Non-Competitive AMPA Receptor Antagonist Perampanel Inhibits Ischemia-Induced Neurodegeneration and Behavioral Deficits in Focal Cortical Pial Vessel Disruption Stroke Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Subjects
2.2. Hippocampal Slice Preparation
2.3. Drug Treatments
2.4. Chemically Induced Long Term Potentiation (cLTP)
2.5. Biochemistry and Western Blotting
2.6. Propidium Iodide Staining
2.7. FluoroJade-C Staining
2.8. Pial Vessel Disruption as a Model of Small-Vessel Stroke
2.9. Y-Maze
2.10. Open Field Test
2.11. Forced Swim Test
2.12. Rotarod
2.13. Structural Modeling and Docking
2.14. Enzyme Linked Immunosorbent Assay (EILSA)
2.15. Statistical Analysis
3. Results
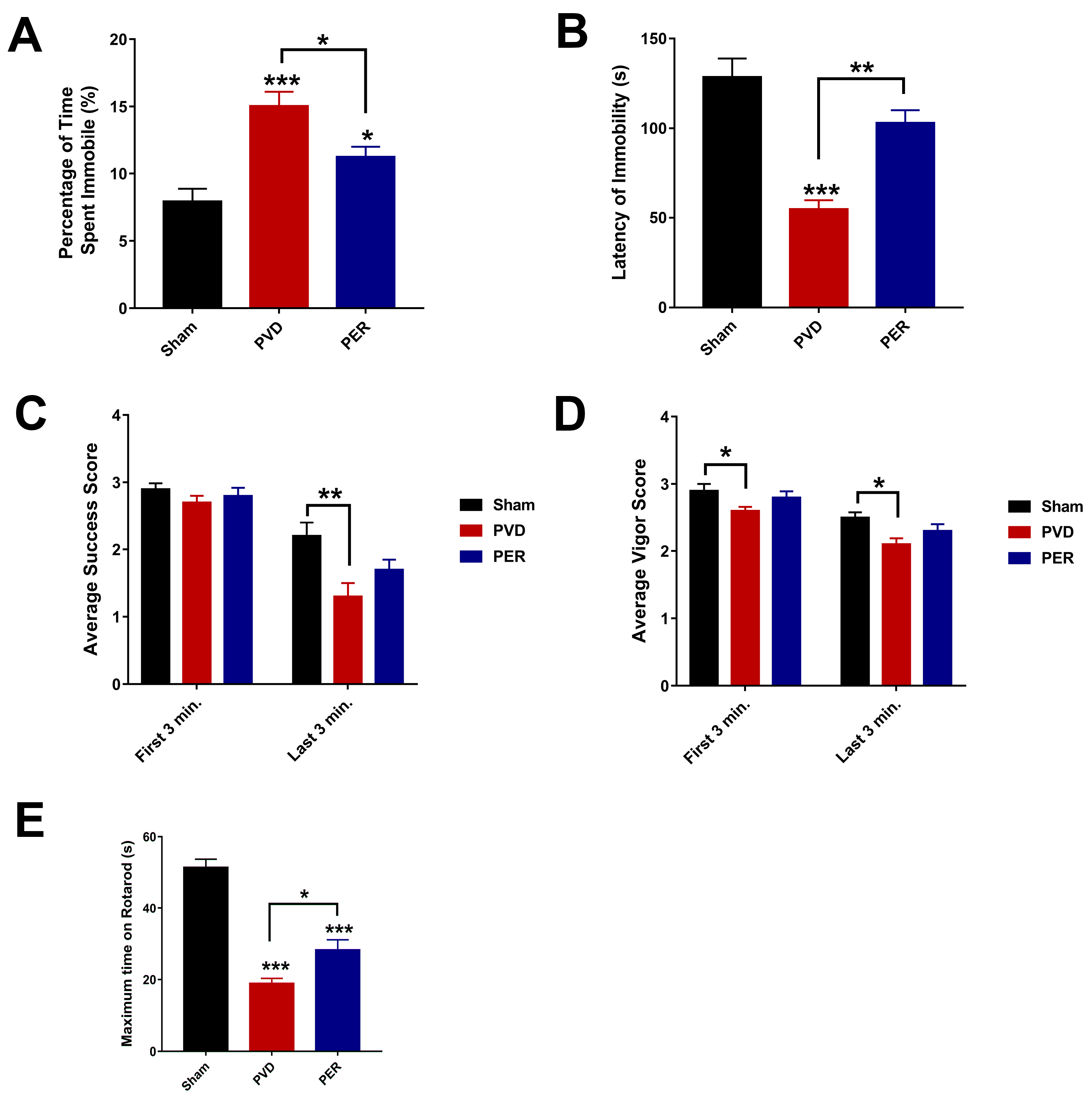
3.1. Perampanel Attenuated PVD-Induced Cognitive Dysfunction
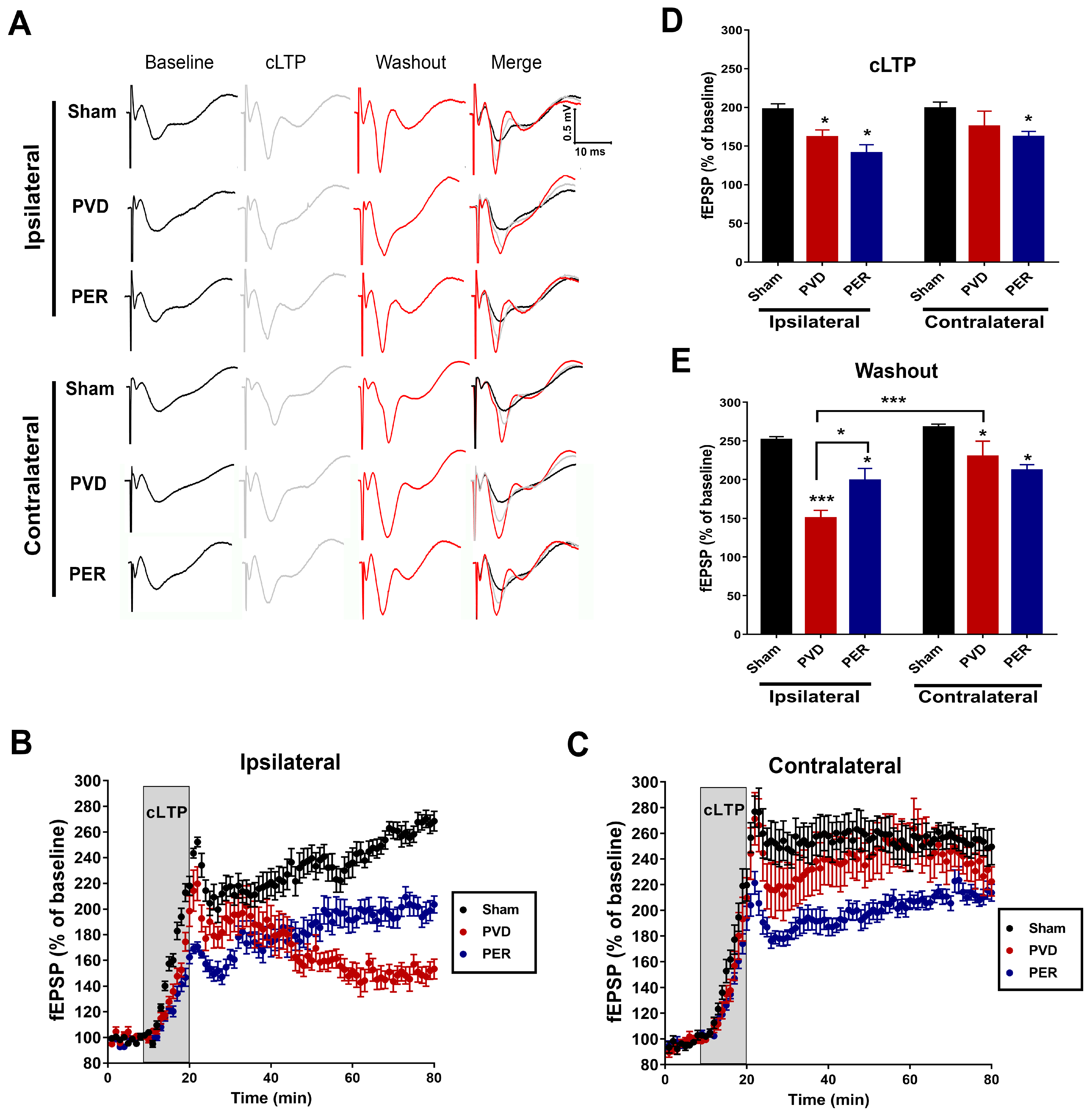
3.2. Perampanel Attenuated LTP Deficits in the Ipsilateral Side of Ischemic Lesion
3.3. Perampanel Inhibited the Depressive-like Behavior of Rats in Forced Swim Test Post Cerebral Ischemia
3.4. Perampanel Prevented Post-Stroke Motor Deficits Caused by PVD Lesion
3.5. Administration of Perampanel Prevented PVD-Induced Anxiety Like-Behavior
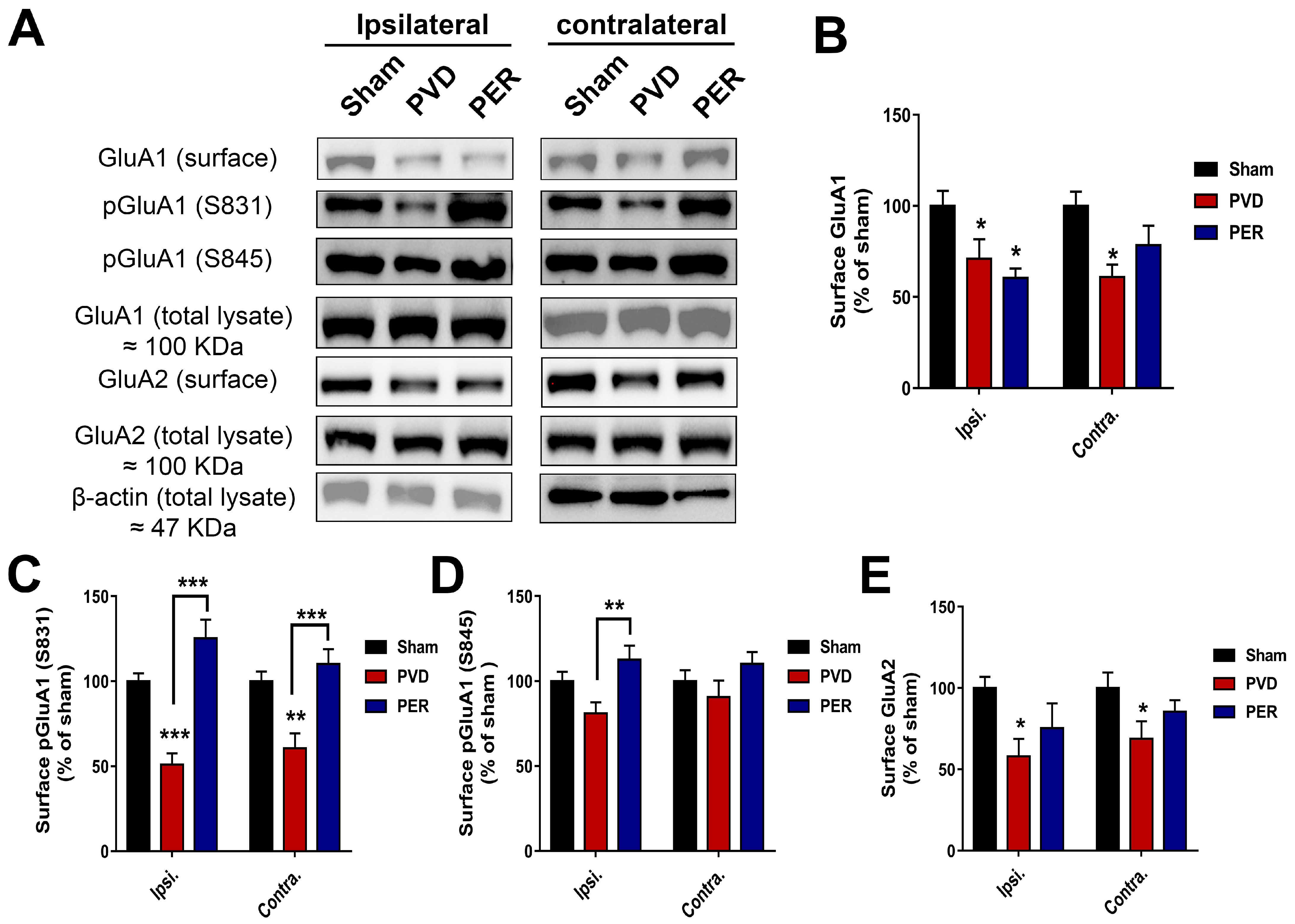
3.6. Perampanel Treatment Partially Prevented PVD-Induced Downregulation of Surface GluA2 While Potentiated Surface Expression of Phosphorylated p-S831 and p-S845 GluA1 in Hippocampus
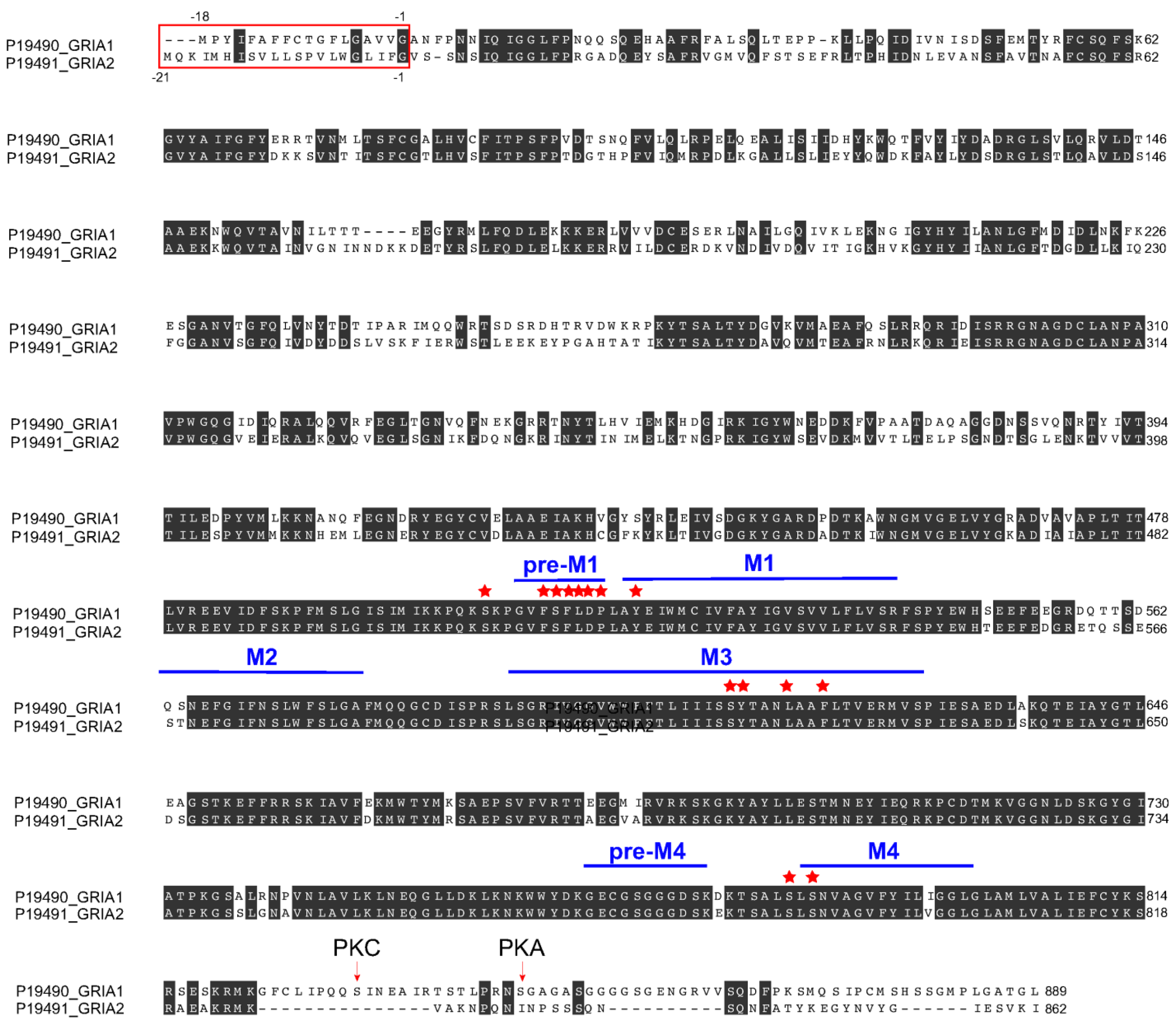
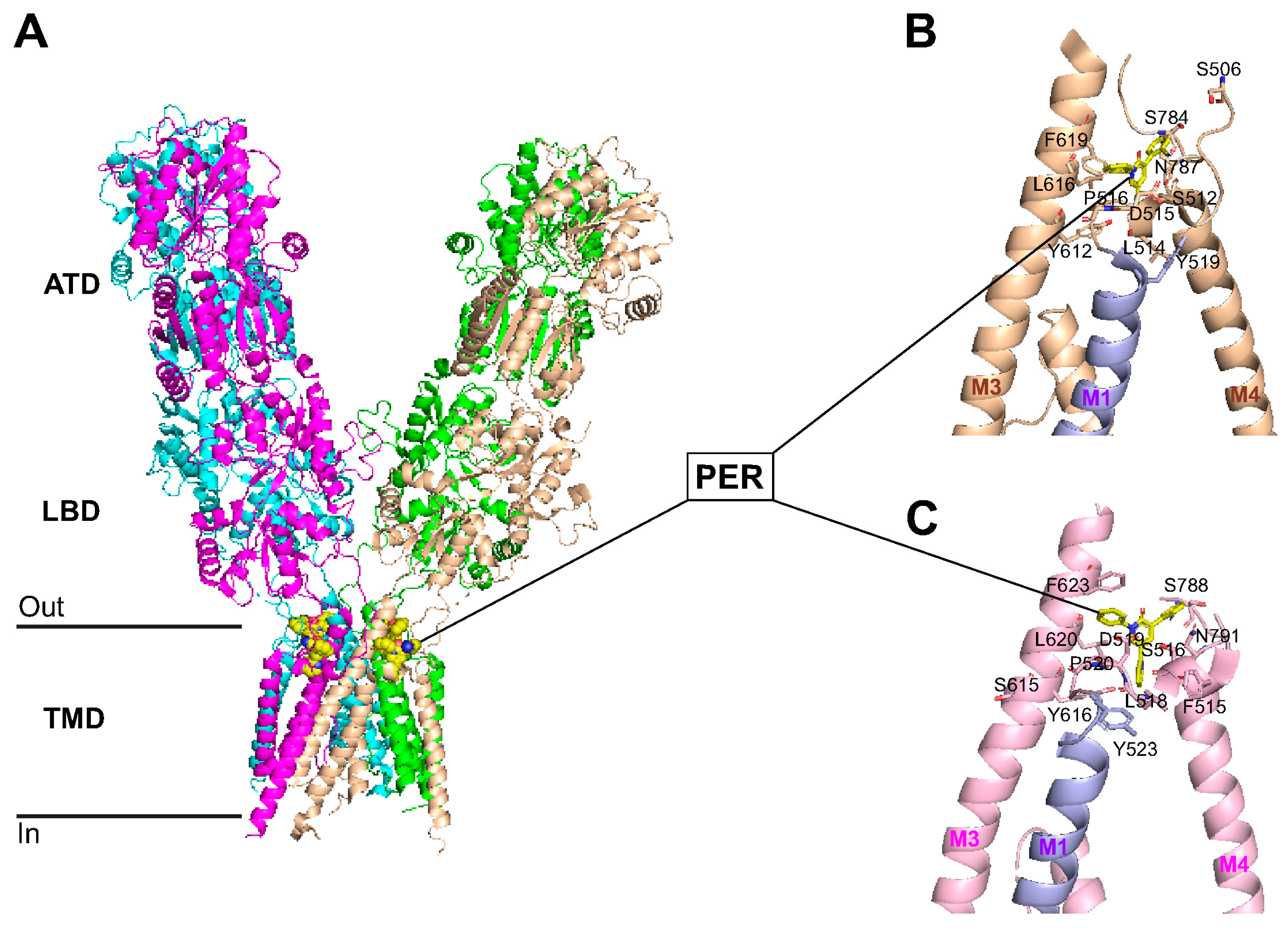
3.7. Perampanel Binding Domains to GluA1 and GluA2 Subunits
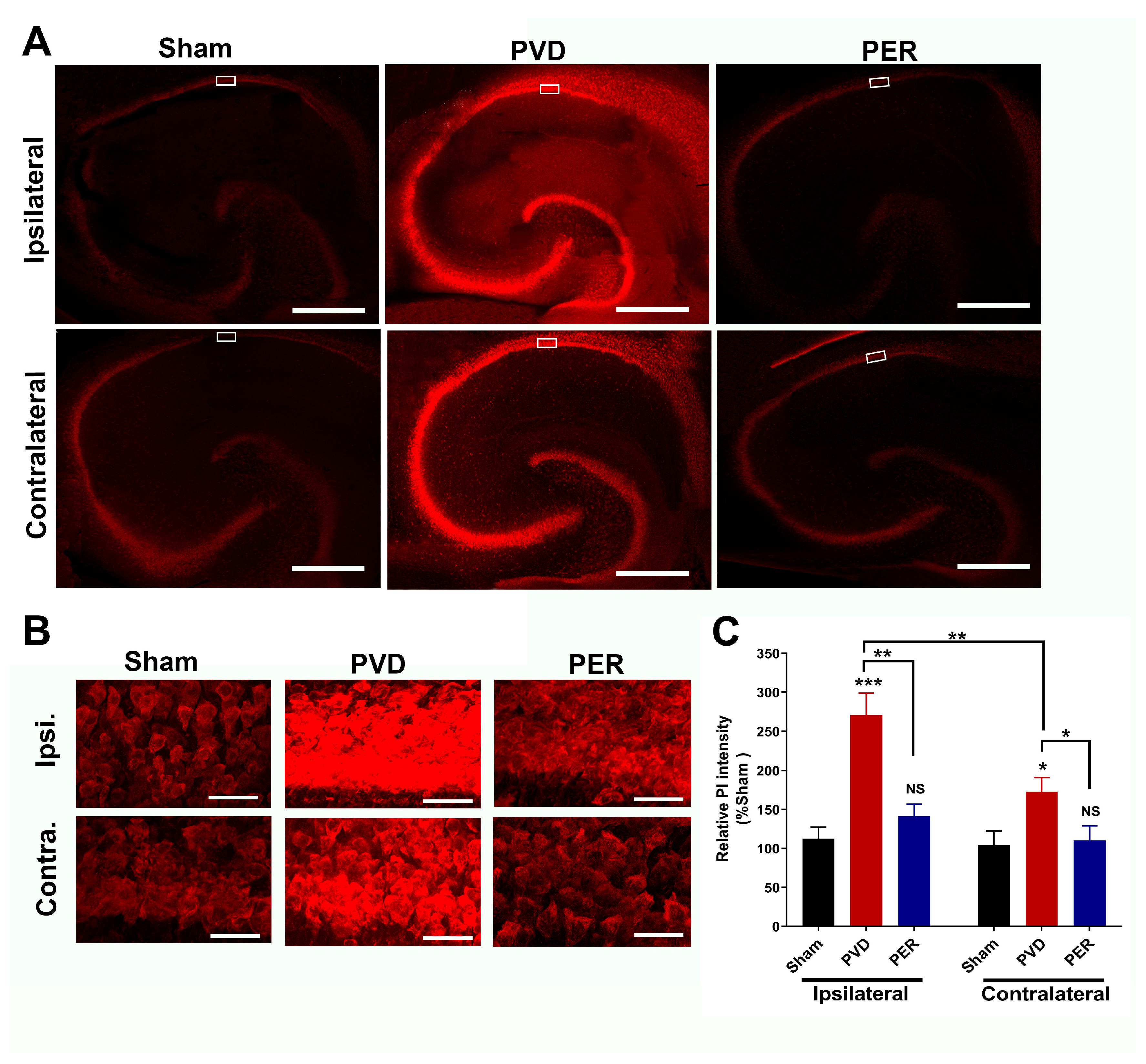
3.8. Administration of Perampanel Inhibited PVD-Induced Hippocampal Cell Death in Both Ipsilateral and Contralateral Sides
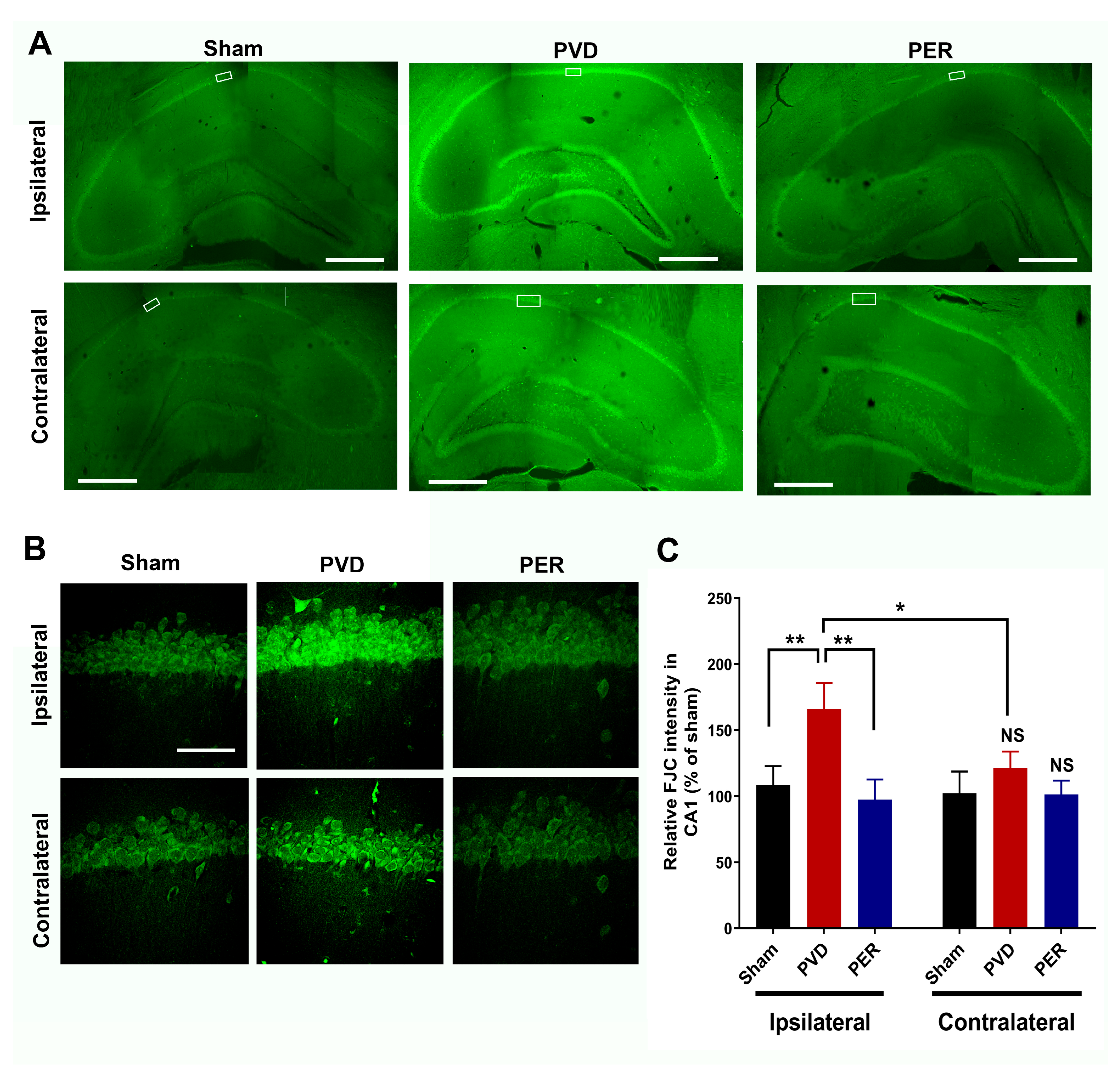
3.9. Perampanel Attenuated PVD-Induced Neurodegeneration in Hippocampus
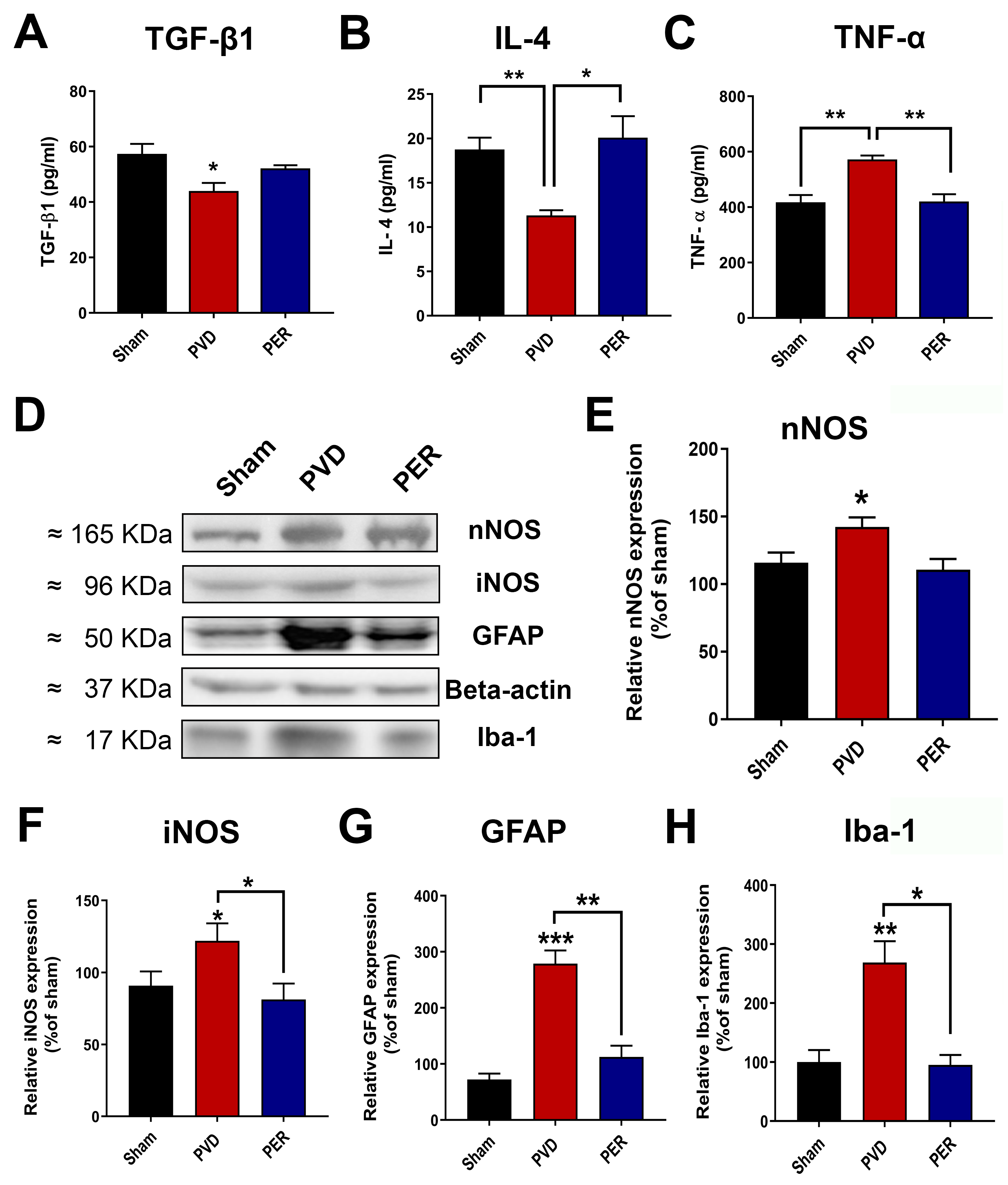
3.10. Perampanel Inhibited Neuroinflammation Mediated by Activated Microglia and Astrocytes
4. Discussion
4.1. Perampanel Binds to GluA1 and GluA2 to Promote Neuroprotection in Pial Vessel Disruption Stroke Model
4.2. Perampanel Attenuates Behavioral and LTP Deficits in Pial Vessel Disruption Stroke Model by Increasing pSer845/pSer831 GluA1
4.3. Perampanel Decreased Microglia/Astrocyte Activation and Neuroinflammation in Pial Vessel Disruption Stroke Model
4.4. Advantages and Limitations of PVD Compared to Photothrombosis and MCAO Models
4.5. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PVD | Pial Vessel Disruption |
| PER | Perampanel |
| cLTP | Chemically induced long term potentiation |
| fEPSP | Field excitatory postsynaptic potential |
| AMPAR | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| A1R | Adenosine-1 receptor |
| A2AR | Adenosine A2-receptor |
References
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef]
- Castillo, J.; Davalos, A.; Noya, M. Progression of ischaemic stroke and excitotoxic aminoacids. Lancet 1997, 349, 79–83. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; de Ferranti, S.D.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Pu, L.; Wang, L.; Zhang, R.; Zhao, T.; Jiang, Y.; Han, L. Projected Global Trends in Ischemic Stroke Incidence, Deaths and Disability-Adjusted Life Years From 2020 to 2030. Stroke 2023, 54, 1330–1339. [Google Scholar] [CrossRef]
- Jeong, H.G.; Kim, B.J.; Choi, J.C.; Hong, K.S.; Yang, M.H.; Jung, C.; Han, M.K.; Bae, H.J. Posttreatment National Institutes of Health Stroke Scale Is Superior to the Initial Score or Thrombolysis in Cerebral Ischemia for 3-Month Outcome. Stroke 2018, 49, 938–944. [Google Scholar] [CrossRef]
