Culture Strategy Determines the Differentiation Status of Sweat Gland Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
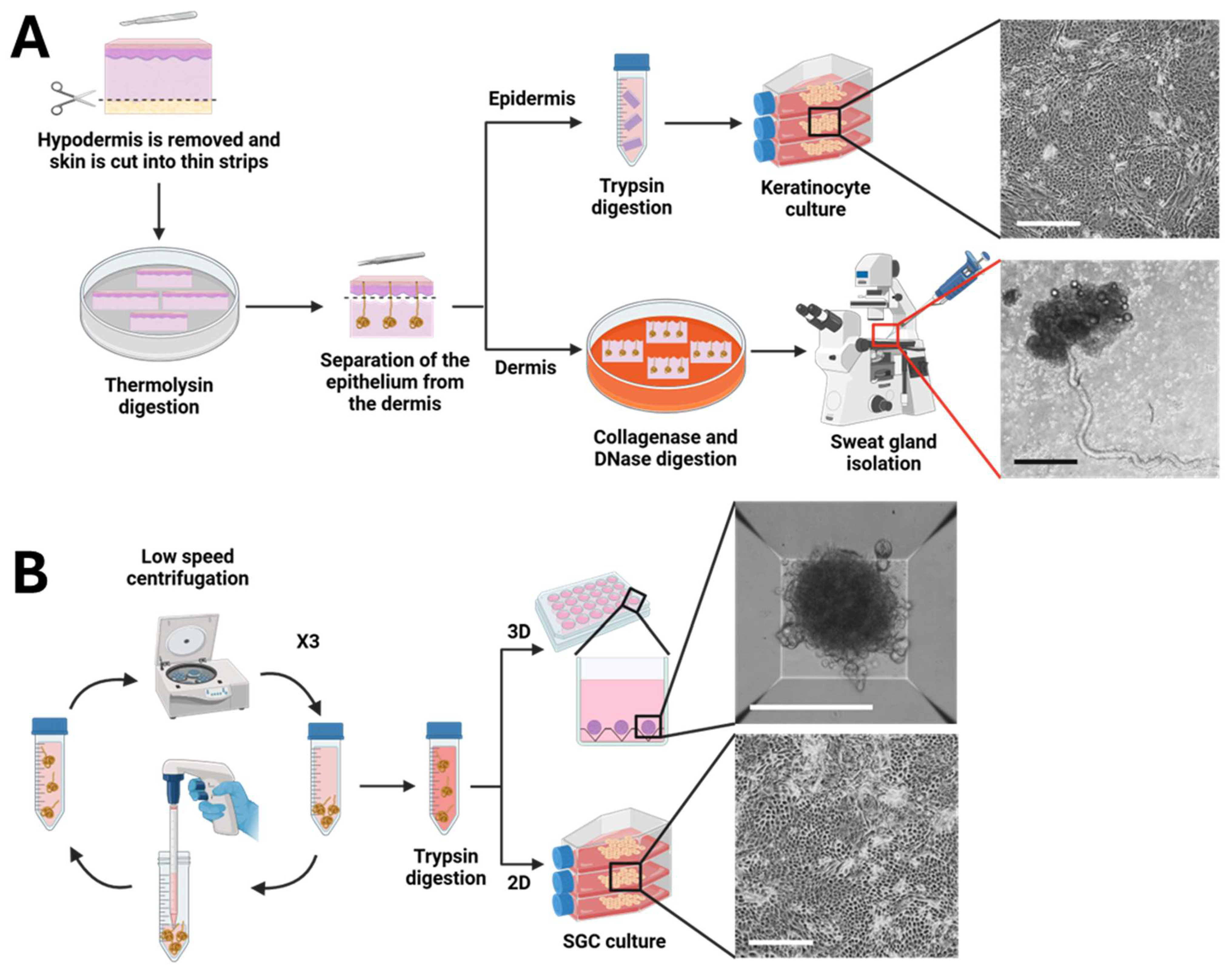
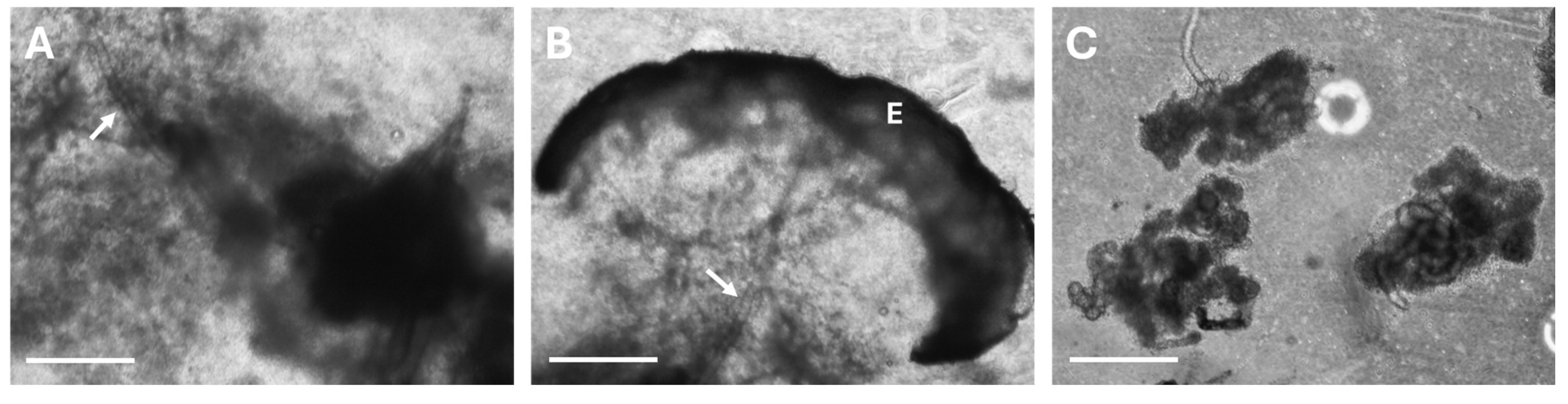
2.2. Sweat Gland Isolation
2.3. Cell Culture
2.4. Spheroid Formation
2.5. Immunofluorescence
3. Results
3.1. Protocol Optimization
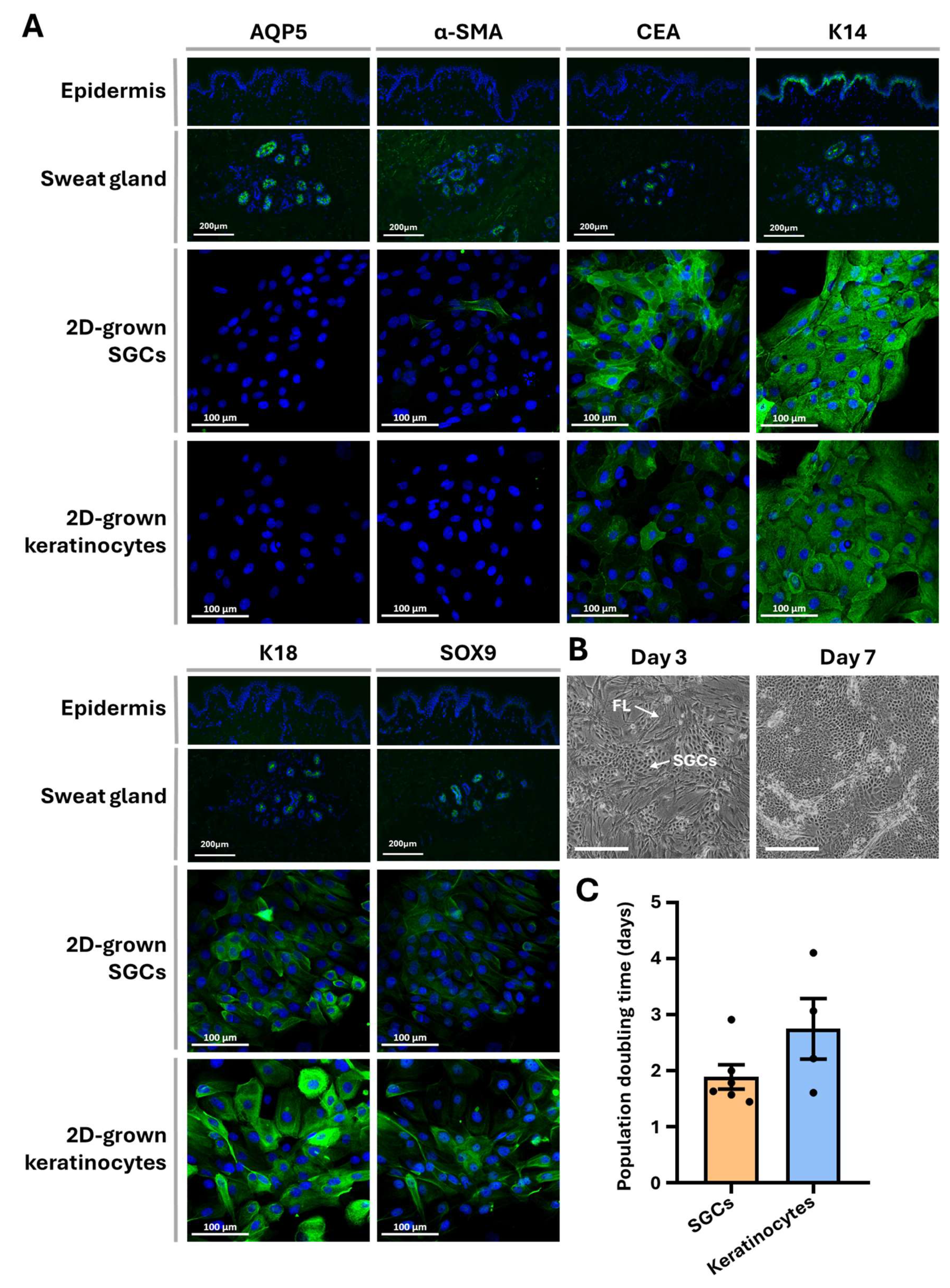
3.2. SGC Immunolabeling and 2D Culture Characterization
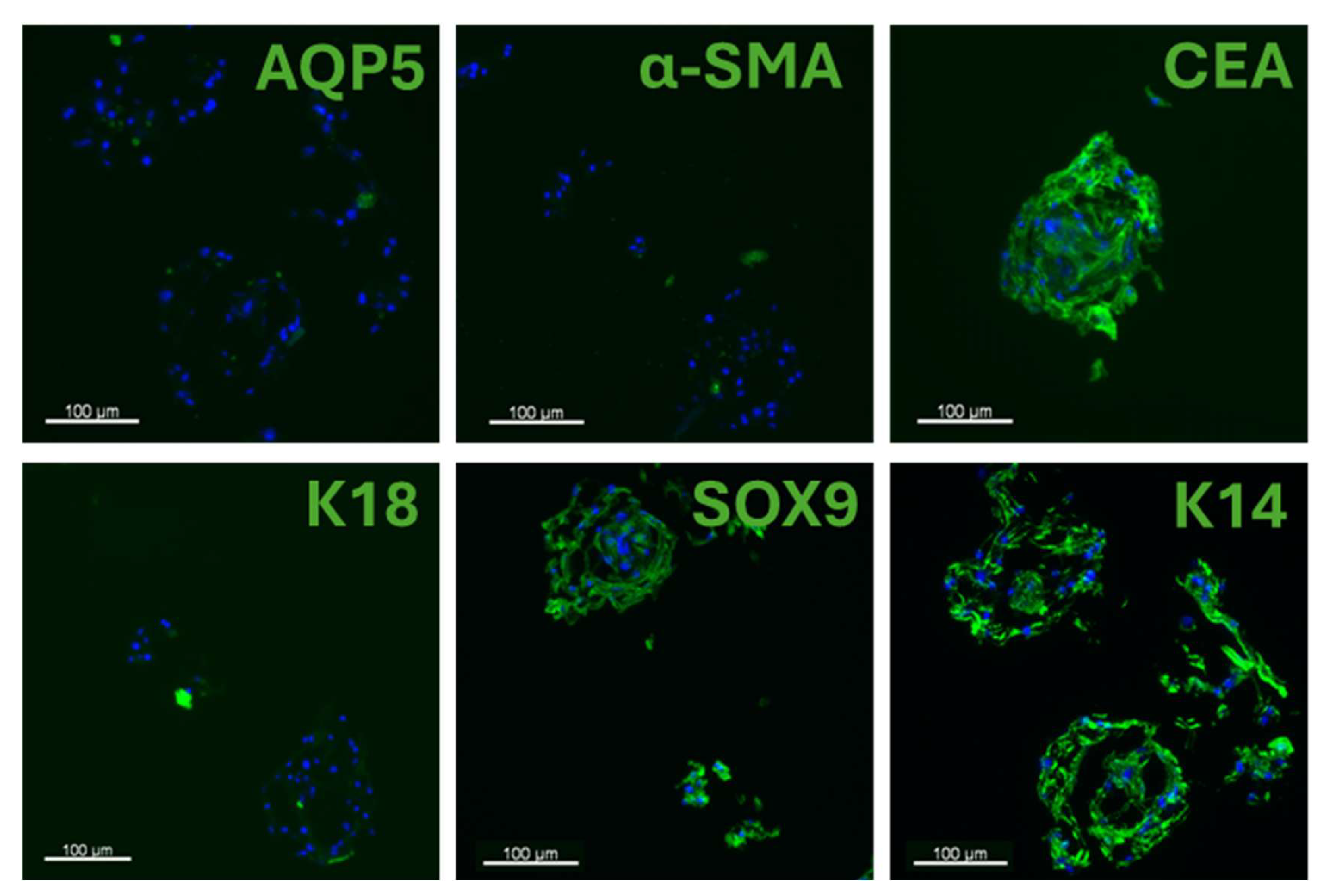
3.3. 3D Culture Characterization
3.4. 3D Culture Phenotype Recovery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SGC | Sweat gland cell |
| TES | Tissue-engineered skin substitute |
| EGF | Epidermal growth factor |
| NHS | Normal human skin |
| AQP5 | Aquaporin 5 |
| α-SMA | α-smooth muscle actin |
| CEA | Carcinoembryonic antigen |
| K14 | Keratin 14 |
| K18 | Keratin 18 |
References
- Sato, K.; Kang, W.H.; Saga, K.; Sato, K.T. Biology of sweat glands and their disorders. I. Normal sweat gland function. J. Am. Acad. Dermatol. 1989, 20, 537–563. [Google Scholar] [CrossRef] [PubMed]
- Watabe, A.; Sugawara, T.; Kikuchi, K.; Yamasaki, K.; Sakai, S.; Aiba, S. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium. J. Dermatol. Sci. 2013, 72, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F.; et al. Dermcidin: A novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2001, 2, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Ohtake, T.; Dorschner, R.A.; Schittek, B.; Garbe, C.; Gallo, R.L. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J. Investig. Dermatol. 2002, 119, 1090–1095. [Google Scholar] [CrossRef]
- Saga, K. Structure and function of human sweat glands studied with histochemistry and cytochemistry. Prog. Histochem. Cytochem. 2002, 37, 323–386. [Google Scholar] [CrossRef]
