcium Channels Contribute to Astrocyte Activation In Vitro. Glia 2016, 64, 1396. [Google Scholar] [CrossRef]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 Proteins. Curr. Mol. Med. 2012, 13, 24. [Google Scholar] [CrossRef]
- Barres, B.A. The Mystery and Magic of Glia: A Perspective on Their Roles in Health and Disease. Neuron 2008, 60, 430. [Google Scholar] [CrossRef] [PubMed]
- Delekate, A.; Füchtemeier, M.; Schumacher, T.; Ulbrich, C.; Foddis, M.; Petzold, G.C. Metabotropic P2Y1 Receptor Signalling Mediates Astrocytic Hyperactivity in Vivo in an Alzheimer’s Disease Mouse Model. Nat. Commun. 2014, 5, 5422. [Google Scholar] [CrossRef] [PubMed]
- Volterra, A.; Meldolesi, J. Astrocytes, from Brain Glue to Communication Elements: The Revolution Continues. Nat. Rev. Neurosci. 2005, 6, 626. [Google Scholar] [CrossRef]
- Guan, P.; Cao, L.; Yang, Y.; Wang, P. Calcium Ions Aggravate Alzheimer’s Disease Through the Aberrant Activation of Neuronal Networks, Leading to Synaptic and Cognitive Deficits. Front. Mol. Neurosci. 2021, 14, 757515. [Google Scholar] [CrossRef]
- Webber, E.K.; Fivaz, M.; Stutzmann, G.E.; Griffioen, G. Cytosolic Calcium: Judge, Jury and Executioner of Neurodegeneration in Alzheimer’s Disease and Beyond. Alzheimer’s Dement. 2023, 19, 3701. [Google Scholar] [CrossRef]
- Baraibar, A.M.; Colomer, T.; Moreno-García, A.; Bernal-Chico, A.; Sánchez-Martín, E.; Utrilla, C.; Serrat, R.; Soria-Gómez, E.; Rodríguez-Antigüedad, A.; Araque, A.; et al. Autoimmune Inflammation Triggers Aberrant Astrocytic Calcium Signaling to Impair Synaptic Plasticity. Brain Behav. Immun. 2024, 121, 192. [Google Scholar] [CrossRef]
- Fan, Y.; Han, J.; Zhao, L.; Wu, C.; Wu, P.; Huang, Z.; Hao, X.; Ji, Y.; Chen, D.; Zhu, M. Experimental Models of Cognitive Impairment for Use in Parkinson’s Disease Research: The Distance Between Reality and Ideal. Front. Aging Neurosci. 2021, 13, 745438. [Google Scholar] [CrossRef]
- Bancroft, E.A.; Srinivasan, R. Emerging Roles for Aberrant Astrocytic Calcium Signals in Parkinson’s Disease. Front. Physiol. 2022, 12, 812212. [Google Scholar] [CrossRef]
- Xu, J.; Minobe, E.; Kameyama, M. Ca2+ Dyshomeostasis Links Risk Factors to Neurodegeneration in Parkinson’s Disease. Front. Cell. Neurosci. 2022, 16, 867385. [Google Scholar] [CrossRef]
- Prunell, G.; Olivera-Bravo, S. A Focus on Astrocyte Contribution to Parkinson’s Disease Etiology. Biomolecules 2022, 12, 1745. [Google Scholar] [CrossRef] [PubMed]
- Vrapciu, A.D.; Rusu, M.C.; Jianu, A.M.; Motoc, A.G.M.; Nicolescu, M.I. Astrocytes—Friends or Foes in Neurodegenerative Disorders. Rom. J. Morphol. Embryol. 2023, 64, 305. [Google Scholar] [CrossRef] [PubMed]
- Lozovaya, N.A.; Eftekhari, S.; Cloarec, R.; Gouty-Colomer, L.; Dufour, A.; Riffault, B.; Billon-Grand, M.; Pons-Bennaceur, A.; Oumar, N.; Burnashev, N.; et al. GABAergic Inhibition in Dual-Transmission Cholinergic and GABAergic Striatal Interneurons Is Abolished in Parkinson Disease. Nat. Commun. 2018, 9, 1422. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Q.; Ma, Y.; Liu, L.; Jia, W.; Chen, L.; Xie, J. Calcium Homeostasis in Parkinson’s Disease: From Pathology to Treatment. Neurosci. Bull. 2022, 38, 1267. [Google Scholar] [CrossRef]
