A Novel, Cell-Compatible Hyaluronidase Activity Assay Identifies Dextran Sulfates and Other Sulfated Polymeric Hydrocarbons as Potent Inhibitors for CEMIP
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Plasmids and Transfection
2.3. Chemicals
2.4. Sulfated Hyaluronan
2.5. CEMIP Hyaluronidase Activity Assay
2.6. Analysis of HA Degradation Products by Agarose Gel Electrophoresis
2.7. Analysis of CEMIP HA Degradation Products by Ultrafiltration and HA-ELISA
2.8. Western Blot
2.9. CPC Pulldown
2.10. Proliferation Assays
2.11. Statistical Analysis
3. Results
3.1. Establishment of a Medium-Throughput Assay for CEMIP-Mediated HA Degradation
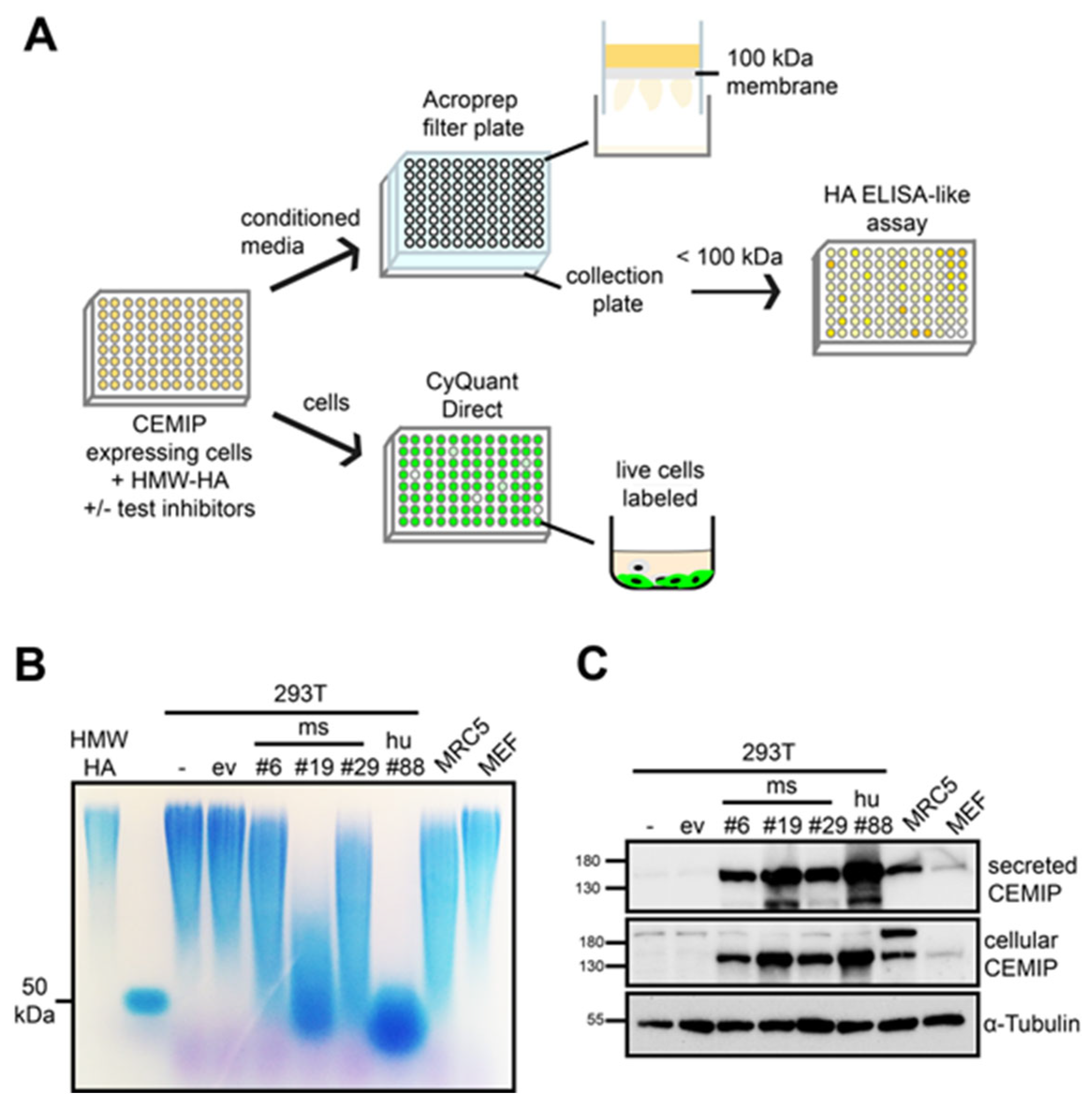
3.2. The Medium-Throughput Assay for CEMIP-Mediated HA Degradation Gives Equivalent Results to Those Obtained with Agarose Gel Electrophoresis
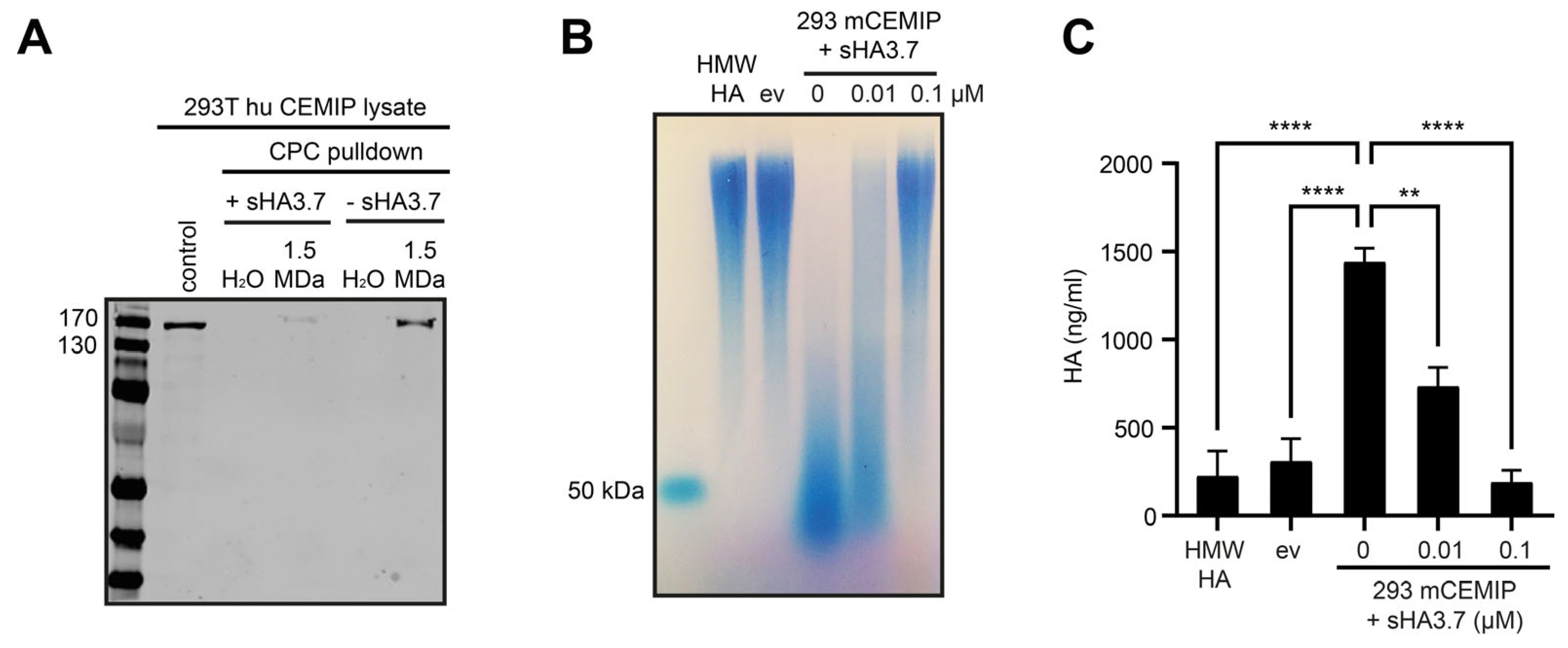
3.3. Characterization of Parameters Required for Inhibition of CEMIP-Mediated HA Degradation by Sulfated HA
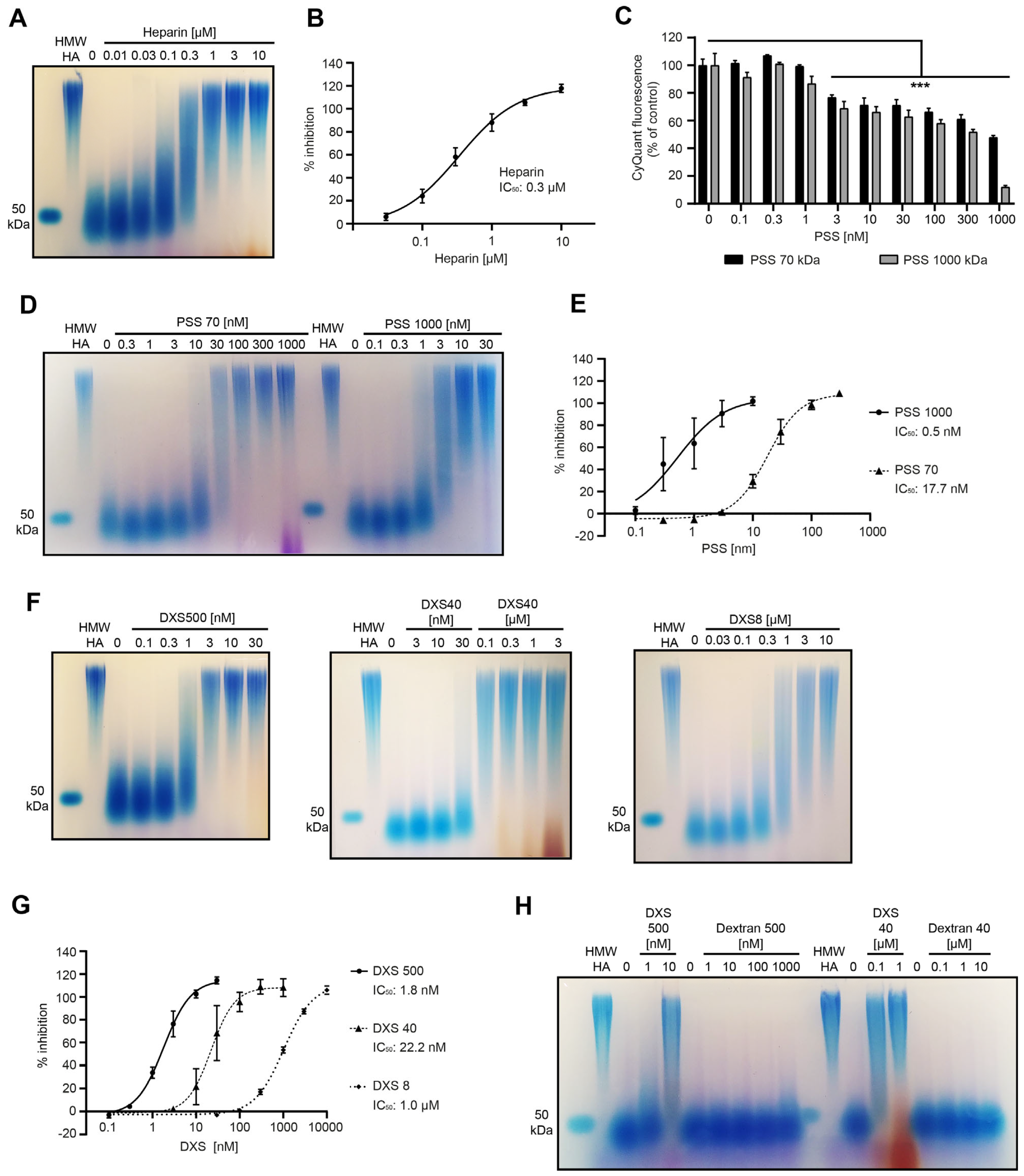
3.4. Screening of a Panel of Hyaluronidase Inhibitors Identifies Dextran Sulfate as a Highly Potent Inhibitor of CEMIP-Mediated HA Degradation
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Spataro, S.; Guerra, C.; Cavalli, A.; Sgrignani, J.; Sleeman, J.; Poulain, L.; Boland, A.; Scapozza, L.; Moll, S.; Prunotto, M. CEMIP (HYBID, KIAA1199): Structure, Function and Expression in Health and Disease. FEBS J. 2022, 290, 3946–3962. [Google Scholar] [CrossRef] [PubMed]
- Domanegg, K.; Sleeman, J.P.; Schmaus, A. CEMIP, a Promising Biomarker That Promotes the Progression and Metastasis of Colorectal and Other Types of Cancer. Cancers 2022, 14, 5093. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Nagaoka, A.; Kusaka-Kikushima, A.; Tobiishi, M.; Kawabata, K.; Sayo, T.; Sakai, S.; Sugiyama, Y.; Enomoto, H.; Okada, Y.; et al. KIAA1199, a Deafness Gene of Unknown Function, Is a New Hyaluronan Binding Protein Involved in Hyaluronan Depolymerization. Proc. Natl. Acad. Sci. USA 2013, 110, 5612–5617. [Google Scholar] [CrossRef] [PubMed]
- Schmaus, A.; Rothley, M.; Schreiber, C.; Möller, S.; Roßwag, S.; Franz, S.; Garvalov, B.K.; Thiele, W.; Spataro, S.; Herskind, C.; et al. Sulfated Hyaluronic Acid Inhibits the Hyaluronidase CEMIP and Regulates the HA Metabolism, Proliferation and Differentiation of Fibroblasts. Matrix Biol. 2022, 109, 173–191. [Google Scholar] [CrossRef]