- Biedermann, T.; Pontiggia, L.; Böttcher-Haberzeth, S.; Tharakan, S.; Braziulis, E.; Schiestl, C.; Meuli, M.; Reichmann, E. Human Eccrine Sweat Gland Cells Can Reconstitute a Stratified Epidermis. J. Investig. Dermatol. 2010, 130, 1996–2009. [Google Scholar] [CrossRef]
- Rittie, L.; Sachs, D.L.; Orringer, J.S.; Voorhees, J.J.; Fisher, G.J. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am. J. Pathol. 2013, 182, 163–171. [Google Scholar] [CrossRef]
- Cui, C.Y.; Schlessinger, D. Eccrine sweat gland development and sweat secretion. Exp. Dermatol. 2015, 24, 644–650. [Google Scholar] [CrossRef]
- Lu Catherine, P.; Polak, L.; Rocha Ana, S.; Pasolli, H.A.; Chen, S.-C.; Sharma, N.; Blanpain, C.; Fuchs, E. Identification of Stem Cell Populations in Sweat Glands and Ducts Reveals Roles in Homeostasis and Wound Repair. Cell 2012, 150, 136–150. [Google Scholar] [CrossRef]
- Germain, L.; Larouche, D.; Nedelec, B.; Perreault, I.; Duranceau, L.; Bortoluzzi, P.; Cloutier, C.B.; Genest, H.; Caouette-Laberge, L.; Dumas, A.; et al. Autologous bilayered self-assembled skin substitutes (SASSs) as permanent grafts:a case series of 14 severely burned patients indicating clinical effectiveness. Eur. Cells Mater. 2018, 36, 128–141. [Google Scholar] [CrossRef]
- Weng, T.; Wu, P.; Zhang, W.; Zheng, Y.; Li, Q.; Jin, R.; Chen, H.; You, C.; Guo, S.; Han, C.; et al. Regeneration of skin appendages and nerves: Current status and further challenges. J. Transl. Med. 2020, 18, 53. [Google Scholar] [CrossRef] [PubMed]
