Diverse Roles of Tubulin Polymerization Promoting Protein 3 (TPPP3) in Human Health and Disease
Abstract
1. Introduction
2. Cancers
2.1. Cervical Cancer
2.2. Non-Small Cell Lung Carcinoma
2.3. Colorectal Cancer
2.4. Breast Cancer
2.5. Endometrial Cancer
2.6. Glioblastoma
2.7. Liver Cancer
2.8. Pancreatic Cancer
2.9. Nasopharyngeal Carcinoma
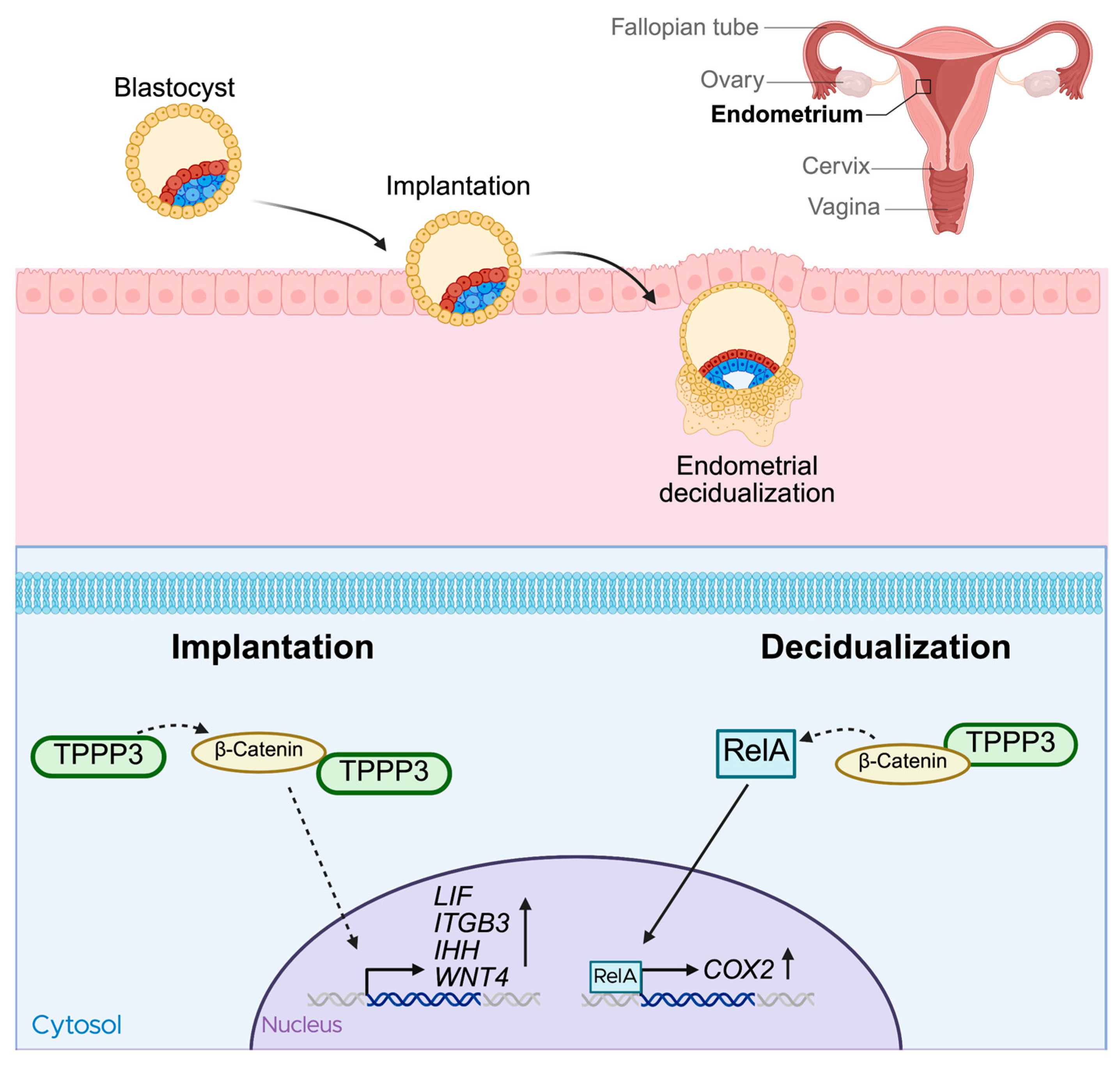
3. Reproduction
4. Musculoskeletal System
5. Endothelial Dysfunction
6. Neurodegenerative Diseases
7. Other Studies
8. Conclusions
9. Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vincze, O.; Tökési, N.; Oláh, J.; Hlavanda, E.; Zotter, Á.; Horváth, I.; Lehotzky, A.; Tirián, L.; Medzihradszky, K.F.; Kovács, J.; et al. Tubulin Polymerization Promoting Proteins (TPPPs): Members of a New Family with Distinct Structures and Functions. Biochemistry 2006, 45, 13818–13826. [Google Scholar] [CrossRef]
- Takahashi, M.; Tomizawa, K.; Ishiguro, K.; Sato, K.; Omori, A.; Sato, S.; Shiratsuchi, A.; Uchida, T.; Imahori, K. A Novel Brain-Specific 25 kDa Protein (P25) Is Phosphorylated by a Ser/Thr-Pro Kinase (TPK II) from Tau Protein Kinase Fractions. FEBS Lett. 1991, 289, 37–43. [Google Scholar] [CrossRef]
- Takahashi, M.; Tomizawa, K.; Fujita, S.C.; Sato, K.; Uchida, T.; Imahori, K. A Brain-Specific Protein P25 Is Localized and Associated with Oligodendrocytes, Neuropil, and Fiber-Like Structures of the CA3 Hippocampal Region in the Rat Brain. J. Neurochem. 1993, 60, 228–235. [Google Scholar] [CrossRef]
- Fu, M.; McAlear, T.S.; Nguyen, H.; Oses-Prieto, J.A.; Valenzuela, A.; Shi, R.D.; Perrino, J.J.; Huang, T.-T.; Burlingame, A.L.; Bechstedt, S.; et al. The Golgi Outpost Protein TPPP Nucleates Microtubules and Is Critical for Myelination. Cell 2019, 179, 132–146.e14. [Google Scholar] [CrossRef]
- Hlavanda, E.; Kovács, J.; Oláh, J.; Orosz, F.; Medzihradszky, K.F.; Ovádi, J. Brain-Specific P25 Protein Binds to Tubulin and Microtubules and Induces Aberrant Microtubule Assemblies at Substoichiometric Concentrations. Biochemistry 2002, 41, 8657–8664. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Lehotzky, A.; Tirián, L.; Tökési, N.; Lénárt, P.; Szabó, B.; Kovács, J.; Ovádi, J. Dynamic Targeting of Microtubules by TPPP/P25 Affects Cell Survival. J. Cell Sci. 2004, 117, 6249–6259. [Google Scholar] [CrossRef] [PubMed]
- Tőkési, N.; Lehotzky, A.; Horváth, I.; Szabó, B.; Oláh, J.; Lau, P.; Ovádi, J. TPPP/P25 Promotes Tubulin Acetylation by Inhibiting Histone Deacetylase 6. J. Biol. Chem. 2010, 285, 17896–17906. [Google Scholar] [CrossRef]
- Mino, R.E.; Rogers, S.L.; Risinger, A.L.; Rohena, C.; Banerjee, S.; Bhat, M.A. Drosophila Ringmaker Regulates Microtubule Stabilization and Axonal Extension during Embryonic Development. J. Cell Sci. 2016, 129, 3282–3294. [Google Scholar] [CrossRef]
- Endres, T.; Duesler, L.; Corey, D.A.; Kelley, T.J. In Vivo Impact of Tubulin Polymerization Promoting Protein (Tppp) Knockout to the Airway Inflammatory Response. Sci. Rep. 2023, 13, 12272. [Google Scholar] [CrossRef]
