A Novel Peptoid Hybrid of Alpha-Calcitonin Gene-Related Peptide (α-CGRP) Ameliorates Cardiac Remodeling in Pressure Overload-Induced Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Pressure Overload-Induced Heart Failure Model
2.2. Immunohistochemistry
2.3. Flow Cytometry
2.4. Elastin Morphology
2.5. Statistical Analysis
3. Results
3.1. NMEG-CGRP Improved Left Ventricular Systolic Function After the Onset of HF Symptoms
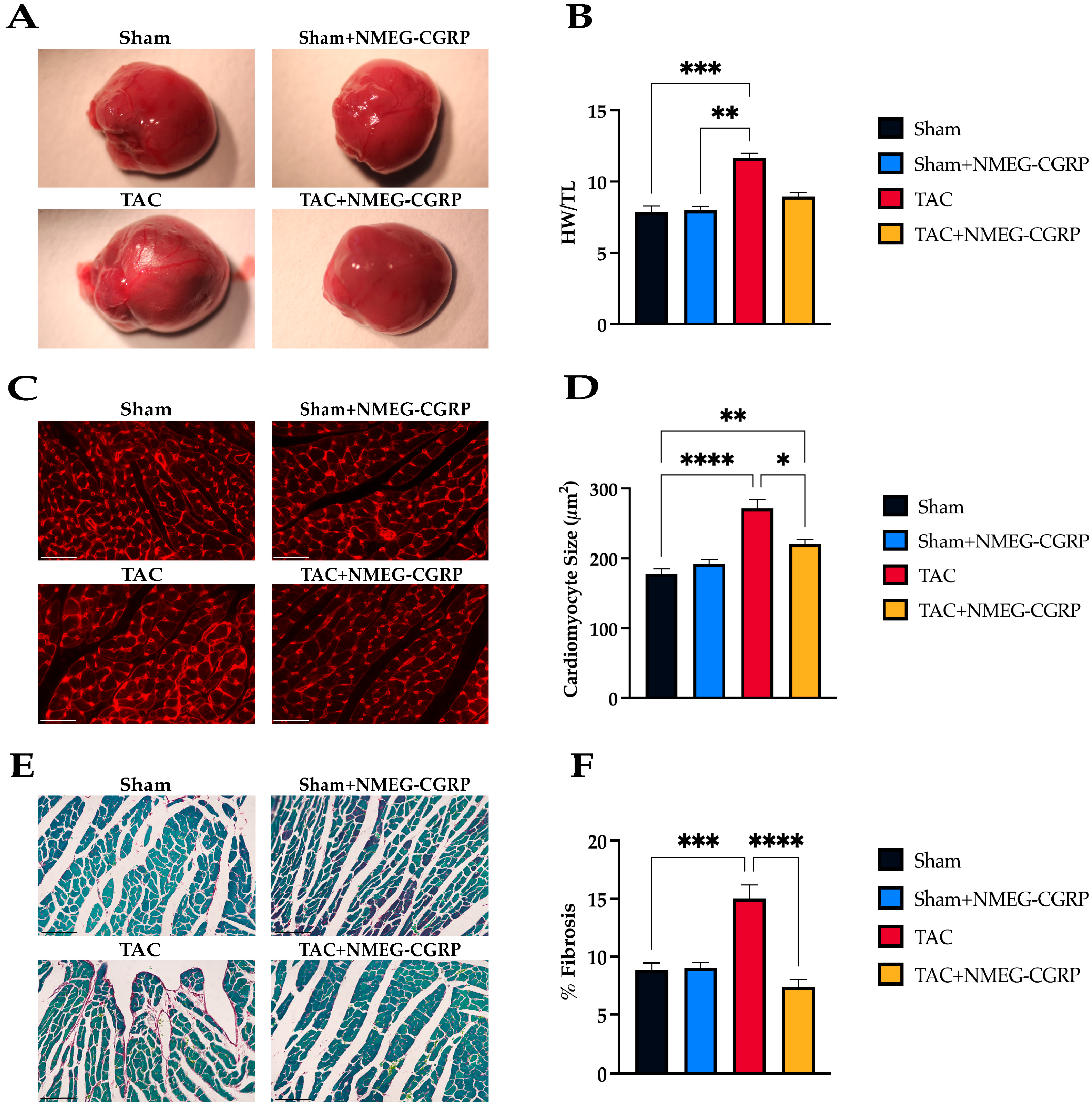
3.2. NMEG-CGRP Reduces Cardiac Remodeling After the Onset of HF Symptoms
3.3. NMEG-CGRP Ameliorates Increased Apoptosis and Oxidative Stress After the Onset of HF Symptoms
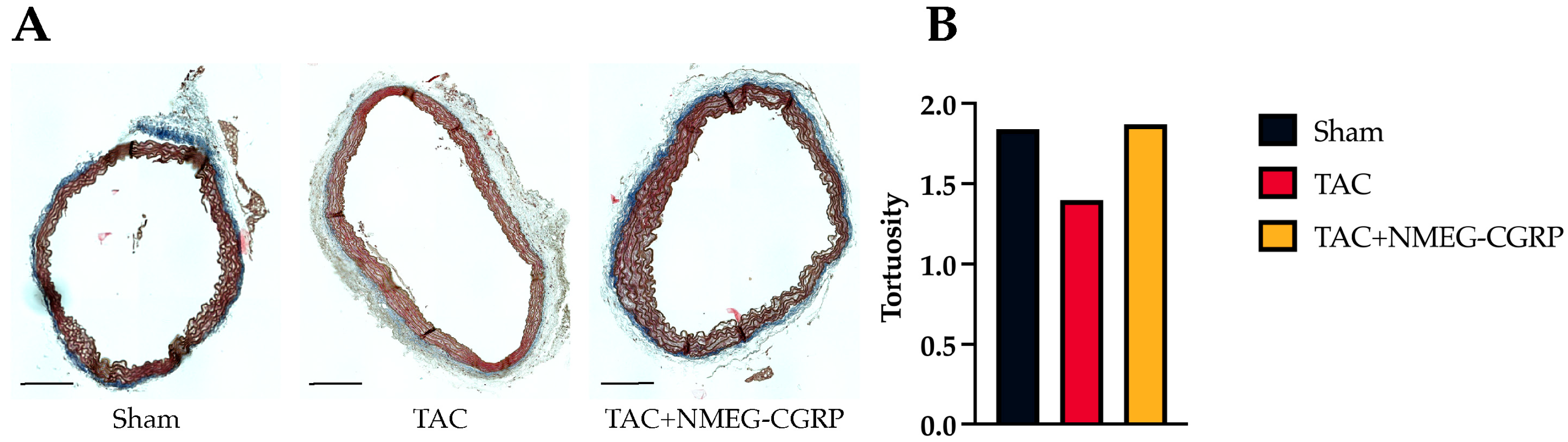
3.4. NMEG-CGRP Protects Aortic Elastin Fiber Tortuosity
3.5. NMEG-CGRP Acts as an Anti-Inflammatory Immunomodulator at the Circulatory and Tissue Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxy-2-nonenal |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| α-CGRP | alpha-calcitonin gene-related peptide |
| ANOVA | analysis of variance |
| BP | blood pressure |
| BSA | bovine serum albumin |
| cDCs | conventional dendritic cells |
| CGRP | calcitonin gene-related peptide |
| CMEG-CGRP | human C-terminal NMEG-α-CGRP |
| DABCO | 1,4-diazabicyclo-2,2,2-octane |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DCs | dendritic cells |
| EDTA | ethylenediaminetetraacetic acid |
| FBS | fetal bovine serum |
| HBSS | Hank’s balanced salt solution |
| HF | heart failure |
| HW/TL | heart weight to tibia length ratio |
| IACUC | Institution Animal Care and Use Committee |
| IgG | immunoglobulin G |
| KO | knockout |
| LV | left ventricle |
| NIH | National Institute of Health |
| NMEG | N-methoxyethyl glycine |
| NMEG-CGRP | human N-terminal NMEG-α-CGRP |
| PBS | phosphate-buffered saline |
| PFA | paraformaldehyde |
| RBC | red blood cell(s) |
| RCF | relative centrifugal force |
| RT | room temperature |
| SEM | standard error of the mean |
| TAC | transverse aortic constriction |
| TUNEL | terminal deoxynucleotidyl transferase dUTP nick-end labeling |
| WGA | wheat germ agglutinin |
References
- Brain, S.D.; Williams, T.J.; Tippins, J.R.; Morris, H.R.; MacIntyre, I. Calcitonin Gene-Related Peptide Is a Potent Vasodilator. Nature 1985, 313, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Supowit, S.C.; Zhao, H.; Wang, D.H.; DiPette, D.J. Regulation of neuronal calcitonin gene-related peptide expression. Hypertension 1995, 26, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubaiee, M.; Gangula, P.R.; Millis, R.M.; Walker, R.K.; Umoh, N.A.; Cousins, V.M.; Jeffress, M.A.; Haddad, G.E. Inotropic and lusitropic effects of calcitonin gene-related peptide in the heart. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1525–H1537. [Google Scholar] [CrossRef]
