MK3 Gene Upregulates Granulosa Cell Apoptosis Through the TNF/P38 MAPK Pathway in Chicken
Abstract
1. Implications
2. Introduction
3. Methods
3.1. Experimental Animals
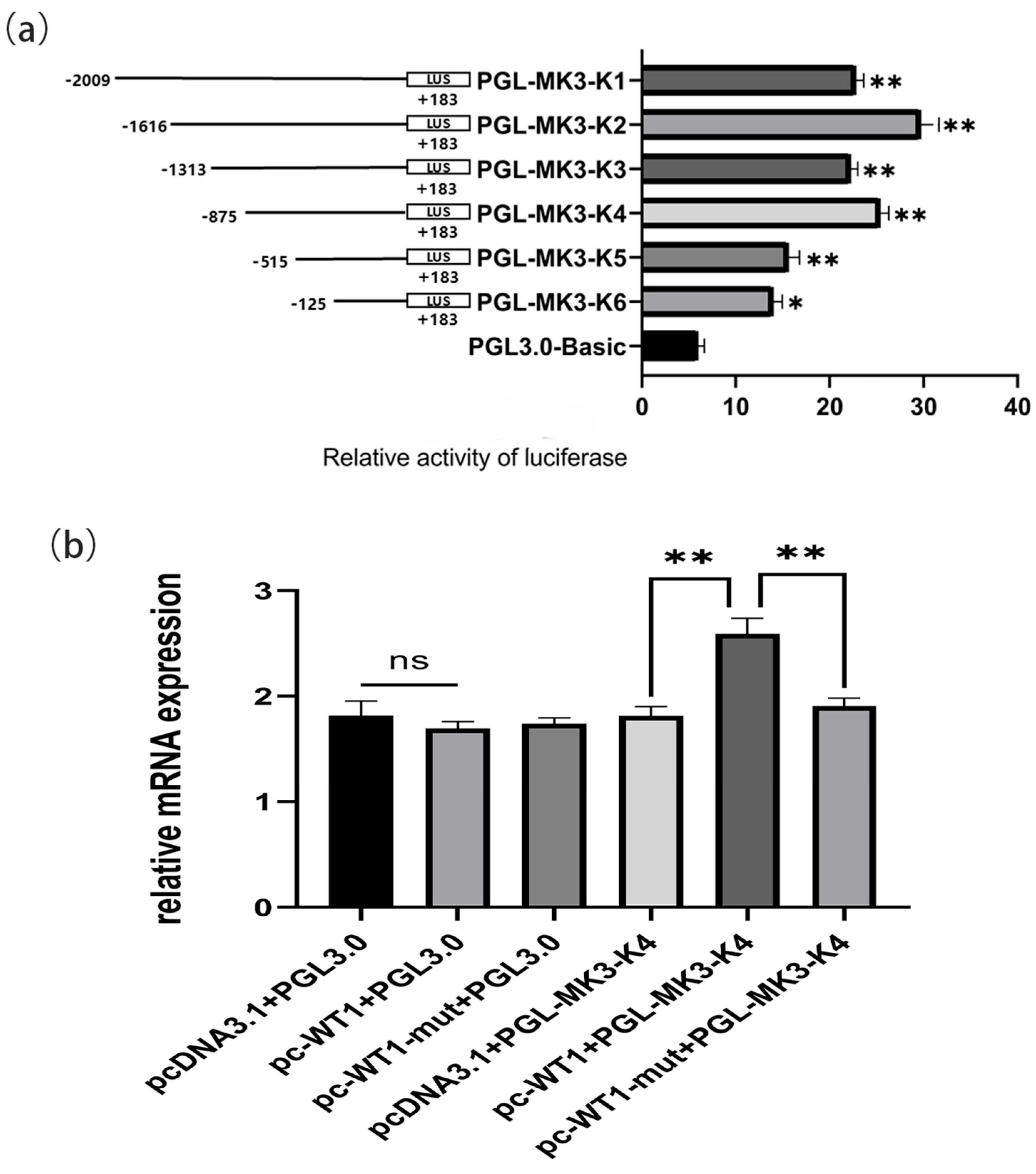
3.2. Isolation, Culture, Transfection, and Dual Luciferase Reporter Gene Assay of Chicken Follicular Granulosa Cells
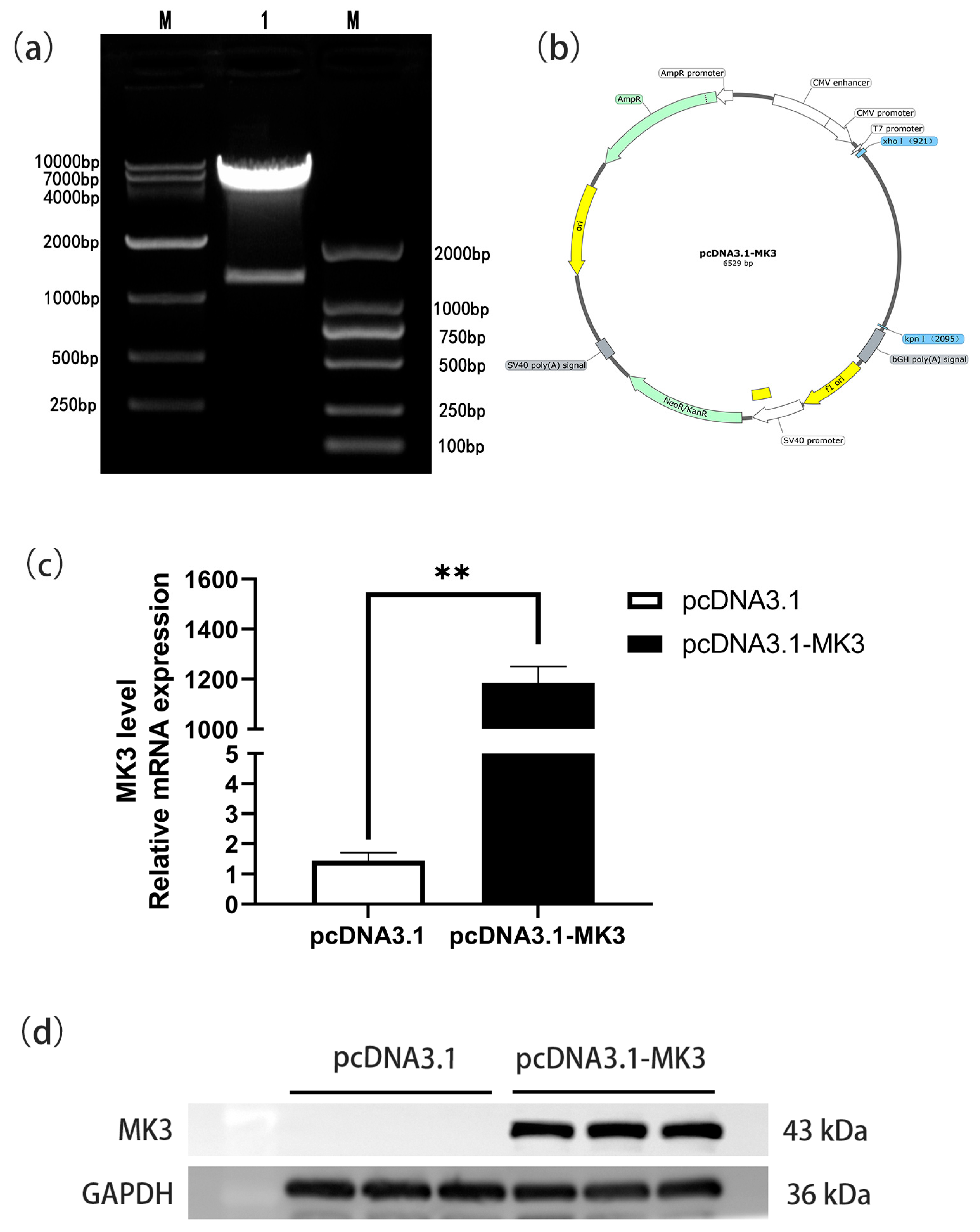
3.3. Recombinant Plasmid Construction
3.4. RNA Interference
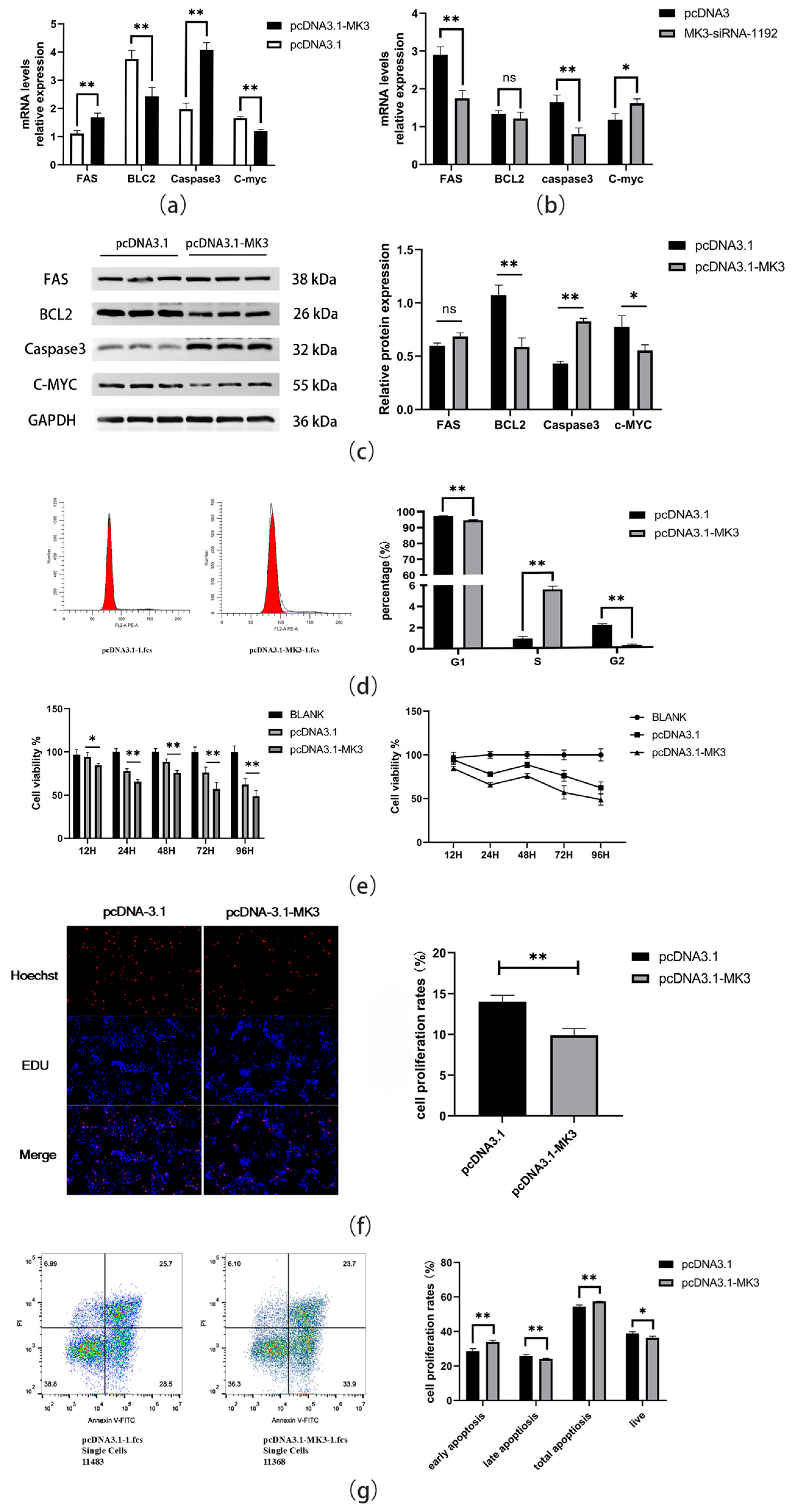
3.5. Quantitative Real-Time PCR
3.6. Western Blotting Analysis
3.7. Cell Counting Kit-8 (CCK-8) Assay
3.8. 5-Ethynyl-2′-Deoxyuridine (EdU) Assay
3.9. Apoptosis and Cell Cycle Analysis
3.10. Data Statistics and Analysis
4. Results
4.1. Construction and Validation of an MK3 Gene Overexpression Vector
4.2. Effect of MK3 Gene Overexpression on Granulosa Cell Proliferation and Apoptosis in Laying Hen Oocytes
4.3. Construction of the Deleted Fragment of the MK3 Gene Promoter and Screening of Transcription Factors
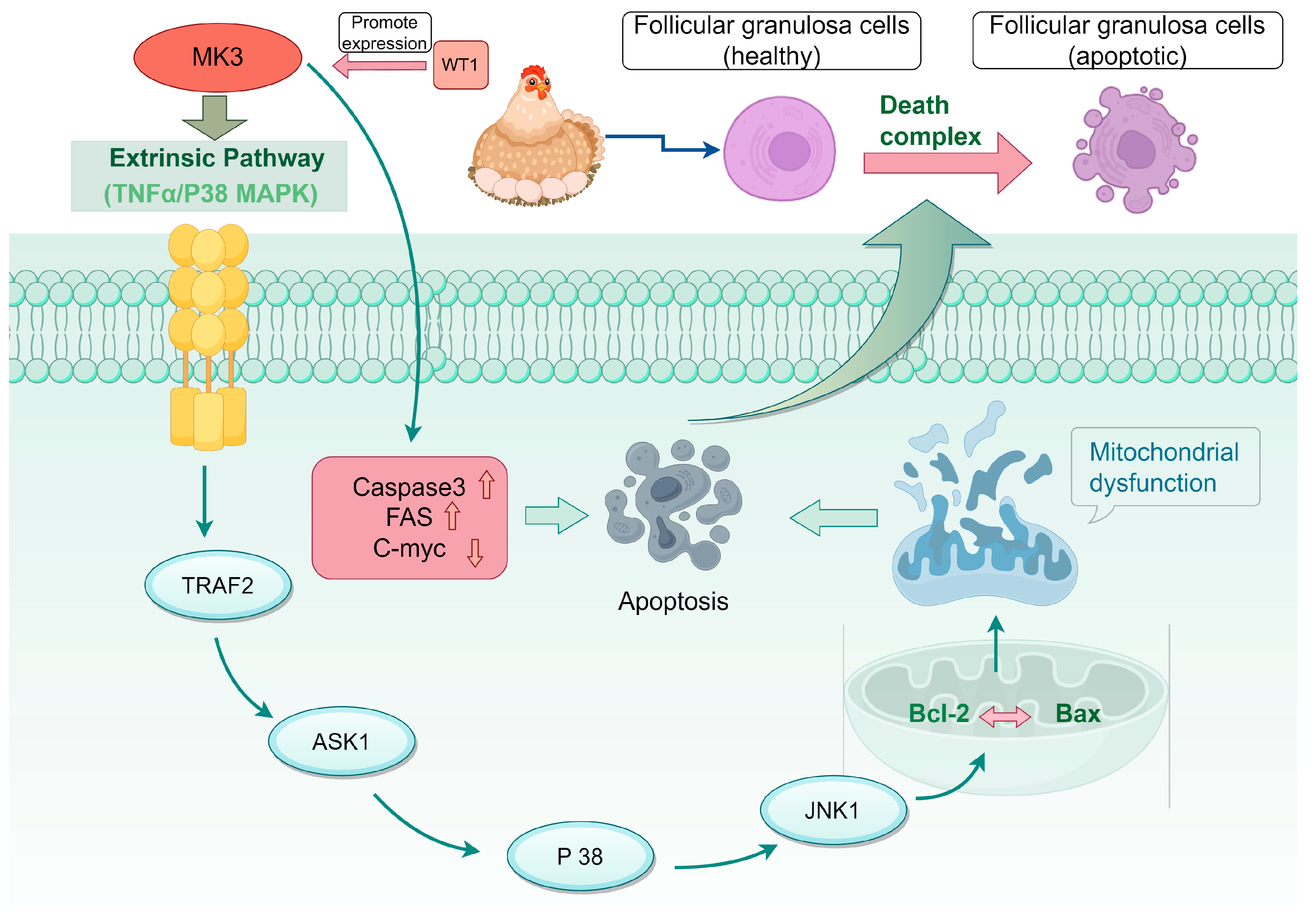
4.4. The MK3 Gene Regulates Granule Cell Apoptosis Through the TNF/P38 MAPK Pathway
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MK3 | mitogen-activated protein kinase activated protein kinase 3 |
| TNF-R1 | TNF receptor superfamily member 1A |
| ASK1 | apoptosis signal regulating kinase-1 |
| P38 | P38 mitogen-activated protein kinase |
| JNK1 | c-Jun N-terminal kinase 1 |
| BAX | BCL-2-associated X protein |
| WT1 | Wilms Tumor 1 |
| FSH | Follicle-stimulating hormone |