- Kovács, G.G.; László, L.; Kovács, J.; Jensen, P.H.; Lindersson, E.; Botond, G.; Molnár, T.; Perczel, A.; Hudecz, F.; Mező, G.; et al. Natively Unfolded Tubulin Polymerization Promoting Protein TPPP/P25 Is a Common Marker of Alpha-Synucleinopathies. Neurobiol. Dis. 2004, 17, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Lindersson, E.; Lundvig, D.; Petersen, C.; Madsen, P.; Nyengaard, J.R.; Højrup, P.; Moos, T.; Otzen, D.; Gai, W.-P.; Blumbergs, P.C.; et al. P25α Stimulates α-Synuclein Aggregation and Is Co-Localized with Aggregated α-Synuclein in α-Synucleinopathies. J. Biol. Chem. 2005, 280, 5703–5715. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, X.; Li, L.; Feng, X.; Yang, Z.; Zhang, W.; Hu, R. Depletion of Tubulin Polymerization Promoting Protein Family Member 3 Suppresses HeLa Cell Proliferation. Mol. Cell. Biochem. 2010, 333, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, K. Centrosome Amplification, Chromosome Instability and Cancer Development. Cancer Lett. 2005, 230, 6–19. [Google Scholar] [CrossRef]
- Nigg, E.A. Origins and Consequences of Centrosome Aberrations in Human Cancers. Int. J. Cancer 2006, 119, 2717–2723. [Google Scholar] [CrossRef]
- Zhou, W.; Li, J.; Wang, X.; Hu, R. Stable Knockdown of TPPP3 by RNA Interference in Lewis Lung Carcinoma Cell Inhibits Tumor Growth and Metastasis. Mol. Cell. Biochem. 2010, 343, 231–238. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Ye, K.; Wu, N.; Li, J.; Liu, N.; He, M.; Lu, B.; Zhou, W.; Hu, R. Knockdown of Tubulin Polymerization Promoting Protein Family Member 3 Suppresses Proliferation and Induces Apoptosis in Non-Small-Cell Lung Cancer. J. Cancer 2016, 7, 1189–1196. [Google Scholar] [CrossRef]
- Xu, N.; Lao, Y.; Zhang, Y.; Gillespie, D.A. Akt: A Double-Edged Sword in Cell Proliferation and Genome Stability. J. Oncol. 2012, 2012, 951724. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.-M.; Meinkoth, J.; Pittman, R.N. Akt Regulates Cell Survival and Apoptosis at a Postmitochondrial Level. J. Cell Biol. 2000, 151, 483–494. [Google Scholar] [CrossRef]
- Tolomeo, M.; Cascio, A. The Multifaced Role of STAT3 in Cancer and Its Implication for Anticancer Therapy. Int. J. Mol. Sci. 2021, 22, 603. [Google Scholar] [CrossRef]
- Li, Y.; Bai, M.; Xu, Y.; Zhao, W.; Liu, N.; Yu, J. TPPP3 Promotes Cell Proliferation, Invasion and Tumor Metastasis via STAT3/ Twist1 Pathway in Non-Small-Cell Lung Carcinoma. Cell. Physiol. Biochem. 2018, 50, 2004–2016. [Google Scholar] [CrossRef]
- Zhao, D.; Besser, A.H.; Wander, S.A.; Sun, J.; Zhou, W.; Wang, B.; Ince, T.; Durante, M.A.; Guo, W.; Mills, G.; et al. Cytoplasmic P27 Promotes Epithelial–Mesenchymal Transition and Tumor Metastasis via STAT3-Mediated Twist1 Upregulation. Oncogene 2015, 34, 5447–5459. [Google Scholar] [CrossRef]
- Ye, K.; Li, Y.; Zhao, W.; Wu, N.; Liu, N.; Li, R.; Chen, L.; He, M.; Lu, B.; Wang, X.; et al. Knockdown of Tubulin Polymerization Promoting Protein Family Member 3 Inhibits Cell Proliferation and Invasion in Human Colorectal Cancer. J. Cancer 2017, 8, 1750–1758. [Google Scholar] [CrossRef]