- Gangula, P.R.; Supowit, S.C.; Wimalawansa, S.J.; Zhao, H.; Hallman, D.M.; DiPette, D.J.; Yallampalli, C. Calcitonin gene-related peptide is a depressor in NG-nitro-L-arginine methyl ester-induced hypertension during pregnancy. Hypertension 1997, 29, 248–253. [Google Scholar] [CrossRef]
- Katki, K.A.; Supowit, S.C.; DiPette, D.J. Role of calcitonin gene-related peptide and substance P in Dahl-salt hypertension. Hypertension 2001, 38, 679–682. [Google Scholar] [CrossRef]
- Supowit, S.C.; Rao, A.; Bowers, M.C.; Zhao, H.; Fink, G.; Steficek, B.; Patel, P.; Katki, K.A.; DiPette, D.J. Calcitonin gene-related peptide protects against hypertension-induced heart and kidney damage. Hypertension 2005, 45, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Mehrotra, S.; Jan Danser, A.H.; Schoemaker, R.G. The role of calcitonin gene-related peptide (CGRP) in ischemic preconditioning in isolated rat hearts. Eur. J. Pharmacol. 2006, 531, 246–253. [Google Scholar] [CrossRef]
- Huang, R.; Karve, A.; Shah, I.; Bowers, M.C.; DiPette, D.J.; Supowit, S.C.; Abela, G.S. Deletion of the mouse α-calcitonin gene-related peptide gene increases the vulnerability of the heart to ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1291–H1297. [Google Scholar] [CrossRef]
- Li, J.; Levick, S.P.; DiPette, D.J.; Janicki, J.S.; Supowit, S.C. Alpha-calcitonin gene-related peptide is protective against pressure overload-induced heart failure. Regul. Pept. 2013, 185, 20–28. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.-J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Kumar, A.; Potts, J.D.; DiPette, D.J. Protective role of α-calcitonin gene-related peptide in cardiovascular diseases. Front. Physiol. 2019, 10, 821. [Google Scholar] [CrossRef]
- Aubdool, A.A.; Thakore, P.; Argunhan, F.; Smillie, S.J.; Schnelle, M.; Srivastava, S.; Alawi, K.M.; Wilde, E.; Mitchell, J.; Farrell-Dillon, K.; et al. A novel alpha-calcitonin gene-related peptide analogue protects against end-organ damage in experimental hypertension, cardiac hypertrophy, and heart failure. Circulation 2017, 136, 367–383. [Google Scholar] [CrossRef]
- Bentsen, S.; Sams, A.; Hasbak, P.; Edvinsson, L.; Kjaer, A.; Ripa, R.S. Myocardial perfusion recovery induced by an alpha-calcitonin gene-related peptide analogue. J. Nucl. Cardiol. 2022, 29, 2090–2099. [Google Scholar] [CrossRef]