| BCL-2 | B-cell lymphoma-2 |
| caspase3 | Caspase3 |
| C-myc | myelocytomatosis viral oncogene homolog |
| FAS | Fas cell surface death receptor |
| TRAF2 | TNF Receptor-Associated Factor 2 |
| TNF-α | Tumor Necrosis Factor-α |
| DMSO | Dimethyl Sulfoxide |
References
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Li, Y.; Zhang, D.; Cui, X.; Dai, K.; Yang, Y.; Liu, S.; Tan, J.; Yan, Q. ANP promotes proliferation and inhibits apoptosis of ovarian granulosa cells by NPRA/PGRMC1/EGFR complex and improves ovary functions of PCOS rats. Cell Death Dis. 2017, 8, e3145. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Liu, H.; Pan, Z. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod. Biol. Endocrinol. 2019, 17, 9. [Google Scholar] [CrossRef]
- Gaestel, M. MAPK-Activated Protein Kinases (MKs): Novel Insights and Challenges. Front. Cell Dev. Biol. 2015, 3, 88. [Google Scholar] [CrossRef]
- Ronkina, N.; Gaestel, M. MAPK-Activated Protein Kinases: Servant or Partner? Annu. Rev. Biochem. 2022, 91, 505–540. [Google Scholar] [CrossRef]
- Shrestha, A.; Bruckmueller, H.; Kildalsen, H.; Kaur, G.; Gaestel, M.; Wetting, H.L.; Mikkola, I.; Seternes, O.M. Phosphorylation of steroid receptor coactivator-3 (SRC-3) at serine 857 is regulated by the p38MAPK-MK2 axis and affects NF-κB-mediated transcription. Sci. Rep. 2020, 10, 11388. [Google Scholar] [CrossRef] [PubMed]
- Holloway, B.A.; Gomez De La Torre Canny, S.; Ye, Y.; Slusarski, D.C.; Freisinger, C.M.; Dosch, R.; Chou, M.M.; Wagner, D.S.; Mullins, M.C. A Novel Role for MAPKAPK2 in Morphogenesis during Zebrafish Development. PLoS Genet. 2009, 5, e1000413. [Google Scholar] [CrossRef]