- Shukla, V.; Kaushal, J.B.; Sankhwar, P.; Manohar, M.; Dwivedi, A. Inhibition of TPPP3 Attenuates β-Catenin/NF-κB/COX-2 Signaling in Endometrial Stromal Cells and Impairs Decidualization. J. Endocrinol. 2019, 240, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.L.; Zhou, S.L.; Qiu, J.; Liu, Y.F.; Hua, H. Correlated Expression of Fas, NF-kappaB, and VEGF-C in Infiltrating Ductal Carcinoma of the Breast. Eur. J. Gynaecol. Oncol. 2012, 33, 633–639. [Google Scholar] [PubMed]
- Huber, M.A.; Azoitei, N.; Baumann, B.; Grünert, S.; Sommer, A.; Pehamberger, H.; Kraut, N.; Beug, H.; Wirth, T. NF-kappaB Is Essential for Epithelial-Mesenchymal Transition and Metastasis in a Model of Breast Cancer Progression. J. Clin. Investig. 2004, 114, 569–581. [Google Scholar] [CrossRef]
- Xu, H.; Lin, F.; Wang, Z.; Yang, L.; Meng, J.; Ou, Z.; Shao, Z.; Di, G.; Yang, G. CXCR2 Promotes Breast Cancer Metastasis and Chemoresistance via Suppression of AKT1 and Activation of COX2. Cancer Lett. 2018, 412, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Zatelli, M.C.; Molè, D.; Tagliati, F.; Minoia, M.; Ambrosio, M.R.; degli Uberti, E. Cyclo-Oxygenase 2 Modulates Chemoresistance in Breast Cancer Cells Involving NF-kappaB. Cell. Oncol. Off. J. Int. Soc. Cell. Oncol. 2009, 31, 457–465. [Google Scholar] [CrossRef]
- Hidalgo, M.; Eckhardt, S.G. Development of Matrix Metalloproteinase Inhibitors in Cancer Therapy. J. Natl. Cancer Inst. 2001, 93, 178–193. [Google Scholar] [CrossRef]
- Huang, Z.; Bu, D.; Yang, N.; Huang, W.; Zhang, L.; Li, X.; Ding, B.-S. Integrated Analyses of Single-Cell Transcriptomics Identify Metastasis-Associated Myeloid Subpopulations in Breast Cancer Lung Metastasis. Front. Immunol. 2023, 14, 1180402. [Google Scholar] [CrossRef]
- Shen, A.; Tong, X.; Li, H.; Chu, L.; Jin, X.; Ma, H.; Ouyang, Y. TPPP3 Inhibits the Proliferation, Invasion and Migration of Endometrial Carcinoma Targeted with miR-1827. Clin. Exp. Pharmacol. Physiol. 2021, 48, 890–901. [Google Scholar] [CrossRef]
- Ren, Q.; Hou, Y.; Li, X.; Fan, X. Silence of TPPP3 Suppresses Cell Proliferation, Invasion and Migration via Inactivating NF-κB/COX2 Signal Pathway in Breast Cancer Cell. Cell Biochem. Funct. 2020, 38, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent Deletions and Down-Regulation of Micro- RNA Genes miR15 and miR16 at 13q14 in Chronic Lymphocytic Leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef]
- Halim, A.; Kim, B.; Kenyon, E.; Moore, A. miR-10b as a Clinical Marker and a Therapeutic Target for Metastatic Breast Cancer. Technol. Cancer Res. Treat. 2025, 24, 15330338251339256. [Google Scholar] [CrossRef]