- Muwanigwa, M.N.; Modamio-Chamarro, J.; Antony, P.; Gomez-Giro, G.; Krüger, R.; Bolognin, S.; Schwamborn, J.C. Alpha-Synuclein Pathology Is Associated with Astrocyte Senescence in a Midbrain Organoid Model of Familial Parkinson’s Disease. Mol. Cell. Neurosci. 2024, 128, 103919. [Google Scholar] [CrossRef]
- Hastings, N.; Rahman, S.M.J.; Stempor, P.; Wayland, M.T.; Kuan, W.; Kotter, M. Connexin 43 Is Downregulated in Advanced Parkinson’s Disease in Multiple Brain Regions Which Correlates with Symptoms. Sci. Rep. 2025, 15, 10250. [Google Scholar] [CrossRef]
- Mallet, N.; Leblois, A.; Maurice, N.; Beurrier, C. Striatal Cholinergic Interneurons: How to Elucidate Their Function in Health and Disease. Front. Pharmacol. 2019, 10, 1488. [Google Scholar] [CrossRef] [PubMed]
- Shigetomi, E.; Jackson-Weaver, O.; Huckstepp, R.T.; O’Dell, T.J.; Khakh, B.S. Cellular/Molecular TRPA1 Channels Are Regulators of Astrocyte Basal Calcium Levels and Long-Term Potentiation via Constitutive D-Serine Release. J. Neurosci. 2013, 33, 10143–10153. [Google Scholar] [CrossRef]
- Charles, A.; Merrill, J.E.; Dirksen, E.R.; Sandersont, M.J. Intercellular Signaling in Glial Cells: Calcium Waves and Oscillations in Response to Mechanical Stimulation and Glutamate. Neuron 1991, 6, 983. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, X.-P.; Song, P.; Hu, Y.; Gong, X.; Peng, X. Complexity-Based Graph Convolutional Neural Network for Epilepsy Diagnosis in Normal, Acute, and Chronic Stages. Front. Comput. Neurosci. 2023, 17, 1211096. [Google Scholar] [CrossRef]
- Ding, S.; Fellin, T.; Zhu, Y.; Lee, S.-Y.; Auberson, Y.P.; Meaney, D.F.; Coulter, D.A.; Carmignoto, G.; Haydon, P.G. Neurobiology of Disease Enhanced Astrocytic Ca2 Signals Contribute to Neuronal Excitotoxicity after Status Epilepticus. J. Neurosci. 2007, 3, 27. [Google Scholar] [CrossRef]
- Morquette, P.; Verdier, D.; Kadala, A.; Féthière, J.; Philippe, A.; Robitaille, R.; Kolta, A. An Astrocyte-Dependent Mechanism for Neuronal Rhythmogenesis. Nat. Neurosci. 2015, 18, 844. [Google Scholar] [CrossRef]
- Agulhon, C.; Petravicz, J.; McMullen, A.B.; Sweger, E.J.; Minton, S.K.; Taves, S.; Casper, K.B.; Fiacco, T.A.; McCarthy, K.D. What Is the Role of Astrocyte Calcium in Neurophysiology? Neuron 2008, 59, 932. [Google Scholar] [CrossRef]
- Ji, Q.; Qie, X.; Ye, M. Dynamical Analysis of Astrocyte-Induced Neuronal Hyper-Excitation. Nonlinear Dyn. 2022, 111, 7713. [Google Scholar] [CrossRef]
- Novakovic, M.; Korshunov, K.S.; Grant, R.A.; Martin, M.E.; Abdala-Valencia, H.; Budinger, G.R.S.; Radulović, J.; Prakriya, M. Astrocyte Reactivity and Inflammation-Induced Depression-like Behaviors Are Regulated by Orai1 Calcium Channels. Nat. Commun. 2023, 14, 5500. [Google Scholar] [CrossRef]