- Tóthová, Z.; Šemeláková, M.; Solárová, Z.; Tomc, J.; Debeljak, N.; Solár, P. The Role of PI3K/AKT and MAPK Signaling Pathways in Erythropoietin Signalization. Int. J. Mol. Sci. 2021, 22, 7682. [Google Scholar] [CrossRef]
- Tepekoy, F.; Akkoyunlu, G. The effect of FSH and activin A on Akt and MAPK1/3 phosphorylation in cultured bovine ovarian cortical strips. J. Ovarian Res. 2016, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Maidarti, M.; Anderson, R.A.; Telfer, E.E. Crosstalk between PTEN/PI3K/Akt Signalling and DNA Damage in the Oocyte: Implications for Primordial Follicle Activation, Oocyte Quality and Ageing. Cells 2020, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Qiao, G.; Luo, X.; Liu, S.; Chen, S.; Ye, G.; Zhang, C.; Yi, J. Ferredoxin 1 regulates granulosa cell apoptosis and autophagy in polycystic ovary syndrome. Clin. Sci. 2023, 137, 453–468. [Google Scholar] [CrossRef]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (MAPKAPKs) in Inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, L.; Cui, X.; Lin, X.; Li, Y.; Wang, Y.; Qin, Y.; Chen, D.; Han, C.; Zhou, B.; et al. Wt1 directs the lineage specification of sertoli and granulosa cells by repressing Sf1 expression. Development 2017, 144, 44–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Ye, Q.; Tong, L.; Jiang, H.; Zhu, X.; Huang, L.; Lin, W.; Fu, H.; Wang, J.; et al. Podocyte apoptosis in diabetic nephropathy by BASP1 activation of the p53 pathway via WT1. Acta Physiol. 