- Nagaoka, A.; Yoshida, H.; Nakamura, S.; Morikawa, T.; Kawabata, K.; Kobayashi, M.; Sakai, S.; Takahashi, Y.; Okada, Y.; Inoue, S. Regulation of Hyaluronan (HA) Metabolism Mediated by HYBID (Hyaluronan-Binding Protein Involved in HA Depolymerization, KIAA1199) and HA Synthases in Growth Factor-Stimulated Fibroblasts. J. Biol. Chem. 2015, 290, 30910–30923. [Google Scholar] [CrossRef]
- Shiozawa, J.; de Vega, S.; Cilek, M.Z.; Yoshinaga, C.; Nakamura, T.; Kasamatsu, S.; Yoshida, H.; Kaneko, H.; Ishijima, M.; Kaneko, K.; et al. Implication of HYBID (Hyaluronan-Binding Protein Involved in Hyaluronan Depolymerization) in Hyaluronan Degradation by Synovial Fibroblasts in Patients with Knee Osteoarthritis. Am. J. Pathol. 2020, 190, 1046–1058. [Google Scholar] [CrossRef]
- Shiozawa, J.; de Vega, S.; Yoshinaga, C.; Ji, X.; Negishi, Y.; Momoeda, M.; Nakamura, T.; Yoshida, H.; Kaneko, H.; Ishijima, M.; et al. Expression and Regulation of Recently Discovered Hyaluronidases, HYBID and TMEM2, in Chondrocytes from Knee Osteoarthritic Cartilage. Sci. Rep. 2022, 12, 17242. [Google Scholar] [CrossRef]
- Soroosh, A.; Albeiroti, S.; West, G.A.; Willard, B.; Fiocchi, C.; de la Motte, C.A. Crohn’s Disease Fibroblasts Overproduce the Novel Protein KIAA1199 to Create Proinflammatory Hyaluronan Fragments. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 358–368.e4. [Google Scholar] [CrossRef]
- Abe, S.; Usami, S.; Nakamura, Y. Mutations in the Gene Encoding KIAA1199 Protein, an Inner-Ear Protein Expressed in Deiters’ Cells and the Fibrocytes, as the Cause of Nonsyndromic Hearing Loss. J. Hum. Genet. 2003, 48, 564–570. [Google Scholar] [CrossRef]
- Marella, M.; Jadin, L.; Keller, G.A.; Sugarman, B.J.; Frost, G.I.; Shepard, H.M. KIAA1199 Expression and Hyaluronan Degradation Colocalize in Multiple Sclerosis Lesions. Glycobiology 2018, 28, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yin, G.; Zhao, H.; Ling, H.; Xie, Z.; Xiao, C.; Chen, Y.; Lin, Y.; Jiang, T.; Jin, S.; et al. Secreted KIAA1199 Promotes the Progression of Rheumatoid Arthritis by Mediating Hyaluronic Acid Degradation in an ANXA1-Dependent Manner. Cell Death Dis. 2021, 12, 102. [Google Scholar] [CrossRef]
- Deroyer, C.; Poulet, C.; Paulissen, G.; Ciregia, F.; Malaise, O.; Plener, Z.; Cobraiville, G.; Daniel, C.; Gillet, P.; Malaise, M.G.; et al. CEMIP (KIAA1199) Regulates Inflammation, Hyperplasia and Fibrosis in Osteoarthritis Synovial Membrane. Cell. Mol. Life Sci. 2022, 79, 260. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Nagaoka, A.; Nakamura, S.; Sugiyama, Y.; Okada, Y.; Inoue, S. Murine Homologue of the Human KIAA1199 Is Implicated in Hyaluronan Binding and Depolymerization. FEBS Open Bio 2013, 3, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Nagaoka, A.; Nakamura, S.; Tobiishi, M.; Sugiyama, Y.; Inoue, S. N-Terminal Signal Sequence Is Required for Cellular Trafficking and Hyaluronan-Depolymerization of KIAA1199. FEBS Lett. 2014, 588, 111–116. [Google Scholar] [CrossRef]