- Georgiou, L.; Echeverría, A.; Georgiou, A.; Kühn, B. Ca2+ Activity Maps of Astrocytes Tagged by Axoastrocytic AAV Transfer. Sci. Adv. 2022, 8, eabe5371. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, G. Calcium Dyshomeostasis Drives Pathophysiology and Neuronal Demise in Age-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 13243. [Google Scholar] [CrossRef]
- Lepore, A.C.; Rauck, B.; Dejea, C.M.; Pardo, A.C.; Rao, M.S.; Rothstein, J.D.; Maragakis, N.J. Focal Transplantation–Based Astrocyte Replacement Is Neuroprotective in a Model of Motor Neuron Disease. Nat. Neurosci. 2008, 11, 1294. [Google Scholar] [CrossRef]
- Windrem, M.S.; Schanz, S.J.; Morrow, C.A.; Munir, J.; Chandler-Militello, D.; Wang, S.; Goldman, S.A. A Competitive Advantage by Neonatally Engrafted Human Glial Progenitors Yields Mice Whose Brains Are Chimeric for Human Glia. J. Neurosci. 2014, 34, 16153. [Google Scholar] [CrossRef]
- Ghuman, H.; Kim, K.S.; Barati, S.; Ganguly, K. Emergence of Task-Related Spatiotemporal Population Dynamics in Transplanted Neurons. Nat. Commun. 2023, 14, 7320. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Pröschel, C.; Zhang, N.; Noble, M.; Mayer-Pröschel, M.; Davies, S.J. Transplanted Astrocytes Derived from BMP- or CNTF-Treated Glial-Restricted Precursors Have Opposite Effects on Recovery and Allodynia after Spinal Cord Injury. J. Biol. 2008, 7, 24. [Google Scholar] [CrossRef]
- Rose, C.R.; Verkhratsky, A. Principles of Sodium Homeostasis and Sodium Signalling in Astroglia. Glia 2016, 64, 1611. [Google Scholar] [CrossRef]
- Nicholson, C.; Hrabětová, S. Brain Extracellular Space: The Final Frontier of Neuroscience. Biophys. J. 2017, 113, 2133. [Google Scholar] [CrossRef]
- Chen, T.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive Fluorescent Proteins for Imaging Neuronal Activity. Nature 2013, 499, 295. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A New Generation of Ca2+ Indicators with Greatly Improved Fluorescence Properties. J. Biol. Chem. 1985, 260, 3440. [Google Scholar] [CrossRef]
- Kim, J.W.; Yong, A.J.H.; Aisenberg, E.E.; Lobel, J.H.; Wang, W.; Dawson, T.M.; Dawson, V.L.; Gao, R.; Jan, Y.N.; Bateup, H.S.; et al. Molecular Recording of Calcium Signals via Calcium-Dependent Proximity Labeling. Nat. Chem. Biol. 2024, 20, 894. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Ichiraku, A.; Matsuda, T.; Sakane, A.; Sasaki, T.; Nagai, T.; Horikawa, K. High-Affinity Tuning of Single Fluorescent Protein-Type Indicators by Flexible Linker Length Optimization in Topology Mutant. Commun. Biol. 2024, 7, 705. [Google Scholar] [CrossRef]
- Farrants, H.; Shuai, Y.; Lemon, W.C.; Hernandez, C.M.; Zhang, D.; Yang, S.F.; Patel, R.; Qiao, G.; Frei, M.S.; Plutkis, S.E.; et al. A Modular Chemigenetic Calcium Indicator for Multiplexed in Vivo Functional Imaging. Nat. Methods 2024, 21, 1916. [Google Scholar] [CrossRef] [PubMed]
- Beier, H.T.; Tolstykh, G.P.; Musick, J.D.; Thomas, R.J.; Ibey, B.L. Plasma Membrane Nanoporation as a Possible Mechanism behind Infrared Excitation of Cells. J. Neural Eng. 2014, 11, 66006. [Google Scholar] [CrossRef]
