Caspase-8 and BID Caught in the Act with Cardiolipin: A New Platform to Provide Mitochondria with Microdomains of Apoptotic Signals
Abstract
1. Introduction
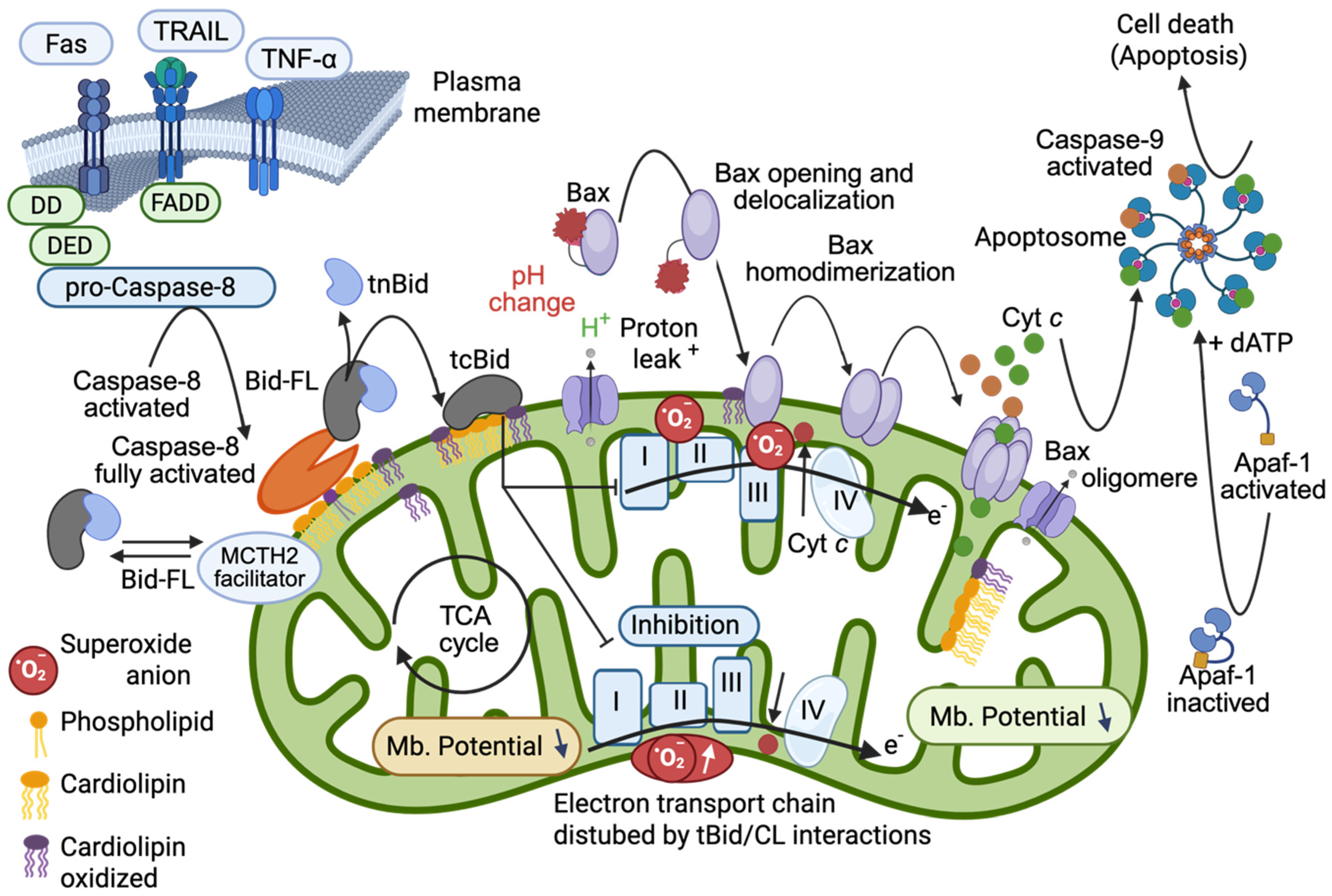
2. The Activation Platform: Cardiolipin, Caspase-8, and BID
2.1. Cardiolipin as a Key Mitochondrial Determinant
2.2. Caspase-8, BID, tBID and Lipid Binding
2.2.1. Interaction with Lipids
2.2.2. Protein–Protein Interactions
2.2.3. Regulatory Cross-Talk
3. MTCH2 at the Mitochondrial Membrane
3.1. MTCH2 as a Facilitator, Not an Enzyme!
3.2. Does MTCH2 Interfere with the tBID-Cardiolipin System?
3.3. Redundant Functions? Or a More Complex Situation
4. Some New Players Enter the Game Together with tBID
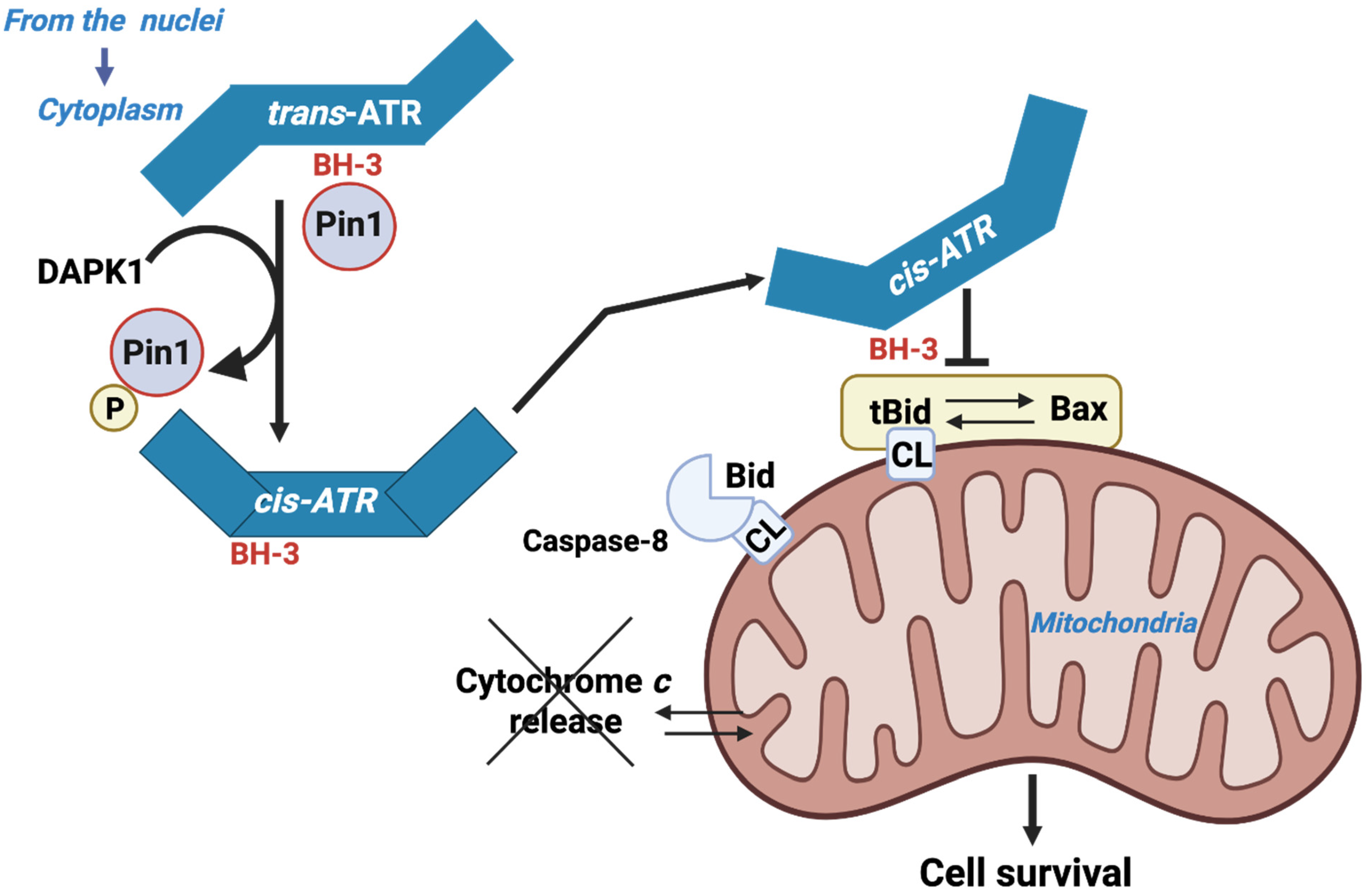
4.1. The ATR Protein Kinase Is a New Player in the Game
4.2. ATM Kinase Is Also a Player in the Game Together with tBID
4.3. MTCH2
4.3.1. MTCH2 as a Facilitator
4.3.2. New Functions of MTCH2, Aside from BID Signaling
4.4. Humanin as an Inhibitor That Sequesters tBID into Fiber Structures
5. In Vitro Systems Demonstrating the Role of Cardiolipin
5.1. Proteoliposome Models and Giant Unilamellar Vesicles
5.2. Purified Mouse Mitochondria
5.3. Yeast Mitochondria
5.4. Biochemical and Biophysical Approach “In Vitro”
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Li, H.; Zhu, H.; Xu, C.J.; Yuan, J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998, 94, 491–501. [Google Scholar] [CrossRef]
- Luo, X.; Budihardjo, I.; Zou, H.; Slaughter, C.; Wang, X. Bid, a bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface receptors. Cell 1998, 94, 481–490. [Google Scholar] [CrossRef]
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000, 102, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Luo, X.; Wang, X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 2001, 412, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Parrish, J.; Li, L.; Klotz, K.; Ledwich, D.; Wang, X.; Xue, D. Mitochondrial endonuclease G is important for apoptosis in C. elegans. Nature 2001, 412, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Widlak, P.; Li, L.Y.; Wang, X.; Garrard, W.T. Action of recombinant human apoptotic endonuclease G on naked DNA and chromatin substrates: Cooperation with exonuclease and DNase I. J. Biol. Chem. 2001, 276, 48404–48409. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Li, X.X.; Gottleib, E.; Hill, R.B.; Thompson, C.B.; Colombini, M. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J. Biol. Chem. 2001, 276, 19414–19419. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Chandel, N.S.; Schumacker, P.T.; Thompson, C.B. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol. Cell 1999, 3, 159–167. [Google Scholar] [CrossRef]
- Mootha, V.K.; Wei, M.C.; Buttle, K.F.; Scorrano, L.; Panoutsakopoulou, V.; Mannella, C.A.; Korsmeyer, S.J. A reversible component of mitochondrial respiratory dysfunction in apoptosis can be rescued by exogenous cytochrome c. EMBO J. 2001, 20, 661–671. [Google Scholar] [CrossRef]