- Yoshida, H.; Aoki, M.; Komiya, A.; Endo, Y.; Kawabata, K.; Nakamura, T.; Sakai, S.; Sayo, T.; Okada, Y.; Takahashi, Y. HYBID (Alias KIAA1199/CEMIP) and Hyaluronan Synthase Coordinately Regulate Hyaluronan Metabolism in Histamine-Stimulated Skin Fibroblasts. J. Biol. Chem. 2020, 295, 2483–2494. [Google Scholar] [CrossRef]
- Su, W.; Matsumoto, S.; Banine, F.; Srivastava, T.; Dean, J.; Foster, S.; Pham, P.; Hammond, B.; Peters, A.; Girish, K.S.; et al. A Modified Flavonoid Accelerates Oligodendrocyte Maturation and Functional Remyelination. Glia 2020, 68, 263–279. [Google Scholar] [CrossRef]
- Narita, T.; Tobisawa, Y.; Bobkov, A.; Jackson, M.; Ohyama, C.; Irie, F.; Yamaguchi, Y. TMEM2 Is a Bona Fide Hyaluronidase Possessing Intrinsic Catalytic Activity. J. Biol. Chem. 2023, 299, 105120. [Google Scholar] [CrossRef]
- Schreiber, C.; Saraswati, S.; Harkins, S.; Gruber, A.; Cremers, N.; Thiele, W.; Rothley, M.; Plaumann, D.; Korn, C.; Armant, O.; et al. Loss of ASAP1 in Mice Impairs Adipogenic and Osteogenic Differentiation of Mesenchymal Progenitor Cells through Dysregulation of FAK/Src and AKT Signaling. PLoS Genet. 2019, 15, 1008216. [Google Scholar] [CrossRef]
- van der Smissen, A.; Hintze, V.; Scharnweber, D.; Moeller, S.; Schnabelrauch, M.; Majok, A.; Simon, J.C.; Anderegg, U. Growth Promoting Substrates for Human Dermal Fibroblasts Provided by Artificial Extracellular Matrices Composed of Collagen I and Sulfated Glycosaminoglycans. Biomaterials 2011, 32, 8938–8946. [Google Scholar] [CrossRef]
- Hintze, V.; Moeller, S.; Schnabelrauch, M.; Bierbaum, S.; Viola, M.; Worch, H.; Scharnweber, D. Modifications of Hyaluronan Influence the Interaction with Human Bone Morphogenetic Protein-4 (hBMP-4). Biomacromolecules 2009, 10, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Cowman, M.K. An Agarose Gel Electrophoretic Method for Analysis of Hyaluronan Molecular Weight Distribution. Anal. Biochem. 1994, 219, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Wisniewski, H.G.; Vilcek, J. A Novel Secretory Tumor Necrosis Factor-Inducible Protein (TSG-6) Is a Member of the Family of Hyaluronate Binding Proteins, Closely Related to the Adhesion Receptor CD44. J. Cell Biol. 1992, 116, 545–557. [Google Scholar] [CrossRef]
- Sleeman, J.P.; Kondo, K.; Moll, J.; Ponta, H.; Herrlich, P. Variant Exons v6 and v7 Together Expand the Repertoire of Glycosaminoglycans Bound by CD44. J. Biol. Chem. 1997, 272, 31837–31844. [Google Scholar] [CrossRef]
