Effect of miR-223-3p and miR-328a-3p Knockdown on Allergic Airway Inflammation in Rat Precision-Cut Lung Slices
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, PCLS Generation, and Transfection
2.1.1. Rats
2.1.2. Rat Model of Allergic Inflammation
2.1.3. Histological Staining
2.1.4. PCLS Generation
2.1.5. PCLS Transfection
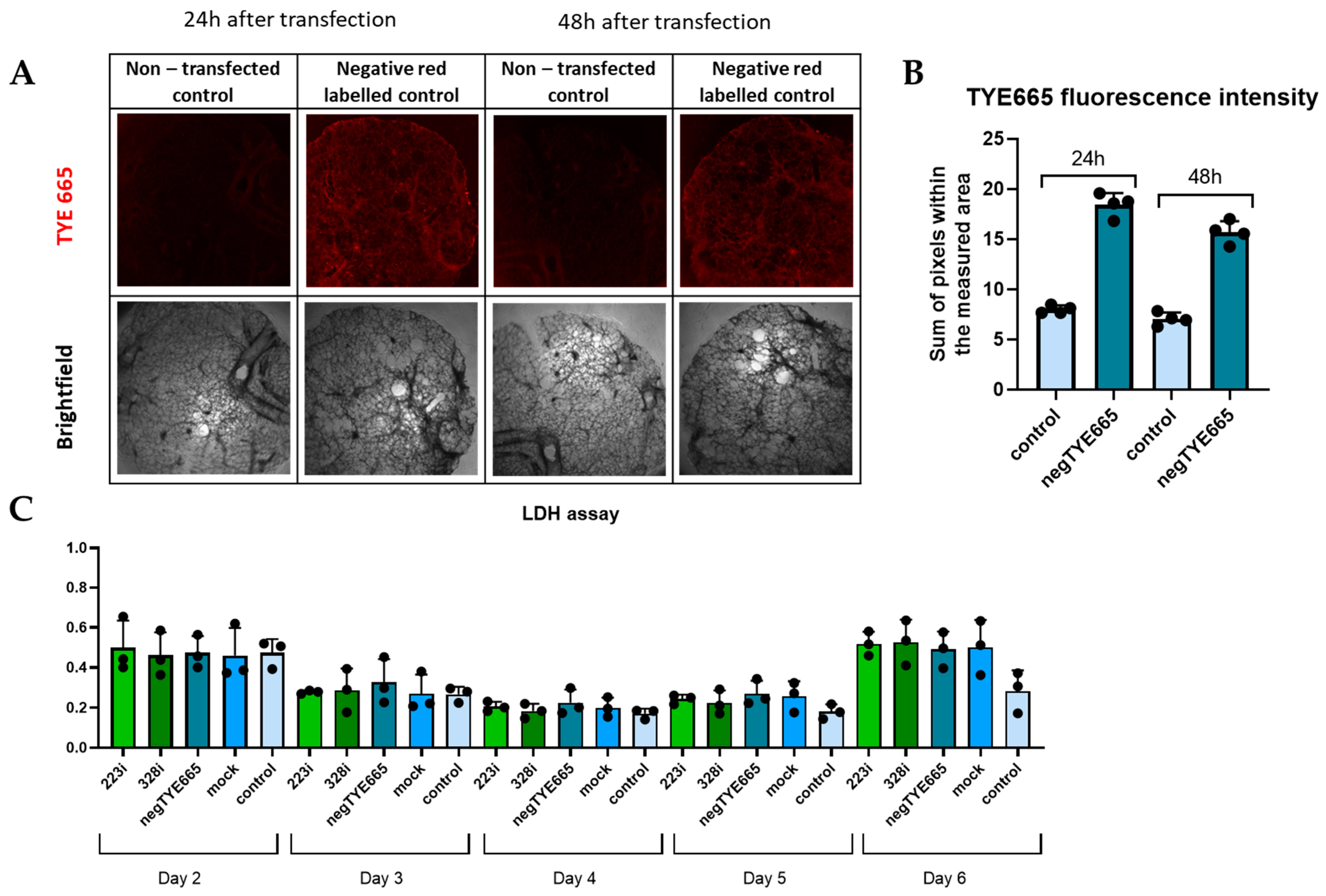
2.2. Lactate Dehydrogenase Assay (LDH)
2.3. Fluorescent Microscope Imaging
2.4. RNA Analysis
2.5. Protein Analysis
2.5.1. Protein Isolation and Western Blotting
2.5.2. Measurement of Proteins Secreted by PCLSs to the Culture Medium
2.6. Statistics
3. Results
3.1. Histological Confirmation of Allergic Inflammation in Rats