- Lutter, M.; Perkins, G.A.; Wang, X. The pro-apoptotic Bcl-2 family member tBid localizes to mitochondrial contact sites. BMC Cell Biol. 2001, 2, 22. [Google Scholar] [CrossRef]
- Lutter, M.; Fang, M.; Luo, X.; Nishijima, M.; Xie, X.; Wang, X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat. Cell Biol. 2000, 2, 754–761. [Google Scholar] [CrossRef]
- Ardail, D.; Lerme, F.; Louisot, P. Further characterization of mitochondrial contact sites: Effect of short-chain alcohols on membrane fluidity and activity. Biochem. Biophys. Res. Commun. 1990, 173, 878–885. [Google Scholar] [CrossRef]
- Ardail, D.; Privat, J.P.; Egret-Charlier, M.; Levrat, C.; Lerme, F.; Louisot, P. Mitochondrial contact sites. Lipid composition and dynamics. J. Biol. Chem. 1990, 265, 18797–18802. [Google Scholar] [CrossRef] [PubMed]
- Esposti, M.D.; Erler, J.T.; Hickman, J.A.; Dive, C. Bid, a widely expressed proapoptotic protein of the Bcl-2 family, displays lipid transfer activity. Mol. Cell Biol. 2001, 21, 7268–7276. [Google Scholar] [CrossRef] [PubMed]
- Esposti, M.D.; Cristea, I.M.; Gaskell, S.J.; Nakao, Y.; Dive, C. Proapoptotic Bid binds to monolysocardiolipin, a new molecular connection between mitochondrial membranes and cell death. Cell Death Differ. 2003, 10, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Gonzalvez, F.; Pariselli, F.; Dupaigne, P.; Budihardjo, I.; Lutter, M.; Antonsson, B.; Diolez, P.; Manon, S.; Martinou, J.C.; Goubern, M.; et al. tBid interaction with cardiolipin primarily orchestrates mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell Death Differ. 2005, 12, 614–626. [Google Scholar] [CrossRef]
- Gonzalvez, F.; Schug, Z.T.; Houtkooper, R.H.; MacKenzie, E.D.; Brooks, D.G.; Wanders, R.J.; Petit, P.X.; Vaz, F.M.; Gottlieb, E. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J. Cell Biol. 2008, 183, 681–696. [Google Scholar] [CrossRef]
- Sorice, M.; Manganelli, V.; Matarrese, P.; Tinari, A.; Misasi, R.; Malorni, W.; Garofalo, T. Cardiolipin-enriched raft-like microdomains are essential activating platforms for apoptotic signals on mitochondria. FEBS Lett. 2009, 583, 2447–2450. [Google Scholar] [CrossRef]
- Gonzalvez, F.; Gottlieb, E. Cardiolipin: Setting the beat of apoptosis. Apoptosis 2007, 12, 877–885. [Google Scholar] [CrossRef]
- Vladimirov, Y.A.; Proskurnina, E.V.; Izmailov, D.Y.; Novikov, A.A.; Brusnichkin, A.V.; Osipov, A.N.; Kagan, V.E. Mechanism of activation of cytochrome C peroxidase activity by cardiolipin. Biochemistry 2006, 71, 989–997. [Google Scholar] [CrossRef]
- Mohammadyani, D.; Yanamala, N.; Samhan-Arias, A.K.; Kapralov, A.A.; Stepanov, G.; Nuar, N.; Planas-Iglesias, J.; Sanghera, N.; Kagan, V.E.; Klein-Seetharaman, J. Structural characterization of cardiolipin-driven activation of cytochrome c into a peroxidase and membrane perturbation. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mandal, A.; Tyurin, V.A.; DeLucia, M.; Ahn, J.; Kagan, V.E.; van der Wel, P.C.A. Surface-Binding to Cardiolipin Nanodomains Triggers Cytochrome c Pro-apoptotic Peroxidase Activity via Localized Dynamics. Structure 2019, 27, 806–815.e4. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Bayir, H.A.; Belikova, N.A.; Kapralov, O.; Tyurina, Y.Y.; Tyurin, V.A.; Jiang, J.; Stoyanovsky, D.A.; Wipf, P.; Kochanek, P.M.; et al. Cytochrome c/cardiolipin relations in mitochondria: A kiss of death. Free Radic. Biol. Med. 2009, 46, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Kantari, C.; Walczak, H. Caspase-8 and Bid: Caught in the act between death receptors and mitochondria. Biochim. Biophys. Acta 2011, 1813, 558–563. [Google Scholar] [CrossRef]
- Zaltsman, Y.; Shachnai, L.; Yivgi-Ohana, N.; Schwarz, M.; Maryanovich, M.; Houtkooper, R.H.; Vaz, F.M.; De Leonardis, F.; Fiermonte, G.; Palmieri, F.; et al. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nat. Cell Biol. 2010, 12, 553–562. [Google Scholar] [CrossRef]
- Hilton, B.A.; Li, Z.; Musich, P.R.; Wang, H.; Cartwright, B.M.; Serrano, M.; Zhou, X.Z.; Lu, K.P.; Zou, Y. ATR Plays a Direct Antiapoptotic Role at Mitochondria, which Is Regulated by Prolyl Isomerase Pin1. Mol. Cell 2015, 60, 35–46. [Google Scholar] [CrossRef]
- Kamer, I.; Sarig, R.; Zaltsman, Y.; Niv, H.; Oberkovitz, G.; Regev, L.; Haimovich, G.; Lerenthal, Y.; Marcellus, R.C.; Gross, A. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell 2005, 122, 593–603. [Google Scholar] [CrossRef]
- Gonzalvez, F.; D’Aurelio, M.; Boutant, M.; Moustapha, A.; Puech, J.P.; Landes, T.; Arnaune-Pelloquin, L.; Vial, G.; Taleux, N.; Slomianny, C.; et al. Barth syndrome: Cellular compensation of mitochondrial dysfunction and apoptosis inhibition due to changes in cardiolipin remodeling linked to tafazzin (TAZ) gene mutation. Biochim. Biophys. Acta 2013, 1832, 1194–1206. [Google Scholar] [CrossRef]
- Saric, A.; Andreau, K.; Armand, A.S.; Moller, I.M.; Petit, P.X. Barth Syndrome: From Mitochondrial Dysfunctions Associated with Aberrant Production of Reactive Oxygen Species to Pluripotent Stem Cell Studies. Front. Genet. 2015, 6, 359. [Google Scholar] [CrossRef]
- Wyzewski, Z.; Gregorczyk-Zboroch, K.P.; Mielcarska, M.B.; Switlik, W.; Niedzielska, A. Bid Protein: A Participant in the Apoptotic Network with Roles in Viral Infections. Int. J. Mol. Sci. 2025, 26, 2385. [Google Scholar] [CrossRef]
- Murphy, M.P.; O’Neill, L.A.J. A break in mitochondrial endosymbiosis as a basis for inflammatory diseases. Nature 2024, 626, 271–279. [Google Scholar] [CrossRef]
- Corcelli, A.; Schlame, M. Cardiolipin as key lipid of mitochondria in health and disease. 2016, 2nd Edition, Florence, Italy, September 30–October 1, 2015. Chem. Phys. Lipids 2016, 198, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Esposti, M.D. Lipids, cardiolipin and apoptosis: A greasy licence to kill. Cell Death Differ. 2002, 9, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.A.; Ryan, R.O. Studies of the cardiolipin interactome. Prog. Lipid Res. 2022, 88, 101195. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, S.; Harner, M.E. The MICOS complex, a structural element of mitochondria with versatile functions. Biol. Chem. 2020, 401, 765–778. [Google Scholar] [CrossRef]