- Bower, A.J.; Renteria, C.; Li, J.; Marjanovic, M.; Barkalifa, R.; Boppart, S.A. High-Speed Label-Free Two-Photon Fluorescence Microscopy of Metabolic Transients during Neuronal Activity. Appl. Phys. Lett. 2021, 118, 081104. [Google Scholar] [CrossRef] [PubMed]
- Kolenc, O.I.; Quinn, K.P. Evaluating Cell Metabolism Through Autofluorescence Imaging of NAD(P)H and FAD. Antioxid. Redox Signal. 2017, 30, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Bard, L.; Reynolds, J.P.; King, C.; Jensen, T.P.; Gourine, A.V.; Rusakov, D.A. Time-Resolved Imaging Reveals Heterogeneous Landscapes of Nanomolar Ca2+ in Neurons and Astroglia. Neuron 2015, 88, 277–288. [Google Scholar] [CrossRef]
- Nicholson, C.; Hrabětová, S. Biophysical Properties of Brain Extracellular Space Explored with Ion-Selective Microelectrodes, Integrative Optical Imaging and Related Techniques. In Frontiers in Neuroengineering Series/Frontiers in Neuroengineering; CRC Press: Boca Raton, FL, USA, 2006; p. 167. [Google Scholar]
- Thorne, R.G.; Nicholson, C. In Vivo Diffusion Analysis with Quantum Dots and Dextrans Predicts the Width of Brain Extracellular Space. Proc. Natl. Acad. Sci. USA 2006, 103, 5567. [Google Scholar] [CrossRef]
- Egelman, D.M.; Montague, P.R. Calcium Dynamics in the Extracellular Space of Mammalian Neural Tissue. Biophys. J. 1999, 76, 1856. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Gabioud, A.A.; Suñer, I.G.; Idziak, A.; Fabritius, A.; Basquin, J.; Angibaud, J.; Nägerl, U.V.; Singh, S.P.; Griesbeck, O. Fluorescent Sensors for Imaging of Interstitial Calcium. Nat. Commun. 2023, 14, 6220. [Google Scholar] [CrossRef]
- Sasaki, T.; Hisada, S.; Kanki, H.; Nunomura, K.; Lin, B.; Nishiyama, K.; Kawano, T.; Matsumura, S.; Mochizuki, H. Modulation of Ca2+ Oscillation Following Ischemia and Nicotinic Acetylcholine Receptors in Primary Cortical Neurons by High-Throughput Analysis. Sci. Rep. 2024, 14, 27667. [Google Scholar] [CrossRef]
- Dana, H.; Sun, Y.; Mohar, B.; Hulse, B.K.; Kerlin, A.; Hasseman, J.; Tsegaye, G.; Tsang, A.; Wong, A.M.; Patel, R.; et al. High-Performance Calcium Sensors for Imaging Activity in Neuronal Populations and Microcompartments. Nat. Methods 2019, 16, 649. [Google Scholar] [CrossRef]
- Yasuda, R.; Nimchinsky, E.A.; Scheuß, V.; Pologruto, T.A.; Oertner, T.G.; Sabatini, B.L.; Svoboda, K. Imaging Calcium Concentration Dynamics in Small Neuronal Compartments. Sci. STKE 2004, 2004, pl5. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, B.L.; Oertner, T.G.; Svoboda, K. The Life Cycle of Ca2+ Ions in Dendritic Spines. Neuron 2002, 33, 439. [Google Scholar] [CrossRef] [PubMed]
- Somjen, G.G. Ions in the Brain: Normal Function, Seizures, and Stroke; Oxford University Press: Oxford, UK, 2004; Volume 11, p. 12. [Google Scholar] [CrossRef]
- Rusakov, D.A.; Fine, A. Extracellular Ca2+ Depletion Contributes to Fast Activity-Dependent Modulation of Synaptic Transmission in the Brain. Neuron 2003, 37, 287. [Google Scholar] [CrossRef]