- Vidale, S.; Agostoni, E. Thrombolysis in acute ischaemic stroke. Brain J. Neurol. 2014, 137 Pt 6, e281. [Google Scholar] [CrossRef][Green Version]
- Wardlaw, J.M.; Murray, V.; Berge, E.; del Zoppo, G.J. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst. Rev. 2014, 2014, Cd000213. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidou, C.; Turski, L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002, 1, 383–386. [Google Scholar] [CrossRef] [PubMed]
- De Keyser, J.; Sulter, G.; Luiten, P.G. Clinical trials with neuroprotective drugs in acute ischaemic stroke: Are we doing the right thing? Trends Neurosci. 1999, 22, 535–540. [Google Scholar] [CrossRef]
- Rajah, G.B.; Ding, Y. Experimental neuroprotection in ischemic stroke: A concise review. Neurosurg. Focus 2017, 42, E2. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Prakash, A.; Medhi, B. Drug therapy in stroke: From preclinical to clinical studies. Pharmacology 2013, 92, 324–334. [Google Scholar] [CrossRef]
- Nito, C.; Ueda, M.; Inaba, T.; Katsura, K.; Katayama, Y. FK506 ameliorates oxidative damage and protects rat brain following transient focal cerebral ischemia. Neurol. Res. 2011, 33, 881–889. [Google Scholar] [CrossRef]
- Akgul, G.; McBain, C.J. Diverse roles for ionotropic glutamate receptors on inhibitory interneurons in developing and adult brain. J. Physiol. 2016, 594, 5471–5490. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W.; Price, M.T.; Samson, L.; Labruyere, J. The role of specific ions in glutamate neurotoxicity. Neurosci. Lett. 1986, 65, 65–71. [Google Scholar] [CrossRef]
- Graham, S.H.; Shiraishi, K.; Panter, S.S.; Simon, R.P.; Faden, A.I. Changes in extracellular amino acid neurotransmitters produced by focal cerebral ischemia. Neurosci. Lett. 1990, 110, 124–130. [Google Scholar] [CrossRef]
- Nishizawa, Y. Glutamate release and neuronal damage in ischemia. Life Sci. 2001, 69, 369–381. [Google Scholar] [CrossRef]
- Hynd, M.R.; Scott, H.L.; Dodd, P.R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2004, 45, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Caudle, W.M.; Zhang, J. Glutamate, excitotoxicity, and programmed cell death in Parkinson disease. Exp. Neurol. 2009, 220, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Wahl, F.; Obrenovitch, T.P.; Hardy, A.M.; Plotkine, M.; Boulu, R.; Symon, L. Extracellular glutamate during focal cerebral ischaemia in rats: Time course and calcium dependency. J. Neurochem. 1994, 63, 1003–1011. [Google Scholar] [CrossRef]
- Lo, E.H.; Pierce, A.R.; Mandeville, J.B.; Rosen, B.R. Neuroprotection with NBQX in rat focal cerebral ischemia. Effects on ADC probability distribution functions and diffusion-perfusion relationships. Stroke 1997, 28, 439–446; discussion 446–447. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki-Yatsugi, S.; Ichiki, C.; Yatsugi, S.; Takahashi, M.; Shimizu-Sasamata, M.; Yamaguchi, T.; Minematsu, K. Neuroprotective effects of an AMPA receptor antagonist YM872 in a rat transient middle cerebral artery occlusion model. Neuropharmacology 2000, 39, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Erdo, F.; Berzsenyi, P.; Nemet, L.; Andrasi, F. Talampanel improves the functional deficit after transient focal cerebral ischemia in rats. A 30-day follow up study. Brain Res. Bull. 2006, 68, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Akins, P.T.; Atkinson, R.P. Glutamate AMPA receptor antagonist treatment for ischaemic stroke. Curr. Med. Res. Opin. 2002, 18 (Suppl. 2), s9–s13. [Google Scholar] [CrossRef]