- Smillie, S.J.; King, R.; Kodji, X.; Outzen, E.; Pozsgai, G.; Fernandes, E.; Marshall, N.; de Winter, P.; Heads, R.J.; Dessapt-Baradez, C.; et al. An ongoing role of alpha calcitonin gene-related peptide as part of the protective network against hypertension, vascular hypertrophy, and oxidative stress. Hypertension 2014, 63, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Z.; Li, X.; Cai, J.; Zhang, B.; Xiang, D.; Li, N.; Li, Y. CGRP derived from cardiac fibroblasts is an endogenous suppressor of cardiac fibroblasts. Cardiovasc. Res. 2019, 116, 1335–1348. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.M.; Takawale, A.; Hulsurkar, M.; Menassa, D.A.; Antanaviciute, A.; Lahiri, S.K.; Mehta, N.; Evans, N.; Psarros, C.; Robinson, P.; et al. Paracrine signaling by cardiac calcitonin controls atrial fibrogenesis and arrhythmia. Nature 2020, 587, 460–465. [Google Scholar] [CrossRef]
- Forrester, E.A.; Benítez-Angeles, M.; Redford, K.E.; Rosenbaum, T.; Abbott, G.W.; Barrese, V.; Albert, A.P.; Johs, D.; Salles-Crawley, I.; Jepps, T.A.; et al. Crucial role for sensory nerves and Na/H exchanger inhibition in dapagliflozin- and empagliflozin-induced arterial relaxation. Cardiovasc. Res. 2024, 120, 1811–1824. [Google Scholar] [CrossRef]
- DiPette, D.J.; Schwarzenberger, K.; Kerr, N.; Holland, O.B. Systemic and regional hemodynamic effects of calcitonin gene-related peptide. Hypertension 1987, 9, III142–III146. [Google Scholar] [CrossRef]
- DiPette, D.J.; Schwarzenberger, K.; Kerr, N.; Holland, O.B. Dose-dependent systemic and regional hemodynamic effects of calcitonin gene-related peptide. Am. J. Med. Sci. 1989, 297, 65–70. [Google Scholar] [CrossRef]
- Dubois-Randé, J.L.; Merlet, P.; Benvenuti, C.; Sediame, S.; Macquin-Mavier, I.; Chabrier, E.; Braquet, P.; Castaigne, A.; Adnot, S. Effects of calcitonin gene-related peptide on cardiac contractility, coronary hemodynamics and myocardial energetics in idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1992, 70, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Supowit, S.C.; Ramana, C.V.; Westlund, K.N.; DiPette, D.J. Calcitonin gene-related peptide gene expression in the spontaneously hypertensive rat. Hypertension 1993, 21, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Supowit, S.; Potts, J.D.; DiPette, D.J. Alpha-calcitonin gene-related peptide prevents pressure-overload induced heart failure: Role of apoptosis and oxidative stress. Physiol. Rep. 2019, 21, e14269. [Google Scholar] [CrossRef] [PubMed]
- Skaria, T.; Mitchell, K.J.; Vogel, O.; Wälchli, T.; Gassmann, M.; Vogel, J. Blood pressure normalization-independent cardioprotective effects of endogenous, physical activity-induced αCGRP (α calcitonin gene-related peptide) in chronically hypertensive mice. Circ. Res. 2019, 125, 1124–1140. [Google Scholar] [CrossRef]
- Massen van den Brink, A.; Meijer, J.; Villalón, C.M.; Ferrari, M.D. Wiping out CGRP: Potential cardiovascular risks. Trends Pharmacol. Sci. 2016, 37, 779–788. [Google Scholar] [CrossRef]