- Qin, H.; He, W.; Yang, C.; Li, J.; Jian, T.; Liang, S.; Chen, T.; Feng, H.; Chen, X.; Liao, X.; et al. Monitoring Astrocytic Ca2+ Activity in Freely Behaving Mice. Front. Cell. Neurosci. 2020, 14, 603095. [Google Scholar] [CrossRef]
- Kim, T.H.; Schnitzer, M.J. Fluorescence Imaging of Large-Scale Neural Ensemble Dynamics. Cell 2022, 185, 9. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Holden, S.; Borovicka, J.; Icardi, J.; O’Niel, A.; Chaklai, A.; Patel, D.; Patel, R.; Kaech, S.; Raber, J.; et al. Large-Scale Recording of Neuronal Activity in Freely-Moving Mice at Cellular Resolution. Nat. Commun. 2023, 14, 6399. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Gezginer, I.; Ding, Q.-P.; Yoshihara, H.A.I.; Deán-Ben, X.L.; Ni, R.; Razansky, D. Non-Invasive Large-Scale Imaging of Concurrent Neuronal, Astrocytic, and Hemodynamic Activity with Hybrid Multiplexed Fluorescence and Magnetic Resonance Imaging (HyFMRI). Light Sci. Appl. 2025, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Narayanan, R. Heterogeneous Off-Target Impact of Ion-Channel Deletion on Intrinsic Properties of Hippocampal Model Neurons That Self-Regulate Calcium. Front. Cell. Neurosci. 2023, 17, 1241450. [Google Scholar] [CrossRef] [PubMed]
- Rondoni, N.A.; Lu, F.; Turner-Evans, D.B.; Gomez, M. Predicting Neuronal Firing from Calcium Imaging Using a Control Theoretic Approach. PLoS Comput. Biol. 2025, 21, e1012603. [Google Scholar] [CrossRef]
- Carbonero, D.; Noueihed, J.; Kramer, M.; White, J.A. Nonnegative Matrix Factorization for Analyzing State Dependent Neuronal Network Dynamics in Calcium Recordings. Sci. Rep. 2024, 14, 27899. [Google Scholar] [CrossRef]
- Toma, J.S.; Karamboulas, K.; Carr, M.; Kolaj, A.; Yuzwa, S.A.; Mahmud, N.; Storer, M.A.; Kaplan, D.R.; Miller, F.D. Peripheral Nerve Single-Cell Analysis Identifies Mesenchymal Ligands That Promote Axonal Growth. eNeuro 2020, 7. [Google Scholar] [CrossRef]
- Moldwin, T.; Azran, L.S.; Segev, I. The Calcitron: A Simple Neuron Model That Implements Many Learning Rules via the Calcium Control Hypothesis. PLoS Comput. Biol. 2025, 21, e1012754. [Google Scholar] [CrossRef]
- Moldwin, T.; Azran, L.S.; Segev, I. A Generalized Mathematical Framework for the Calcium Control Hypothesis Describes Weight-Dependent Synaptic Plasticity. J. Comput. Neurosci. 2025, 53, 333–357. [Google Scholar] [CrossRef]
- Cahill, M.K.; Collard, M.; Tse, V.; Reitman, M.E.; Etchenique, R.; Kirst, C.; Poskanzer, K.E. Network-Level Encoding of Local Neurotransmitters in Cortical Astrocytes. Nature 2024, 629, 146. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Pasqualetti, F.; Papouin, T.; Ching, S. Astrocytes as a Mechanism for Contextually-Guided Network Dynamics and Function. PLoS Comput. Biol. 2024, 20, e1012186. [Google Scholar] [CrossRef] [PubMed]
| Mechanism/Source | Mode of Activation | Functional Output | References |
|---|---|---|---|
