Alcohol Consumption and Cervical Carcinogenesis: Time to Draw Conclusions
Abstract
1. Introduction
2. Alcohol and Cancer
3. Human Papillomavirus and Other Risk Factors in Cervical Cancer
4. Molecular Mechanism of Alcohol-Mediated Carcinogenicity
4.1. Acetaldehyde Production
4.2. Oxidative Stress
4.3. Altered Retinoid Metabolism
4.4. Changes to Estrogen Regulation
4.5. Immune Suppression Compromises HPV Clearance
5. Synergistic Effects of Tobacco and Alcohol on Cervical Cancer
6. Synergistic Effects of HPV-HIV Co-Infection and Alcohol Use on Cervical Cancer
7. Epidemiologic Evidence for Alcohol Consumption and Cervical Carcinogenesis
8. Inconsistent Findings and Study Limitations
8.1. Methodological Heterogeneity
8.2. Sample Size and Statistical Power
8.3. Confounding and Bias Considerations
9. Clinical Implications and Public Health Perspectives
9.1. Screening and Prevention Strategies
9.2. Integration with HPV Vaccination Programs
9.3. Treatment Considerations
10. Future Research Directions and Recommendations
10.1. Mechanistic Studies
10.2. Large-Scale Prospective Cohorts
10.3. Intervention Studies
11. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, V.K.; Dan, N.; Chauhan, N.; Wang, Q.; Setua, S.; Nagesh, P.K.B.; Malik, S.; Batra, V.; Yallapu, M.M.; Miller, D.D.; et al. VERU-111 suppresses tumor growth and metastatic phenotypes of cervical cancer cells through the activation of p53 signaling pathway. Cancer Lett. 2020, 470, 64–74. [Google Scholar] [CrossRef]
- Karuri, A.R.; Kashyap, V.K.; Yallapu, M.M.; Zafar, N.; Kedia, S.K.; Jaggi, M.; Chauhan, S.C. Disparity in rates of HPV infection and cervical cancer in underserved US populations. Front. Biosci. (Schol. Ed.) 2017, 9, 254–269. [Google Scholar]
- Li, M.; Wang, J.; Geng, Y.; Li, Y.; Wang, Q.; Liang, Q.; Qi, Q. A strategy of gene overexpression based on tandem repetitive promoters in Escherichia coli. Microb. Cell Fact. 2012, 11, 19. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Connor, J. Alcohol consumption as a cause of cancer. Addiction 2017, 112, 222–228. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Alcohol Drinking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1988; Volume 44, ISBN 978-92-832-1244-7. [Google Scholar]
- Alcohol and Cancer Risk: The, U.S. Surgeon General’s Advisory. 2025. Available online: https://www.hhs.gov/surgeongeneral/reports-and-publications/addiction-and-substance-misuse/index.html (accessed on 11 April 2025).
- Seitz, H.K.; Becker, P. Alcohol metabolism and cancer risk. Alcohol. Res. Health 2007, 30, 38–41, 44–47. [Google Scholar]
- Dinis-Oliveira, R.J. Oxidative and Non-Oxidative Metabolomics of Ethanol. Curr. Drug Metab. 2016, 17, 327–335. [Google Scholar] [CrossRef]
- Shukla, S.D.; Lim, R.W. Epigenetic effects of ethanol on the liver and gastrointestinal system. Alcohol. Res. 2013, 35, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Weiderpass, E.; Ye, W.; Tamimi, R.; Trichopolous, D.; Nyren, O.; Vainio, H.; Adami, H.O. Alcoholism and risk for cancer of the cervix uteri, vagina, and vulva. Cancer Epidemiol. Biomark. Prev. 2001, 10, 899–901. [Google Scholar]
- Mayadev, J.; Li, C.S.; Lim, J.; Valicenti, R.; Alvarez, E.A. Alcohol Abuse Decreases Pelvic Control and Survival in Cervical Cancer: An Opportunity of Lifestyle Intervention for Outcome Improvement. Am. J. Clin. Oncol. 2017, 40, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Praud, D.; Rota, M.; Rehm, J.; Shield, K.; Zatoński, W.; Hashibe, M.; La Vecchia, C.; Boffetta, P. Cancer incidence and mortality attributable to alcohol consumption. Int. J. Cancer 2016, 138, 1380–1387. [Google Scholar] [CrossRef]
- Nelson, D.E.; Jarman, D.W.; Rehm, J.; Greenfield, T.K.; Rey, G.; Kerr, W.C.; Miller, P.; Shield, K.D.; Ye, Y.; Naimi, T.S. Alcohol-attributable cancer deaths and years of potential life lost in the United States. Am. J. Public. Health 2013, 103, 641–648. [Google Scholar] [CrossRef]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Esser, M.B.; Sherk, A.; Liu, Y.; Naimi, T.S. Deaths from Excessive Alcohol Use—United States, 2016–2202. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 154–161. [Google Scholar] [CrossRef]
- Alcohol and Cancer Risk. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/alcohol/alcohol-fact-sheet#r4 (accessed on 11 April 2025).
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef]
- Zhao, J.; Stockwell, T.; Roemer, A.; Chikritzhs, T. Is alcohol consumption a risk factor for prostate cancer? A systematic review and meta-analysis. BMC Cancer 2016, 16, 845. [Google Scholar] [CrossRef]
- Moore, A.A.; Gould, R.; Reuben, D.B.; Greendale, G.A.; Carter, M.K.; Zhou, K.; Karlamangla, A. Longitudinal patterns and predictors of alcohol consumption in the United States. Am. J. Public Health 2005, 95, 458–465. [Google Scholar] [CrossRef]
