Erythropoiesis-Stimulating Agent Protects Against Kidney Fibrosis by Inhibiting G2/M Cell Cycle Arrest
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Kidney 2 (HK-2) Cell Culture and DARB Treatment
2.2. Animal Experiments
2.3. Analysis of Gene Expression by Quantitative RT-PCR
2.4. Histopathological Evaluation, Immunohistochemical Analysis, and Immunofluorescence Analysis
2.5. Immunoblotting of Renal Fibrosis Marker and Cell Cycle Proteins
2.6. Flow Cytometry and Fluorescence-Activated Cell Sorting (FACS)
2.7. Statistical Analysis
3. Results
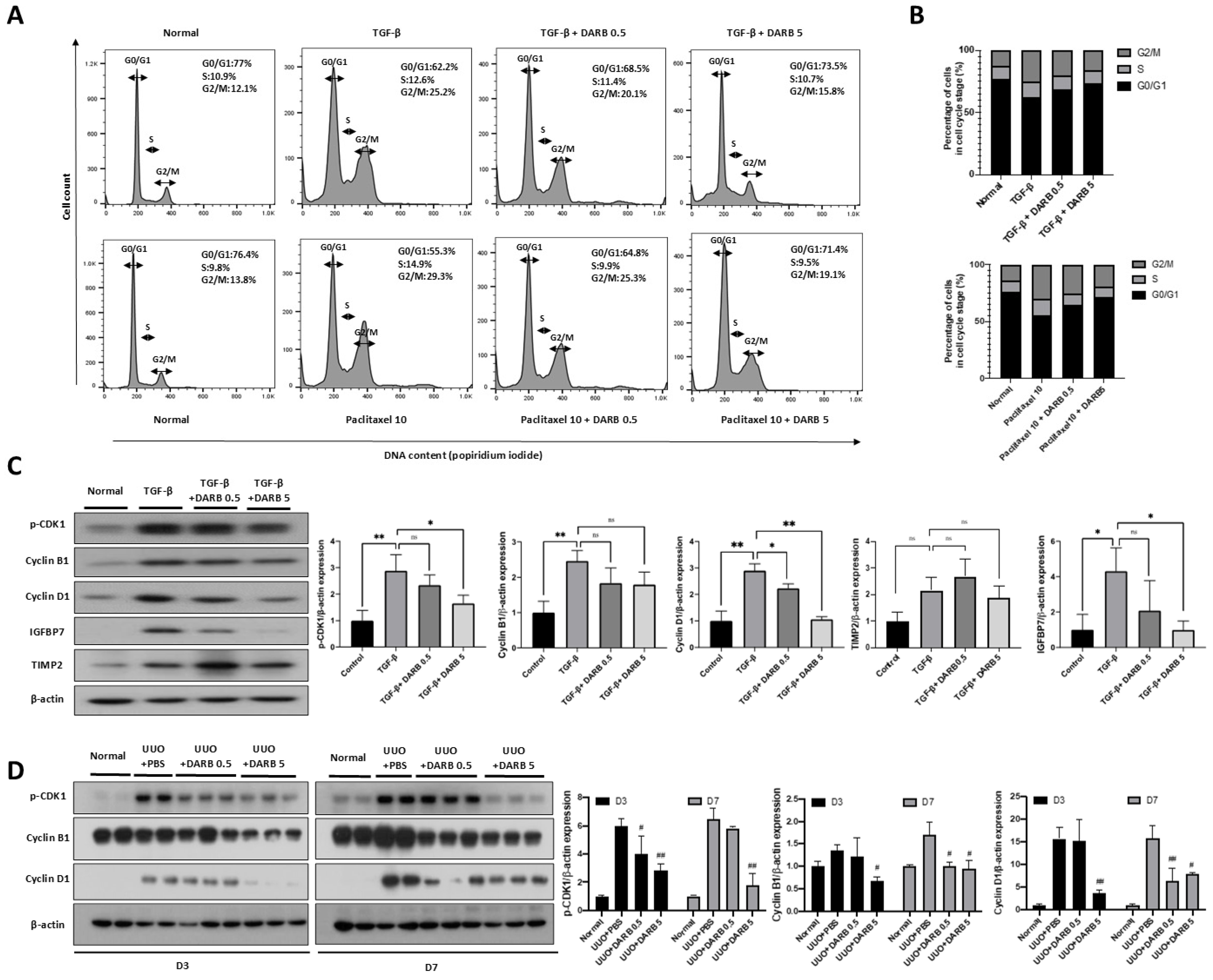
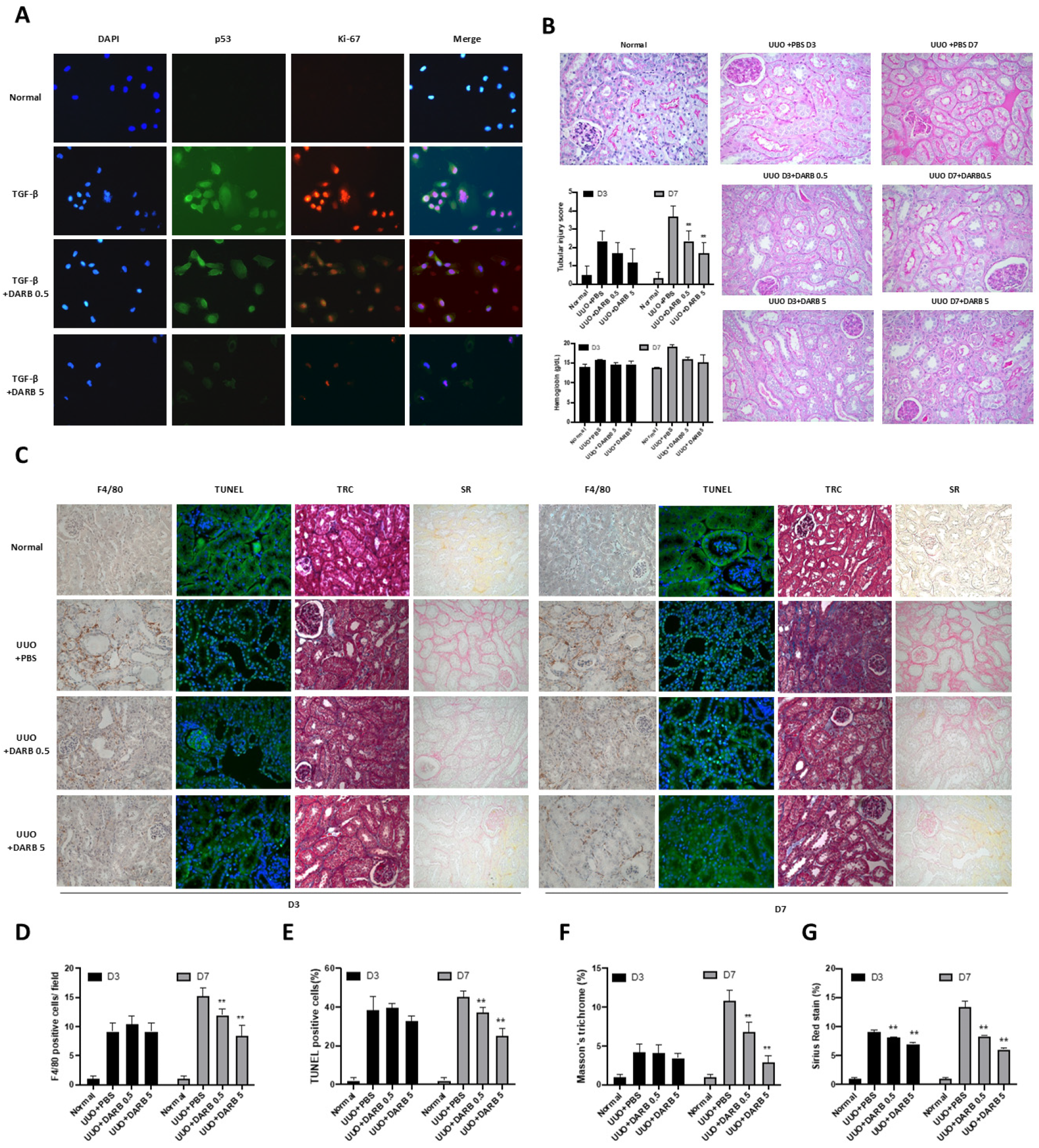
3.1. ESA Reduced G2/M Phase Cell Cycle Arrest in the Kidney
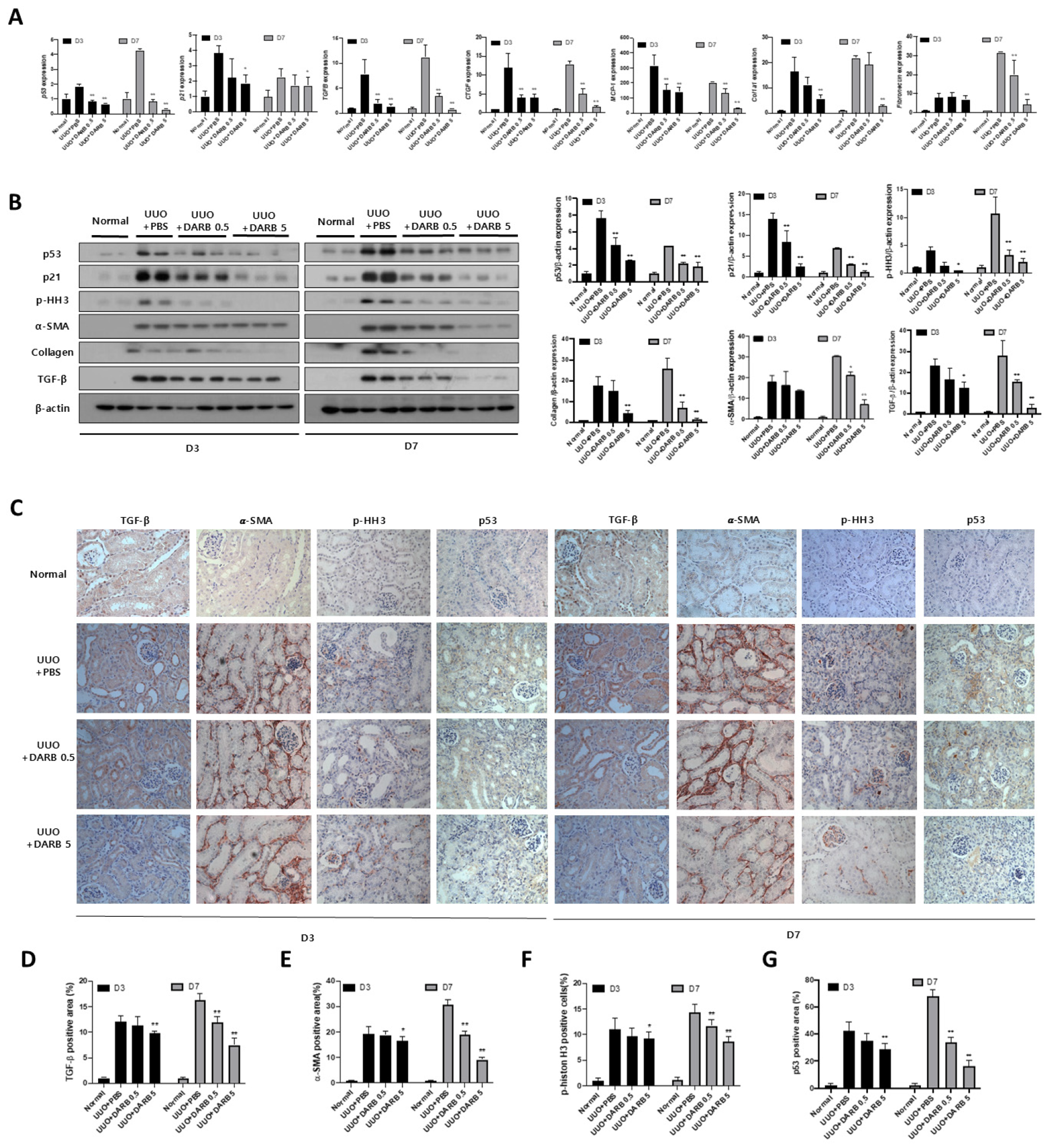
3.2. ESA Exhibited Anti-Fibrotic Activity in the Kidney
3.3. ESA Protects Against Kidney Fibrosis by Attenuating G2/M Cell Cycle Arrest
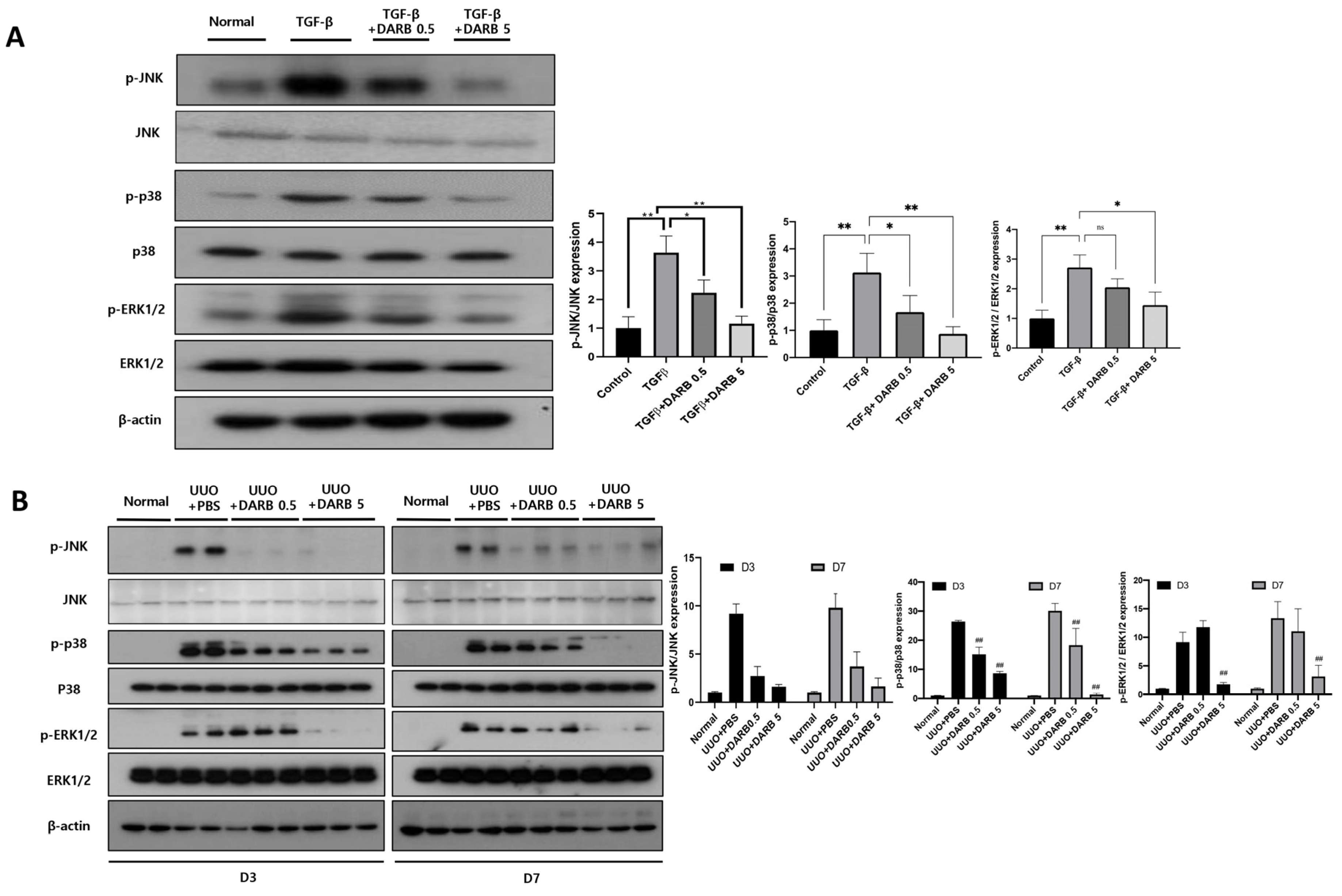
3.4. ESA Attenuates Kidney Fibrosis by Modulating G2/M Cell Cycle Arrest Through JNK and p38-MAPK Signaling Pathways
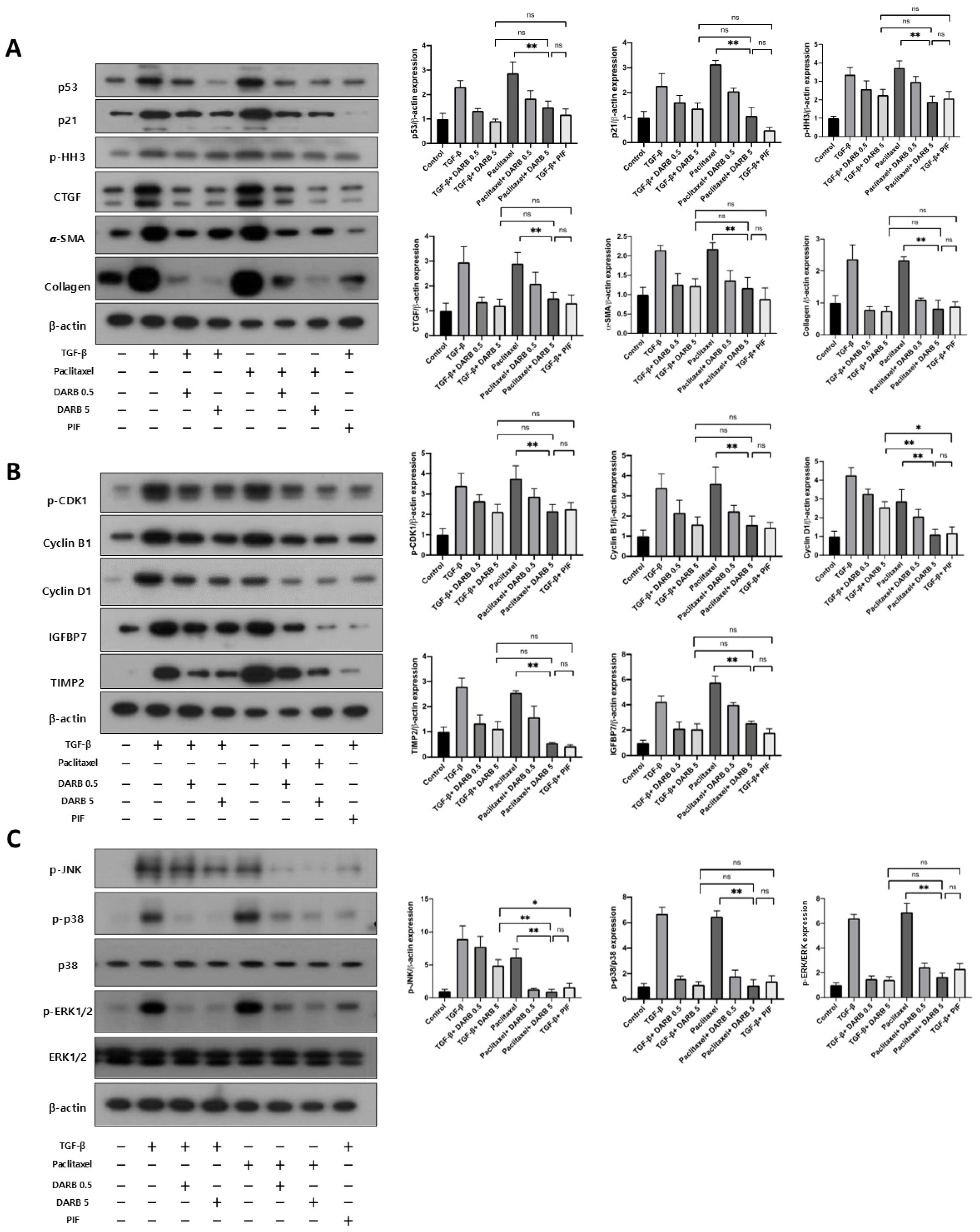
3.5. Comparable Effects of ESA and p53 Inhibitors on Cell Cycle Modulation and Kidney Fibrosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Locatelli, F.; Del Vecchio, L. New Strategies for Anaemia Management in Chronic Kidney Disease. Contrib. Nephrol. 2017, 189, 184–188. [Google Scholar] [CrossRef]
- Sun, Y.B.; Qu, X.; Caruana, G.; Li, J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation 2016, 92, 102–107. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Huang, X.; Wei, X.; Zhao, D.; Jiang, L.; Zhao, X.; Du, Y. Advances in Understanding the Effects of Erythropoietin on Renal Fibrosis. Front. Med. 2020, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Vesey, D.A.; Johnson, D.W.; Wei, M.Q.; Gobe, G.C. Apoptosis of