2021, 232, e13634. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Guan, J.; Liu, L.; Zhang, S.; An, P.; Fan, A.; Song, G.; Zhang, P.; Zhao, T.; Tang, B.; et al. Effects of WT1down-regulation on oocyte maturation and preimplantation embryo development in pigs. Reproduction 2014, 148, 377–387. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, W.; Chen, M.; Liu, C.; Wang, L.; Wei, H.; Zhang, R.; Ren, Z.; Chen, Y.; Luo, M.; Zhao, J.; et al. Epg5 deficiency leads to primary ovarian insufficiency due to WT1 accumulation in mouse granulosa cells. Autophagy 2023, 19, 644–659. [Google Scholar] [CrossRef]
- Shao, T.; Ke, H.; Liu, R.; Xu, L.; Han, S.; Zhang, X.; Dang, Y.; Jiao, X.; Li, W.; Chen, Z.J.; et al. Autophagy regulates differentiation of ovarian granulosa cells through degradation of WT1. Autophagy 2022, 18, 1864–1878. [Google Scholar] [CrossRef]
- Ketelut-Carneiro, N.; Fitzgerald, K.A. Apoptosis, Pyroptosis, and Necroptosis—Oh My! The Many Ways a Cell Can Die. J. Mol. Biol. 2022, 434, 167378. [Google Scholar]
- Han, Y.-H.; Wang, Y.; Lee, S.-J.; Jin, M.-H.; Sun, H.-N.; Kwon, T. Regulation of anoikis by extrinsic death receptor pathways. Cell Commun. Signal. 2023, 21, 227. [Google Scholar] [CrossRef]
- Costigan, A.; Hollville, E.; Martin, S.J. Discriminating Between Apoptosis, Necrosis, Necroptosis, and Ferroptosis by Microscopy and Flow Cytometry. Curr. Protoc. 2023, 3, e951. [Google Scholar] [CrossRef]