| IP3R (Inositol 1,4,5-trisphosphate receptors) | G-protein-coupled receptor activation → phospholipase C → IP3 production → ER Ca2+ release | Gliotransmitter release (glutamate, ATP, GABA, D-serine), metabolic coupling | [4,6,9,13,14] |
| SOCE (Store-operated calcium entry) | ER Ca2+ depletion → STIM sensor activation → Orai channel opening at ER-plasma membrane junctions | ER Ca2+ store replenishment, sustained Ca2+ signaling | [6,9,13] |
| mGluR (Metabotropic glutamate receptors) | Glutamate binding → phospholipase C activation → IP3-mediated Ca2+ mobilization | Gliotransmission, synaptic modulation | [4,8,9,12] |
| P2Y (Purinergic P2Y receptors) | ATP/ADP binding → phospholipase C activation → IP3-mediated Ca2+ mobilization | Gliotransmission, intercellular Ca2+ wave propagation | [4,9,13,14] |
| VGCC (Voltage-gated calcium channels) | Membrane depolarization → channel opening → Ca2+ influx | Localized Ca2+ entry, depolarization-linked responses | [5,9,13,14] |
| NCX (Na+-Ca2+ exchanger) | Reverse mode operation during elevated intracellular Na+ (e.g., after neurotransmitter uptake) | Ca2+ entry independent of ER stores, contribution to ionic homeostasis (with Na+,K+-ATPase) | [7,9,13] |
| Ionotropic receptors | Ligand binding (e.g., AMPA, NMDA, P2X) → direct Ca2+ influx | Microdomain signaling in fine processes | [4,9,13,14] |
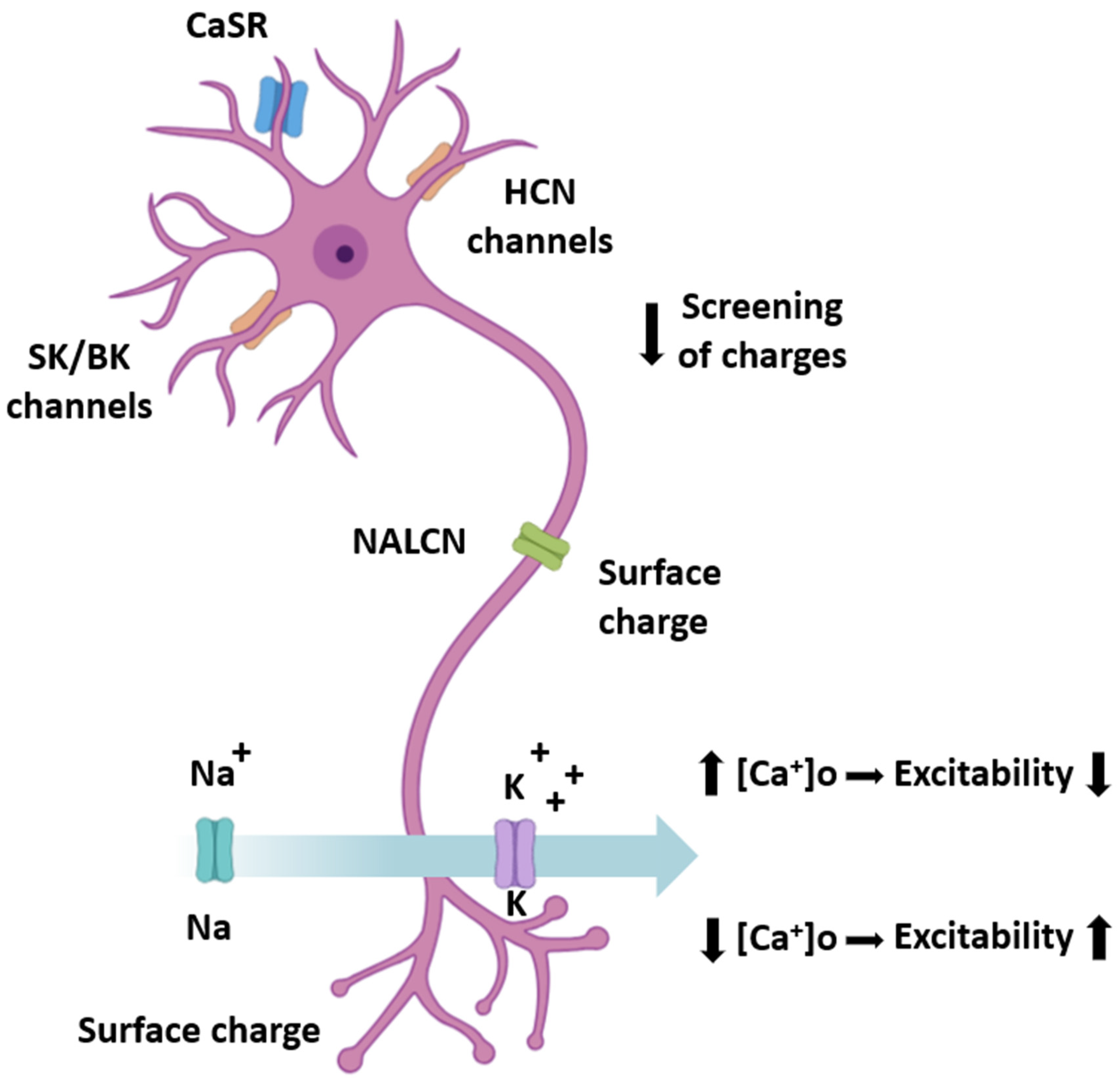
| Neuronal Target | Mechanism of Sensitivity to [Ca2+]o | Functional Outcome | References |
|---|---|---|---|
| CaSR (Calcium-sensing receptor) | G-protein-coupled receptor activation by extracellular Ca2+ → intracellular cascades regulating ion channels, neurotransmitter release, and gene expression | Coupling of extracellular calcium availability to neuronal physiology | [39,57,60,61] |
| HCN channels (Hyperpolarization-activated cyclic nucleotide-gated) | Surface charge screening by Ca2+ ions → shielding of negative membrane charges → shifted voltage dependence | Altered pacemaker currents and excitability | [59,62] |
| SK/BK channels (Small/large-conductance Ca2+-activated K+ channels) | Indirect modulation through [Ca2+]o effects on local depolarization and intracellular Ca2+ entry | Modulation of afterhyperpolarization and firing patterns | [47,59,62] |
| NALCN (Sodium leak channel) | Proposed response to [Ca2+]o changes through mechanisms involving auxiliary subunits | Altered baseline excitability and membrane potential | [27,63,64] |
| Voltage-gated Na+/K+ channels | Screening of fixed negative charges by divalent Ca2+ alters gating of fast voltage-gated channels | Depolarized neuronal thresholds, increased excitability (with [Ca2+]o reduction) | [59,62,65] |
| NMDA receptors | Direct modulation by extracellular Ca2+ availability affecting receptor activity | Altered synaptic plasticity induction (LTP/LTD) | [30,47,66] |
| Presynaptic Ca2+ channels | [Ca2+]o changes alter driving force for Ca2+ entry during action potentials | Modified neurotransmitter release probability and short-term plasticity | [47,52,59] |