tubulointerstitial chronic inflammatory cells in progressive renal fibrosis after cancer therapies. Transl. Res. 2007, 150, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Leask, A.; Abraham, D.J. TGF-beta signaling and the fibrotic response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef]
- Toda, N.; Mukoyama, M.; Yanagita, M.; Yokoi, H. CTGF in kidney fibrosis and glomerulonephritis. Inflamm. Regen. 2018, 38, 14. [Google Scholar] [CrossRef]
- Yang, L.; Besschetnova, T.Y.; Brooks, C.R.; Shah, J.V.; Bonventre, J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010, 16, 535–543. [Google Scholar] [CrossRef]
- Ming, W.-H.; Wen, L.; Hu, W.-J.; Qiao, R.-F.; Zhou, Y.; Su, B.-W.; Bao, Y.-N.; Gao, P.; Luan, Z.-L. The crosstalk of Wnt/β-catenin signaling and p53 in acute kidney injury and chronic kidney disease. Kidney Res. Clin. Pract. 2024, 43, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Ishani, A.; Xue, J.L.; Himmelfarb, J.; Eggers, P.W.; Kimmel, P.L.; Molitoris, B.A.; Collins, A.J. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 2009, 20, 223–228. [Google Scholar] [CrossRef]
- Cho, N.-J.; Jeong, I.; Kim, Y.; Kim, D.O.; Ahn, S.-J.; Kang, S.-H.; Gil, H.-W.; Lee, H. A machine learning-based approach for predicting renal function recovery in general ward patients with acute kidney injury. Kidney Res. Clin. Pract. 2024, 43, 538–547. [Google Scholar] [CrossRef]
- Koh, E.S.; Chung, S. Recent Update on Acute Kidney Injury-to-Chronic Kidney Disease Transition. Yonsei Med. J. 2024, 65, 247–256. [Google Scholar] [CrossRef]
- Park, Y.; Hwang, W.M. Management of Elderly Patients with Chronic Kidney Disease. Yonsei Med. J. 2025, 66, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Tampe, D.; Zeisberg, M. Potential approaches to reverse or repair renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 226–237. [Google Scholar] [CrossRef]