- Breslow, R.A.; Graubard, B.I. Prospective study of alcohol consumption in the United States: Quantity, frequency, and cause-specific mortality. Alcohol. Clin. Exp. Res. 2008, 32, 513–521. [Google Scholar] [CrossRef]
- Edelman, E.J.; Fiellin, D.A. In the Clinic. Alcohol Use. Ann. Intern. Med. 2016, 164, Itc1-16. [Google Scholar] [CrossRef] [PubMed]
- WHO. No Level of Alcohol Consumption is Safe for Our Health. 4 January 2023; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Hingson, R.W.; Zha, W.; White, A.M. Drinking Beyond the Binge Threshold: Predictors, Consequences, and Changes in the U.S. Am. J. Prev. Med. 2017, 52, 717–727. [Google Scholar] [CrossRef]
- Esser, M.B.; Hedden, S.L.; Kanny, D.; Brewer, R.D.; Gfroerer, J.C.; Naimi, T.S. Prevalence of alcohol dependence among US adult drinkers, 2009–2011. Prev. Chronic Dis. 2014, 11, E206. [Google Scholar] [CrossRef] [PubMed]
- CDC. Alcohol Use and Your Health; CDC: Atlanta, GA, USA, 2023. [Google Scholar]
- Molina, P.E.; Happel, K.I.; Zhang, P.; Kolls, J.K.; Nelson, S. Focus on: Alcohol and the immune system. Alcohol. Res. Health 2010, 33, 97–108. [Google Scholar] [PubMed]
- Li, J.; Li, S. From Viral Infection to Genome Reshaping: The Triggering Role of HPV Integration in Cervical Cancer. Int. J. Mol. Sci. 2025, 26, 9214. [Google Scholar] [CrossRef] [PubMed]
- de Sanjosé, S.; Diaz, M.; Castellsagué, X.; Clifford, G.; Bruni, L.; Muñoz, N.; Bosch, F.X. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect. Dis. 2007, 7, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.P.; Li, N.; Smith, J.S.; Qiao, Y.L. Human papillomavirus type distribution in women from Asia: A meta-analysis. Int. J. Gynecol. Cancer 2008, 18, 71–79. [Google Scholar] [CrossRef]
- Eckert, L.O.N.; Moscicki, A.B. Committee Opinion No. 704: Human Papillomavirus Vaccination. Obstet. Gynecol. 2017, 129, 1. [Google Scholar]
- Adam, E.; Berkova, Z.; Daxnerova, Z.; Icenogle, J.; Reeves, W.C.; Kaufman, R.H. Papillomavirus detection: Demographic and behavioral characteristics influencing the identification of cervical disease. Am. J. Obstet. Gynecol. 2000, 182, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, M.; Koskela, P.; Jellum, E.; Bloigu, A.; Anttila, T.; Hallmans, G.; Luukkaala, T.; Thoresen, S.; Youngman, L.; Dillner, J.; et al. Herpes simplex virus and risk of cervical cancer: A longitudinal, nested case-control study in the nordic countries. Am. J. Epidemiol. 2002, 156, 687–692. [Google Scholar] [CrossRef]
- Muñoz, N.; Franceschi, S.; Bosetti, C.; Moreno, V.; Herrero, R.; Smith, J.S.; Shah, K.V.; Meijer, C.J.; Bosch, F.X. Role of parity and human papillomavirus in cervical cancer: The IARC multicentric case-control study. Lancet 2002, 359, 1093–1101. [Google Scholar] [CrossRef]
- Urbute, A.; Frederiksen, K.; Thomsen, L.T.; Kesmodel, U.S.; Kjaer, S.K. Overweight and obesity as risk factors for cervical cancer and detection of precancers among screened women: A nationwide, population-based cohort study. Gynecol. Oncol. 2024, 181, 20–27. [Google Scholar] [CrossRef]
- Sugawara, Y.; Tsuji, I.; Mizoue, T.; Inoue, M.; Sawada, N.; Matsuo, K.; Ito, H.; Naito, M.; Nagata, C.; Kitamura, Y.; et al. Cigarette smoking and cervical cancer risk: An evaluation based on a systematic review and meta-analysis among Japanese women. Jpn. J. Clin. Oncol. 2018, 49, 77–86. [Google Scholar] [CrossRef]
- Collins, S.; Rollason, T.P.; Young, L.S.; Woodman, C.B. Cigarette smoking is an independent risk factor for cervical intraepithelial neoplasia in young women: A longitudinal study. Eur. J. Cancer 2010, 46, 405–411. [Google Scholar] [CrossRef]
- Louie, K.S.; Castellsague, X.; de Sanjose, S.; Herrero, R.; Meijer, C.J.; Shah, K.; Munoz, N.; Bosch, F.X. Smoking and passive smoking in cervical cancer risk: Pooled analysis of couples from the IARC multicentric case-control studies. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1379–1390. [Google Scholar] [CrossRef]
- Aguayo, F.; Muñoz, J.P.; Perez-Dominguez, F.; Carrillo-Beltrán, D.; Oliva, C.; Calaf, G.M.; Blanco, R.; Nuñez-Acurio, D. High-Risk Human Papillomavirus and Tobacco Smoke Interactions in Epithelial Carcinogenesis. Cancers 2020, 12, 2201. [Google Scholar] [CrossRef]
- Burk, R.D.; Kelly, P.; Feldman, J.; Bromberg, J.; Vermund, S.H.; DeHovitz, J.A.; Landesman, S.H. Declining prevalence of cervicovaginal human papillomavirus infection with age is independent of other risk factors. Sex. Transm. Dis. 1996, 23, 333–341. [Google Scholar] [CrossRef]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Unger, E.R.; Barr, E. Human papillomavirus and cervical cancer. Emerg. Infect. Dis. 2004, 10, 2031–2032. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- DiGiuseppe, S.; Bienkowska-Haba, M.; Guion, L.G.M.; Keiffer, T.R.; Sapp, M. Human Papillomavirus Major Capsid Protein L1 Remains Associated with the Incoming Viral Genome throughout the Entry Process. J. Virol. 2017, 91, e00537-17. [Google Scholar] [CrossRef]