3.2. Analysis of Transfection Efficacy and Lack of Cytotoxicity
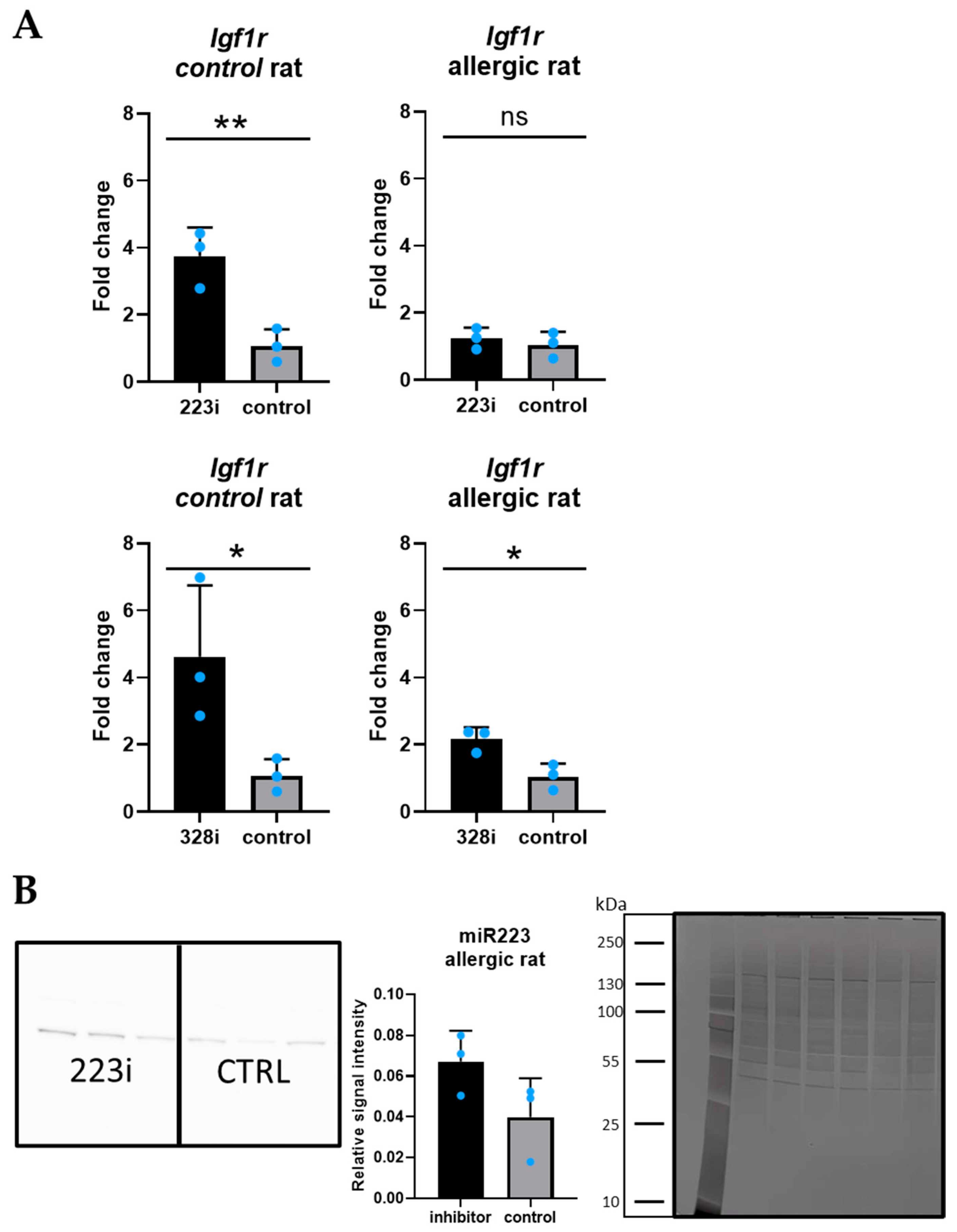
3.3. Igf1R: A Target Gene for Both miR-223-3p and miR-328a-3p
3.4. Analysis of Culture Medium—Panel of Cytokines Associated with Allergic Inflammation
3.5. The Effect of Silencing miR223-3p and miR328a-3p Expression on Genes Associated with Allergic Inflammation in PCLSs from the Rat with Allergic Inflammation and the Control Rat
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Lond. Engl. 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- The Global Asthma Report 2022. [Internet]. Available online: https://globalasthmareport.org/burden/deaths.php (accessed on 15 October 2024).