- Sun, J.; Zuckermann, R.N. Peptoid polymers: A highly designable bioinspired material. ACS Nano 2013, 7, 4715–4732. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, T.J.; Wu, C.W.; Zuckermann, R.N.; Barron, A.E. Extreme stability of helices formed by water-soluble poly-N-substituted glycines (polypeptoids) with alpha-chiral side chains. Biopolymers 2001, 63, 12–20. [Google Scholar] [CrossRef]
- Park, M.; Jardetzky, T.S.; Barron, A.E. NMEGylation: A novel modification to enhance the bioavailability of therapeutic peptides. Biopolymers 2011, 96, 688–693. [Google Scholar] [CrossRef]
- Giorgio, A.; Del Gatto, A.; Pennacchio, S.; Saviano, M.; Zaccaro, L. Peptoids: Smart and Emerging Candidates for the Diagnosis of Cancer, Neurological and Autoimmune Disorders. Int. J. Mol. Sci. 2023, 24, 16333. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, S.; Liu, Z.; Guo, J.; Cao, S.; Long, S. Recent advances in anticancer peptoids. Bioorg. Chem. 2023, 139, 106686. [Google Scholar] [CrossRef]
- Nyembe, P.L.; Ntombela, T.; Makatini, M.M. Review: Structure-Activity Relationship of Antimicrobial Peptoids. Pharmaceutics 2023, 15, 1506. [Google Scholar] [CrossRef]
- Dohm, M.T.; Kapoor, R.; Barron, A.E. Peptoids: Bio-inspired polymers as Potential Pharmaceuticals. Curr. Pharm. Des. 2011, 17, 2732–2747. [Google Scholar] [CrossRef]
- Yeo, X.Y.; Cunliffe, G.; Ho, R.C.; Lee, S.S.; Jung, S. Potentials of Neuropeptides as Therapeutic Agents for Neurological Diseases. Biomedicines 2022, 10, 343. [Google Scholar] [CrossRef]
- Cafaro, V.; Bosso, A.; Di Nardo, I.; D’Amato, A.; Izzo, I.; De Riccardis, F.; Siepi, M.; Culurciello, R.; D’Urzo, N.; Chiarot, E.; et al. The Antimicrobial, Antibiofilm and Anti-Inflammatory Activities of P13#1, a Cathelicidin-like Achiral Peptoid. Pharmaceuticals 2023, 16, 1386. [Google Scholar] [CrossRef]
- Kapoor, R.; Eimerman, P.R.; Hardy, J.W.; Cirillo, J.D.; Contag, C.H.; Barron, A.E. Efficacy of antimicrobial peptoids against mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2011, 55, 3058–3062. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; McDermott, G.; Wetzler, M.; Le Gros, M.A.; Myllys, M.; Knoechel, C.; Barron, A.E.; Larabell, C.A. Soft X-ray tomography of phenotypic switching and cellular response to antifungal peptoids in Candida albicans. Proc. Natl. Acad. Sci. USA 2009, 106, 19375–19380. [Google Scholar] [CrossRef]
- Bolt, H.L.; Denny, P.W.; Cobb, S.L. An efficient method for the synthesis of peptoids with mixed lysine-type/arginine-type monomers and evaluation of their anti-leishmanial activity. J. Vis. Exp. 2016, 117, 54750. [Google Scholar] [CrossRef]
- Turner, J.P.; Lutz-Rechtin, T.; Moore, K.A.; Rogers, L.; Bhave, O.; Moss, M.A.; Servoss, S.L. Rationally designed peptoids modulate aggregation of amyloid-beta 40. ACS Chem. Neurosci. 2014, 5, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Deloach, S.; DiPette, D.J.; Potts, J.D. A novel bioactive peptide-peptoid hybrid of alpha-calcitonin gene-related peptide protects against pressure-overload induced heart failure. Front. Pharmacol. 2025; submitted. [Google Scholar]