- Ginsberg, M.D. Current status of neuroprotection for cerebral ischemia: Synoptic overview. Stroke 2009, 40 (Suppl. 3), S111–S114. [Google Scholar] [CrossRef]
- Dale, N.; Pearson, T.; Frenguelli, B.G. Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice. J. Physiol. 2000, 526 Pt 1, 143–155. [Google Scholar] [CrossRef]
- Zhang, D.; Xiong, W.; Albensi, B.C.; Parkinson, F.E. Expression of human equilibrative nucleoside transporter 1 in mouse neurons regulates adenosine levels in physiological and hypoxic-ischemic conditions. J. Neurochem. 2011, 118, 4–11. [Google Scholar] [CrossRef]
- Cunha, R.A. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005, 1, 111–134. [Google Scholar] [CrossRef]
- Chen, Z.; Xiong, C.; Pancyr, C.; Stockwell, J.; Walz, W.; Cayabyab, F.S. Prolonged adenosine A1 receptor activation in hypoxia and pial vessel disruption focal cortical ischemia facilitates clathrin-mediated AMPA receptor endocytosis and long-lasting synaptic inhibition in rat hippocampal CA3-CA1 synapses: Differential regulation of GluA2 and GluA1 subunits by p38 MAPK and JNK. J. Neurosci. 2014, 34, 9621–9643. [Google Scholar] [CrossRef]
- Stockwell, J.; Jakova, E.; Cayabyab, F.S. Adenosine A1 and A2A Receptors in the Brain: Current Research and Their Role in Neurodegeneration. Molecules 2017, 22, 676. [Google Scholar] [CrossRef]
- Qin, X.; Zaki, M.G.; Chen, Z.; Jakova, E.; Ming, Z.; Cayabyab, F.S. Adenosine Signaling and Clathrin-Mediated Endocytosis of Glutamate AMPA Receptors in Delayed Hypoxic Injury in Rat Hippocampus: Role of Casein Kinase 2. Mol. Neurobiol. 2021, 58, 1932–1951. [Google Scholar] [CrossRef]
- Jakova, E.; Moutaoufik, M.T.; Lee, J.S.; Babu, M.; Cayabyab, F.S. Adenosine A1 receptor ligands bind to alpha-synuclein: Implications for alpha-synuclein misfolding and alpha-synucleinopathy in Parkinson’s disease. Transl. Neurodegener. 2022, 11, 9. [Google Scholar] [CrossRef]
- Zaki, M.G.; Jakova, E.; Pordeli, M.; Setork, E.; Taghibiglou, C.; Cayabyab, F.S. The Anti-Parkinsonian A2A Receptor Antagonist Istradefylline (KW-6002) Attenuates Behavioral Abnormalities, Neuroinflammation, and Neurodegeneration in Cerebral Ischemia: An Adenosinergic Signaling Link Between Stroke and Parkinson’s Disease. Int. J. Mol. Sci. 2025, 26, 5680. [Google Scholar] [CrossRef]
- Newcombe, J.; Uddin, A.; Dove, R.; Patel, B.; Turski, L.; Nishizawa, Y.; Smith, T. Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol. 2008, 18, 52–61. [Google Scholar] [CrossRef]
- Cheng, X.-L.; Ding, F.; Li, H.; Tan, X.-Q.; Liu, X.; Cao, J.-M.; Gao, X. Activation of AMPA receptor promotes TNF-α release via the ROS-cSrc-NFκB signaling cascade in RAW264.7 macrophages. Biochem. Biophys. Res. Commun. 2015, 461, 275–280. [Google Scholar] [CrossRef]
- Levite, M. Glutamate, T cells and multiple sclerosis. J. Neural Transm. 2017, 124, 775–798. [Google Scholar] [CrossRef] [PubMed]
- Hanada, T. Ionotropic Glutamate Receptors in Epilepsy: A Review Focusing on AMPA and NMDA Receptors. Biomolecules 2020, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Rohracher, A.; Höfler, J.; Kalss, G.; Leitinger, M.; Kuchukhidze, G.; Deak, I.; Dobesberger, J.; Novak, H.; Pilz, G.; Zerbs, A. Perampanel in patients with refractory and super-refractory status epilepticus in a neurological intensive care unit. Epilepsy Behav. 2015, 49, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Perampanel: A review in drug-resistant epilepsy. Drugs 2015, 75, 1657–1668. [Google Scholar] [CrossRef]
- Patel, N.C. Perampanel (Fycompa): AMPA Receptor Antagonist for the Treatment of Seizure. In Innovative Drug Synthesis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 271–282. [Google Scholar]