- Zeisberg, M.; Bonner, G.; Maeshima, Y.; Colorado, P.; Müller, G.A.; Strutz, F.; Kalluri, R. Renal fibrosis: Collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am. J. Pathol. 2001, 159, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.G.; Sun, W.X.; Gao, B.S.; Lian, X.; Zhou, H.L. Cell Cycle Arrest as a Therapeutic Target of Acute Kidney Injury. Curr. Protein Pept. Sci. 2017, 18, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V. Primary proximal tubule injury leads to epithelial cell cycle arrest, fibrosis, vascular rarefaction, and glomerulosclerosis. Kidney Int. Suppl. 2014, 4, 39–44. [Google Scholar] [CrossRef]
- Canaud, G.; Bonventre, J.V. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol. Dial. Transplant. 2015, 30, 575–583. [Google Scholar] [CrossRef]
- Park, S.-H.; Choi, M.-J.; Song, I.-K.; Choi, S.-Y.; Nam, J.-O.; Kim, C.-D.; Lee, B.-H.; Park, R.-W.; Park, K.M.; Kim, Y.-J.; et al. Erythropoietin decreases renal fibrosis in mice with ureteral obstruction: Role of inhibiting TGF-beta-induced epithelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 2007, 18, 1497–1507. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Choi, D.E.; Na, K.-R.; Lee, S.-J.; Suh, K.-S.; Kim, S.Y.; Shin, Y.-T.; Lee, K.W. Erythropoietin attenuates renal injury in an experimental model of rat unilateral ureteral obstruction via anti-inflammatory and anti-apoptotic effects. J. Urol. 2009, 181, 1434–1443. [Google Scholar] [CrossRef]
- Shih, H.M.; Wu, C.J.; Lin, S.L. Physiology and pathophysiology of renal erythropoietin-producing cells. J. Formos. Med. Assoc. 2018, 117, 955–963. [Google Scholar] [CrossRef]