- Wagener, A.H.; Zwinderman, A.H.; Luiten, S.; Fokkens, W.J.; Bel, E.H.; Sterk, P.J.; van Drunen, C.M. The impact of allergic rhinitis and asthma on human nasal and bronchial epithelial gene expression. PLoS ONE 2013, 8, e80257. [Google Scholar] [CrossRef][Green Version]
- Kim, D.H.; Gu, A.; Lee, J.-S.; Yang, E.J.; Kashif, A.; Hong, M.H.; Kim, G.; Park, B.S.; Lee, S.J.; Kim, I.S. Suppressive effects of S100A8 and S100A9 on neutrophil apoptosis by cytokine release of human bronchial epithelial cells in asthma. Int. J. Med. Sci. 2020, 17, 498–509. [Google Scholar] [CrossRef]
- Bartel, S.; La Grutta, S.; Cilluffo, G.; Perconti, G.; Bongiovanni, A.; Giallongo, A.; Behrends, J.; Kruppa, J.; Hermann, S.; Chiang, D.; et al. Human airway epithelial extracellular vesicle miRNA signature is altered upon asthma development. Allergy 2020, 75, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Yagi, H.; Ota, M.; Tanaka, A.; Sato, K.; Inoue, T.; Yamada, S.; Arakawa, N.; Ishige, T.; Kobayashi, Y.; et al. Oxidative stress induces MUC5AC expression through mitochondrial damage-dependent STING signaling in human bronchial epithelial cells. FASEB BioAdv. 2023, 5, 171–181. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, J.; Sun, H.; Wei, Q.; Nong, G. miR-29a-3p regulates the epithelial-mesenchymal transition via the SPARC/ERK signaling pathway in human bronchial epithelial cells. Int. J. Mol. Med. 2021, 48, 171. [Google Scholar] [CrossRef]
- Jia, M.; Fu, H.; Jiang, X.; Wang, L.; Xu, J.; Barnes, P.J.; Adcock, I.M.; Liu, Y.; He, S.; Zhang, F.; et al. DEL-1, as an anti-neutrophil transepithelial migration molecule, inhibits airway neutrophilic inflammation in asthma. Allergy 2024, 79, 1180–1194. [Google Scholar] [CrossRef]
- Viana, F.; O’Kane, C.M.; Schroeder, G.N. Precision-cut lung slices: A powerful ex vivo model to investigate respiratory infectious diseases. Mol. Microbiol. 2022, 117, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Alsafadi, H.N.; Uhl, F.E.; Pineda, R.H.; Bailey, K.E.; Rojas, M.; Wagner, D.E.; Königshoff, M. Applications and Approaches for Three-Dimensional Precision-Cut Lung Slices. Disease Modeling and Drug Discovery. Am. J. Respir. Cell Mol. Biol. 2020, 62, 681–691. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, A.A. Trials and Tribulations of MicroRNA Therapeutics. Int. J. Mol. Sci. 2024, 25, 1469. [Google Scholar] [CrossRef] [PubMed]
- RNA-Based Medicine: From Molecular Mechanisms to Therapy [Internet]. Available online: https://www.embopress.org/doi/epdf/10.15252/embj.2023114760 (accessed on 26 October 2024).