- Rogawski, M.A.; Hanada, T. Preclinical pharmacology of perampanel, a selective non-competitive AMPA receptor antagonist. Acta Neurol. Scand. 2013, 127, 19–24. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Brust, T.B.; Cayabyab, F.S.; MacVicar, B.A. C-Jun N-terminal kinase regulates adenosine A1 receptor-mediated synaptic depression in the rat hippocampus. Neuropharmacology 2007, 53, 906–917. [Google Scholar] [CrossRef]
- Kim, J.E.; Choi, H.C.; Song, H.K.; Kang, T.C. Perampanel Affects Up-Stream Regulatory Signaling Pathways of GluA1 Phosphorylation in Normal and Epileptic Rats. Front. Cell. Neurosci. 2019, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.X.; Wang, J.Z.; Wang, D.L.; Miao, J.J.; Li, H.; Liu, Z.G.; Yuan, X.; Liu, W.; Zhou, J.R. The Orally Active Noncompetitive AMPAR Antagonist Perampanel Attenuates Focal Cerebral Ischemia Injury in Rats. Cell. Mol. Neurobiol. 2018, 38, 459–466. [Google Scholar] [CrossRef]
- Nakajima, M.; Suda, S.; Sowa, K.; Sakamoto, Y.; Nito, C.; Nishiyama, Y.; Aoki, J.; Ueda, M.; Yokobori, S.; Yamada, M.; et al. AMPA Receptor Antagonist Perampanel Ameliorates Post-Stroke Functional and Cognitive Impairments. Neuroscience 2018, 386, 256–264. [Google Scholar] [CrossRef]
- Mohammad, H.; Sekar, S.; Wei, Z.; Moien-Afshari, F.; Taghibiglou, C. Perampanel but Not Amantadine Prevents Behavioral Alterations and Epileptogenesis in Pilocarpine Rat Model of Status Epilepticus. Mol. Neurobiol. 2019, 56, 2508–2523. [Google Scholar] [CrossRef]
- Chen, T.; Dai, S.H.; Jiang, Z.Q.; Luo, P.; Jiang, X.F.; Fei, Z.; Gui, S.B.; Qi, Y.L. The AMPAR Antagonist Perampanel Attenuates Traumatic Brain Injury Through Anti-Oxidative and Anti-Inflammatory Activity. Cell. Mol. Neurobiol. 2017, 37, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A.M.; Traini, C.; Cipriani, S.; Gianfriddo, M.; Mello, T.; Giovannini, M.G.; Galli, A.; Pedata, F. The adenosine A2A receptor antagonist ZM241385 enhances neuronal survival after oxygen-glucose deprivation in rat CA1 hippocampal slices. Br. J. Pharmacol. 2009, 157, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Stockwell, J.; Cayabyab, F.S. Adenosine A1 Receptor-Mediated Endocytosis of AMPA Receptors Contributes to Impairments in Long-Term Potentiation (LTP) in the Middle-Aged Rat Hippocampus. Neurochem. Res. 2016, 41, 1085–1097. [Google Scholar] [CrossRef]
- Cayabyab, F.S.; Gowribai, K.; Walz, W. Involvement of matrix metalloproteinases-2 and -9 in the formation of a lacuna-like cerebral cavity. J. Neurosci. Res. 2013, 91, 920–933. [Google Scholar] [CrossRef]
- Wang, K.; Walz, W. Unusual topographical pattern of proximal astrogliosis around a cortical devascularizing lesion. J. Neurosci. Res. 2003, 73, 497–506. [Google Scholar] [CrossRef]
- Wang, K.; Bekar, L.K.; Furber, K.; Walz, W. Vimentin-expressing proximal reactive astrocytes correlate with migration rather than proliferation following focal brain injury. Brain Res. 2004, 1024, 193–202. [Google Scholar] [CrossRef]
- Hua, R.; Walz, W. Minocycline treatment prevents cavitation in rats after a cortical devascularizing lesion. Brain Res. 2006, 1090, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini-Giampietro, D.E.; Zukin, R.S.; Bennett, M.V.; Cho, S.; Pulsinelli, W.A. Switch in glutamate receptor subunit gene expression in CA1 subfield of hippocampus following global ischemia in rats. Proc. Natl. Acad. Sci. USA 1992, 89, 10499–10503. [Google Scholar] [CrossRef]