- Swartling, C.; Naver, H.; Pihl-Lundin, I.; Hagforsen, E.; Vahlquist, A. Sweat gland morphology and periglandular innervation in essential palmar hyperhidrosis before and after treatment with intradermal botulinum toxin. J. Am. Acad. Dermatol. 2004, 51, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, L.; Zhang, M.; Tang, S.; Fu, X. Three-dimensional culture and identification of human eccrine sweat glands in matrigel basement membrane matrix. Cell Tissue Res. 2013, 354, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, M.; Zhang, X.; Bai, T.; Chi, G.; Liu, J.Y.; Li, Y. Isolation, culture and phenotypic characterization of human sweat gland epithelial cells. Int. J. Mol. Med. 2014, 34, 997–1003. [Google Scholar] [CrossRef]
- Tao, R.; Han, Y.; Chai, J.; Li, D.; Sun, T. Isolation, culture, and verification of human sweat gland epithelial cells. Cytotechnology 2010, 62, 489–495. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Liu, J.; Pang, C.; Zhang, J.; Li, Y.; Fu, X. Location, Isolation, and Identification of Mesenchymal Stem Cells from Adult Human Sweat Glands. Stem Cells Int. 2018, 2018, 2090276. [Google Scholar] [CrossRef]
- Lee, C.M.; Jones, C.J.; Kealey, T. Biochemical and ultrastructural studies of human eccrine sweat glands isolated by shearing and maintained for seven days. J. Cell Sci. 1984, 72, 259–274. [Google Scholar] [CrossRef]
- Jimenez, F.; Alam, M.; Hernandez, I.; Poblet, E.; Hardman, J.A.; Paus, R. An efficient method for eccrine gland isolation from human scalp. Exp. Dermatol. 2018, 27, 678–681. [Google Scholar] [CrossRef]
- Nagel, S.; Rohr, F.; Weber, C.; Kier, J.; Siemers, F.; Kruse, C.; Danner, S.; Brandenburger, M.; Matthiessen, A.E. Multipotent nestin-positive stem cells reside in the stroma of human eccrine and apocrine sweat glands and can be propagated robustly in vitro. PLoS ONE 2013, 8, e78365. [Google Scholar] [CrossRef]