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three Isoforms of Mammalian Hyaluronan Synthases Have Distinct Enzymatic Properties. J. Biol. Chem. 1999, 274, 25085–25092. [Google Scholar] [CrossRef]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its Nature, Distribution, Functions and Turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Schmaus, A.; Klusmeier, S.; Rothley, M.; Dimmler, A.; Sipos, B.; Faller, G.; Thiele, W.; Allgayer, H.; Hohenberger, P.; Post, S.; et al. Accumulation of Small Hyaluronan Oligosaccharides in Tumour Interstitial Fluid Correlates with Lymphatic Invasion and Lymph Node Metastasis. Br. J. Cancer 2014, 111, 559–567. [Google Scholar] [CrossRef]
- Hofinger, E.S.A.; Bernhardt, G.; Buschauer, A. Kinetics of Hyal-1 and PH-20 Hyaluronidases: Comparison of Minimal Substrates and Analysis of the Transglycosylation Reaction. Glycobiology 2007, 17, 963–971. [Google Scholar] [CrossRef]
- Isoyama, T.; Thwaites, D.; Selzer, M.G.; Carey, R.I.; Barbucci, R.; Lokeshwar, V.B. Differential Selectivity of Hyaluronidase Inhibitors toward Acidic and Basic Hyaluronidases. Glycobiology 2006, 16, 11–21. [Google Scholar] [CrossRef]
- Udabage, L.; Brownlee, G.R.; Stern, R.; Brown, T.J. Inhibition of Hyaluronan Degradation by Dextran Sulphate Facilitates Characterisation of Hyaluronan Synthesis: An in Vitro and in Vivo Study. Glycoconj. J. 2004, 20, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, K.; Suresh, P.; Santhosh, M.S.; Hemshekhar, M.; Thushara, R.M.; Marathe, G.K.; Thirunavukkarasu, C.; Kemparaju, K.; Kumar, M.S.; Girish, K.S. Inhibition of Hyaluronidase by N-Acetyl Cysteine and Glutathione: Role of Thiol Group in Hyaluronan Protection. Int. J. Biol. Macromol. 2013, 55, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Orlando, Z.; Lengers, I.; Melzig, M.F.; Buschauer, A.; Hensel, A.; Jose, J. Autodisplay of Human Hyaluronidase Hyal-1 on Escherichia Coli and Identification of Plant-Derived Enzyme Inhibitors. Molecules 2015, 20, 15449–15468. [Google Scholar] [CrossRef]
- Botzki, A.; Rigden, D.J.; Braun, S.; Nukui, M.; Salmen, S.; Hoechstetter, J.; Bernhardt, G.; Dove, S.; Jedrzejas, M.J.; Buschauer, A. L-Ascorbic Acid 6-Hexadecanoate, a Potent Hyaluronidase Inhibitor. X-Ray Structure and Molecular Modeling of Enzyme-Inhibitor Complexes. J. Biol. Chem. 2004, 279, 45990–45997. [Google Scholar] [CrossRef]
- Olgen, S.; Kaessler, A.; Nebioğlu, D.; Jose, J. New Potent Indole Derivatives as Hyaluronidase Inhibitors. Chem. Biol. Drug Des. 2007, 70, 547–551. [Google Scholar] [CrossRef]