- Prosser-Loose, E.J.; Verge, V.M.; Cayabyab, F.S.; Paterson, P.G. Protein-energy malnutrition alters hippocampal plasticity-associated protein expression following global ischemia in the gerbil. Curr. Neurovasc. Res. 2010, 7, 341–360. [Google Scholar] [CrossRef]
- Simon, P.; Dupuis, R.; Costentin, J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 1994, 61, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 97, 52587. [Google Scholar] [CrossRef]
- Lv, Y.-C.; Gao, A.-B.; Yang, J.; Zhong, L.-Y.; Jia, B.; Ouyang, S.-H.; Gui, L.; Peng, T.-H.; Sun, S.; Cayabyab, F.S. Long-term adenosine A1 receptor activation-induced sortilin expression promotes α-synuclein upregulation in dopaminergic neurons. Neural Regen. Res. 2020, 15, 712–723. [Google Scholar] [CrossRef]
- Deacon, R.M. Measuring motor coordination in mice. J. Vis. Exp. 2013, 75, e2609. [Google Scholar] [CrossRef]
- Yang, J.; Anishchenko, I.; Park, H.; Peng, Z.; Ovchinnikov, S.; Baker, D. Improved protein structure prediction using predicted interresidue orientations. Proc. Natl. Acad. Sci. USA 2020, 117, 1496–1503. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef]
- Moutaoufik, M.T.; Morrow, G.; Maaroufi, H.; Férard, C.; Finet, S.; Tanguay, R.M. Oligomerization and chaperone-like activity of Drosophila melanogaster small heat shock protein DmHsp27 and three arginine mutants in the alpha-crystallin domain. Cell Stress Chaperones 2017, 22, 455–466. [Google Scholar] [CrossRef]
- Chater, T.E.; Goda, Y. The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front. Cell. Neurosci. 2014, 8, 401. [Google Scholar] [CrossRef]
- Malinow, R. AMPA receptor trafficking and long-term potentiation. Philos. Trans. R. Soc. B Biol. Sci. 2003, 358, 707–714. [Google Scholar] [CrossRef]
- Towfighi, A.; Ovbiagele, B.; Husseini, N.E.; Hackett, M.L.; Jorge, R.E.; Kissela, B.M.; Mitchell, P.H.; Skolarus, L.E.; Whooley, M.A.; Williams, L.S. Poststroke Depression: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2017, 48, e30–e43. [Google Scholar] [CrossRef]
- Paolucci, S. Epidemiology and treatment of post-stroke depression. Neuropsychiatr. Dis. Treat. 2008, 4, 145–154. [Google Scholar] [CrossRef]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.-Y.Y.; Whiteley, W.N.; Dennis, M.S.; Mead, G.E.; Carson, A.J. Anxiety After Stroke: The Importance of Subtyping. Stroke 2018, 49, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, M.; O’Shea-Greenfield, A.; Rogers, S.W.; Heinemann, S. Cloning by functional expression of a member of the glutamate receptor family. Nature 1989, 342, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Roche, K.W.; O’Brien, R.J.; Mammen, A.L.; Bernhardt, J.; Huganir, R.L. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 1996, 16, 1179–1188. [Google Scholar] [CrossRef]
- Yelshanskaya, M.V.; Singh, A.K.; Sampson, J.M.; Narangoda, C.; Kurnikova, M.; Sobolevsky, A.I. Structural Bases of Noncompetitive Inhibition of AMPA-Subtype Ionotropic Glutamate Receptors by Antiepileptic Drugs. Neuron 2016, 91, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.D.; Al-Khoury, L.; Zivin, J.A. Neuroprotection for Ischemic Stroke: Two Decades of Success and Failure. NeuroRx 2004, 1, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Labiche, L.A.; Grotta, J.C. Clinical trials for cytoprotection in stroke. NeuroRx 2004, 1, 46–70. [Google Scholar] [CrossRef]
- Sun, J.-H.; Tan, L.; Yu, J.-T. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann. Transl. Med. 2014, 2, 80. [Google Scholar] [CrossRef]