- Benaroya, H. Mitochondria and MICOS–Function and modeling. Rev. Neurosci. 2024, 35, 503–531. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Ikonen, E. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 2001, 13, 470–477. [Google Scholar] [CrossRef]
- Hakomori, S.I. Structure and function of glycosphingolipids and sphingolipids: Recollections and future trends. Biochim. Biophys. Acta 2008, 1780, 325–346. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Garofalo, T.; Misasi, R.; Mattei, V.; Giammarioli, A.M.; Malorni, W.; Pontieri, G.M.; Pavan, A.; Sorice, M. Association of the death-inducing signaling complex with microdomains after triggering through CD95/Fas. Evidence for caspase-8-ganglioside interaction in T cells. J. Biol. Chem. 2003, 278, 8309–8315. [Google Scholar] [CrossRef]
- Scaffidi, C.; Fulda, S.; Srinivasan, A.; Friesen, C.; Li, F.; Tomaselli, K.J.; Debatin, K.M.; Krammer, P.H.; Peter, M.E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998, 17, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Scaffidi, C.; Pietsch, T.; Krammer, P.H.; Peter, M.E.; Debatin, K.M. Activation of the CD95 (APO-1/Fas) pathway in drug- and gamma-irradiation-induced apoptosis of brain tumor cells. Cell Death Differ. 1998, 5, 884–893. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malorni, W.; Garofalo, T.; Tinari, A.; Manganelli, V.; Misasi, R.; Sorice, M. Analyzing lipid raft dynamics during cell apoptosis. Methods Enzymol. 2008, 442, 125–140. [Google Scholar] [PubMed]
- Jalmar, O.; Garcia-Saez, A.J.; Berland, L.; Gonzalvez, F.; Petit, P.X. Giant unilamellar vesicles (GUVs) as a new tool for analysis of caspase-8/Bid-FL complex binding to cardiolipin and its functional activity. Cell Death Dis. 2010, 1, e103. [Google Scholar] [CrossRef]
- Jalmar, O.; Francois-Moutal, L.; Garcia-Saez, A.J.; Perry, M.; Granjon, T.; Gonzalvez, F.; Gottlieb, E.; Ayala-Sanmartin, J.; Klosgen, B.; Schwille, P.; et al. Caspase-8 binding to cardiolipin in giant unilamellar vesicles provides a functional docking platform for bid. PLoS ONE 2013, 8, e55250. [Google Scholar] [CrossRef]
- Bleicken, S.; Garcia-Saez, A.J.; Conte, E.; Bordignon, E. Dynamic interaction of cBid with detergents, liposomes and mitochondria. PLoS ONE 2012, 7, e35910. [Google Scholar] [CrossRef]
- Unsay, J.D.; Cosentino, K.; Sporbeck, K.; Garcia-Saez, A.J. Pro-apoptotic cBid and Bax exhibit distinct membrane remodeling activities: An AFM study. Biochim. Biophys. Acta Biomembr. 2017, 1859, 17–27. [Google Scholar] [CrossRef]
- Schug, Z.T.; Gonzalvez, F.; Houtkooper, R.H.; Vaz, F.M.; Gottlieb, E. BID is cleaved by caspase-8 within a native complex on the mitochondrial membrane. Cell Death Differ. 2011, 18, 538–548. [Google Scholar] [CrossRef]
- Flores-Romero, H.; Hohorst, L.; John, M.; Albert, M.C.; King, L.E.; Beckmann, L.; Szabo, T.; Hertlein, V.; Luo, X.; Villunger, A.; et al. BCL-2-family protein tBID can act as a BAX-like effector of apoptosis. EMBO J. 2022, 41, e108690. [Google Scholar] [CrossRef]