- Weidner, J.; Bartel, S.; Kılıç, A.; Zissler, U.M.; Renz, H.; Schwarze, J.; Schmidt-Weber, C.B.; Maes, T.; Rebane, A.; Krauss-Etschmann, S.; et al. Spotlight on microRNAs in allergy and asthma. Allergy 2021, 76, 1661–1678. [Google Scholar] [CrossRef] [PubMed]
- Langwiński, W.; Szczepankiewicz, D.; Narożna, B.; Stegmayr, J.; Wagner, D.; Alsafadi, H.; Lindstedt, S.; Stachowiak, Z.; Nowakowska, J.; Skrzypski, M.; et al. Allergic inflammation in lungs and nasal epithelium of rat model is regulated by tissue-specific miRNA expression. Mol. Immunol. 2022, 147, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Roffel, M.P.; Bracke, K.R.; Heijink, I.H.; Maes, T. miR-223: A Key Regulator in the Innate Immune Response in Asthma and COPD. Front. Med. 2020, 7, 196. [Google Scholar] [CrossRef]
- Shi, M.; Lu, Q.; Zhao, Y.; Ding, Z.; Yu, S.; Li, J.; Ji, M.; Fan, H.; Hou, S. miR-223: A key regulator of pulmonary inflammation. Front. Med. 2023, 10, 1187557. Available online: https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2023.1187557/full (accessed on 26 October 2024). [CrossRef]
- Xu, W.; Wang, Y.; Ma, Y.; Yang, J. MiR-223 plays a protecting role in neutrophilic asthmatic mice through the inhibition of NLRP3 inflammasome. Respir. Res. 2020, 21, 116. [Google Scholar] [CrossRef] [PubMed]
- Narożna, B.; Langwinski, W.; Jackson, C.; Lackie, P.; Holloway, J.W.; Szczepankiewicz, A. MicroRNA-328 is involved in wound repair process in human bronchial epithelial cells. Respir. Physiol. Neurobiol. 2017, 242, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Wang, A.L.; Li, J.; Lutz, S.M.; Kho, A.T.; Weiss, S.T.; Tantisira, K.G.; McGeachie, M.J. Seasonal Variation in miR-328-3p and let-7d-3p Are Associated with Seasonal Allergies and Asthma Symptoms in Children. Allergy Asthma Immunol. Res. 2021, 13, 576. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Tiwari, A.; McGeachie, M.J. Recent miRNA Research in Asthma. Curr. Allergy Asthma Rep. 2022, 22, 231–258. [Google Scholar] [CrossRef]
- Gao, J.; Wu, X.-L. MiR-328-3p promotes TGF-β1-induced proliferation, migration, and inflammation of airway smooth muscle cells by regulating the PTEN/Akt pathway. Allergol. Immunopathol. 2023, 51, 151–159. [Google Scholar] [CrossRef]
- Nowakowska, J.; Gvazava, N.; Langwinski, W.; Ziarniak, K.; Silva, I.A.N.; Stegmayr, J.; Wagner, D.E.; Szczepankiewicz, A. Optimizing miRNA transfection for screening in precision cut lung slices. Am. J. Physiol. Lung Cell. Mol. Physiol. 2024, 327, L712–L723. [Google Scholar] [CrossRef]
- Langwiński, W.; Nowakowska, J.; Sakrajda, K.; Ziarniak, K.; Stachowiak, Z.; Kachel, M.; Narożna, B.; Szczepankiewicz, A. An optimized QIAzol-based protocol for simultaneous miRNA, RNA, and protein isolation from precision-cut lung slices (PCLS). Respir. Res. 2024, 25, 422. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Lin, Y.-C.-D.; Cui, S.; Huang, Y.; Tang, Y.; Xu, J.; Bao, J.; Li, Y.; Wen, J.; Zuo, H.; et al. miRTarBase update 2022: An informative resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2022, 50, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.-J.; Zhang, X.-Q.; Li, J.-T.; Dai, X.-M.; Zhao, F. miRNA-223 regulates ischemic neuronal injury by targeting the type 1 insulin-like growth factor receptor (IGF1R). Folia Neuropathol. 2018, 56, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Qiu, Z.; Wei, L.; Yu, X.; Gao, X.; Jiang, S.; Tian, H.; Jiang, C.; Zhu, D. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-α1C. Hypertens. Dallas Tex 1979 2012, 59, 1006–1013. [Google Scholar] [CrossRef]