- Li, H.; Chen, L.; Zeng, S.; Li, X.; Zhang, X.; Lin, C.; Zhang, M.; Xie, S.; He, Y.; Shu, S.; et al. Matrigel basement membrane matrix induces eccrine sweat gland cells to reconstitute sweat gland-like structures in nude mice. Exp. Cell Res. 2015, 332, 67–77. [Google Scholar] [CrossRef]
- Klaka, P.; Grüdl, S.; Banowski, B.; Giesen, M.; Sättler, A.; Proksch, P.; Welss, T.; Förster, T. A novel organotypic 3D sweat gland model with physiological functionality. PLoS ONE 2017, 12, e0182752. [Google Scholar] [CrossRef]
- Diao, J.; Liu, J.; Wang, S.; Chang, M.; Wang, X.; Guo, B.; Yu, Q.; Yan, F.; Su, Y.; Wang, Y. Sweat gland organoids contribute to cutaneous wound healing and sweat gland regeneration. Cell Death Dis. 2019, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.; Zhang, B.; Zhang, M.; Chen, W.; Tang, S.; Fu, X. Changes in keratins and alpha-smooth muscle actin during three-dimensional reconstitution of eccrine sweat glands. Cell Tissue Res. 2016, 365, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Larouche, D.; Cantin-Warren, L.; Desgagne, M.; Guignard, R.; Martel, I.; Ayoub, A.; Lavoie, A.; Gauvin, R.; Auger, F.A.; Moulin, V.J.; et al. Improved Methods to Produce Tissue-Engineered Skin Substitutes Suitable for the Permanent Closure of Full-Thickness Skin Injuries. BioRes. Open Access 2016, 5, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Ferland, K.; De Koninck, H.; Cattier, B.; Cortez Ghio, S.; Larouche, D.; Germain, L. Initiating Separate Cultures of Keratinocytes and Melanocytes from a Single Biopsy. In Skin Tissue Engineering: Methods and Protocols; Biedermann, T., Böttcher-Haberzeth, S., Eds.; Springer: New York, NY, USA, 2025; pp. 35–48. [Google Scholar] [CrossRef]