- Ho, C.S.; Noor, S.M.; Nagoor, N.H. MiR-378 and MiR-1827 Regulate Tumor Invasion, Migration and Angiogenesis in Human Lung Adenocarcinoma by Targeting RBX1 and CRKL, Respectively. J. Cancer 2018, 9, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Sun, J.; Liao, J.; Pu, C.; Yuan, S.; Li, C.; Chen, J.; Shang, L.; Pan, Z.; Chen, J. Down-Regulation of circMUC16 Inhibited the Progression of ALL via Interaction with miR-1182 and TPPP3. Ann. Hematol. 2025, 104, 3389–3401. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Hu, J.; Li, F.; Yuan, H.; Yan, M.; Liao, G.; Xu, L.; Pang, B.; Ping, Y.; Xiao, Y.; et al. Discovering Rare Genes Contributing to Cancer Stemness and Invasive Potential by GBM Single-Cell Transcriptional Analysis. Cancers 2019, 11, 2025. [Google Scholar] [CrossRef]
- Xu, X.; Hou, Y.; Long, N.; Jiang, L.; Yan, Z.; Xu, Y.; Lv, Y.; Xiang, X.; Yang, H.; Liu, J.; et al. TPPP3 Promote Epithelial-Mesenchymal Transition via Snail1 in Glioblastoma. Sci. Rep. 2023, 13, 17960. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH Factors in Tumour Progression: An Alliance against the Epithelial Phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, Z.; Zhou, Z.; Zhou, Z.; He, H.; Sun, J.; Zhou, X.; Chin, Y.R.; Zhang, L.; Yang, M. Distinguishing High-Metastasis-Potential Circulating Tumor Cells through Fluidic Shear Stress in a Bloodstream-like Microfluidic Circulatory System. Oncogene 2024, 43, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Ansari, D.; Pawłowski, K.; Zhou, Q.; Sasor, A.; Welinder, C.; Kristl, T.; Bauden, M.; Rezeli, M.; Jiang, Y.; et al. Proteomic Analyses Identify Prognostic Biomarkers for Pancreatic Ductal Adenocarcinoma. Oncotarget 2018, 9, 9789–9807. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Li, J.; Su, Q.; Qiu, Y.; Zhang, Z.; Zhang, L.; Mo, W. TPPP3 Associated with Prognosis and Immune Infiltrates in Head and Neck Squamous Carcinoma. BioMed Res. Int. 2020, 2020, 3962146. [Google Scholar] [CrossRef]
- Xiao, T.; Lin, F.; Zhou, J.; Tang, Z. The Expression and Role of Tubulin Polymerization-Promoting Protein 3 in Oral Squamous Cell Carcinoma. Arch. Oral Biol. 2022, 143, 105519. [Google Scholar] [CrossRef]
- Liu, C.; Ni, C.; Li, C.; Tian, H.; Jian, W.; Zhong, Y.; Zhou, Y.; Lyu, X.; Zhang, Y.; Xiang, X.-J.; et al. Lactate-Related Gene Signatures as Prognostic Predictors and Comprehensive Analysis of Immune Profiles in Nasopharyngeal Carcinoma. J. Transl. Med. 2024, 22, 1116. [Google Scholar] [CrossRef]
- He, Y.; Song, T.; Ning, J.; Wang, Z.; Yin, Z.; Jiang, P.; Yuan, Q.; Yu, W.; Cheng, F. Lactylation in Cancer: Mechanisms in Tumour Biology and Therapeutic Potentials. Clin. Transl. Med. 2024, 14, e70070. [Google Scholar] [CrossRef] [PubMed]
- Manohar, M.; Khan, H.; Sirohi, V.K.; Das, V.; Agarwal, A.; Pandey, A.; Siddiqui, W.A.; Dwivedi, A. Alteration in Endometrial Proteins during Early- and Mid-Secretory Phases of the Cycle in Women with Unexplained Infertility. PLoS ONE 2014, 9, e111687. [Google Scholar] [CrossRef]
- Ferenczy, A.; Mutter, G.L. The Endometrial Cycle|GLOWM. Available online: http://www.glowm.com/section-view/heading/TheEndometrialCycle/item/292 (accessed on 8 July 2025).