- Danov, O.; Lasswitz, L.; Obernolte, H.; Hesse, C.; Braun, A.; Wronski, S.; Sewald, K. Rupintrivir reduces RV-induced TH-2 cytokine IL-4 in precision-cut lung slices (PCLS) of HDM-sensitized mice ex vivo. Respir. Res. 2019, 20, 228. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, R.K.; Geillinger-Kästle, K.E.; Fuentes-Mateos, R.; van Orsoy, R.; Al-Alyan, N.; Burgess, J.K.; Gosens, R. The disruptive effects of COPD exacerbation-associated factors on epithelial repair responses. Front. Immunol. 2024, 15, 1346491. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Simon, D.; Ait Yahia, S.; Audousset, C.; Fanton d’Andon, M.; Chamaillard, M.; Gomperts Boneca, I.; Tsicopoulos, A. Local receptor-interacting protein kinase 2 inhibition mitigates house dust mite-induced asthma. Eur. Respir. J. 2024, 64, 2302288. [Google Scholar] [CrossRef]
- Stephenson, K.E.; Porte, J.; Kelly, A.; Wallace, W.A.; Huntington, C.E.; Overed-Sayer, C.L.; Cohen, E.S.; Jenkins, R.G.; John, A.E. The IL-33:ST2 axis is unlikely to play a central fibrogenic role in idiopathic pulmonary fibrosis. Respir. Res. 2023, 24, 89. [Google Scholar] [CrossRef]


| Rat Protein Name | Mean Protein Level ± SD (Rat with Allergic Inflammation) | Mean Protein Level ± SD (Control Rat) |
|---|---|---|
| CXCL2 | 1.95 ± 0.58 | 1.76 ± 0.73 |
| ICAM-1 | 5.91 ± 1.22 | 5.80 ± 2.34 |
| IFNγ | 0.96 ± 1.82 | 0.32 ± 0.95 |
| IL-10 | 0.05 ± 0.06 | 0.04 ± 0.07 |
| IL-13 | 0.04 ± 0.01 | 0.04 ± 0.02 |
| IL-18 | 0.17 ± 0.03 | 0.22 ± 0.07 |
| IL-2 | 0.08 ± 0.04 | 0.07 ± 0.05 |
| IL-6 | 8.49 ± 3.45 | 7.62 ± 4.08 |
| VEGF | 0.78 ± 0.45 | 0.72 ± 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowakowska, J.; Kachel, M.; Langwiński, W.; Ziarniak, K.; Szczepankiewicz, A. Effect of miR-223-3p and miR-328a-3p Knockdown on Allergic Airway Inflammation in Rat Precision-Cut Lung Slices. Cells 2025, 14, 104. https://doi.org/10.3390/cells14020104
Nowakowska J, Kachel M, Langwiński W, Ziarniak K, Szczepankiewicz A. Effect of miR-223-3p and miR-328a-3p Knockdown on Allergic Airway Inflammation in Rat Precision-Cut Lung Slices. Cells. 2025; 14(2):104. https://doi.org/10.3390/cells14020104
Chicago/Turabian StyleNowakowska, Joanna, Maria Kachel, Wojciech Langwiński, Kamil Ziarniak, and Aleksandra Szczepankiewicz. 2025. "Effect of miR-223-3p and miR-328a-3p Knockdown on Allergic Airway Inflammation in Rat Precision-Cut Lung Slices" Cells 14, no. 2: 104. https://doi.org/10.3390/cells14020104
APA StyleNowakowska, J., Kachel, M., Langwiński, W., Ziarniak, K., & Szczepankiewicz, A. (2025). Effect of miR-223-3p and miR-328a-3p Knockdown on Allergic Airway Inflammation in Rat Precision-Cut Lung Slices. Cells, 14(2), 104. https://doi.org/10.3390/cells14020104