- Stanley, M.A.; Pett, M.R.; Coleman, N. HPV: From infection to cancer. Biochem. Soc. Trans. 2007, 35 Pt 6, 1456–1460. [Google Scholar] [CrossRef]
- Apt, D.; Watts, R.M.; Suske, G.; Bernard, H.U. High Sp1/Sp3 ratios in epithelial cells during epithelial differentiation and cellular transformation correlate with the activation of the HPV-16 promoter. Virology 1996, 224, 281–291. [Google Scholar] [CrossRef]
- Cripe, T.P.; Alderborn, A.; Anderson, R.D.; Parkkinen, S.; Bergman, P.; Haugen, T.H.; Pettersson, U.; Turek, L.P. Transcriptional activation of the human papillomavirus-16 P97 promoter by an 88-nucleotide enhancer containing distinct cell-dependent and AP-1-responsive modules. New Biol. 1990, 2, 450–463. [Google Scholar] [PubMed]
- Gravitt, P.E.; Winer, R.L. Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses 2017, 9, 267. [Google Scholar] [CrossRef]
- Doorbar, J. The papillomavirus life cycle. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2005, 32 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front. Microbiol. 2019, 10, 3116. [Google Scholar]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef]
- Li, S.; Hong, X.; Wei, Z.; Xie, M.; Li, W.; Liu, G.; Guo, H.; Yang, J.; Wei, W.; Zhang, S. Ubiquitination of the HPV Oncoprotein E6 Is Critical for E6/E6AP-Mediated p53 Degradation. Front. Microbiol. 2019, 10, 2483. [Google Scholar] [CrossRef] [PubMed]
- Rasi Bonab, F.; Baghbanzadeh, A.; Ghaseminia, M.; Bolandi, N.; Mokhtarzadeh, A.; Amini, M.; Dadashzadeh, K.; Hajiasgharzadeh, K.; Baradaran, B.; Bannazadeh Baghi, H. Molecular pathways in the development of HPV-induced cervical cancer. Excli J. 2021, 20, 320–337. [Google Scholar]
- Auborn, K.J.; Woodworth, C.; DiPaolo, J.A.; Bradlow, H.L. The interaction between HPV infection and estrogen metabolism in cervical carcinogenesis. Int. J. Cancer 1991, 49, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Espina, N.; Lima, V.; Lieber, C.S.; Garro, A.J. In vitro and in vivo inhibitory effect of ethanol and acetaldehyde on O6-methylguanine transferase. Carcinogenesis 1988, 9, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef]
- Mizumoto, A.; Ohashi, S.; Hirohashi, K.; Amanuma, Y.; Matsuda, T.; Muto, M. Molecular Mechanisms of Acetaldehyde-Mediated Carcinogenesis in Squamous Epithelium. Int. J. Mol. Sci. 2017, 18, 1943. [Google Scholar] [CrossRef] [PubMed]
- Varela-Rey, M.; Woodhoo, A.; Martinez-Chantar, M.L.; Mato, J.M.; Lu, S.C. Alcohol, DNA methylation, and cancer. Alcohol. Res. 2013, 35, 25–35. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Acetaldehyde as an underestimated risk factor for cancer development: Role of genetics in ethanol metabolism. Genes Nutr. 2010, 5, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Fang, F.; Cui, W.; Liu, Y.; Liu, Y. Effect of Ethanol-Induced Methyl Donors Consumption on the State of Hypomethylation in Cervical Cancer. Int. J. Mol. Sci. 2023, 24, 7729. [Google Scholar] [CrossRef]
- Oze, I.; Matsuo, K.; Suzuki, T.; Kawase, T.; Watanabe, M.; Hiraki, A.; Ito, H.; Hosono, S.; Ozawa, T.; Hatooka, S.; et al. Impact of multiple alcohol dehydrogenase gene polymorphisms on risk of upper aerodigestive tract cancers in a Japanese population. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3097–3102. [Google Scholar] [CrossRef]
- Imani, M.M.; Moradi, M.M.; Rezaei, F.; Mozaffari, H.R.; Sharifi, R.; Safaei, M.; Azizi, F.; Basamtabar, M.; Sohrabi, Z.; Shalchi, M.; et al. Association between alcohol dehydrogenase polymorphisms (rs1229984, rs1573496, rs1154460, and rs284787) and susceptibility to head and neck cancers: A systematic review and meta-analysis. Arch. Oral Biol. 2024, 160, 105898. [Google Scholar] [CrossRef]
- Chang, T.G.; Yen, T.T.; Wei, C.Y.; Hsiao, T.H.; Chen, I.C. Impacts of ADH1B rs1229984 and ALDH2 rs671 polymorphisms on risks of alcohol-related disorder and cancer. Cancer Med. 2023, 12, 747–759. [Google Scholar] [CrossRef]
- Chen, C.C.; Lu, R.B.; Chen, Y.C.; Wang, M.F.; Chang, Y.C.; Li, T.K.; Yin, S.J. Interaction between the functional polymorphisms of the alcohol-metabolism genes in protection against alcoholism. Am. J. Hum. Genet. 1999, 65, 795–807. [Google Scholar] [CrossRef]
- Coutelle, C.; Höhn, B.; Benesova, M.; Oneta, C.M.; Quattrochi, P.; Roth, H.J.; Schmidt-Gayk, H.; Schneeweiss, A.; Bastert, G.; Seitz, H.K. Risk factors in alcohol associated breast cancer: Alcohol dehydrogenase polymorphism and estrogens. Int. J. Oncol. 2004, 25, 1127–1132. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Rajendran, P. The Multifarious Link between Cytochrome P450s and Cancer. Oxid. Med. Cell. Longev. 2020, 2020, 3028387. [Google Scholar] [CrossRef]