- Koike, H.; Nishida, Y.; Shinomura, T.; Ohkawara, B.; Ohno, K.; Zhuo, L.; Kimata, K.; Ushida, T.; Imagama, S. Possible Repositioning of an Oral Anti-Osteoporotic Drug, Ipriflavone, for Treatment of Inflammatory Arthritis via Inhibitory Activity of KIAA1199, a Novel Potent Hyaluronidase. Int. J. Mol. Sci. 2022, 23, 4089. [Google Scholar] [CrossRef]
- Schmaus, A.; Sleeman, J.P. Hyaluronidase-1 Expression Promotes Lung Metastasis in Syngeneic Mouse Tumor Models without Affecting Accumulation of Small Hyaluronan Oligosaccharides in Tumor Interstitial Fluid. Glycobiology 2015, 25, 258–268. [Google Scholar] [CrossRef]
- Nagaraju, S.; Girish, K.S.; Pan, Y.; Easely, K.A.; Kemparaju, K. Estimation of Serum Hyaluronidase Activity Overcoming the Turbidity Interference. Clin. Lab. Sci. 2011, 24, 172–177. [Google Scholar] [CrossRef]
- Reissig, J.L.; Storminger, J.L.; Leloir, L.F. A Modified Colorimetric Method for the Estimation of N-Acetylamino Sugars. J. Biol. Chem. 1955, 217, 959–966. [Google Scholar] [CrossRef]
- Frost, G.I.; Stern, R. A Microtiter-Based Assay for Hyaluronidase Activity Not Requiring Specialized Reagents. Anal. Biochem. 1997, 251, 263–269. [Google Scholar] [CrossRef]
- Yang, W.; Ni, J.; Luo, F.; Weng, W.; Wei, Q.; Lin, Z.; Chen, G. Cationic Carbon Dots for Modification-Free Detection of Hyaluronidase via an Electrostatic-Controlled Ratiometric Fluorescence Assay. Anal. Chem. 2017, 89, 8384–8390. [Google Scholar] [CrossRef] [PubMed]
- Al’Qteishat, A.; Gaffney, J.; Krupinski, J.; Rubio, F.; West, D.; Kumar, S.; Kumar, P.; Mitsios, N.; Slevin, M. Changes in Hyaluronan Production and Metabolism Following Ischaemic Stroke in Man. Brain 2006, 129, 2158–2176. [Google Scholar] [CrossRef] [PubMed]
- Declèves, A.-E.; Caron, N.; Voisin, V.; Legrand, A.; Bouby, N.; Kultti, A.; Tammi, M.I.; Flamion, B. Synthesis and Fragmentation of Hyaluronan in Renal Ischaemia. Nephrol. Dial. Transplant. 2012, 27, 3771–3781. [Google Scholar] [CrossRef] [PubMed]
- Haserodt, S.; Aytekin, M.; Dweik, R.A. A Comparison of the Sensitivity, Specificity, and Molecular Weight Accuracy of Three Different Commercially Available Hyaluronan ELISA-like Assays. Glycobiology 2011, 21, 175–183. [Google Scholar] [CrossRef]