- Bleicken, S.; Landeta, O.; Landajuela, A.; Basanez, G.; Garcia-Saez, A.J. Proapoptotic Bax and Bak form stable protein-permeable pores of tunable size. J Biol Chem. 2013, 15, 33241–33252. [Google Scholar] [CrossRef]
- Hockings, C.; Anwari, K.; Ninnis, R.L.; Brouwer, J.; O’Hely, M.; Evangelista, M.; Hinds, M.G.; Czabotar, P.E.; Lee, E.F.; Fairlie, W.D.; et al. Bid chimeras indicate that most BH3-only proteins can directly activate Bak and Bax, and show no preference for Bak versus Bax. Cell Death Dis. 2015, 6, e1735. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, T.; Grace, C.R.; Llambi, F.; Nourse, A.; Fitzgerald, P.; Gehring, K.; Kriwacki, R.W.; Green, D.R. BID-induced structural changes in BAK promote apoptosis. Nat. Struct. Mol. Biol. 2013, 20, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Sarosiek, K.A.; Chi, X.; Bachman, J.A.; Sims, J.J.; Montero, J.; Patel, L.; Flanagan, A.; Andrews, D.W.; Sorger, P.; Letai, A. BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response. Mol. Cell 2013, 51, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Petit, P.X.; Dupaigne, P.; Pariselli, F.; Gonzalvez, F.; Etienne, F.; Rameau, C.; Bernard, S. Interaction of the alpha-helical H6 peptide from the pro-apoptotic protein tBid with cardiolipin. FEBS J. 2009, 276, 6338–6354. [Google Scholar] [CrossRef]
- Gonzalvez, F.; Pariselli, F.; Jalmar, O.; Dupaigne, P.; Sureau, F.; Dellinger, M.; Hendrickson, E.A.; Bernard, S.; Petit, P.X. Mechanistic issues of the interaction of the hairpin-forming domain of tBid with mitochondrial cardiolipin. PLoS ONE 2010, 5, e9342. [Google Scholar] [CrossRef]
- Cartron, P.F.; Gallenne, T.; Bougras, G.; Gautier, F.; Manero, F.; Vusio, P.; Meflah, K.; Vallette, F.M.; Juin, P. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol. Cell 2004, 16, 807–818. [Google Scholar] [CrossRef]
- Juin, P.; Cartron, P.F.; Vallette, F.M. Activation of Bax by BH3 domains during apoptosis: The unfolding of a deadly plot. Cell Cycle 2005, 4, 637–642. [Google Scholar] [CrossRef]
- Garcia-Saez, A.J.; Mingarro, I.; Perez-Paya, E.; Salgado, J. Membrane-insertion fragments of Bcl-xL, Bax, and Bid. Biochemistry 2004, 43, 10930–10943. [Google Scholar] [CrossRef]
- Garcia-Saez, A.J.; Coraiola, M.; Dalla Serra, M.; Mingarro, I.; Menestrina, G.; Salgado, J. Peptides derived from apoptotic Bax and Bid reproduce the poration activity of the parent full-length proteins. Biophys. J. 2005, 88, 3976–3990. [Google Scholar] [CrossRef]
- Cartron, P.F.; Oliver, L.; Mayat, E.; Meflah, K.; Vallette, F.M. Impact of pH on Bax alpha conformation, oligomerisation and mitochondrial integration. FEBS Lett. 2004, 578, 41–46. [Google Scholar] [CrossRef]
- Guna, A.; Stevens, T.A.; Inglis, A.J.; Replogle, J.M.; Esantsi, T.K.; Muthukumar, G.; Shaffer, K.C.L.; Wang, M.L.; Pogson, A.N.; Jones, J.J.; et al. MTCH2 is a mitochondrial outer membrane protein insertase. Science 2022, 378, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Bartos, L.; Menon, A.K.; Vacha, R. Insertases scramble lipids: Molecular simulations of MTCH2. Structure 2024, 32, 505–510.e4. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yang, Y.; Hou, R.; Zhang, L.; Shen, C.; Yang, X.; Luo, Z.; Yin, Z.; Cao, Y. MTCH2 in Metabolic Diseases, Neurodegenerative Diseases, Cancers, Embryonic Development and Reproduction. Drug Des. Devel. Ther. 2024, 18, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Raemy, E.; Montessuit, S.; Pierredon, S.; van Kampen, A.H.; Vaz, F.M.; Martinou, J.C. Cardiolipin or MTCH2 can serve as tBID receptors during apoptosis. Cell Death Differ. 2016, 23, 1165–1174. [Google Scholar] [CrossRef]
- Cimprich, K.A.; Cortez, D. ATR: An essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 2008, 9, 616–627. [Google Scholar] [CrossRef]
- Zeman, M.K.; Cimprich, K.A. Causes and consequences of replication stress. Nat. Cell Biol. 2014, 16, 2–9. [Google Scholar] [CrossRef]
- Sancar, A.; Lindsey-Boltz, L.A.; Unsal-Kacmaz, K.; Linn, S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004, 73, 39–85. [Google Scholar] [CrossRef]
- Bartkova, J.; Horejsi, Z.; Koed, K.; Kramer, A.; Tort, F.; Zieger, K.; Guldberg, P.; Sehested, M.; Nesland, J.M.; Lukas, C.; et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 2005, 434, 864–870. [Google Scholar] [CrossRef]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald, E.R., III; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef]
- Cortez, D.; Guntuku, S.; Qin, J.; Elledge, S.J. ATR and ATRIP: Partners in checkpoint signaling. Science 2001, 294, 1713–1716. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ravanan, P.; Talwar, P. Death Associated Protein Kinase 2016, 1 (DAPK1): A Regulator of Apoptosis and Autophagy. Front. Mol. Neurosci. 2016, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Zinkel, S.S.; Hurov, K.E.; Ong, C.; Abtahi, F.M.; Gross, A.; Korsmeyer, S.J. A role for proapoptotic BID in the DNA-damage response. Cell 2005, 122, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Maryanovich, M.; Oberkovitz, G.; Niv, H.; Vorobiyov, L.; Zaltsman, Y.; Brenner, O.; Lapidot, T.; Jung, S.; Gross, A. The ATM-BID pathway regulates quiescence and survival of haematopoietic stem cells. Nat. Cell Biol. 2012, 14, 535–541. [Google Scholar] [CrossRef]
- Tasdogan, A.; Kumar, S.; Allies, G.; Bausinger, J.; Beckel, F.; Hofemeister, H.; Mulaw, M.; Madan, V.; Scharffetter-Kochanek, K.; Feuring-Buske, M.; et al. DNA Damage-Induced HSPC Malfunction Depends on ROS Accumulation Downstream of IFN-1 Signaling and Bid Mobilization. Cell Stem Cell 2016, 19, 752–767. [Google Scholar] [CrossRef]
- Cao, F.; Zhou, T.; Simpson, D.; Zhou, Y.; Boyer, J.; Chen, B.; Jin, T.; Cordeiro-Stone, M.; Kaufmann, W. p53-Dependent but ATM-independent inhibition of DNA synthesis and G2 arrest in cadmium-treated human fibroblasts. Toxicol. Appl. Pharmacol. 2007, 218, 174–185. [Google Scholar] [CrossRef]
- Bar-Lev, Y.; Moshitch-Moshkovitz, S.; Tsarfaty, G.; Kaufman, D.; Horev, J.; Resau, J.H.; Tsarfaty, I. Mimp/Mtch2, an Obesity Susceptibility Gene, Induces Alteration of Fatty Acid Metabolism in Transgenic Mice. PLoS ONE 2016, 11, e0157850. [Google Scholar] [CrossRef]