- Psychoyos, A. Hormonal Control of Ovoimplantation. In Vitamins & Hormones; Harris, R.S., Diczfalusy, E., Munson, P.L., Glover, J., Thimann, K.V., Wool, I.G., Lorawe, J.A., Eds.; Academic Press: Cambridge, MA, USA, 1974; Volume 31, pp. 201–256. [Google Scholar]
- Cakmak, H.; Taylor, H.S. Implantation Failure: Molecular Mechanisms and Clinical Treatment. Hum. Reprod. Update 2011, 17, 242–253. [Google Scholar] [CrossRef]
- Shukla, V.; Popli, P.; Kaushal, J.B.; Gupta, K.; Dwivedi, A. Uterine TPPP3 Plays Important Role in Embryo Implantation via Modulation of β-Catenin†. Biol. Reprod. 2018, 99, 982–999. [Google Scholar] [CrossRef]
- Aghajanova, L. Leukemia Inhibitory Factor and Human Embryo Implantation. Ann. N. Y. Acad. Sci. 2004, 1034, 176–183. [Google Scholar] [CrossRef]
- Lee, K.; Jeong, J.; Kwak, I.; Yu, C.-T.; Lanske, B.; Soegiarto, D.W.; Toftgard, R.; Tsai, M.-J.; Tsai, S.; Lydon, J.P.; et al. Indian Hedgehog Is a Major Mediator of Progesterone Signaling in the Mouse Uterus. Nat. Genet. 2006, 38, 1204–1209. [Google Scholar] [CrossRef]
- Peng, J.; Monsivais, D.; You, R.; Zhong, H.; Pangas, S.A.; Matzuk, M.M. Uterine Activin Receptor-like Kinase 5 Is Crucial for Blastocyst Implantation and Placental Development. Proc. Natl. Acad. Sci. USA 2015, 112, E5098–E5107. [Google Scholar] [CrossRef]
- Tei, C.; Maruyama, T.; Kuji, N.; Miyazaki, T.; Mikami, M.; Yoshimura, Y. Reduced Expression of Avβ3 Integrin in the Endometrium of Unexplained Infertility Patients with Recurrent IVF-ET Failures: Improvement by Danazol Treatment. J. Assist. Reprod. Genet. 2003, 20, 13–20. [Google Scholar] [CrossRef]
- Tepekoy, F.; Akkoyunlu, G.; Demir, R. The Role of Wnt Signaling Members in the Uterus and Embryo during Pre-Implantation and Implantation. J. Assist. Reprod. Genet. 2015, 32, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, O.A.; Jonnaert, M.; Labelle-Dumais, C.; Kuroda, K.; Clarke, H.J.; Dufort, D. Uterine Wnt/β-Catenin Signaling Is Required for Implantation. Proc. Natl. Acad. Sci. USA 2005, 102, 8579–8584. [Google Scholar] [CrossRef] [PubMed]
- Gellersen, B.; Brosens, J.J. Cyclic Decidualization of the Human Endometrium in Reproductive Health and Failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Paria, B.C.; Das, S.K.; Dinchuk, J.E.; Langenbach, R.; Trzaskos, J.M.; Dey, S.K. Multiple Female Reproductive Failures in Cyclooxygenase 2–Deficient Mice. Cell 1997, 91, 197–208. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Aitkenhead, M.; Hughes, C.C.W.; Levin, E.R. Integration of the Non-Genomic and Genomic Actions of Estrogen: MEMBRANE-INITIATED SIGNALING BY STEROID TO TRANSCRIPTION AND CELL BIOLOGY. J. Biol. Chem. 2002, 277, 50768–50775. [Google Scholar] [CrossRef]
- Schmedtje, J.F.; Ji, Y.-S.; Liu, W.-L.; DuBois, R.N.; Runge, M.S. Hypoxia Induces Cyclooxygenase-2 via the NF-κB P65 Transcription Factor in Human Vascular Endothelial Cells. J. Biol. Chem. 1997, 272, 601–608. [Google Scholar] [CrossRef]
- Staverosky, J.A.; Pryce, B.A.; Watson, S.S.; Schweitzer, R. Tubulin Polymerization-Promoting Protein Family Member 3, Tppp3, Is a Specific Marker of the Differentiating Tendon Sheath and Synovial Joints. Dev. Dyn. 2009, 238, 685–692. [Google Scholar] [CrossRef]
- Harvey, T.; Flamenco, S.; Fan, C.-M. A Tppp3+Pdgfra+ Tendon Stem Cell Population Contributes to Regeneration and Reveals a Shared Role for PDGF Signalling in Regeneration and Fibrosis. Nat. Cell Biol. 2019, 21, 1490–1503. [Google Scholar] [CrossRef]