- Li, X.-J.; Liang, L.; Shi, H.-X.; Sun, X.-P.; Wang, J.; Zhang, L.-S. Neuroprotective effects of curdione against focal cerebral ischemia reperfusion injury in rats. Neuropsychiatr. Dis. Treat. 2017, 13, 1733–1740. [Google Scholar] [CrossRef]
- Sakai, N.; Yanai, K.; Ryu, J.H.; Nagasawa, H.; Hasegawa, T.; Sasaki, T.; Kogure, K.; Watanabe, T. Behavioral studies on rats with transient cerebral ischemia induced by occlusion of the middle cerebral artery. Behav. Brain Res. 1996, 77, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Bouet, V.; Freret, T.; Toutain, J.; Divoux, D.; Boulouard, M.; Schumann-Bard, P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp. Neurol. 2007, 203, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Gyertyan, I.; Gigler, G.; Simo, A. The neuroprotective and hypothermic effect of GYKI-52466, a non-competitive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-antagonist on histological and behavioural variables in the gerbil global ischemia model. Brain Res. Bull. 1999, 50, 179–186. [Google Scholar] [CrossRef]
- Block, F.; Schmitt, W.; Schwarz, M. Pretreatment but not posttreatment with GYKI 52466 reduces functional deficits and neuronal damage after global ischemia in rats. J. Neurol. Sci. 1996, 139, 167–172. [Google Scholar] [CrossRef]
- Hunter, A.J.; Hatcher, J.; Virley, D.; Nelson, P.; Irving, E.; Hadingham, S.J.; Parsons, A.A. Functional assessments in mice and rats after focal stroke. Neuropharmacology 2000, 39, 806–816. [Google Scholar] [CrossRef]
- Wang, Z.; Tsai, L.-K.; Munasinghe, J.; Leng, Y.; Fessler, E.B.; Chibane, F.; Leeds, P.; Chuang, D.-M. Chronic Valproate Treatment Enhances Postischemic Angiogenesis and Promotes Functional Recovery in a Rat Model of Ischemic Stroke. Stroke 2012, 43, 2430–2436. [Google Scholar] [CrossRef]
- Hanada, T.; Hashizume, Y.; Tokuhara, N.; Takenaka, O.; Kohmura, N.; Ogasawara, A.; Hatakeyama, S.; Ohgoh, M.; Ueno, M.; Nishizawa, Y. Perampanel: A novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia 2011, 52, 1331–1340. [Google Scholar] [CrossRef]
- Vahid-Ansari, F.; Lagace, D.C.; Albert, P.R. Persistent post-stroke depression in mice following unilateral medial prefrontal cortical stroke. Transl. Psychiatry 2016, 6, e863. [Google Scholar] [CrossRef]
- Kiselycznyk, C.; Zhang, X.; Huganir, R.L.; Holmes, A.; Svenningsson, P. Reduced phosphorylation of GluA1 subunits relates to anxiety-like behaviours in mice. Int. J. Neuropsychopharmacol. 2013, 16, 919–924. [Google Scholar] [CrossRef]
- Kapus, G.L.; Gacsalyi, I.; Vegh, M.; Kompagne, H.; Hegedus, E.; Leveleki, C.; Harsing, L.G.; Barkoczy, J.; Bilkei-Gorzo, A.; Levay, G. Antagonism of AMPA receptors produces anxiolytic-like behavior in rodents: Effects of GYKI 52466 and its novel analogues. Psychopharmacology 2008, 198, 231–241. [Google Scholar] [CrossRef]
- Wolak, M.; Siwek, A.; Szewczyk, B.; Poleszak, E.; Pilc, A.; Popik, P.; Nowak, G. Involvement of NMDA and AMPA receptors in the antidepressant-like activity of antidepressant drugs in the forced swim test. Pharmacol. Rep. 2013, 65, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Nakanishi, H.; Tamura, A.; Urae, A.; Mine, K.; Yamamoto, K.; Fujiwara, M. Long-term spatial cognitive impairment after middle cerebral artery occlusion in rats: No involvement of the hippocampus. J. Cereb. Blood Flow Metab. 1995, 15, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, R.; Shetty, R.A.; Thangthaeng, N.; Liu, R.; Chen, Z.; Sumien, N.; Rutledge, M.; Dillon, G.H.; Yuan, F.; et al. Transient focal cerebral ischemia induces long-term cognitive function deficit in an experimental ischemic stroke model. Neurobiol. Dis. 2013, 59, 18–25. [Google Scholar] [CrossRef]