- Zhang, Y.; Bauersachs, J.; Langer, H.F. Immune mechanisms in heart failure. Eur. J. Heart Fail. 2017, 19, 1379–1389. [Google Scholar] [CrossRef]
- Moskalik, A.; Niderla-Bielińska, J.; Ratajska, A. Multiple roles of cardiac macrophages in heart homeostasis and failure. Heart Fail. Rev. 2021, 27, 1413–1430. [Google Scholar] [CrossRef]
- Assas, B.M.; Pennock, J.I.; Miyan, J.A. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front. Neurosci. 2014, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.J.; Downing, J.E.; Miyan, J.A. Without nerves, immunology remains incomplete -in vivo veritas. Immunology 2005, 116, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.F.; Dhanasekaran, S.; Howarth, F.C. Neuropeptides in the rat corpus cavernosum and seminal vesicle: Effects of age and two types of diabetes. Auton. Neurosci. 2009, 146, 76–80. [Google Scholar] [CrossRef] [PubMed]
- De Winter, B.Y.; Bredenoord, A.J.; Van Nassauw, L.; De Man, J.G.; De Schepper, H.U.; Timmermans, J.P.; Pelckmans, P.A. Involvement of afferent neurons in the pathogenesis of endotoxin-inducd ileus in mice: Role of CGRP and TRPV1 receptors. Eur. J. Pharmacol. 2009, 615, 177–184. [Google Scholar] [CrossRef]
- Reinshagen, M.; Flämig, G.; Ernst, S.; Geerling, I.; Wong, H.; Walsh, J.H.; Eysselein, V.E.; Adler, G. Calcitonin gene-related peptide mediates the protective effect of sensory nerves in a model of colonic injury. J. Pharmacol. Exp. Ther. 1998, 286, 657–661. [Google Scholar] [CrossRef]
- Smith, A.S.; Smid, S.D. Impaired capsaicin and neurokinin-evoked colonic motility in inflammatory bowel disease. J. Pharmacol. Exp. Ther. 1998, 286, 657–661. [Google Scholar] [CrossRef]
- Russo, A.; Hay, D.L. CGRP physiology, pharmacology, and therapeutic targets: Migraine and beyond. Physiol. Rev. 2023, 103, 1565–1644. [Google Scholar] [CrossRef]
- Lu, Y.-Z.; Nayer, B.; Singh, S.K.; Alshoubaki, Y.K.; Yuan, E.; Park, A.J.; Maruyama, K.; Akira, S.; Martino, M.M. CGRP sensory neurons promote tissue healing via neutrophils and macrophages. Nature 2024, 628, 604–611. [Google Scholar] [CrossRef]
- Broome, C.S.; Miyan, J.A. Neuropeptide control of bone marrow neutrophil production: A key axis for neuroimmunomodulation. Ann. N. Y. Acad. Sci. 2000, 917, 424–434. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Stringer, C.; Wang, T.; Michaelos, M.; Pachitariu, M. Cellpose: A generalist algorithm for cellular segmentation. Nat. Methods 2020, 18, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L. Vascular Remodeling in Hypertension. Hypertension 2012, 59, 367–374. [Google Scholar] [CrossRef]