- Sakamoto, T.; Hara, M.; Higaki, Y.; Ichiba, M.; Horita, M.; Mizuta, T.; Eguchi, Y.; Yasutake, T.; Ozaki, I.; Yamamoto, K.; et al. Influence of alcohol consumption and gene polymorphisms of ADH2 and ALDH2 on hepatocellular carcinoma in a Japanese population. Int. J. Cancer 2006, 118, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Watanabe, J.; Kawajiri, K. Genetic polymorphisms in the 5’-flanking region change transcriptional regulation of the human cytochrome P450IIE1 gene. J. Biochem. 1991, 110, 559–565. [Google Scholar] [CrossRef]
- Persson, I.; Johansson, I.; Bergling, H.; Dahl, M.L.; Seidegård, J.; Rylander, R.; Rannug, A.; Högberg, J.; Sundberg, M.I. Genetic polymorphism of cytochrome P4502E1 in a Swedish population. Relationship to incidence of lung cancer. FEBS Lett. 1993, 319, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tian, F.; Dai, L.; Chai, Y. Cytochrome P450 2E1 gene polymorphism and alcohol drinking on the risk of hepatocellular carcinoma: A meta-analysis. Mol. Biol. Rep. 2014, 41, 7645–7650. [Google Scholar] [CrossRef] [PubMed]
- Orywal, K.; Jelski, W.; Zdrodowski, M.; Szmitkowski, M. The Diagnostic Value of Alcohol Dehydrogenase Isoenzymes and Aldehyde Dehydrogenase Measurement Sera of Cervical Cancer Patients. Anticancer Res. 2016, 36, 2265–2269. [Google Scholar]
- Orywal, K.; Jelski, W.; Zdrodowski, M.; Szmitkowski, M. The activity of class I, II, III and IV alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in cervical cancer. Clin. Biochem. 2011, 44, 1231–1234. [Google Scholar] [CrossRef]
- Orywal, K.; Jelski, W.; Zdrodowski, M.; Szmitkowski, M. The diagnostic value of alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase measurement in the sera of patients with endometrial cancer. Anticancer Res. 2013, 33, 3725–3730. [Google Scholar]
- Seitz, H.K.; Stickel, F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol. Chem. 2006, 387, 349–360. [Google Scholar] [CrossRef]
- Letafati, A.; Taghiabadi, Z.; Zafarian, N.; Tajdini, R.; Mondeali, M.; Aboofazeli, A.; Chichiarelli, S.; Saso, L.; Jazayeri, S.M. Emerging paradigms: Unmasking the role of oxidative stress in HPV-induced carcinogenesis. Infect. Agent. Cancer 2024, 19, 30. [Google Scholar] [CrossRef]
- Wu, W.S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006, 25, 695–705. [Google Scholar] [CrossRef]
- Despot, A.; Fureš, R.; Despot, A.M.; Mikuš, M.; Zlopaša, G.; D’Amato, A.; Chiantera, V.; Serra, P.; Etrusco, A.; Laganà, A.S. Reactive oxygen species within the vaginal space: An additional promoter of cervical intraepithelial neoplasia and uterine cervical cancer development? Open Med. 2023, 18, 20230826. [Google Scholar] [CrossRef]
- Aleynik, S.I.; Leo, M.A.; Aleynik, M.K.; Lieber, C.S. Increased circulating products of lipid peroxidation in patients with alcoholic liver disease. Alcohol. Clin. Exp. Res. 1998, 22, 192–196. [Google Scholar] [CrossRef]
- el Ghissassi, F.; Barbin, A.; Nair, J.; Bartsch, H. Formation of 1,N6-ethenoadenine and 3,N4-ethenocytosine by lipid peroxidation products and nucleic acid bases. Chem. Res. Toxicol. 1995, 8, 278–283. [Google Scholar] [CrossRef]
- Haorah, J.; Ramirez, S.H.; Floreani, N.; Gorantla, S.; Morsey, B.; Persidsky, Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic. Biol. Med. 2008, 45, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Feng, Z.; Eveleigh, J.; Iyer, G.; Pan, J.; Amin, S.; Chung, F.L.; Tang, M.S. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis 2002, 23, 1781–1789. [Google Scholar] [CrossRef]
- Shinohara, M.; Adachi, Y.; Mitsushita, J.; Kuwabara, M.; Nagasawa, A.; Harada, S.; Furuta, S.; Zhang, Y.; Seheli, K.; Miyazaki, H.; et al. Reactive oxygen generated by NADPH oxidase 1 (Nox1) contributes to cell invasion by regulating matrix metalloprotease-9 production and cell migration. J. Biol. Chem. 2010, 285, 4481–4488. [Google Scholar] [CrossRef] [PubMed]
- Kwaśniewska, A.; Goździcka-Józefiak, A.; Borzecki, A.; Baranowski, W. DNA adducts in squamous cell cervical carcinomas associated with HPV infection. Eur. J. Gynaecol. Oncol. 2004, 25, 359–361. [Google Scholar] [PubMed]
- Villéger, R.; Chulkina, M.; Mifflin, R.C.; Powell, D.W.; Pinchuk, I.V. Disruption of retinol-mediated IL-6 expression in colon cancer-associated fibroblasts: New perspectives on the role of vitamin A metabolism. Oncotarget 2023, 14, 377–381. [Google Scholar] [CrossRef]
- Helm, C.W.; Lorenz, D.J.; Meyer, N.J.; Rising, W.W.; Wulff, J.L. Retinoids for preventing the progression of cervical intra-epithelial neoplasia. Cochrane Database Syst. Rev. 2013, 2013, Cd003296. [Google Scholar]
- Choo, C.K.; Rorke, E.A.; Eckert, R.L. Retinoid regulation of cell differentiation in a series of human papillomavirus type 16-immortalized human cervical epithelial cell lines. Carcinogenesis 1995, 16, 375–381. [Google Scholar] [CrossRef]
- Wang, X.D. Retinoids and alcohol-related carcinogenesis. J. Nutr. 2003, 133, 287s–290s. [Google Scholar] [CrossRef]