- Moll, I.; Moll, R. Changes of expression of intermediate filament proteins during ontogenesis of eccrine sweat glands. J. Investig. Dermatol. 1992, 98, 777–785. [Google Scholar] [CrossRef]
- Metze, D.; Bhardwaj, R.; Amann, U.; Eades-Perner, A.-M.; Neumaier, M.; Wagener, C.; Jantscheff, P.; Grunert, F.; Luger, T.A. Glycoproteins of the Carcinoembryonic Antigen (CEA) Family Are Expressed in Sweat and Sebaceous Glands of Human Fetal and Adult Skin. J. Investig. Dermatol. 1996, 106, 64–69. [Google Scholar] [CrossRef]
- Schön, M.; Benwood, J.; O’Connell-Willstaedt, T.; Rheinwald, J.G. Human sweat gland myoepithelial cells express a unique set of cytokeratins and reveal the potential for alternative epithelial and mesenchymal differentiation states in culture. J. Cell Sci. 1999, 112, 1925–1936. [Google Scholar] [CrossRef]
- Kurokawa, I.; Takahashi, K.; Moll, I.; Moll, R. Expression of keratins in cutaneous epithelial tumors and related disorders–distribution and clinical significance. Exp. Dermatol. 2011, 20, 217–228. [Google Scholar] [CrossRef]
- Kadaja, M.; Keyes, B.E.; Lin, M.; Pasolli, H.A.; Genander, M.; Polak, L.; Stokes, N.; Zheng, D.; Fuchs, E. SOX9: A stem cell transcriptional regulator of secreted niche signaling factors. Genes Dev. 2014, 28, 328–341. [Google Scholar] [CrossRef]
- Bisson, F.; Paquet, C.; Bourget, J.-M.; Zaniolo, K.; Rochette, P.J.; Landreville, S.; Damour, O.; Boudreau, F.; Auger, F.A.; Guérin, S.L.; et al. Contribution of Sp1 to Telomerase Expression and Activity in Skin Keratinocytes Cultured with a Feeder Layer. J. Cell. Physiol. 2015, 230, 308–317. [Google Scholar] [CrossRef]