- Mair, J.; Lechleitner, P.; Längle, T.; Wiedermann, C.; Dienstl, F.; Saria, A. Plasma CGRP in Acute Myocardial Infarction. Lancet 1995, 335, 168. [Google Scholar] [CrossRef]
- Uren, N.G.; Seydoux, C.; Davies, G.J. Effect of intravenous calcitonin gene related peptide on ischaemia threshold and coronary stenosis severity in humans. Cardiovasc. Res. 1993, 27, 1477–1481. [Google Scholar] [CrossRef]
- Gennari, C.; Nami, R.; Fischer, J.A. Improved cardiac performance with human calcitonin gene related peptide in patients with congestive heart failure. Cardiovasc. Res. 1990, 24, 239–241. [Google Scholar] [CrossRef]
- Kratofil, R.M.; Kubes, P.; Deniset, J.F. Monocyte Conversion during Inflammation and Injury. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 35–42. [Google Scholar] [CrossRef]
- Vicheth, V.; Zhong, C.; Guan, J.; Zhang, X.; Chen, D.; Yang, P. Causal Roles of Immune Cells in Cardiovascular Diseases: A Mendelian Randomization (MR) Study. JRSM Cardiovasc. Dis. 2024, 13, 20480040241271777. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Arnott, C.; Beale, A.L.; Chandramouli, C.; Hilfiker-Kleiner, D.; Kaye, D.M.; Ky, B.; Santema, B.T.; Sliwa, K.; Voors, A.A. Sex Differences in Heart Failure. Eur. Heart J. 2019, 40, 3859–3868c. [Google Scholar] [CrossRef]
- Gangula, P.R.R.; Chauhan, M.; Reed, L.; Yallampalli, C. Age-Related Changes in Dorsal Ganglia, Circulating and Vascular Calcitonin Gene-Related Peptide (CGRP) Concentrations in Female Rats: Effects of Female Sex Steroid Hormones. Neurosci. Lett. 2009, 454, 118–123. [Google Scholar] [CrossRef] [PubMed]
- de Vries Lentsch, S.; Rubio-Beltrán, E.; Massen van den Brink, A. Changing Levels of Sex Hormones and Calcitonin Gene-Related Peptide (CGRP) during a Woman’s Life: Implications for the Efficacy and Safety of Novel Antimigraine Medications. Maturitas 2021, 145, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Bosch, L.; de Haan, J.J.; Bastemeijer, M.; van der Burg, J.; van der Worp, E.; Wesseling, M.; Viola, M.; Odille, G.; Sluijter, J.P.G.; Wever, K.E.; et al. The transverse aortic constriction heart failure animal model: A systemic review and meta-analysis. Heart Fail. Rev. 2021, 26, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Menendez, L.; Karamanlidis, G.; Kolwicz, S.; Tian, R. Substrain specific response to cardiac pressure overload in C57BL/6 mice. Am. J. Phsiol. Heart Circ. Physiol. 2013, 305, H397–H402. [Google Scholar] [CrossRef]
- Richards, D.A.; Aronovitz, M.J.; Calamaras, T.D.; Tam, K.; Martin, G.L.; Liu, P.; Bowditch, H.K.; Zhang, P.; Huggins, G.S.; Blanton, R.M. Distinct Phenotypes Induced by Three Degrees of Transverse Aortic Constriction in Mice. Sci. Rep. 2019, 9, 5844. [Google Scholar] [CrossRef]