- Ratna, A.; Mandrekar, P. Alcohol and Cancer: Mechanisms and Therapies. Biomolecules 2017, 7, 61. [Google Scholar] [CrossRef]
- Duester, G. Alcohol dehydrogenase as a critical mediator of retinoic acid synthesis from vitamin A in the mouse embryo. J. Nutr. 1998, 128 (Suppl. 2), 459s–462s. [Google Scholar] [CrossRef]
- Chute, J.P.; Muramoto, G.G.; Whitesides, J.; Colvin, M.; Safi, R.; Chao, N.J.; McDonnell, D.P. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 11707–11712. [Google Scholar] [CrossRef]
- Berlin Grace, V.M.; Niranjali Devaraj, S.; Radhakrishnan Pillai, M.; Devaraj, H. HPV-induced carcinogenesis of the uterine cervix is associated with reduced serum ATRA level. Gynecol. Oncol. 2006, 103, 113–119. [Google Scholar] [CrossRef]
- Behbakht, K.; DeGeest, K.; Turyk, M.E.; Wilbanks, G.D. All-trans-retinoic acid inhibits the proliferation of cell lines derived from human cervical neoplasia. Gynecol. Oncol. 1996, 61, 31–39. [Google Scholar] [CrossRef]
- Liu, Y.; Nguyen, N.; Colditz, G.A. Links between alcohol consumption and breast cancer: A look at the evidence. Womens Health 2015, 11, 65–77. [Google Scholar]
- James, C.D.; Morgan, I.M.; Bristol, M.L. The Relationship between Estrogen-Related Signaling and Human Papillomavirus Positive Cancers. Pathogens 2020, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Franceschi, S.; Lambert, P.F. Estrogen and ERalpha: Culprits in cervical cancer? Trends Endocrinol. Metab. TEM 2010, 21, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Hellberg, D. Sex steroids and cervical cancer. Anticancer Res. 2012, 32, 3045–3054. [Google Scholar] [PubMed]
- Mitrani-Rosenbaum, S.; Tsvieli, R.; Tur-Kaspa, R. Oestrogen stimulates differential transcription of human papillomavirus type 16 in SiHa cervical carcinoma cells. J. Gen. Virol. 1989, 70 Pt 8, 2227–2232. [Google Scholar] [CrossRef]
- Sun, Q.; Liang, Y.; Zhang, T.; Wang, K.; Yang, X. ER-α36 mediates estrogen-stimulated MAPK/ERK activation and regulates migration, invasion, proliferation in cervical cancer cells. Biochem. Biophys. Res. Commun. 2017, 487, 625–632. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Roszak, A.; Pawlik, P.; Sajdak, S.; Jagodziński, P.P. Increased 17ß-hydroxysteroid dehydrogenase type 1 levels in primary cervical cancer. Biomed. Pharmacother. 2015, 72, 179–183. [Google Scholar] [CrossRef]
- Meadows, G.G.; Zhang, H. Effects of Alcohol on Tumor Growth, Metastasis, Immune Response, and Host Survival. Alcohol. Res. 2015, 37, 311–322. [Google Scholar] [CrossRef]
- Min, K.-J.; Lee, J.-K.; Lee, S.; Kim, M.K. Alcohol Consumption and Viral Load Are Synergistically Associated with CIN1. PLoS ONE 2013, 8, e72142. [Google Scholar] [CrossRef]
- Ben-Eliyahu, S.; Page, G.G.; Yirmiya, R.; Taylor, A.N. Acute alcohol intoxication suppresses natural killer cell activity and promotes tumor metastasis. Nat. Med. 1996, 2, 457–460. [Google Scholar] [CrossRef]
- Laso, F.J.; Madruga, J.I.; Girón, J.A.; López, A.; Ciudad, J.; San Miguel, J.F.; Alvarez-Mon, M.; Orfao, A. Decreased natural killer cytotoxic activity in chronic alcoholism is associated with alcohol liver disease but not active ethanol consumption. Hepatology 1997, 25, 1096–1100. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, Y.; Guo, D.; Zhang, Z.; Liu, M. Reduced NK Cell Cytotoxicity by Papillomatosis-Derived TGF-β Contributing to Low-Risk HPV Persistence in JORRP Patients. Front. Immunol. 2022, 13, 849493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, M.; An, Y.; Gao, C.; Wang, T.; Zhang, Z.; Zhang, G.; Li, S.; Li, W.; Li, M.; et al. Targeting immune microenvironment in cervical cancer: Current research and advances. J. Transl. Med. 2025, 23, 888. [Google Scholar] [CrossRef]
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients 2021, 13, 3173. [Google Scholar] [CrossRef]
- Turati, F.; Garavello, W.; Tramacere, I.; Pelucchi, C.; Galeone, C.; Bagnardi, V.; Corrao, G.; Islami, F.; Fedirko, V.; Boffetta, P.; et al. A meta-analysis of alcohol drinking and oral and pharyngeal cancers: Results from subgroup analyses. Alcohol Alcohol. 2013, 48, 107–118. [Google Scholar] [CrossRef]
- Pelucchi, C.; Gallus, S.; Garavello, W.; Bosetti, C.; La Vecchia, C. Cancer risk associated with alcohol and tobacco use: Focus on upper aero-digestive tract and liver. Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2006, 29, 193–198. [Google Scholar]
- Boyle, P. Tobacco: Science, Policy and Public Health; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Salaspuro, V.; Salaspuro, M. Synergistic effect of alcohol drinking and smoking on in vivo acetaldehyde concentration in saliva. Int. J. Cancer 2004, 111, 480–483. [Google Scholar] [CrossRef]