- Li, H.; Witkos, T.M.; Umlauf, S.; Thompson, C. Potency Assay Variability Estimation in Practice. Pharm. Stat. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Kohi, S.; Sato, N.; Koga, A.; Matayoshi, N.; Hirata, K. KIAA1199 Is Induced by Inflammation and Enhances Malignant Phenotype in Pancreatic Cancer. Oncotarget 2017, 8, 17156–17163. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular Size-Dependent Signaling and Biological Functions in Inflammation and Cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Schmaus, A.; Bauer, J.; Sleeman, J.P. Sugars in the Microenvironment: The Sticky Problem of HA Turnover in Tumors. Cancer Metastasis Rev. 2014, 33, 1059–1079. [Google Scholar] [CrossRef]
- Ke, C.; Wang, D.; Sun, Y.; Qiao, D.; Ye, H.; Zeng, X. Immunostimulatory and Antiangiogenic Activities of Low Molecular Weight Hyaluronic Acid. Food Chem. Toxicol. 2013, 58, 401–407. [Google Scholar] [CrossRef]
- Kuang, D.-M.; Wu, Y.; Chen, N.; Cheng, J.; Zhuang, S.-M.; Zheng, L. Tumor-Derived Hyaluronan Induces Formation of Immunosuppressive Macrophages through Transient Early Activation of Monocytes. Blood 2007, 110, 587–595. [Google Scholar] [CrossRef]
- Scheibner, K.A.; Lutz, M.A.; Boodoo, S.; Fenton, M.J.; Powell, J.D.; Horton, M.R. Hyaluronan Fragments Act as an Endogenous Danger Signal by Engaging TLR2. J. Immunol. 2006, 177, 1272–1281. [Google Scholar] [CrossRef]
- Zhang, B.; Du, Y.; He, Y.; Liu, Y.; Zhang, G.; Yang, C.; Gao, F. INT-HA Induces M2-like Macrophage Differentiation of Human Monocytes via TLR4-miR-935 Pathway. Cancer Immunol. Immunother. 2019, 68, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-F.; Qiao, S.-P.; Shi, S.-L.; Yao, L.-F.; Hou, X.-L.; Li, C.-F.; Lin, F.-H.; Guo, K.; Acharya, A.; Chen, X.-B.; et al. Modulating Three-Dimensional Microenvironment with Hyaluronan of Different Molecular Weights Alters Breast Cancer Cell Invasion Behavior. ACS Appl. Mater. Interfaces 2017, 9, 9327–9338. [Google Scholar] [CrossRef] [PubMed]
- Tolg, C.; Telmer, P.; Turley, E. Specific Sizes of Hyaluronan Oligosaccharides Stimulate Fibroblast Migration and Excisional Wound Repair. PLoS ONE 2014, 9, e88479. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, K.; Deng-Pichon, U.; Warnet, J.-M.; Rat, P. Hyaluronan Fragments Improve Wound Healing on in Vitro Cutaneous Model through P2X7 Purinoreceptor Basal Activation: Role of Molecular Weight. PLoS ONE 2012, 7, e48351. [Google Scholar] [CrossRef]
- Damodarasamy, M.; Johnson, R.S.; Bentov, I.; MacCoss, M.J.; Vernon, R.B.; Reed, M.J. Hyaluronan Enhances Wound Repair and Increases Collagen III in Aged Dermal Wounds. Wound Repair Regen. 2014, 22, 521–526. [Google Scholar] [CrossRef]
- Engelberg, H. Plasma Heparin Levels in Normal Man. Circulation 1961, 23, 578–581. [Google Scholar] [CrossRef]