- Rheinwatd, J.G.; Green, H. Seria cultivation of strains of human epidemal keratinocytes: The formation keratinizin colonies from single cell is. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Nejsum, L.N.; Kwon, T.-H.; Jensen, U.B.; Fumagalli, O.; Frøkiaer, J.; Krane, C.M.; Menon, A.G.; King, L.S.; Agre, P.C.; Nielsen, S. Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc. Natl. Acad. Sci. USA 2002, 99, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Markey, A.C.; Lane, E.B.; Churchill, L.J.; MacDonald, D.M.; Leigh, I.M. Expression of Simple Epithelial Keratins 8 and 18 in Epidermal Neoplasia. J. Investig. Dermatol. 1991, 97, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Scurry, J.; de Boer, W.G.R.M. Carcinoembryonic antigen in skin and related tumours as determined by immunohistological techniques. Pathology 1983, 15, 379–384. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shi, G.; Sohn, K.-C.; Li, Z.; Choi, D.-K.; Fan, Y.-M.; Nam, Y.H.; Kim, S.; Im, M.; Kim, C.D. Expression and functional role of Sox9 in human epidermal keratinocytes. J. Dermatol. Sci. 2013, 69, e42. [Google Scholar] [CrossRef]
- Vidal, V.P.I.; Chaboissier, M.-C.; Lützkendorf, S.; Cotsarelis, G.; Mill, P.; Hui, C.-C.; Ortonne, N.; Ortonne, J.-P.; Schedl, A. Sox9 Is Essential for Outer Root Sheath Differentiation and the Formation of the Hair Stem Cell Compartment. Curr. Biol. 2005, 15, 1340–1351. [Google Scholar] [CrossRef]
- Nowak, J.A.; Polak, L.; Pasolli, H.A.; Fuchs, E. Hair Follicle Stem Cells Are Specified and Function in Early Skin Morphogenesis. Cell Stem Cell 2008, 3, 33–43. [Google Scholar] [CrossRef]