- Oki, E.; Zhao, Y.; Yoshida, R.; Egashira, A.; Ohgaki, K.; Morita, M.; Kakeji, Y.; Maehara, Y. The difference in p53 mutations between cancers of the upper and lower gastrointestinal tract. Digestion 2009, 79 (Suppl. 1), 33–39. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-S.; Oh, H.Y.; Kim, M.K.; Lee, D.O.; Chung, Y.K.; Kim, J.-Y.; Lee, C.W. Combined Effect of Secondhand Smoking and Alcohol Drinking on Risk of Persistent Human Papillomavirus Infection. BioMed Res. Int. 2019, 2019, 5829676. [Google Scholar] [CrossRef]
- Schabath, M.B.; Thompson, Z.J.; Egan, K.M.; Torres, B.N.; Nguyen, A.; Papenfuss, M.R.; Abrahamsen, M.E.; Giuliano, A.R. Alcohol consumption and prevalence of human papillomavirus (HPV) infection among US men in the HPV in Men (HIM) study. Sex. Transm. Infect. 2015, 91, 61–67. [Google Scholar] [CrossRef]
- Kashyap, V.K.; Nagesh, P.K.B.; Singh, A.K.; Massey, A.; Darkwah, G.P.; George, A.; Khan, S.; Hafeez, B.B.; Zafar, N.; Kumar, S.; et al. Curcumin attenuates smoking and drinking activated NF-κB/IL-6 inflammatory signaling axis in cervical cancer. Cancer Cell Int. 2024, 24, 343. [Google Scholar] [CrossRef] [PubMed]
- Houlihan, C.F.; Larke, N.L.; Watson-Jones, D.; Smith-McCune, K.K.; Shiboski, S.; Gravitt, P.E.; Smith, J.S.; Kuhn, L.; Wang, C.; Hayes, R. Human papillomavirus infection and increased risk of HIV acquisition. A systematic review and meta-analysis. Aids 2012, 26, 2211–2222. [Google Scholar] [CrossRef]
- WHO. Women Living with HIV Have a Six-Fold Increased Risk of Cervical Cancer When Compared to Women Without HIV; WHO: Geneva, Switzerland, 16 November 2020; Available online: https://www.who.int/news/item/16-11-2020-who-releases-new-estimates-of-the-global-burden-of-cervical-cancer-associated-with-hiv (accessed on 11 April 2025).
- Wen, X.J.; Balluz, L.; Town, M. Prevalence of HIV risk behaviors between binge drinkers and non-binge drinkers aged 18- to 64-years in US, 2008. J. Community Health 2012, 37, 72–79. [Google Scholar] [CrossRef]
- Chersich, M.F.; Luchters, S.M.; Malonza, I.M.; Mwarogo, P.; King’ola, N.; Temmerman, M. Heavy episodic drinking among Kenyan female sex workers is associated with unsafe sex, sexual violence and sexually transmitted infections. Int. J. STD AIDS 2007, 18, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Chersich, M.F.; Bosire, W.; King’ola, N.; Temmerman, M.; Luchters, S. Effects of hazardous and harmful alcohol use on HIV incidence and sexual behaviour: A cohort study of Kenyan female sex workers. Glob. Health 2014, 10, 22. [Google Scholar] [CrossRef]
- Chiao, C.; Morisky, D.E.; Rosenberg, R.; Ksobiech, K.; Malow, R. The relationship between HIV/Sexually Transmitted Infection risk and alcohol use during commercial sex episodes: Results from the study of female commercial sex workers in the Philippines. Subst. Use Misuse 2006, 41, 1509–1533. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, O.O.; Wigfall, L.T.; Rossheim, M.E.; Tomar, A.; Barry, A.E. Binge drinking, HIV/HPV co-infection risk, and HIV testing: Factors associated with HPV vaccination among young adults in the United States. Prev. Med. 2020, 134, 106023. [Google Scholar] [CrossRef]
- Oh, H.Y.; Kim, M.K.; Seo, S.; Lee, D.O.; Chung, Y.K.; Lim, M.C.; Kim, J.; Lee, C.W.; Park, S. Alcohol consumption and persistent infection of high-risk human papillomavirus. Epidemiol. Infect. 2014, 143, 1442–1450, Erratum in PLoS ONE 2015, 10, e0124991. [Google Scholar] [CrossRef]
- Oh, H.Y.; Seo, S.-S.; Kim, M.K.; Lee, D.O.; Chung, Y.K.; Lim, M.C.; Kim, J.-Y.; Lee, C.W.; Park, S.-Y. Synergistic Effect of Viral Load and Alcohol Consumption on the Risk of Persistent High-Risk Human Papillomavirus Infection. PLoS ONE 2015, 9, e104374. [Google Scholar] [CrossRef]
- Mekonnen, A.G.; Mittiku, Y.M. Early-onset of sexual activity as a potential risk of cervical cancer in Africa: A review of literature. PLoS Glob. Public Health 2023, 3, e0000941. [Google Scholar] [CrossRef] [PubMed]
- Lytvynenko, M.; Bocharova, T.; Zhelezniakova, N.; Narbutova, T.; Gargin, V. Cervical transformation in alcohol abuse patients. Georgian Med. News 2017, 10, 12–17. [Google Scholar]
- Nielsen, A.; Munk, C.; Jørgensen, H.O.; Winther, J.F.; van den Brule, A.J.; Kjaer, S.K. Multiple-type human papillomavirus infection in younger uncircumcised men. Int. J. STD AIDS 2013, 24, 128–133. [Google Scholar] [CrossRef]
- Ho, G.Y.; Bierman, R.; Beardsley, L.; Chang, C.J.; Burk, R.D. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 1998, 338, 423–428. [Google Scholar] [CrossRef]
- Burkett, B.J.; Peterson, C.M.; Birch, L.M.; Brennan, C.; Nuckols, M.L.; Ward, B.E.; Crum, C.P. The relationship between contraceptives, sexual practices, and cervical human papillomavirus infection among a college population. J. Clin. Epidemiol. 1992, 45, 1295–1302. [Google Scholar] [CrossRef]