- Wang, J.; Matsushita, K.; Zhong, J.; Ma, L.J.; Yang, H.C.; Fogo, A.B. Low-Dose Erythropoietin Amplifies Beneficial Effects of Angiotensin II Blockade on Glomerulosclerosis. Lab. Investig. 2023, 103, 100015. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.E.; Jeong, J.Y.; Lim, B.J.; Lee, K.W.; Shin, Y.T.; Na, K.R. Pretreatment with darbepoetin attenuates renal injury in a rat model of cisplatin-induced nephrotoxicity. Korean J. Intern. Med. 2009, 24, 238–246. [Google Scholar] [CrossRef]
- Ceol, M.; Del Prete, D.; Tosetto, E.; Graziotto, R.; Gambaro, G.; D'Angelo, A.; Anglani, F. GAPDH as housekeeping gene at renal level. Kidney Int. 2004, 65, 1972–1973. [Google Scholar] [CrossRef]
- Guh, J.-Y.; Chuang, T.-D.; Chen, H.-C.; Hung, W.-C.; Lai, Y.-H.; Shin, S.-J.; Chuang, L.-Y. Beta-hydroxybutyrate-induced growth inhibition and collagen production in HK-2 cells are dependent on TGF-beta and Smad3. Kidney Int. 2003, 64, 2041–2051. [Google Scholar] [CrossRef]
- Huang, P.-Y.; Juan, Y.-H.; Hung, T.-W.; Tsai, Y.-P.; Ting, Y.-H.; Lee, C.-C.; Tsai, J.-P.; Hsieh, Y. β-Mangostin Alleviates Renal Tubulointerstitial Fibrosis via the TGF-β1/JNK Signaling Pathway. Cells 2024, 13, 1701. [Google Scholar] [CrossRef]
- Mendoza-Soto, P.; Jara, C.; Torres-Arévalo, Á.; Oyarzún, C.; Mardones, G.A.; Quezada-Monrás, C.; Martín, R.S. Pharmacological Blockade of the Adenosine A(2B) Receptor Is Protective of Proteinuria in Diabetic Rats, through Affecting Focal Adhesion Kinase Activation and the Adhesion Dynamics of Podocytes. Cells 2024, 13, 846. [Google Scholar] [CrossRef]
- Sun, S.; Ning, X.; Zhang, Y.; Lu, Y.; Nie, Y.; Han, S.; Liu, L.; Du, R.; Xia, L.; He, L.; et al. Hypoxia-inducible factor-1alpha induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int. 2009, 75, 1278–1287. [Google Scholar] [CrossRef]