- Almufleh, A.; Marbach, J.; Chih, S.; Stadnick, E.; Davies, R.; Liu, P.; Mielniczuk, L. Ejection fraction improvement and reverse remodeling achieved with Sacubitril/Valsartan in heart failure with reduced ejection fraction patients. Am. J. Cardiovasc. Dis. 2017, 20, 108–113. [Google Scholar]
- Sandhu, A.T.; Tisdale, R.L.; Rodriguez, F.; Stafford, R.S.; Maron, D.J.; Hernandez-Boussard, T.; Lewis, E.; Heidenreich, P.A. Disparity in the Setting of Incident Heart Failure Diagnosis. Circ. Heart Fail. 2021, 14, e008538. [Google Scholar] [CrossRef] [PubMed]



| Antibody | Fluorophore | Catalog Number |
|---|---|---|
| CD11c | Brilliant Violet 421™ | 117329 1 |
| CD4 | Brilliant Violet 570™ | 100542 1 |
| I-A/I-E | Brilliant Violet 605™ | 107639 1 |
| CD192 (CCR2) | Brilliant Violet 650™ | 150613 1 |
| CD68 | Brilliant Violet 711™ | 137029 1 |
| CD11b | Brilliant Violet 750™ | 101267 1 |
| CD117 (c-kit) | Brilliant Violet 785™ | 135138 1 |
| CD8a | PerCP/Cyanine5.5 | 155014 1 |
| Ly-6C | APC/Cyanine7 | 128026 1 |
| CD106 | APC | 105718 1 |
| CD31 | Alexa Fluor 700 | 102444 1 |
| TER-119 | FITC | 116206 1 |
| NK1.1 | Spark YG™ 581 | 156531 1 |
| CD34 | PE/Dazzle™ 594 | 128615 1 |
| CD41 | PE/Cyanine7 | 133916 1 |
| CD45 | NovaFluor™ Yellow 730 | M005T02Y07-A 2 |
| CD3 | BUV563 | 741235 3 |
| CD45R/B220 | BUV661 | 612972 3 |
| CD19 | BUV737 | 612781 3 |
| Ly-6G | BUV805 | 741994 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deloach, S.; Kumar, A.; Ruggiero, E.; Ball, R.; Gleason, K.; Kubinak, J.; DiPette, D.J.; Potts, J.D. A Novel Peptoid Hybrid of Alpha-Calcitonin Gene-Related Peptide (α-CGRP) Ameliorates Cardiac Remodeling in Pressure Overload-Induced Heart Failure. Cells 2025, 14, 1580. https://doi.org/10.3390/cells14201580
Deloach S, Kumar A, Ruggiero E, Ball R, Gleason K, Kubinak J, DiPette DJ, Potts JD. A Novel Peptoid Hybrid of Alpha-Calcitonin Gene-Related Peptide (α-CGRP) Ameliorates Cardiac Remodeling in Pressure Overload-Induced Heart Failure. Cells. 2025; 14(20):1580. https://doi.org/10.3390/cells14201580
Chicago/Turabian StyleDeloach, Sarah, Ambrish Kumar, Emily Ruggiero, Ryan Ball, Kamryn Gleason, Jason Kubinak, Donald J. DiPette, and Jay D. Potts. 2025. "A Novel Peptoid Hybrid of Alpha-Calcitonin Gene-Related Peptide (α-CGRP) Ameliorates Cardiac Remodeling in Pressure Overload-Induced Heart Failure" Cells 14, no. 20: 1580. https://doi.org/10.3390/cells14201580
APA StyleDeloach, S., Kumar, A., Ruggiero, E., Ball, R., Gleason, K., Kubinak, J., DiPette, D. J., & Potts, J. D. (2025). A Novel Peptoid Hybrid of Alpha-Calcitonin Gene-Related Peptide (α-CGRP) Ameliorates Cardiac Remodeling in Pressure Overload-Induced Heart Failure. Cells, 14(20), 1580. https://doi.org/10.3390/cells14201580