- Buzaglo-Azriel, L.; Kuperman, Y.; Tsoory, M.; Zaltsman, Y.; Shachnai, L.; Zaidman, S.L.; Bassat, E.; Michailovici, I.; Sarver, A.; Tzahor, E.; et al. Loss of Muscle MTCH2 Increases Whole-Body Energy Utilization and Protects from Diet-Induced Obesity. Cell Rep. 2016, 14, 1602–1610. [Google Scholar] [CrossRef]
- Rottiers, V.; Francisco, A.; Platov, M.; Zaltsman, Y.; Ruggiero, A.; Lee, S.S.; Gross, A.; Libert, S. MTCH2 is a conserved regulator of lipid homeostasis. Obesity 2017, 25, 616–625. [Google Scholar] [CrossRef]
- Bahat, A.; Goldman, A.; Zaltsman, Y.; Khan, D.H.; Halperin, C.; Amzallag, E.; Krupalnik, V.; Mullokandov, M.; Silberman, A.; Erez, A.; et al. MTCH2-mediated mitochondrial fusion drives exit from naive pluripotency in embryonic stem cells. Nat. Commun. 2018, 9, 5132. [Google Scholar] [CrossRef]
- Goldman, A.; Mullokandov, M.; Zaltsman, Y.; Regev, L.; Levin-Zaidman, S.; Gross, A. MTCH2 cooperates with MFN2 and lysophosphatidic acid synthesis to sustain mitochondrial fusion. EMBO Rep. 2024, 25, 45–67. [Google Scholar] [CrossRef]
- Chourasia, S.; Petucci, C.; Shoffler, C.; Abbasian, D.; Wang, H.; Han, X.; Sivan, E.; Brandis, A.; Mehlman, T.; Malitsky, S.; et al. MTCH2 controls energy demand and expenditure to fuel anabolism during adipogenesis. EMBO J. 2025, 44, 1007–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Zhao, B.C.; Li, H.L.; Liu, Y.; Wang, B.; Li, A.Q.; Zeng, T.S.; Hui, H.X.; Sun, J.; Cikes, D.; et al. MTCH2 Suppresses Thermogenesis by Regulating Autophagy in Adipose Tissue. Adv. Sci. 2025, 12, e2416598. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.M.; Baty, C.J.; Li, N.; Ding, W.X.; Gao, W.; Li, M.; Chen, X.; Ma, J.; Michalopoulos, G.K.; Yin, X.M. Bid agonist regulates murine hepatocyte proliferation by controlling endoplasmic reticulum calcium homeostasis. Hepatology 2010, 52, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhou, J.; Zhang, H.; Lin, Z.; Khambu, B.; Liu, G.; Ma, M.; Chen, X.; Chalasani, N.; Yin, X.M. Promotion of diet-induced obesity and metabolic syndromes by BID is associated with gut microbiota. Hepatol. Commun. 2022, 6, 3349–3362. [Google Scholar] [CrossRef]
- Degli Esposti, M. Sequence and functional similarities between pro-apoptotic Bid and plant lipid transfer proteins. Biochim. Biophys. Acta 2002, 1553, 331–340. [Google Scholar] [CrossRef]
- Wu, C.; Wang, T.; Ghosh, A.; Long, F.; Sharma, A.K.; Dahlby, T.; Noe, F.; Severi, I.; Colleluori, G.; Cinti, S.; et al. MTCH2 modulates CPT1 activity to regulate lipid metabolism of adipocytes. Nat. Commun. 2025, 16, 8831. [Google Scholar] [CrossRef]
- Zhang, X.; Li, E.; Kuang, Y.; Gai, Y.; Feng, Y.; Huang, Y.; Wei, Z.; Niu, J.; Yu, S.; Yang, Z.; et al. MTCH2 regulates NRF2-mediated RRM1 expression to promote melanoma proliferation and dacarbazine insensitivity. Cell Death Dis. 2025, 16, 268. [Google Scholar] [CrossRef]
- Guo, B.; Zhai, D.; Cabezas, E.; Welsh, K.; Nouraini, S.; Satterthwait, A.C.; Reed, J.C. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 2003, 423, 456–461. [Google Scholar] [CrossRef]