- Sugg, K.B.; Markworth, J.F.; Disser, N.P.; Rizzi, A.M.; Talarek, J.R.; Sarver, D.C.; Brooks, S.V.; Mendias, C.L. Postnatal Tendon Growth and Remodeling Require Platelet-Derived Growth Factor Receptor Signaling. Am. J. Physiol. Cell Physiol. 2018, 314, C389–C403. [Google Scholar] [CrossRef]
- Yea, J.-H.; Gomez-Salazar, M.; Onggo, S.; Li, Z.; Thottappillil, N.; Cherief, M.; Negri, S.; Xing, X.; Qin, Q.; Tower, R.J.; et al. Tppp3+ Synovial/Tendon Sheath Progenitor Cells Contribute to Heterotopic Bone after Trauma. Bone Res. 2023, 11, 1–12. [Google Scholar] [CrossRef]
- Meyers, C.; Lisiecki, J.; Miller, S.; Levin, A.; Fayad, L.; Ding, C.; Sono, T.; McCarthy, E.; Levi, B.; James, A.W. Heterotopic Ossification: A Comprehensive Review. JBMR Plus 2019, 3, e10172. [Google Scholar] [CrossRef] [PubMed]
- Kendal, A.R.; Layton, T.; Al-Mossawi, H.; Appleton, L.; Dakin, S.; Brown, R.; Loizou, C.; Rogers, M.; Sharp, R.; Carr, A. Multi-Omic Single Cell Analysis Resolves Novel Stromal Cell Populations in Healthy and Diseased Human Tendon. Sci. Rep. 2020, 10, 13939. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.; Khor, W.; Burr, N.; Sivakumar, B. Flexor Tendon Repairs in Children: Outcomes from a Specialist Tertiary Centre. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 717–723. [Google Scholar] [CrossRef]
- Goto, A.; Komura, S.; Kato, K.; Maki, R.; Hirakawa, A.; Aoki, H.; Tomita, H.; Taguchi, J.; Ozawa, M.; Matsushima, T.; et al. PI3K-Akt Signalling Regulates Scx-Lineage Tenocytes and Tppp3-Lineage Paratenon Sheath Cells in Neonatal Tendon Regeneration. Nat. Commun. 2025, 16, 3734. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, B.; Moran, S.L.; Bravo, C.; Amadio, P. Factors That Influence the Outcome of Zone I and Zone II Flexor Tendon Repairs in Children. J. Hand Surg. 2006, 31, 1661–1666. [Google Scholar] [CrossRef]
- Franke, T.F.; Kaplan, D.R.; Cantley, L.C. PI3K: Downstream AKTion Blocks Apoptosis. Cell 1997, 88, 435–437. [Google Scholar] [CrossRef]
- Shih, B.; Bayat, A. Scientific Understanding and Clinical Management of Dupuytren Disease. Nat. Rev. Rheumatol. 2010, 6, 715–726. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Khan, M.J.; Rizwan Alam, M.; Waldeck-Weiermair, M.; Karsten, F.; Groschner, L.; Riederer, M.; Hallström, S.; Rockenfeller, P.; Konya, V.; Heinemann, A.; et al. Inhibition of Autophagy Rescues Palmitic Acid-Induced Necroptosis of Endothelial Cells. J. Biol. Chem. 2012, 287, 21110–21120. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, Y.; Nan, W.; Zhou, W.; Huang, J.; Li, R.; Zhou, L.; Hu, R. Interaction of TPPP3 with VDAC1 Promotes Endothelial Injury through Activation of Reactive Oxygen Species. Oxid. Med. Cell. Longev. 2020, 2020, 5950195. [Google Scholar] [CrossRef] [PubMed]
- Camara, A.K.S.; Zhou, Y.; Wen, P.-C.; Tajkhorshid, E.; Kwok, W.-M. Mitochondrial VDAC1: A Key Gatekeeper as Potential Therapeutic Target. Front. Physiol. 2017, 8, 460. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive Oxygen Species in Cancer: Current Findings and Future Directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Chen, M.; Yang, L.; Wagle, M.; Guo, S.; Hu, B. MicroRNA-133b Negatively Regulates Zebrafish Single Mauthner-Cell Axon Regeneration through Targeting Tppp3 in Vivo. Front. Mol. Neurosci. 2017, 10, 375. [Google Scholar] [CrossRef]
- Rao, M.; Luo, Z.; Liu, C.-C.; Chen, C.-Y.; Wang, S.; Nahmou, M.; Tanasa, B.; Virmani, A.; Byrne, L.; Goldberg, J.L.; et al. Tppp3 Is a Novel Molecule for Retinal Ganglion Cell Identification and Optic Nerve Regeneration. Acta Neuropathol. Commun. 2024, 12, 204. [Google Scholar] [CrossRef]
- Kelliher, M.T.; Saunders, H.A.J.; Wildonger, J. Microtubule Control of Functional Architecture in Neurons. Curr. Opin. Neurobiol. 2019, 57, 39–45. [Google Scholar] [CrossRef]