- Sikström, B.; Hellberg, D.; Nilsson, S.; Mårdh, P.A. Smoking, alcohol, sexual behaviour and drug use in women with cervical human papillomavirus infection. Arch. Gynecol. Obstet. 1995, 256, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Tolstrup, J.; Munk, C.; Thomsen, B.L.; Svare, E.; van den Brule, A.J.; Grønbaek, M.; Meijer, C.; Kjaer Krüger, S. The role of smoking and alcohol intake in the development of high-grade squamous intraepithelial lesions among high-risk HPV-positive women. Acta Obstet. Gynecol. Scand. 2006, 85, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Deng, Y.; Boakye, D.; Tin, M.S.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.J.; Elcarte, E.; et al. Global distribution, risk factors, and recent trends for cervical cancer: A worldwide country-level analysis. Gynecol. Oncol. 2022, 164, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Chincha Lino, O.J.; Chinchihualpa Paredes, N.O.; Samalvides Cuba, F. Factors associated with normal or abnormal Papanicolaou smear among HIV-infected women at a national hospital in Lima, Peru, 2012–2015. AIDS Care 2022, 34, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Hayumbu, V.; Hangoma, J.; Hamooya, B.M.; Malumani, M.; Masenga, S.K. Cervical cancer and precancerous cervical lesions detected using visual inspection with acetic acid at Livingstone Teaching Hospital. Pan Afr. Med. J. 2021, 40, 235. [Google Scholar] [CrossRef] [PubMed]
- Abebe, M.; Eshetie, S.; Tessema, B. Prevalence of sexually transmitted infections among cervical cancer suspected women at University of Gondar Comprehensive Specialized Hospital, North-west Ethiopia. BMC Infect. Dis. 2021, 21, 378. [Google Scholar] [CrossRef]
- Mancuso, P.; Djuric, O.; Collini, G.; Serventi, E.; Massari, M.; Zerbini, A.; Giorgi Rossi, P.; Vicentini, M. Risk of cancer in individuals with alcohol and drug use disorders: A registry-based study in Reggio Emilia, Italy. Eur. J. Cancer Prev. 2020, 29, 270–278. [Google Scholar] [CrossRef]
- Allen, N.E.; Beral, V.; Casabonne, D.; Kan, S.W.; Reeves, G.K.; Brown, A.; Green, J. Moderate alcohol intake and cancer incidence in women. J. Natl. Cancer Inst. 2009, 101, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Sigvardsson, S.; Hardell, L.; Przybeck, T.R.; Cloninger, R. Increased cancer risk among Swedish female alcoholics. Epidemiology 1996, 7, 140–143. [Google Scholar] [CrossRef]
- Kjaerheim, K.; Andersen, A. Cancer incidence among waitresses in Norway. Cancer Causes Control 1994, 5, 31–37. [Google Scholar] [CrossRef]
- Tønnesen, H.; Møller, H.; Andersen, J.R.; Jensen, E.; Juel, K. Cancer morbidity in alcohol abusers. Br. J. Cancer 1994, 69, 327–332. [Google Scholar] [CrossRef]
- Adami, H.O.; McLaughlin, J.K.; Hsing, A.W.; Wolk, A.; Ekbom, A.; Holmberg, L.; Persson, I. Alcoholism and cancer risk: A population-based cohort study. Cancer Causes Control 1992, 3, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Alcohol Consumption and Cervical Cancer Associations Among Women in Los Angeles, California. Available online: https://scholarworks.waldenu.edu/cgi/viewcontent.cgi?article=6154&context=dissertations (accessed on 11 September 2025).
- Abdalla AE, T.T.; Gallagher, J.; Schmitt, J.W. Alcohol Consumption and the Development of High-Grade Cervical Dysplasia. Gynecol. Obstet. 2020, 10, 519. [Google Scholar]
- Kingsley, K.; Struthers, M.; Freel, N.; Enyeart, J.; Munford, W.; Miller, C.N.; Spangelo, B.; Steffen, J.; Park, G.; Keiserman, M.A.; et al. Ethanol and acetaldehyde mediate folic acid and human papillomavirus-induced proliferation of oral squamous cell carcinoma cells in vitro. BMC Proc. 2012, 6 (Suppl. 3), P64. [Google Scholar] [CrossRef]
- Arroyo Mühr, L.S.; Gini, A.; Yilmaz, E.; Hassan, S.S.; Lagheden, C.; Hultin, E.; Garcia Serrano, A.; Ure, A.E.; Andersson, H.; Merino, R.; et al. Concomitant human papillomavirus (HPV) vaccination and screening for elimination of HPV and cervical cancer. Nat. Commun. 2024, 15, 3679. [Google Scholar] [CrossRef]
- El-Zein, M.; Richardson, L.; Franco, E.L. Cervical cancer screening of HPV vaccinated populations: Cytology, molecular testing, both or none. J. Clin. Virol. 2016, 76 (Suppl. 1), S62–S68. [Google Scholar] [CrossRef]
- Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 30 September 2025).
- Luu, X.Q.; Jun, J.K.; Suh, M.; Oh, J.K.; Yu, S.Y.; Choi, K.S. Cervical Cancer Screening, HPV Vaccination, and Cervical Cancer Elimination. JAMA Netw. Open 2025, 8, e2526683. [Google Scholar] [CrossRef] [PubMed]
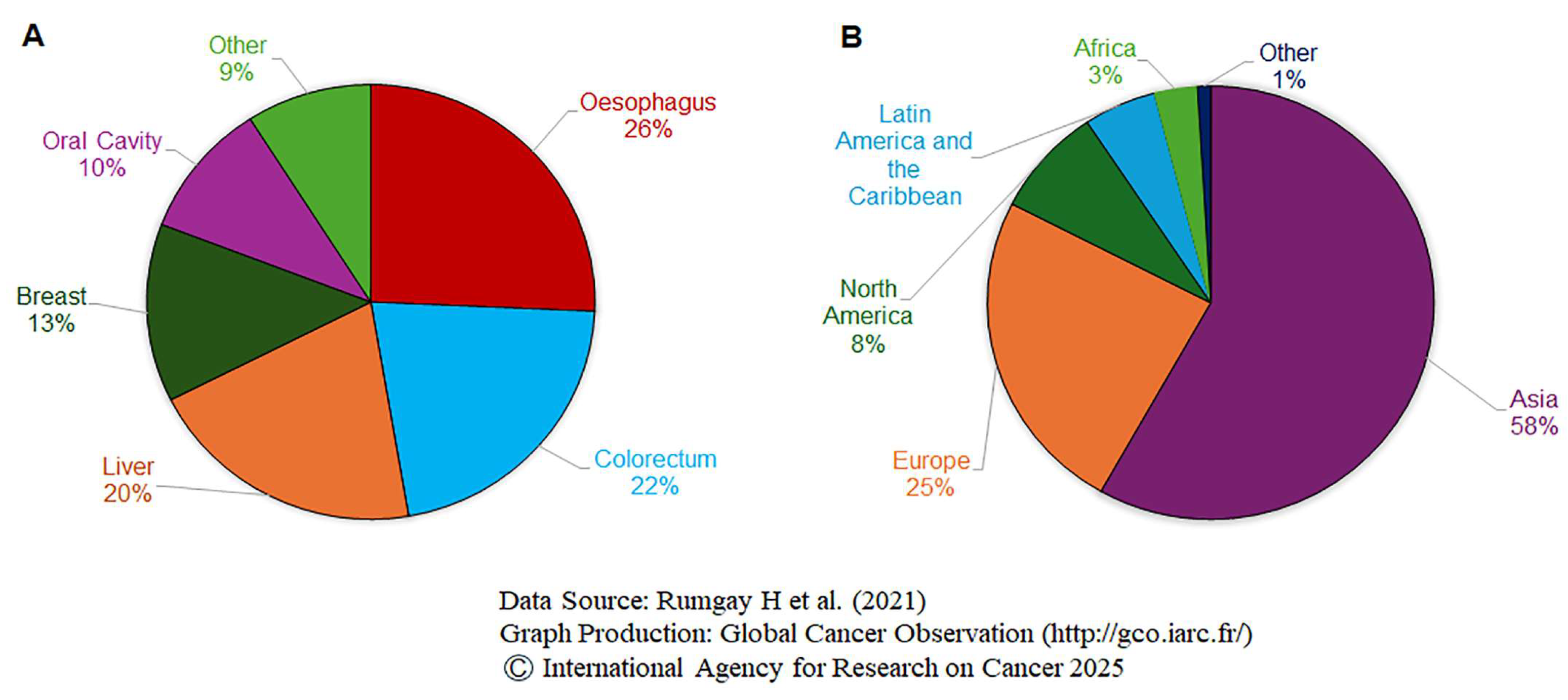
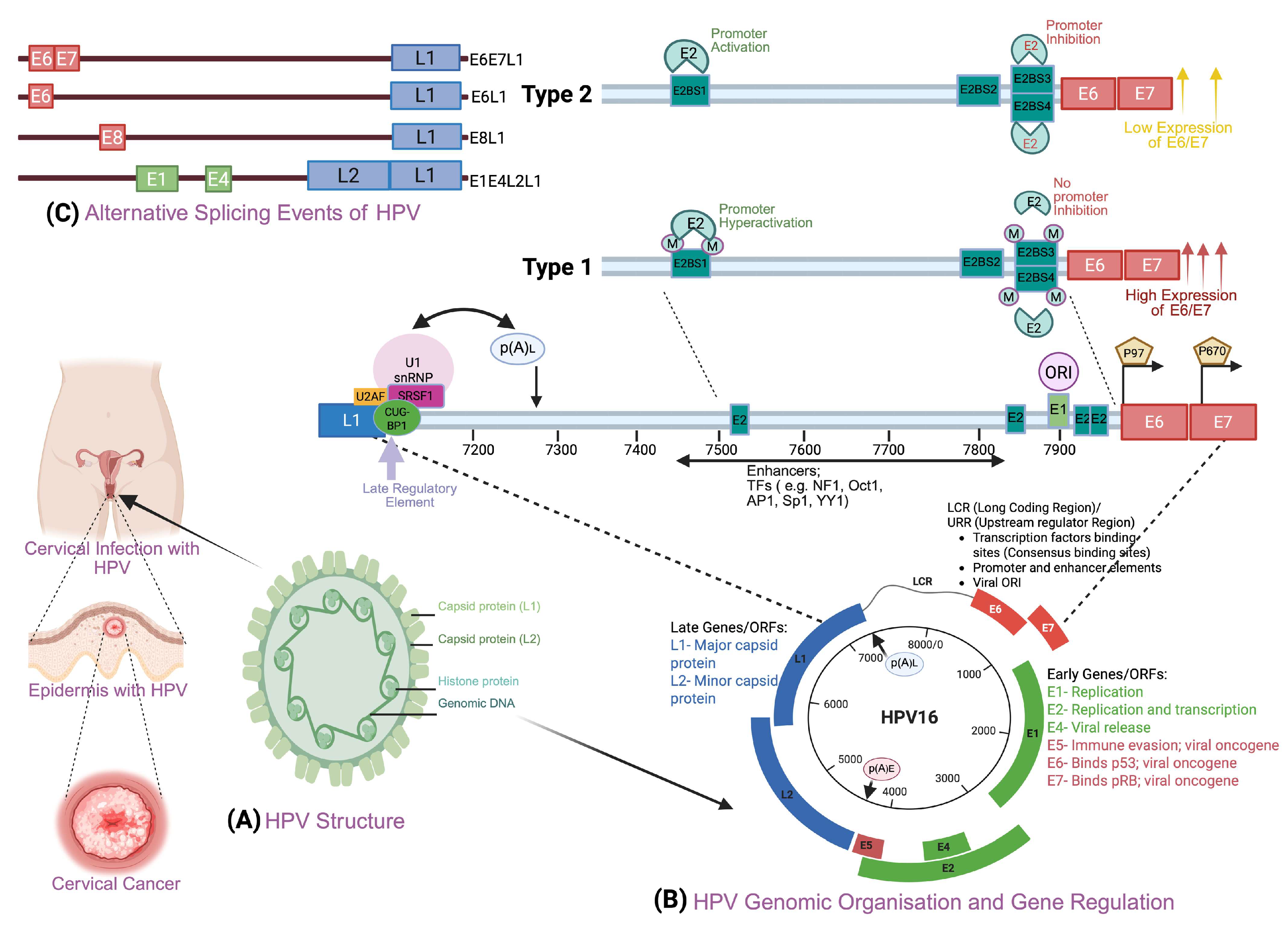
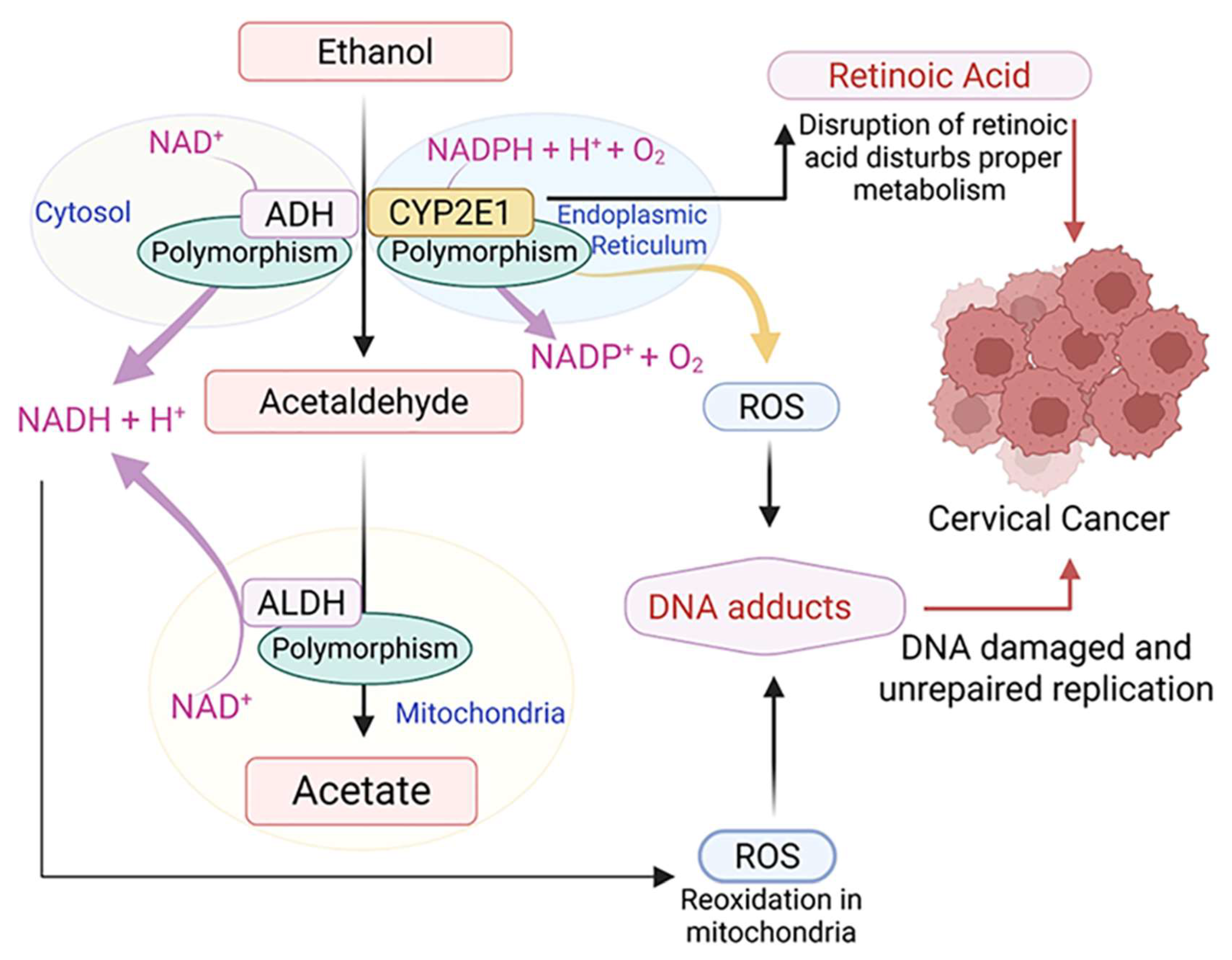
| Research Title (Publishing Year) | Study Type | Country (Study Period) | Cases/Study Size | Alcohol Consumption | RR (95% CI) | References |
|---|---|---|---|---|---|---|
| Global distribution, risk factors, and recent trends for cervical cancer: A worldwide country-level analysis (2022) | GLOBOCAN database | Globally in 2018 | 311,365 deaths/568,847 cases | Alcohol consumption | β = 1.89, 95% CI = 0.59–3.19, p = 0.005; β = 0.98, CI = 0.08–1.88, p = 0.033 | [133] |