- Zapala, B.; Kaczynski, L.; Kiec-Wilk, B.; Staszel, T.; Knapp, A.; Thoresen, G.H.; Wybranska, I.; Dembinska-Kiec, A. Humanins, the neuroprotective and cytoprotective peptides with antiapoptotic and anti-inflammatory properties. Pharmacol. Rep. 2010, 62, 767–777. [Google Scholar] [CrossRef]
- Morris, D.L.; Kastner, D.W.; Johnson, S.; Strub, M.P.; He, Y.; Bleck, C.K.E.; Lee, D.Y.; Tjandra, N. Humanin induces conformational changes in the apoptosis regulator BAX and sequesters it into fibers, preventing mitochondrial outer-membrane permeabilization. J. Biol. Chem. 2019, 294, 19055–19065. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L.; Johnson, S.; Bleck, C.K.E.; Lee, D.Y.; Tjandra, N. Humanin selectively prevents the activation of pro-apoptotic protein BID by sequestering it into fibers. J. Biol. Chem. 2020, 295, 18226–18238. [Google Scholar] [CrossRef] [PubMed]
- Sunami, T.; Caschera, F.; Morita, Y.; Toyota, T.; Nishimura, K.; Matsuura, T.; Suzuki, H.; Hanczyc, M.M.; Yomo, T. Detection of association and fusion of giant vesicles using a fluorescence-activated cell sorter. Langmuir 2010, 26, 15098–15103. [Google Scholar] [CrossRef] [PubMed]
- Lacronique, V.; Mignon, A.; Fabre, M.; Viollet, B.; Rouquet, N.; Molina, T.; Porteu, A.; Henrion, A.; Bouscary, D.; Varlet, P.; et al. Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat. Med. 1996, 2, 80–86. [Google Scholar] [CrossRef]
- de la Coste, A.; Fabre, M.; McDonell, N.; Porteu, A.; Gilgenkrantz, H.; Perret, C.; Kahn, A.; Mignon, A. Differential protective effects of Bcl-xL and Bcl-2 on apoptotic liver injury in transgenic mice. Am. J. Physiol. 1999, 277, G702–G708. [Google Scholar] [CrossRef]
- Ciarlo, L.; Manganelli, V.; Matarrese, P.; Garofalo, T.; Tinari, A.; Gambardella, L.; Marconi, M.; Grasso, M.; Misasi, R.; Sorice, M.; et al. Raft-like microdomains play a key role in mitochondrial impairment in lymphoid cells from patients with Huntington’s disease. J. Lipid Res. 2012, 53, 2057–2068. [Google Scholar] [CrossRef]
- Garofalo, T.; Manganelli, V.; Grasso, M.; Mattei, V.; Ferri, A.; Misasi, R.; Sorice, M. Role of mitochondrial raft-like microdomains in the regulation of cell apoptosis. Apoptosis 2015, 20, 621–634. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petit, P.X. Caspase-8 and BID Caught in the Act with Cardiolipin: A New Platform to Provide Mitochondria with Microdomains of Apoptotic Signals. Cells 2025, 14, 1678. https://doi.org/10.3390/cells14211678
Petit PX. Caspase-8 and BID Caught in the Act with Cardiolipin: A New Platform to Provide Mitochondria with Microdomains of Apoptotic Signals. Cells. 2025; 14(21):1678. https://doi.org/10.3390/cells14211678
Chicago/Turabian StylePetit, Patrice X. 2025. "Caspase-8 and BID Caught in the Act with Cardiolipin: A New Platform to Provide Mitochondria with Microdomains of Apoptotic Signals" Cells 14, no. 21: 1678. https://doi.org/10.3390/cells14211678
APA StylePetit, P. X. (2025). Caspase-8 and BID Caught in the Act with Cardiolipin: A New Platform to Provide Mitochondria with Microdomains of Apoptotic Signals. Cells, 14(21), 1678. https://doi.org/10.3390/cells14211678