- Zheng, D.H.; Han, Z.Q.; Wang, X.X.; Ma, D.; Zhang, J. Erythropoietin attenuates high glucose-induced oxidative stress and inhibition of osteogenic differentiation in periodontal ligament stem cell (PDLSCs). Chem. Biol. Interact. 2019, 305, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hamano, Y.; Aoki, T.; Shirai, R.; Hatano, M.; Kimura, R.; Ogawa, M.; Yokosuka, O.; Ueda, S. Low-dose darbepoetin alpha attenuates progression of a mouse model of aristolochic acid nephropathy through early tubular protection. Nephron Exp. Nephrol. 2010, 114, e69–e81. [Google Scholar] [CrossRef]
- Yuksel, O.H.; Yildirim, C.; Urkmez, A.; Ozbay, N.; Uruc, F.; Sahin, A.; Akan, S.; Verit, A. The effect of darbepoetin alfa on renal fibrosis in rats with acute unilateral ureteral obstruction. Arch. Esp. Urol. 2018, 71, 212–221. [Google Scholar] [PubMed]
- Qi, R.; Wang, J.; Jiang, Y.; Qiu, Y.; Xu, M.; Rong, R.; Zhu, T. Snai1-induced partial epithelial-mesenchymal transition orchestrates p53-p21-mediated G2/M arrest in the progression of renal fibrosis via NF-κB-mediated inflammation. Cell. Death Dis. 2021, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Hornsveld, M.; Feringa, F.M.; Krenning, L.; Berg, J.v.D.; Smits, L.M.; Nguyen, N.B.; Rodríguez-Colman, M.J.; Dansen, T.B.; Medema, R.H.; Burgering, B.M. A FOXO-dependent replication checkpoint restricts proliferation of damaged cells. Cell Rep. 2021, 34, 108675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jiang, N.; Han, Y.; Yang, M.; Gao, P.; Xiong, X.; Xiong, S.; Zeng, L.; Xiao, Y.; Wei, L.; et al. Aristolochic acid induces renal fibrosis by arresting proximal tubular cells in G2/M phase mediated by HIF-1α. FASEB J. 2020, 34, 12599–12614. [Google Scholar] [CrossRef]
- Bonventre, J.V. Maladaptive proximal tubule repair: Cell cycle arrest. Nephron Clin. Pract. 2014, 127, 61–64. [Google Scholar] [CrossRef]