- Noh, K.-M.; Yokota, H.; Mashiko, T.; Castillo, P.E.; Zukin, R.S.; Bennett, M.V. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc. Natl. Acad. Sci. USA 2005, 102, 12230–12235. [Google Scholar] [CrossRef] [PubMed]
- Park, J. Phosphorylation of the AMPAR-TARP Complex in Synaptic Plasticity. Proteomes 2018, 6, 40. [Google Scholar] [CrossRef]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef]
- Wood, P.L. Microglia as a unique cellular target in the treatment of stroke: Potential neurotoxic mediators produced by activated microglia. Neurol. Res. 1995, 17, 242–248. [Google Scholar] [CrossRef]
- Kaushal, V.; Schlichter, L.C. Mechanisms of Microglia-Mediated Neurotoxicity in a New Model of the Stroke Penumbra. J. Neurosci. 2008, 28, 2221–2230. [Google Scholar] [CrossRef]
- Taylor, R.A.; Sansing, L.H. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin. Dev. Immunol. 2013, 2013, 746068. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, L.; Xapelli, S.; Silva, A.P.; Jakobsen, B.; Poulsen, F.R.; Oliveira, C.R.; Vezzani, A.; Malva, J.O.; Zimmer, J. Modulator Effects of Interleukin-1β and Tumor Necrosis Factor-α on AMPA-Induced Excitotoxicity in Mouse Organotypic Hippocampal Slice Cultures. J. Neurosci. 2005, 25, 6734–6744. [Google Scholar] [CrossRef]
- Barone, F.C.; Arvin, B.; White, R.F.; Miller, A.; Webb, C.L.; Willette, R.N.; Lysko, P.G.; Feuerstein, G.Z. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke 1997, 28, 1233–1244. [Google Scholar] [CrossRef]
- Maddahi, A.; Kruse, L.S.; Chen, Q.W.; Edvinsson, L. The role of tumor necrosis factor-alpha and TNF-alpha receptors in cerebral arteries following cerebral ischemia in rat. J. Neuroinflamm. 2011, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Aida, V.; Niedzielko, T.L.; Szaflarski, J.P.; Floyd, C.L. Acute administration of perampanel, an AMPA receptor antagonist, reduces cognitive impairments after traumatic brain injury in rats. Exp. Neurol. 2020, 327, 113222. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, A.; Akyol, A.; Yenisey, C.; Arpaci, E.; Kiylioglu, N.; Tataroglu, C. Oxidative stress in acute ischemic stroke. J. Clin. Neurosci. 2007, 14, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Zaremba, J.; Skrobanski, P.; Losy, J. Tumour necrosis factor-alpha is increased in the cerebrospinal fluid and serum of ischaemic stroke patients and correlates with the volume of evolving brain infarct. Biomed. Pharmacother. 2001, 55, 258–263. [Google Scholar] [CrossRef]
- Slevin, M.; Krupinski, J.; Slowik, A.; Kumar, P.; Szczudlik, A.; Gaffney, J. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke 2000, 31, 1863–1870. [Google Scholar] [CrossRef]
- Shin, H.J.; Lee, K.Y.; Kang, J.W.; Choi, S.G.; Kim, D.W.; Yi, Y.Y. Perampanel Reduces Brain Damage via Induction of M2 Microglia in a Neonatal Rat Stroke Model. Int. J. Nanomed. 2022, 17, 2791–2804. [Google Scholar] [CrossRef] [PubMed]
- Labat-gest, V.; Tomasi, S. Photothrombotic ischemia: A minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J. Vis. Exp. 2013, 76, 50370. [Google Scholar] [CrossRef]
- Rosenblum, W.I.; El-Sabban, F. Platelet aggregation in the cerebral microcirculation: Effect of aspirin and other agents. Circ. Res. 1977, 40, 320–328. [Google Scholar] [CrossRef]
- Watson, B.D.; Dietrich, W.D.; Busto, R.; Wachtel, M.S.; Ginsberg, M.D. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann. Neurol. 1985, 17, 497–504. [Google Scholar] [CrossRef]