- Menzel-Severing, J.; Zenkel, M.; Polisetti, N.; Sock, E.; Wegner, M.; Kruse, F.E.; Schlötzer-Schrehardt, U. Transcription factor profiling identifies Sox9 as regulator of proliferation and differentiation in corneal epithelial stem/progenitor cells. Sci. Rep. 2018, 8, 10268. [Google Scholar] [CrossRef]
- Chakravarty, G.; Rider, B.; Mondal, D. Cytoplasmic compartmentalization of SOX9 abrogates the growth arrest response of breast cancer cells that can be rescued by trichostatin A treatment. Cancer Biol. Ther. 2011, 11, 71–83. [Google Scholar] [CrossRef]
- Huang, Z.; Zhen, Y.; Yin, W.; Ma, Z.; Zhang, L. Shh promotes sweat gland cell maturation in three-dimensional culture. Cell Tissue Bank. 2016, 17, 317–325. [Google Scholar] [CrossRef]
- Huang, S.; Xu, Y.; Wu, C.; Sha, D.; Fu, X. In vitro constitution and in vivo implantation of engineered skin constructs with sweat glands. Biomaterials 2010, 31, 5520–5525. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Koninck, H.; Ferland, K.; Barbier, M.A.; Larouche, D.; Germain, L. Culture Strategy Determines the Differentiation Status of Sweat Gland Cells. Cells 2025, 14, 1643. https://doi.org/10.3390/cells14201643
De Koninck H, Ferland K, Barbier MA, Larouche D, Germain L. Culture Strategy Determines the Differentiation Status of Sweat Gland Cells. Cells. 2025; 14(20):1643. https://doi.org/10.3390/cells14201643
Chicago/Turabian StyleDe Koninck, Henri, Karel Ferland, Martin A. Barbier, Danielle Larouche, and Lucie Germain. 2025. "Culture Strategy Determines the Differentiation Status of Sweat Gland Cells" Cells 14, no. 20: 1643. https://doi.org/10.3390/cells14201643
APA StyleDe Koninck, H., Ferland, K., Barbier, M. A., Larouche, D., & Germain, L. (2025). Culture Strategy Determines the Differentiation Status of Sweat Gland Cells. Cells, 14(20), 1643. https://doi.org/10.3390/cells14201643