- Oláh, J.; Lehotzky, A.; Szénási, T.; Berki, T.; Ovádi, J. Modulatory Role of TPPP3 in Microtubule Organization and Its Impact on Alpha-Synuclein Pathology. Cells 2022, 11, 3025. [Google Scholar] [CrossRef]
- Ross, C.A.; Poirier, M.A. Protein Aggregation and Neurodegenerative Disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef]
- Mo, Q.; Liu, X.; Gong, W.; Wang, Y.; Yuan, Z.; Sun, X.; Wang, S. Pinpointing Novel Plasma and Brain Proteins for Common Ocular Diseases: A Comprehensive Cross-Omics Integration Analysis. Int. J. Mol. Sci. 2024, 25, 10236. [Google Scholar] [CrossRef]
- Zhu, T.; Zhou, Y.; Zhang, L.; Kong, L.; Tang, H.; Xiao, Q.; Sun, X.; Shen, F.; Zhou, H.; Ni, W.; et al. A Transcriptomic and Proteomic Analysis and Comparison of Human Brain Tissue from Patients with and without Epilepsy. Sci. Rep. 2025, 15, 16369. [Google Scholar] [CrossRef] [PubMed]
- Pastor, M.D.; Nogal, A.; Molina-Pinelo, S.; Meléndez, R.; Salinas, A.; González De la Peña, M.; Martín-Juan, J.; Corral, J.; García-Carbonero, R.; Carnero, A.; et al. Identification of Proteomic Signatures Associated with Lung Cancer and COPD. J. Proteomics 2013, 89, 227–237. [Google Scholar] [CrossRef]
- Zeller, T.; Schurmann, C.; Schramm, K.; Müller, C.; Kwon, S.; Wild, P.S.; Teumer, A.; Herrington, D.; Schillert, A.; Iacoviello, L.; et al. Transcriptome-Wide Analysis Identifies Novel Associations with Blood Pressure. Hypertens. Dallas Tex 1979 2017, 70, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Li, C.; Huang, P.; Liu, Z.; He, Y.; Liu, B. Transcriptional Landscape of Intestinal Environment in DSS-Induced Ulcerative Colitis Mouse Model. BMC Gastroenterol. 2024, 24, 60. [Google Scholar] [CrossRef]
- Akshay, A.; Besic, M.; Kuhn, A.; Burkhard, F.C.; Bigger-Allen, A.; Adam, R.M.; Monastyrskaya, K.; Hashemi Gheinani, A. Machine Learning-Based Classification of Transcriptome Signatures of Non-Ulcerative Bladder Pain Syndrome. Int. J. Mol. Sci. 2024, 25, 1568. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Z.; Hu, X.; Zhang, Y. Epigenetic Characterization of Sarcopenia-Associated Genes Based on Machine Learning and Network Screening. Eur. J. Med. Res. 2024, 29, 54. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, M.; Zhao, X.; Bin, E.; Hu, Y.; Tang, N.; Dai, H.; Wang, C. Telomere Shortening Impairs Alveolar Regeneration. Cell Prolif. 2022, 55, e13211. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Tan, C.; Yue, X.; Zhao, Y.; Peng, J.; Wang, X.; Laddha, S.V.; Chan, C.S.; Zheng, S.; et al. microRNA-1827 Represses MDM2 to Positively Regulate Tumor Suppressor P53 and Suppress Tumorigenesis. Oncotarget 2016, 7, 8783–8796. [Google Scholar] [CrossRef]
- Pan, Y.; Bush, E.C.; Toonen, J.A.; Ma, Y.; Solga, A.C.; Sims, P.A.; Gutmann, D.H. Whole Tumor RNA-Sequencing and Deconvolution Reveal a Clinically-Prognostic PTEN/PI3K-Regulated Glioma Transcriptional Signature. Oncotarget 2017, 8, 52474–52487. [Google Scholar] [CrossRef]
- Lee, S.W.L.; Paoletti, C.; Campisi, M.; Osaki, T.; Adriani, G.; Kamm, R.D.; Mattu, C.; Chiono, V. MicroRNA Delivery through Nanoparticles. J. Control. Release 2019, 313, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Song, K.; Lu, X.; Feng, W.; Di, W. Liposomal Delivery of MicroRNA-7 Targeting EGFR to Inhibit the Growth, Invasion, and Migration of Ovarian Cancer. ACS Omega 2021, 6, 11669–11678. [Google Scholar] [CrossRef] [PubMed]
- Segal, M.; Slack, F.J. Challenges Identifying Efficacious miRNA Therapeutics for Cancer. Expert Opin. Drug Discov. 2020, 15, 987–992. [Google Scholar] [CrossRef] [PubMed]