| Disorder | Astrocytic Ca2+ Abnormality | Consequences for Neurons/Networks | Translational Implications | Refs. |
|---|---|---|---|---|
| Alzheimer’s disease (AD) | Abnormal intracellular Ca2+ oscillations; exaggerated Ca2+ transients; impaired extracellular Ca2+ buffering (due to amyloid-β effects on receptors/channels) | Destabilized gliotransmission; synaptic and cognitive decline | Excitotoxic cascades; impaired amyloid clearance via disrupted Ca2+-dependent endocytosis; self-reinforcing pathological cycle | [88,89,93,95,96] |
| Parkinson’s disease (PD) | Disrupted astrocytic Ca2+ handling in striatum (due to α-synuclein accumulation); weakened ability to maintain ionic stability particularly affecting astrocyte-cholinergic interneuron interactions | Altered balance between dopaminergic and cholinergic signaling; compromised striatal circuit function | Motor dysfunction and cognitive decline; selective vulnerability of striatal cholinergic interneurons | [99,100,101,103,104,105,106,107] |
| Epilepsy | Rapid [Ca2+]o drops during seizures; impaired astrocytic Ca2+ buffering; aberrant Ca2+ oscillations | Compromised ionic homeostasis restoration; prolonged hyperexcitability; facilitated recurrent ictal activity | Lowered seizure thresholds; network instability driven by astrocytic dysfunction; potential therapeutic target for stabilizing [Ca2+]o and restoring excitability balance | [35,82,110,111,114] |
| Autism spectrum disorders (ASD) | Immature astrocytic Ca2+ signaling profiles (due to failed astrocyte maturation) | Impaired synapse formation, pruning, and plasticity; altered circuit wiring | Synaptic maturation deficits leading to atypical circuit development and behavioral phenotypes | [36,67,68,83] |
| Intellectual disabilities (ID) | Mutations in Ca2+-handling proteins (IP3 receptors, STIM/Orai components, exchangers); disrupted intracellular Ca2+ mobilization and extracellular modulation | Impaired astrocytic support to neuronal networks; long-lasting deficits in synaptic connectivity | Cognitive dysfunction resulting in persistent impairments in learning and memory functions | [6,28,115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka, A.; Śniegocki, M.; Ziółkowska, E.A. Calcium Dynamics in Astrocyte-Neuron Communication from Intracellular to Extracellular Signaling. Cells 2025, 14, 1709. https://doi.org/10.3390/cells14211709
Nowacka A, Śniegocki M, Ziółkowska EA. Calcium Dynamics in Astrocyte-Neuron Communication from Intracellular to Extracellular Signaling. Cells. 2025; 14(21):1709. https://doi.org/10.3390/cells14211709
Chicago/Turabian StyleNowacka, Agnieszka, Maciej Śniegocki, and Ewa A. Ziółkowska. 2025. "Calcium Dynamics in Astrocyte-Neuron Communication from Intracellular to Extracellular Signaling" Cells 14, no. 21: 1709. https://doi.org/10.3390/cells14211709
APA StyleNowacka, A., Śniegocki, M., & Ziółkowska, E. A. (2025). Calcium Dynamics in Astrocyte-Neuron Communication from Intracellular to Extracellular Signaling. Cells, 14(21), 1709. https://doi.org/10.3390/cells14211709