- Mielke, C.H.; Starr, C.M.; Klock, J.C.; Devereaux, D.; Mielke, M.R.; Baker, D.E.; Broemeling, L.; Wacksman, M.; White, J.R.; Oliver, S.A.; et al. Direct Measurement of Unfractionated Heparin Using a Biochemical Assay. Clin Appl Thromb Hemost 1999, 5, 267–276. [Google Scholar] [CrossRef]
- Herrera-Heredia, S.A.; Hsu, H.-P.; Kao, C.-Y.; Tsai, Y.-H.; Yamaguchi, Y.; Roers, A.; Hsu, C.-L.; Dzhagalov, I.L. Heparin Is Required for the Formation of Granules in Connective Tissue Mast Cells. Front. Immunol. 2022, 13, 1000405. [Google Scholar] [CrossRef]
- Takano, H.; Furuta, K.; Yamashita, K.; Sakanaka, M.; Itano, N.; Gohda, E.; Nakayama, K.; Kimata, K.; Sugimoto, Y.; Ichikawa, A.; et al. Restriction of Mast Cell Proliferation through Hyaluronan Synthesis by Co-Cultured Fibroblasts. Biol. Pharm. Bull. 2012, 35, 408–412. [Google Scholar] [CrossRef]
- Nardo, A.D.; Chang, Y.-L.; Alimohammadi, S.; Masuda-Kuroki, K.; Wang, Z.; Sriram, K.; Insel, P.A. Mast Cell Tolerance in the Skin Microenvironment to Commensal Bacteria Is Controlled by Fibroblasts. Cell Rep. 2023, 42, 112453. [Google Scholar] [CrossRef]
- Dong, L.; Xu, W.; Deng, Y.; Tan, J.; Qin, W. Efficacy and Safety of Potassium Binders in the Treatment of Patients with Chronic Kidney Disease and Hyperkalemia. Eur. J. Pharmacol. 2022, 931, 175174. [Google Scholar] [CrossRef] [PubMed]
- Petrovici, A.R.; Pinteala, M.; Simionescu, N. Dextran Formulations as Effective Delivery Systems of Therapeutic Agents. Molecules 2023, 28, 1086. [Google Scholar] [CrossRef] [PubMed]
- Ramasundaram, S.; Saravanakumar, G.; Sobha, S.; Oh, T.H. Dextran Sulfate Nanocarriers: Design, Strategies and Biomedical Applications. Int. J. Mol. Sci. 2022, 24, 355. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, J.; Tang, X.; Huang, K.; Chen, L. Polyelectrolyte Three Layer Nanoparticles of Chitosan/Dextran Sulfate/Chitosan for Dual Drug Delivery. Colloids Surf. B Biointerfaces 2020, 190, 110925. [Google Scholar] [CrossRef]
- Ammassam Veettil, R.; Marcano, D.C.; Yuan, X.; Zaheer, M.; Adumbumkulath, A.; Lee, R.; Isenhart, L.C.; Soriano, N.; Mhatre, K.; Joseph, R.; et al. Dextran Sulfate Polymer Wafer Promotes Corneal Wound Healing. Pharmaceutics 2021, 13, 1628. [Google Scholar] [CrossRef]
- Gupta, S.; Puttaiahgowda, Y.M.; Deiglmayr, L. Recent Advances in the Design and Immobilization of Heparin for Biomedical Application: A Review. Int. J. Biol. Macromol. 2024, 264, 130743. [Google Scholar] [CrossRef]
- Beurskens, D.M.H.; Huckriede, J.P.; Schrijver, R.; Hemker, H.C.; Reutelingsperger, C.P.; Nicolaes, G.A.F. The Anticoagulant and Nonanticoagulant Properties of Heparin. Thromb. Haemost. 2020, 120, 1371–1383. [Google Scholar] [CrossRef]