- Evans, A.C.O.; Ireland, J.L.H.; Winn, M.E.; Lonergan, P.; Smith, G.W.; Coussens, P.M.; Ireland, J.J. Identification of genes involved in apoptosis and dominant follicle development during follicular waves in cattle. Biol. Reprod. 2004, 70, 1475–1484. [Google Scholar] [CrossRef]
- Nakayama, M.; Manabe, N.; Inoue, N.; Matsui, T.; Miyamoto, H. Changes in the expression of tumor necrosis factor (TNF) alpha, TNFalpha receptor (TNFR) 2, and TNFR-associated factor 2 in granulosa cells during atresia in pig ovaries. Biol. Reprod. 2003, 68, 530–535. [Google Scholar] [CrossRef][Green Version]
- Jääskeläinen, M.; Kyrönlahti, A.; Anttonen, M.; Nishi, Y.; Yanase, T.; Secchiero, P.; Zauli, G.; Tapanainen, J.S.; Heikinheimo, M.; Vaskivuo, T.E. TRAIL pathway components and their putative role in granulosa cell apoptosis in the human ovary. Differentiation 2009, 77, 369–376. [Google Scholar] [CrossRef]
- Sovilj, D.; Kelemen, C.D.; Dvorakova, S.; Zobalova, R.; Raabova, H.; Kriska, J.; Hermanova, Z.; Knotek, T.; Anderova, M.; Klener, P.; et al. Cell-specific modulation of mitochondrial respiration and metabolism by the pro-apoptotic Bcl-2 family members Bax and Bak. Apoptosis 2024, 29, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Li, A.; Lou, A.; Zhang, X.; Chen, Y.; Yang, L.; Li, Y.; Yang, S.; Hou, F.F. Advanced oxidation protein products promote NADPH oxidase-dependent β-cell destruction and dysfunction through the Bcl-2/Bax apoptotic pathway. Lab. Investig. 2017, 97, 792–805. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kugu, K.; Ratts, V.S.; Piquette, G.N.; Tilly, K.I.; Tao, X.J.; Martimbeau, S.; Aberdeen, G.W.; Krajewski, S.; Reed, J.C.; Pepe, G.J.; et al. Analysis of apoptosis and expression of bcl-2 gene family members in the human and baboon ovary. Cell Death Differ. 1998, 5, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Sai, T.; Goto, Y.; Yoshioka, R.; Maeda, A.; Matsuda, F.; Sugimoto, M.; Wongpanit, K.; Jin, H.Z.; Li, J.Y.; Manabe, N. Bid and Bax are involved in granulosa cell apoptosis during follicular atresia in porcine ovaries. J. Reprod. Dev. 2011, 57, 421–427. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Liu, J.; Zhang, Y.; Pi, J.; Wu, Y. MK3 Gene Upregulates Granulosa Cell Apoptosis Through the TNF/P38 MAPK Pathway in Chicken. Cells 2025, 14, 1630. https://doi.org/10.3390/cells14201630
Chen L, Liu J, Zhang Y, Pi J, Wu Y. MK3 Gene Upregulates Granulosa Cell Apoptosis Through the TNF/P38 MAPK Pathway in Chicken. Cells. 2025; 14(20):1630. https://doi.org/10.3390/cells14201630
Chicago/Turabian StyleChen, Li, Jia Liu, Ying Zhang, Jinsong Pi, and Yan Wu. 2025. "MK3 Gene Upregulates Granulosa Cell Apoptosis Through the TNF/P38 MAPK Pathway in Chicken" Cells 14, no. 20: 1630. https://doi.org/10.3390/cells14201630
APA StyleChen, L., Liu, J., Zhang, Y., Pi, J., & Wu, Y. (2025). MK3 Gene Upregulates Granulosa Cell Apoptosis Through the TNF/P38 MAPK Pathway in Chicken. Cells, 14(20), 1630. https://doi.org/10.3390/cells14201630