- Cosentino, C.C.; Skrypnyk, N.I.; Brilli, L.L.; Chiba, T.; Novitskaya, T.; Woods, C.; West, J.; Korotchenko, V.N.; McDermott, L.; Day, B.W.; et al. Histone deacetylase inhibitor enhances recovery after AKI. J. Am. Soc. Nephrol. 2013, 24, 943–953. [Google Scholar] [CrossRef]
- Tang, J.; Liu, N.; Tolbert, E.; Ponnusamy, M.; Ma, L.; Gong, R.; Bayliss, G.; Yan, H.; Zhuang, S. Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am. J. Pathol. 2013, 183, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fu, P.; Huang, X.R.; Liu, F.; Lai, K.N.; Lan, H.Y. Activation of p53 promotes renal injury in acute aristolochic acid nephropathy. J. Am. Soc. Nephrol. 2010, 21, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Finn, W.F. Enhanced recovery from postischemic acute renal failure. Micropuncture studies in the rat. Circ. Res. 1980, 46, 440–448. [Google Scholar] [CrossRef]
- Yang, Q.H.; Liu, D.W.; Long, Y.; Liu, H.Z.; Chai, W.Z.; Wang, X.T. Acute renal failure during sepsis: Potential role of cell cycle regulation. J. Infect. 2009, 58, 459–464. [Google Scholar] [CrossRef]
- Taylor, W.R.; Stark, G.R. Regulation of the G2/M transition by p53. Oncogene 2001, 20, 1803–1815. [Google Scholar] [CrossRef]
- Yan, M.; Tang, C.; Ma, Z.; Huang, S.; Dong, Z. DNA damage response in nephrotoxic and ischemic kidney injury. Toxicol. Appl. Pharmacol. 2016, 313, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Astuti, P.; Pike, T.; Widberg, C.; Payne, E.; Harding, A.; Hancock, J.; Gabrielli, B. MAPK pathway activation delays G2/M progression by destabilizing Cdc25B. J. Biol. Chem. 2009, 284, 33781–33788. [Google Scholar] [CrossRef]
- Vera, J.; Raatz, Y.; Wolkenhauer, O.; Kottek, T.; Bhattacharya, A.; Simon, J.C.; Kunz, M. Chk1 and Wee1 control genotoxic-stress induced G2-M arrest in melanoma cells. Cell. Signal. 2015, 27, 951–960. [Google Scholar] [CrossRef]