| Cell Type | Cell Line | Role of TPPP3 | Altered Pathways | Ref |
|---|---|---|---|---|
| Cervical cancer | HeLa | Tumorigenic Proliferation Mitosis | [14] | |
| Lewis lung carcinoma | Tumorigenic Proliferation Mitosis Suppression induces apoptosis | [17] | ||
| Non-small cell lung cancer | A549 H1229 | Tumorigenic Proliferation Suppression induces apoptosis | STAT3/AKT | [18] |
| Non-small cell lung cancer | 95-C SPC-A1 A549 H1229 Patient Samples (treatment naive) | Tumorigenic Proliferation Invasion Migration Metastasis Suppression induces apoptosis | STAT3/Twist1 | [22] |
| Colorectal cancer | SW480 HT29 RKO LOVO SW620 HUVEC | Tumorigenic Proliferation Invasion Migration Metastasis Angiogenesis | STAT3 VEGF/P21 | [24] |
| Breast cancer | MCF-7 T47D Patient samples (treatment naïve) | Tumorigenic Proliferation Invasion Migration | NF-kB/COX2 | [33] |
| Endometrial cancer | Ishikawa KLE RL-95 AN3CA Patient Samples (treatment naïve) | Tumorigenic Proliferation Invasion Migration TPPP3 is targeted by mIR-1827 | [32] | |
| Glioblastoma | U87ZMG U118 A172 LN229 U251 | Tumorigenic Proliferation Invasion Migration EMT Suppression induces apoptosis | SNAIL1 | [39] |
| Hepatocellular carcinoma | HepG2 SK-Hep-1 | Tumorigenic Metastasis | [22] | |
| Pancreatic ductal adenocarcinoma | FFPE patient samples | Protective High expression associated with longer survival. | [43] | |
| Nasopharyngeal carcinoma | Patient samples | Protective Lower TPPP3 expression in samples compared to normal tissue. Higher expression associated with better survival | Immune cell infiltration into tumors | [44] |
| Oral squamous cell carcinoma | Patient samples | Protective Lower TPPP3 expression in samples compared to normal tissue. Higher expression associated with better survival | Immune cell infiltration into tumors | [45] |
| Disease/Context | Data Type | Regulation | Ref |
|---|---|---|---|
| Diabetic Retinopathy | Proteome-wide associate studies | [84] | |
| Epilepsy | RNA-seq iTRAQ proteomics | Downregulated in patients with epilepsy | [85] |
| Lung cancer and COPD | Proteomics | Upregulated in COPD, lung cancer, and COPD and lung cancer groups | [86] |
| High blood pressure | microarray | Upregulated in monocytes associated with increased blood pressure. | [87] |
| Ulcerative colitis | RNA-seq | Downregulated in ulcerative colitis group compared to control | [88] |
| Non-ulcerative bladder pain syndrome | RNA-seq | Biomarker for non-ulcerative bladder pain syndrome | [89] |
| Sarcopenia | RNA-seq | Upregulated in patients with sarcopenia | [90] |
| Alveolar regeneration | RNA-seq | Upregulated during AT2 cell differentiation. TPPP3 was reduced in Tert−\− mice. | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lord, J.W.; Horibata, S. Diverse Roles of Tubulin Polymerization Promoting Protein 3 (TPPP3) in Human Health and Disease. Cells 2025, 14, 1573. https://doi.org/10.3390/cells14201573
Lord JW, Horibata S. Diverse Roles of Tubulin Polymerization Promoting Protein 3 (TPPP3) in Human Health and Disease. Cells. 2025; 14(20):1573. https://doi.org/10.3390/cells14201573
Chicago/Turabian StyleLord, James W., and Sachi Horibata. 2025. "Diverse Roles of Tubulin Polymerization Promoting Protein 3 (TPPP3) in Human Health and Disease" Cells 14, no. 20: 1573. https://doi.org/10.3390/cells14201573
APA StyleLord, J. W., & Horibata, S. (2025). Diverse Roles of Tubulin Polymerization Promoting Protein 3 (TPPP3) in Human Health and Disease. Cells, 14(20), 1573. https://doi.org/10.3390/cells14201573