| Factors associated with normal or abnormal Papanicolaou smear among HIV women at a national hospital in Lima, Peru, 2012–2015 (2021) | Case–control | Lima, Peru (2012–2015) | 368 patients | Alcohol consumption | Abnormal Pap smear OR, 1.77; 95% CI = 1.06–2.95 | [134] |
| Cervical cancer and precancerous cervical lesions detected using visual inspection with acetic acid at Livingstone Teaching Hospital (2021) | Cross-sectional | Zambia (2019–2020) | 329 women’s | Alcohol consumption | OR, 0.30; 95% CI = 0.12–0.74 | [135] |
| Prevalence of sexually transmitted infections among cervical cancer suspected women at University of Gondar Comprehensive | Cross-sectional | Ethiopia (February–April 2017) | 403 women’s | Alcohol addiction | OR, 2.2; 95% CI = 1.07–4.5, p = 0.031 | [136] |
| Specialized Hospital, North-west Ethiopia (2021) | ||||||
| Risk of cancer in individuals with alcohol and drug use disorders: a registry-based study in Reggio Emilia, Italy (2020) | Population-based cohort | Italy (1996–2014) | 4373 patients | Alcohol use | SIR = 8.6; 95% CI = 2.8–26.7 | [137] |
| Alcohol Abuse Decreases Pelvic Control and Survival in Cervical Cancer: An Opportunity of Lifestyle Intervention for Outcome Improvement (2017) | Retrospective | USA (2007–2013) | 95 patients | Alcohol abuse decreases the DFS | p = 0.005; HR, 10.57; 95% CI = 2.07–53.93 and OS p = 0.001; HR, 10.80; 95% CI: 2.57–45.40. | [13] |
| Synergistic effect of viral load and alcohol consumption on the risk of persistent high-risk human papillomavirus infection (2014) | Prospective cohort | Korea (2002–2011) | 11,140 patients | Diagnosis of alcohol | HR-HPV, 1 year follow-up OR, 4.14; 95% CI = 1.89–9.05; 2-year persistence (OR, 6.61; 95% CI = 2.09–20.9) | [125]. |
| Alcohol consumption and viral load are synergistically associated with CIN1 (2013) | Cohort | Korea (2006–2009) | 1243 patients | Alcohol drinker | Increased risk of CINI; OR, 2.18; 95% CI = 1.22–3.89; High HPV viral load (≥100 RLU/PC) with high risk of CINI, OR, 19.1; 95% CI= 6.60–55.3 | [103] |
| Moderate alcohol intake and cancer incidence in women (2009) | Prospective cohort | United Kingdom (1996–2001) | 1,280,296 middle-aged women | Women drinking several drinks/week | RR, 95% floated CI varies across different sample sizes. | [138] |
| Alcoholism and risk for cancer of the cervix uteri, vagina, and vulva (2001) | Population-based cohort | Sweden (1965–1995) | 36,856 patients | Diagnosis of alcoholism | In situ cervical cancer (SIR, 1.7; 95% CI = 1.6–1.9, Invasive cervical cancer SIR, 2.9; 95% CI = 2.4–3.5); Cancer of vagina (SIR, 4 4.6; 95% CI = 2.2–8.5) | [12] |
| Increased cancer risk among Swedish female alcoholics (1996) | Population-based cohort | Sweden (1917–1977) | 187/15,508 alcoholic women’s | Alcohol abuse | Cervix uteri, RR, 3.9; 95% CI = 2.8–5.4 vulva, vagina, and unspecified female genital organs (RR,4.0; 95% CI = 1.3–12) | [139] |
| Cancer incidence among waitresses in Norway (1994) | Population-based cohort | Norway (1959–1991) | 5314 waitresses (21/38 cases) | Alcohol consumption | SIR, 1.8; 95% CI | [140] |
| Cancer morbidity in alcohol abusers (1994) | Population-based cohort | Denmark (1954–1987) | 22/3093 | Alcohol consumption | RR, 2.0; 95% CI = 1.2–3.0 | [141] |
| Alcoholism and cancer risk: a population-based cohort study (1992) | Population-based cohort | Sweden (1965–1983) | 6/1013 | Diagnosis of alcohol | SIR, 4.2; 95% CI = 1.5–9.1 | [142] |
| Natural history of cervicovaginal papillomavirus infection in young women, 1998 | Prospective study | State University in New Brunswick, New Jersey | 608 female students | High alcohol consumption | RR, 2.0; 95% CI = 1.2–3.1 | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashyap, V.K.; Kenchappa, D.B.; Singh, A.K.; Singh, B.; Yallapu, M.M.; Cobos, E.; Chauhan, S.C. Alcohol Consumption and Cervical Carcinogenesis: Time to Draw Conclusions. Cells 2025, 14, 1639. https://doi.org/10.3390/cells14201639
Kashyap VK, Kenchappa DB, Singh AK, Singh B, Yallapu MM, Cobos E, Chauhan SC. Alcohol Consumption and Cervical Carcinogenesis: Time to Draw Conclusions. Cells. 2025; 14(20):1639. https://doi.org/10.3390/cells14201639
Chicago/Turabian StyleKashyap, Vivek K., Divya B. Kenchappa, Ajay K. Singh, Bhupesh Singh, Murali M. Yallapu, Everardo Cobos, and Subhash C. Chauhan. 2025. "Alcohol Consumption and Cervical Carcinogenesis: Time to Draw Conclusions" Cells 14, no. 20: 1639. https://doi.org/10.3390/cells14201639
APA StyleKashyap, V. K., Kenchappa, D. B., Singh, A. K., Singh, B., Yallapu, M. M., Cobos, E., & Chauhan, S. C. (2025). Alcohol Consumption and Cervical Carcinogenesis: Time to Draw Conclusions. Cells, 14(20), 1639. https://doi.org/10.3390/cells14201639