- de Raucourt, E.; Mauray, S.; Chaubet, F.; Maiga-Revel, O.; Jozefowicz, M.; Fischer, A.M. Anticoagulant Activity of Dextran Derivatives. J. Biomed. Mater. Res. 1998, 41, 49–57. [Google Scholar] [CrossRef]
- Miura, T.; Kawano, M.; Takahashi, K.; Yuasa, N.; Habu, M.; Kimura, F.; Imamura, T.; Nakayama, F. High-Sulfated Hyaluronic Acid Ameliorates Radiation-Induced Intestinal Damage Without Blood Anticoagulation. Adv. Radiat. Oncol. 2022, 7, 100900. [Google Scholar] [CrossRef]
- Möller, S.; Schmidtke, M.; Weiss, D.; Schiller, J.; Pawlik, K.; Wutzler, P.; Schnabelrauch, M. Synthesis and Antiherpetic Activity of Carboxymethylated and Sulfated Hyaluronan Derivatives. Carbohydr. Polym. 2012, 90, 608–615. [Google Scholar] [CrossRef]
- Hauck, S.; Zager, P.; Halfter, N.; Wandel, E.; Torregrossa, M.; Kakpenova, A.; Rother, S.; Ordieres, M.; Räthel, S.; Berg, A.; et al. Collagen/Hyaluronan Based Hydrogels Releasing Sulfated Hyaluronan Improve Dermal Wound Healing in Diabetic Mice via Reducing Inflammatory Macrophage Activity. Bioact. Mater. 2021, 6, 4342–4359. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan in Inflammatory Bowel Disease: Cross-Linking Inflammation and Coagulation. Matrix Biol. 2019, 78–79, 314–323. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmaus, A.; Spataro, S.; Sallmann, P.; Möller, S.; Scapozza, L.; Prunotto, M.; Sleeman, J.P. A Novel, Cell-Compatible Hyaluronidase Activity Assay Identifies Dextran Sulfates and Other Sulfated Polymeric Hydrocarbons as Potent Inhibitors for CEMIP. Cells 2025, 14, 101. https://doi.org/10.3390/cells14020101
Schmaus A, Spataro S, Sallmann P, Möller S, Scapozza L, Prunotto M, Sleeman JP. A Novel, Cell-Compatible Hyaluronidase Activity Assay Identifies Dextran Sulfates and Other Sulfated Polymeric Hydrocarbons as Potent Inhibitors for CEMIP. Cells. 2025; 14(2):101. https://doi.org/10.3390/cells14020101
Chicago/Turabian StyleSchmaus, Anja, Sofia Spataro, Paul Sallmann, Stephanie Möller, Leonardo Scapozza, Marco Prunotto, and Jonathan P. Sleeman. 2025. "A Novel, Cell-Compatible Hyaluronidase Activity Assay Identifies Dextran Sulfates and Other Sulfated Polymeric Hydrocarbons as Potent Inhibitors for CEMIP" Cells 14, no. 2: 101. https://doi.org/10.3390/cells14020101
APA StyleSchmaus, A., Spataro, S., Sallmann, P., Möller, S., Scapozza, L., Prunotto, M., & Sleeman, J. P. (2025). A Novel, Cell-Compatible Hyaluronidase Activity Assay Identifies Dextran Sulfates and Other Sulfated Polymeric Hydrocarbons as Potent Inhibitors for CEMIP. Cells, 14(2), 101. https://doi.org/10.3390/cells14020101