- Pabla, N.; Bhatt, K.; Dong, Z. Checkpoint kinase 1 (Chk1)-short is a splice variant and endogenous inhibitor of Chk1 that regulates cell cycle and DNA damage checkpoints. Proc. Natl. Acad. Sci. USA 2012, 109, 197–202. [Google Scholar] [CrossRef]
- Abraham, R.T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001, 15, 2177–2196. [Google Scholar] [CrossRef]
- Goodarzi, A.A.; Block, W.D.; Lees-Miller, S.P. The role of ATM and ATR in DNA damage-induced cell cycle control. Prog. Cell. Cycle Res. 2003, 5, 393–411. [Google Scholar]
- Wang, R.; He, G.; Nelman-Gonzalez, M.; Ashorn, C.L.; Gallick, G.E.; Stukenberg, P.T.; Kirschner, M.W.; Kuang, J. Regulation of Cdc25C by ERK-MAP kinases during the G2/M transition. Cell 2007, 128, 1119–1132. [Google Scholar] [CrossRef]
- Harvey, S.L.; Charlet, A.; Haas, W.; Gygi, S.P.; Kellogg, D.R. Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell 2005, 122, 407–420. [Google Scholar] [CrossRef]
- Matheson, C.J.; Backos, D.S.; Reigan, P. Targeting WEE1 Kinase in Cancer. Trends Pharmacol. Sci. 2016, 37, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Hirakawa, Y.; Nangaku, M. The role of oxidative stress and hypoxia in renal disease. Kidney Res. Clin. Pract. 2019, 38, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Grynberg, K.; Ma, F.Y.; Nikolic-Paterson, D.J. The JNK Signaling Pathway in Renal Fibrosis. Front. Physiol. 2017, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- de Borst, M.H.; Prakash, J.; Sandovici, M.; Klok, P.A.; Hamming, I.; Kok, R.J.; Navis, G.; van Goor, H. c-Jun NH2-terminal kinase is crucially involved in renal tubulo-interstitial inflammation. J. Pharmacol. Exp. Ther. 2009, 331, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Arany, I.; Megyesi, J.K.; Kaneto, H.; Tanaka, S.; Safirstein, R.L. Activation of ERK or inhibition of JNK ameliorates H2O2 cytotoxicity in mouse renal proximal tubule cells. Kidney Int. 2004, 65, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.