- Schmid-Elsaesser, R.; Zausinger, S.; Hungerhuber, E.; Baethmann, A.; Reulen, H.J. A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: Evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke 1998, 29, 2162–2170. [Google Scholar] [CrossRef]
- Dittmar, M.; Spruss, T.; Schuierer, G.; Horn, M. External carotid artery territory ischemia impairs outcome in the endovascular filament model of middle cerebral artery occlusion in rats. Stroke 2003, 34, 2252–2257. [Google Scholar] [CrossRef]
- Dittmar, M.S.; Fehm, N.P.; Vatankhah, B.; Bogdahn, U.; Schlachetzki, F. Adverse effects of the intraluminal filament model of middle cerebral artery occlusion. Stroke 2005, 36, 530–532; author reply 530–532. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Omae, T.; Fisher, M. Spontaneous hyperthermia and its mechanism in the intraluminal suture middle cerebral artery occlusion model of rats. Stroke 1999, 30, 2464–2470; discussion 2470–2471. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhen, G.; Meloni, B.P.; Campbell, K.; Winn, H.R. RODENT STROKE MODEL GUIDELINES FOR PRECLINICAL STROKE TRIALS (1ST EDITION). J. Exp. Stroke Transl. Med. 2009, 2, 2–27. [Google Scholar] [CrossRef]
- Reglodi, D.; Somogyvari-Vigh, A.; Maderdrut, J.L.; Vigh, S.; Arimura, A. Postischemic spontaneous hyperthermia and its effects in middle cerebral artery occlusion in the rat. Exp. Neurol. 2000, 163, 399–407. [Google Scholar] [CrossRef]
- Gerriets, T.; Stolz, E.; Walberer, M.; Kaps, M.; Bachmann, G.; Fisher, M. Neuroprotective effects of MK-801 in different rat stroke models for permanent middle cerebral artery occlusion: Adverse effects of hypothalamic damage and strategies for its avoidance. Stroke 2003, 34, 2234–2239. [Google Scholar] [CrossRef]
- Steele, E.C., Jr.; Guo, Q.; Namura, S. Filamentous middle cerebral artery occlusion causes ischemic damage to the retina in mice. Stroke 2008, 39, 2099–2104. [Google Scholar] [CrossRef]
- Fluri, F.; Schuhmann, M.K.; Kleinschnitz, C. Animal models of ischemic stroke and their application in clinical research. Drug Des. Dev. Ther. 2015, 9, 3445–3454. [Google Scholar] [CrossRef] [PubMed]
- Walz, W.; Cayabyab, F.S. Neutrophil Infiltration and Matrix Metalloproteinase-9 in Lacunar Infarction. Neurochem. Res. 2017, 42, 2560–2565. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaki, M.G.; Moutaoufik, M.T.; Pordeli, M.; Babu, M.; Taghibiglou, C.; Cayabyab, F.S. Non-Competitive AMPA Receptor Antagonist Perampanel Inhibits Ischemia-Induced Neurodegeneration and Behavioral Deficits in Focal Cortical Pial Vessel Disruption Stroke Model. Cells 2025, 14, 1628. https://doi.org/10.3390/cells14201628
Zaki MG, Moutaoufik MT, Pordeli M, Babu M, Taghibiglou C, Cayabyab FS. Non-Competitive AMPA Receptor Antagonist Perampanel Inhibits Ischemia-Induced Neurodegeneration and Behavioral Deficits in Focal Cortical Pial Vessel Disruption Stroke Model. Cells. 2025; 14(20):1628. https://doi.org/10.3390/cells14201628
Chicago/Turabian StyleZaki, Michael G., Mohamed Taha Moutaoufik, Mahboubeh Pordeli, Mohan Babu, Changiz Taghibiglou, and Francisco S. Cayabyab. 2025. "Non-Competitive AMPA Receptor Antagonist Perampanel Inhibits Ischemia-Induced Neurodegeneration and Behavioral Deficits in Focal Cortical Pial Vessel Disruption Stroke Model" Cells 14, no. 20: 1628. https://doi.org/10.3390/cells14201628
APA StyleZaki, M. G., Moutaoufik, M. T., Pordeli, M., Babu, M., Taghibiglou, C., & Cayabyab, F. S. (2025). Non-Competitive AMPA Receptor Antagonist Perampanel Inhibits Ischemia-Induced Neurodegeneration and Behavioral Deficits in Focal Cortical Pial Vessel Disruption Stroke Model. Cells, 14(20), 1628. https://doi.org/10.3390/cells14201628