-M.; Wang, Q.; Wan, Q.; Lin, J.-G.; Hu, M.-S.; Liu, Y.-X.; Wang, R. The role of the p38 MAPK signaling pathway in high glucose-induced epithelial-mesenchymal transition of cultured human renal tubular epithelial cells. PLoS ONE 2011, 6, e22806. [Google Scholar] [CrossRef]
- Liu, Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 2010, 21, 212–222. [Google Scholar] [CrossRef]
- Yang, L.; Ma, L.; Fu, P.; Nie, J. Update of cellular senescence in kidney fibrosis: From mechanism to potential interventions. Front. Med. 2025, 19, 250–264. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, D.; Jhee, J.H.; Kim, S.H.; Kim, T.Y.; Kim, H.J.; Bae, W.; Choi, H.Y.; Park, H.C. Erythropoiesis-Stimulating Agent Protects Against Kidney Fibrosis by Inhibiting G2/M Cell Cycle Arrest. Cells 2025, 14, 1662. https://doi.org/10.3390/cells14211662
Oh D, Jhee JH, Kim SH, Kim TY, Kim HJ, Bae W, Choi HY, Park HC. Erythropoiesis-Stimulating Agent Protects Against Kidney Fibrosis by Inhibiting G2/M Cell Cycle Arrest. Cells. 2025; 14(21):1662. https://doi.org/10.3390/cells14211662
Chicago/Turabian StyleOh, Donghwan, Jong Hyun Jhee, Soo Hyun Kim, Tae Yeon Kim, Hyo Jeong Kim, Wooram Bae, Hoon Young Choi, and Hyeong Cheon Park. 2025. "Erythropoiesis-Stimulating Agent Protects Against Kidney Fibrosis by Inhibiting G2/M Cell Cycle Arrest" Cells 14, no. 21: 1662. https://doi.org/10.3390/cells14211662
APA StyleOh, D., Jhee, J. H., Kim, S. H., Kim, T. Y., Kim, H. J., Bae, W., Choi, H. Y., & Park, H. C. (2025). Erythropoiesis-Stimulating Agent Protects Against Kidney Fibrosis by Inhibiting G2/M Cell Cycle Arrest. Cells, 14(21), 1662. https://doi.org/10.3390/cells14211662




